Abstract
Kir1.1 and Kir4.1 channels may be involved in the maintenance of pH and K+ homeostasis in renal epithelial cells and CO2 chemoreception in brainstem neurons. To understand the molecular determinants for their characteristic differences, the structure-function relationship was studied using site-directed mutagenesis.
According to previous studies, Glu158 in Kir4.1 is likely to be the major rectification controller. This was confirmed in both Kir1.1 and Kir4.1. Mutation of Gly210, the second potential rectification controller, to glutamate did not show any additional effect on the inward rectification.
More interestingly, we found that Glu158 in Kir4.1 was also an important residue contributing to single channel conductance and pH sensitivity. The E158N Kir4.1 mutant had a unitary conductance of 35 pS and a midpoint pH for channel inhibition (pKa) value of 6.72, both of which were almost identical to those of the wild-type (WT) Kir1.1. Flickering channel activity was clearly seen in the E158N mutant at positive membrane potentials, which is typical in the WT Kir1.1 but absent in the WT Kir4.1.
Reverse mutation in Kir1.1 (N171E) reduced the unitary conductance to 27 pS (23 pS in WT Kir4.1). However, the pH sensitivity of this mutant did not show a marked difference from the WT Kir1.1. Therefore, it is possible that a residue(s) in addition to Asn171 is also involved. Thus we studied several other residues in both M2 and H5 regions. We found that joint mutations of Val140 and Asn171 to residues seen in Kir4.1 greatly reduced the pH sensitivity (pKa 6.08).
The V140T mutation in Kir1.1 led to a unitary conductance of ∼70 pS, and the G210E mutation in Kir4.1 caused a decrease in pH sensitivity of 0.4 pH units.
These results indicate that the pore-forming sequences are targets for modulations of multiple channel-biophysical properties and demonstrate a site contributing to rectification, unitary conductance and proton sensitivity in these Kir channels.
Inward rectifier K+ channels (Kir) are primary regulators of membrane potential and cellular excitability. A large number of Kir channels have been cloned and characterized, which can be divided into seven sub-families (Kir1-7). They are made of heterotetramers or homotetramers, giving rise to a large diversity of Kir channels. The biophysical properties of these channels such as rectification, single channel conductance, and sensitivities to membrane-bound and cytosolic factors such as G proteins, nucleotides, second messengers, protons, etc. (Nichols & Lopatin, 1997) vary in a subunit-dependent manner.
Kir1.1 (ROMK1) and Kir4.1 (BIR10) are two close relatives expressed in several tissues including the kidney and brainstem (Ho et al. 1993; Bredt et al. 1995). Previous studies have suggested that they may be involved in the maintenance of pH and K+ homeostasis in renal epithelial cells and CO2 chemoreception in brainstem neurons (Doi et al. 1996; Xu et al. 2000b). Although these channels share ∼75 % homology in their amino acid sequences, Kir1.1 differs from Kir4.1 in at least three biophysical characteristics: (1) the pHi sensitivity of Kir1.1 is much higher (pKa≈ 6.8) than that of Kir4.1 (pKa≈ 6.0) (Fakler et al. 1996; Xu et al. 2000b); (2) Kir1.1 has a weaker inward rectification than Kir4.1 (Ho et al. 1993; Bond et al. 1994); and (3) the single channel conductance is ∼37 pS in Kir1.1 and ∼22 pS in Kir4.1 (measured with 150 mm K+ on both sides of the membrane; see Repunte et al. 1999; Yang & Jiang, 1999). Whereas the stronger rectification in Kir4.1 seems to be related to an acidic residue in the second putative transmembrane domain or the M2 region (Fakler et al. 1994; Lu & MacKinnon, 1994; Stanfield et al. 1994; Wible et al. 1994; Taglialatela et al. 1995; Yang et al. 1995), a member of the Kir4 family (sWIRK) carrying this negative residue with a high sequence homology to Kir4.1 shows a weak inward rectification (Kubo et al. 1996). Thus the molecular mechanisms for all these differences remain to be understood.
Considering some of these measurements obtained in cell-free excised patches, intrinsic amino acid sequences and tertiary structures between these channels seem to underlie their distinct channel properties. To understand the structure-function relationship of these properties, we performed mutation analysis experiments on Kir1.1 and Kir4.1. Our results showed that a residue in the M2 region was a major determinant of all the differences (i.e. proton sensitivity, conductance and rectification), indicating that the channel pore region is also a target area in the modulation of multiple channel-biophysical properties.
METHODS
Construction of WT and mutant cDNAs
Kir1.1 (ROMK1, GenBank accession number X72341) and Kir4.1 (BIR10, GenBank accession number X83585) cDNAs were generously provided by Dr Steven Hebert and Dr John Adelman, respectively. These cDNAs were inserted into a eukaryotic expression vector, pcDNA3.1 (Invitrogen, Carlsbad, CA, USA). Site-specific mutations were made using a site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Amino acid residues studied are shown in Fig. 1. Double or triple mutations were constructed using the template carrying a single mutation. Correct mutations were confirmed with DNA sequencing.
Figure 1. Sequence comparison of pore-forming domains in Kir4.1, Kir1.1 and other close relatives in the Kir family.
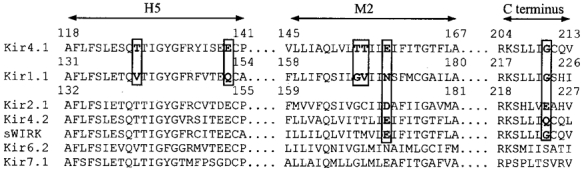
Amino acid sequences in the H5, M2 and part of the C-terminus are aligned using the BLAST two-sequence comparison (http://www.ncbi.nlm.nih.gov/BLAST/, National Center for Biotechnology Information, USA). Residues emboldened and boxed in Kir1.1 and Kir4.1 were examined in these studies.
Preparation and injection of Xenopus oocytes
All experimental procedures were subject to the Animal Welfare Assurance of Georgia State University (no. A97008). Frogs (Xenopus laevis) were anaesthetized by bathing them in 0.3 % 3-aminobenzoic acid ethyl ester. A few lobes of ovaries were removed after a small abdominal incision (∼5 mm) and the surgical incision was then closed. Frogs were humanely killed after the final collection. Oocytes were treated with 2 mg ml−1 of collagenase (Type I, Sigma Chemical Co.) in OR2 solution (82 mm NaCl, 2 mm KCl, 1 mm MgCl2 and 5 mm Hepes (pH 7.4)) for 90 min at room temperature (∼25°C). After three washes with OR2 solution, cDNA in the pcDNA3.1 vector (40–50 ng in 50 nl double distilled water) was injected into the oocytes. The oocytes were then incubated at 18°C in ND-96 solution containing (mm): NaCl 96, KCl 2, MgCl2 1, CaCl2 1.8, Hepes 5, sodium pyruvate 2.5; and 100 mg l−1 geneticin (pH 7.4).
CO2 exposure, intracellular acidification and intracellular pH measurements
Xenopus oocytes were placed in a semi-closed recording chamber (Medical System, Greenvale, NY, USA), where perfusion solution bathed both the top and bottom surface of the oocytes. The perfusate and the superfusion gas entered the chamber from the inlet at one end and flowed out at the other end. There was a 3 mm × 15 mm gap on the top cover of the chamber, which served as the gas outlet and the access to the oocytes for recording microelectrodes. The perfusate (KD 90) contained 90 mm KCl, 3 mm MgCl2 and 5 mm Hepes (pH 7.4). At baseline, the chamber was ventilated with atmospheric air. Exposure of the oocytes to CO2 was carried out by switching the superfusion air to a gas mixture containing CO2 (15 %) balanced with 21 % O2 and N2. The high solubility of CO2 resulted in a detectable change in intra- or extracellular acidification as fast as 10 s in these oocytes.
Intracellular pH (pHi) and extracellular pH (pHo) were measured using ion-selective microelectrodes. Details of procedures were described in our previous paper (Xu et al. 2000b). In brief, two single-barrelled microelectrodes were employed. The tip of the ion-selective microelectrode was filled with H+ liquid exchanger (Hydrogen Ion Ionophore I, Cocktail A, Fluka Chemie AG, Switzerland) and the remainder of the microelectrode was backfilled with phosphate buffer (pH 7.00). The other microelectrode was filled with 3 M KCl. Membrane potential was eliminated by subtracting records between these two channels. Serial calibrations of ion-selective microelectrodes were made with potassium phosphate buffer at pH 6.0, 7.0 and 8.0 (Fisher Scientific, Pittsburgh, PA, USA).
Electrophysiology
Whole-cell currents were studied on the oocytes 2–4 days after injection. Two-electrode voltage clamp was performed using an amplifier (Geneclamp 500, Axon Instruments) at ∼25°C. The extracellular solution contained: 90 mm KCl, 3 mm MgCl2 and 5 mm Hepes (pH 7.4). In some experiments, inward rectification was measured using low K+ extracellular solution (10 mm with 80 mm Na+). Cells were impaled using electrodes filled with 3 M KCl. One of the electrodes (1.0–2.0 MΩ) served for voltage measurements and the other (0.3–0.6 MΩ) was used for current recording. Current records were low-pass filtered (Bessel, 4-pole filter, 3 dB at 5 kHz), digitized at 5 kHz (12-bit resolution) and stored on computer disk for later analysis (pCLAMP 6, Axon Instruments) (Yang & Jiang, 1999; Zhu et al. 1999). Patch clamp experiments were performed at room temperature as described previously (Yang & Jiang, 1999; Zhu et al. 1999). In brief, fire-polished patch pipettes (0.5–2 MΩ) were made from 1.2 mm borosilicate capillary glass. The oocyte vitelline membranes were mechanically removed after exposure to hypertonic solution (400 mosmol l−1) for 5 min. The stripped oocytes were placed in a solution containing (mm): 40 KCl, 75 potassium gluconate, 5 potassium fluoride, 0.1 sodium vanadate, 10 potassium pyrophosphate, 1 EGTA, 0.2 adenosine diphosphate (ADP), 10 Pipes (pH 7.4), 10 glucose and 0.1 spermine; i.e. FVPP solution (fluoride, vanadate and pyrophosphate). Macroscopic currents were recorded from giant inside-out patches using the FVPP solution applied to the bath and recording pipettes. In a control experiment, we found that the macroscopic currents showed less than 10 % reduction over a 20 min period of recording in the FVPP solution. Current records were low-pass filtered (2000 Hz, Bessel, 4-pole filter, −3 dB), digitized (10 kHz, 12-bit resolution), and stored on computer disk for later analysis (pCLAMP 6, Axon Instruments). Single channel conductance was measured as a slope conductance with at least two voltage points. The channel open-state probability (Popen) was calculated as described previously (Yang & Jiang, 1999; Zhu et al. 1999). Since the pipette resistance is low, small junction potentials will produce detectable currents. Under our experimental conditions when the same solution was applied to both bath and pipette, there should be no electrical potential between the recording pipette and the grounding. Therefore, any electrical potentials recorded should be considered as junction potentials that were eliminated by adding a counterbalance voltage through the amplifier.
A parallel perfusion system was used to administer agents to patches at a rate of ∼1 ml min−1 with no dead space (Yang & Jiang, 1999; Zhu et al. 1999). Low pH exposures were carried out using FVPP solutions that had been titrated to various pH levels as required. Data are presented as means ± standard error of the mean (s.e.m.), and differences in means were tested with Student's t test or ANOVA and considered as significant if P≤ 0.05. Data were empirically fitted using the Hill equation. Statistical differences between fittings were not tested.
RESULTS
Inward rectification
Whole-cell currents were studied in Xenopus oocytes that had received a cDNA injection of Kir1.1, Kir4.1 or one of their mutants. In the two-electrode voltage clamp mode, inward rectifying currents as large as 10 μA were seen in most injected oocytes. These currents were sensitive to micromolar concentrations of Ba2+ and Cs+ (Xu et al. 2000b; Zhu et al. 2000), and showed evident rectification although the rectification was much weaker in Kir1.1 than in Kir4.1.
It is known that inward rectification in Kir channels is controlled by two acidic residues with one (Asp172 in Kir2.1) in the M2 region and another (Glu224 in Kir2.1) in the C-terminus (Fig. 1) (Fakler et al. 1994; Lu & MacKinnon, 1994; Stanfield et al. 1994; Wible et al. 1994; Taglialatela et al. 1995; Yang et al. 1995; Shyng et al. 1997). Consistent with previous studies (Fakler et al. 1994; Lu & MacKinnon, 1994), our data showed that the N171E Kir1.1 mutant became a strong rectifier. Likewise, the E158N Kir4.1 mutant exhibited a typical Kir1.1-like weak rectification. Interestingly, the inward rectification of the N171E mutant was stronger than that of the wild-type (WT) Kir4.1, indicating that there is an additional rectification controller(s) in Kir1.1. Several residues in the pore-forming sequences that differ between Kir1.1 and Kir4.1 were studied, i.e. V140, Q152, G167 and V168 in Kir1.1 (Fig. 1). Functional expression was only seen in the V140T Kir1.1 mutant, which did not show any effect on the rectification (not shown). Introduction of a negative residue to the second rectification-controlling site (G210E) did not enhance the inward rectification of Kir4.1 with high (90 mm, Fig. 2A–C) or low (10 mm, Fig. 2D–F) K+ in the bath solution, suggesting a less important role of this site (Gly210) in Kir4.1 than in Kir2 channels.
Figure 2. Lack of effect of the G210E mutation on inward rectification in Kir4.1.
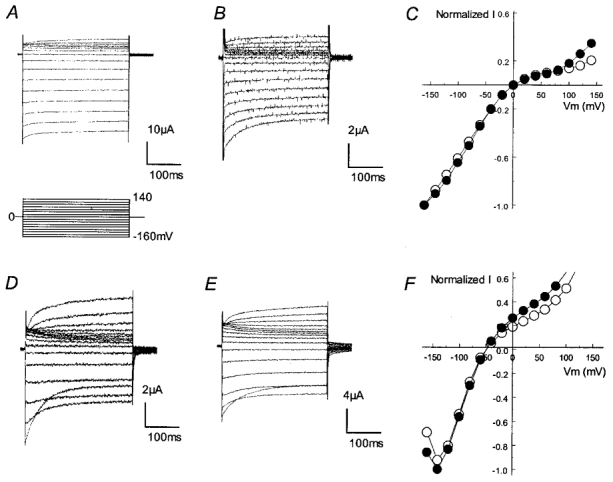
Inward rectifying currents were recorded in the voltage clamp mode from −160 to 140 mV with a 20 mV increment using 90 mm (A and B) and 10 mm (D and E) K+ in the bath solution. A, currents from the wild-type (WT) Kir4.1 show an intermediate inward rectification with only modest outward currents. B, mutation of Gly210 to glutamate did not have any detectable effect on the rectification. C the I–V plot of WT (○) and G210E-mutant Kir4.1 (•) shows a similar I–V relationship. D–F, a similar phenomenon was observed when the extracellular K+ concentration was reduced to 10 mm.
Single channel conductance
It is known that several residues within, or near to, the pore-lining sequences are involved in the single channel conductance (Choe et al. 1997; Repunte et al. 1999; Shieh et al. 1999). In Kir channels, the conductive pore involves certain N-terminal residues, M2, H5, some C-terminal domains and even several extracellular sites (Kubo et al. 1998; Repunte et al. 1999; Lu et al. 1999b). We have noticed that the strength of inward rectification appears to be correlated to the single channel conductance: Kir channels with strong rectification such as Kir2 and Kir4 have smaller conductance (15–25 pS) than the weak rectifier Kir channels such as Kir1 and Kir6 (40–80 pS; Nichols & Lopatin, 1997). Therefore, it is possible that the rectification-controlling site affects the single channel conductance. We thus measured the single channel conductance in inside-out patches. With 150 mm K+ on both sides of the inside-out patch membranes, the unitary conductance was 38.9 ± 0.7 pS (n= 18) in WT Kir1.1 and 22.8 ± 0.6 pS (n= 22) in WT Kir4.1. Under such a condition, the E158N Kir4.1 mutant showed a single channel conductance of 35.4 ± 0.5 pS (n= 20), which was much larger than its WT counterpart and became similar to the WT Kir1.1 (Fig. 3A and B). Flickering channel activity was clearly seen in the E158N mutant at positive membrane potentials (Fig. 3A), which is typical in the WT Kir1.1 but absent in the WT Kir4.1. Reverse mutation in Kir1.1 (N171E) resulted in an average conductance of 27.1 ± 0.9 pS (n= 18) (Fig. 3C and D), which was more like the WT Kir4.1 than the WT Kir1.1.
Figure 3. Unitary conductance of the E158N and N171E mutants.
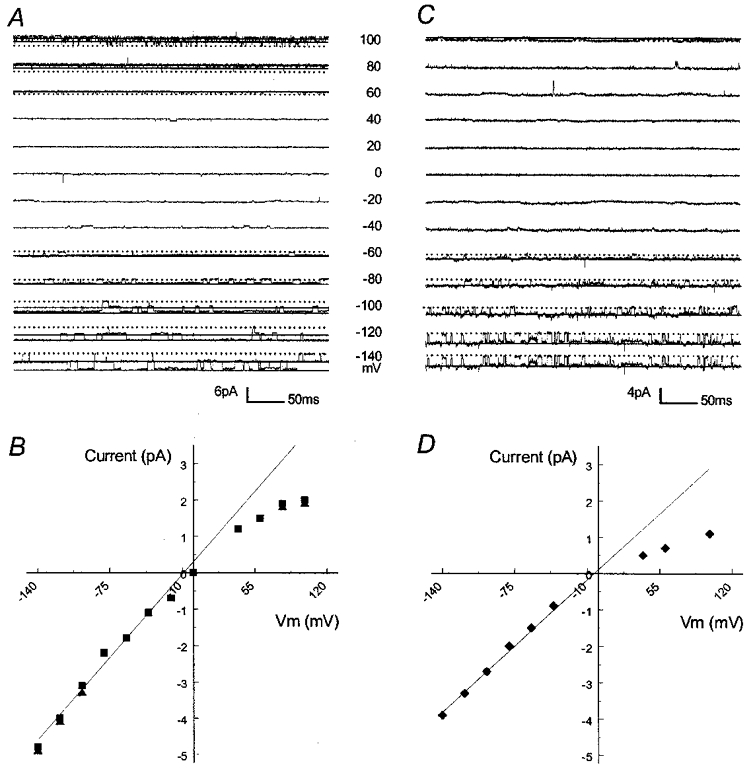
Single channel currents were recorded from two inside-out patches using symmetrical concentrations of K+ (150 mm) on both sides of the patch at various membrane potentials (Vm) listed between panels A and C. A, the E158N mutation was created in Kir4.1. Two active channels are seen at negative Vm. These channels show flickering openings at positive Vm. Continuous line, opening; dotted line, closure. B, I–V relationship of the single channel currents from A. The straight line represents a conductance of 37 pS. C, an active channel was recorded from an oocyte after an injection of Kir1.1 carrying the N171E mutation. D, this current has a slope conductance of 28 pS.
A large body of evidence favours the idea that the pore-lining residues are potential candidates affecting the inner diameter of the pore as well as the conductance, as demonstrated previously with Glu179 in the sWIRK channel (Kubo et al. 1996), Glu224 (Yang et al. 1995) and Asp172 in Kir2.1 (Kubo et al. 1993; Oishi et al. 1998). To see if the unitary conductance is related to residues other than Asn171, mutations were constructed in which residues found in Kir1.1 but not in Kir4.1 were mutated in combination with N171E in Kir1.1. We focused on sites in the H5 and M2 regions with a clear contrast in amino acid characteristics, namely V140, Q152, G167 and V168 in Kir1.1 (see Fig. 1). Whereas the Q152E-N171E and G167T-V168T-N171E mutants failed to yield functional channels, the N171E-V140T mutant showed an increase in the unitary conductance (54.0 ± 2.1 pS, n= 8) (Fig. 4A). The increased conductance was likely to be a result of the V140T mutation, since the single V140T mutation raised the unitary conductance to 71.0 ± 1.8 pS (n= 4) (Fig. 4B), suggesting that Val140 is another residue involved in single channel conductance. These results therefore are consistent with our observations indicating that the presence of glutamate at position 171 in Kir1.1 or 158 in Kir4.1 causes a marked reduction in single channel conductance.
Figure 4. Unitary conductance of the N171E-V140T and V140T mutants.
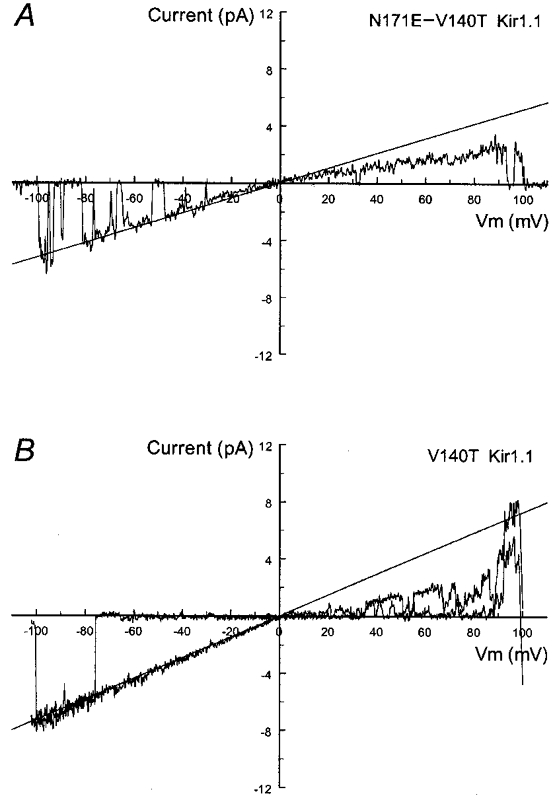
Single channel currents were recorded from two inside-out patches under the same conditions as Fig. 3. With a ramp command potential from 100 to −100 mV added, an active channel is seen in both A and B. These currents show a clear inward rectification. Straight line represents a slope conductance of 52 pS for the N171E-V140T Kir1.1 mutant (A) and 73 pS for the V140T Kir1.1 mutant (B).
pH sensitivity
Our results showed that Glu158 was involved in inward rectification and single channel conductance in Kir4.1. Previous studies have demonstrated that an equivalent site in Kir6 is important for ATP sensitivity (Shyng et al. 1997). Since both Kir1.1 and Kir4.1 are pH sensitive, we asked whether this site was also related to the proton sensitivity of these channels.
The proton sensitivity was examined using CO2 in whole-cell voltage clamp and low pH buffers in excised inside-out patches (Zhu et al. 1999, 2000; Xu et al. 2000b). With 15 % CO2, 69.4 ± 4.3 % (n= 6) and 22.4 ± 5.5 % (n= 7) of the whole-cell currents were inhibited in Kir1.1 and Kir4.1, respectively. The current inhibition was mediated by protons, since both currents were inhibited to a similar degree by selectively lowering intra- but not extracellular pH to the levels (pHi 6.6, pHo 6.2) seen during 15 % CO2 exposure (Xu et al. 2000; Zhu et al. 2000). Macroscopic currents recorded in inside-out patches were inhibited by lowering pHi, suggesting that this inhibition is independent of cytosol-soluble factors, and that the pH-sensing machinery is located in the intracellular domains of the channel proteins. The pHi sensitivity of Kir1.1, however, was much higher with an apparent pK (pKa) of 6.73 and a Hill coefficient (nH) of 3.7 compared with that of Kir4.1 (pKa 6.00, nH 2.3) (Fig 5A and Fig 6A, and Table 1).
Figure 5. Effects of site-directed mutations on channel sensitivity to pHi.
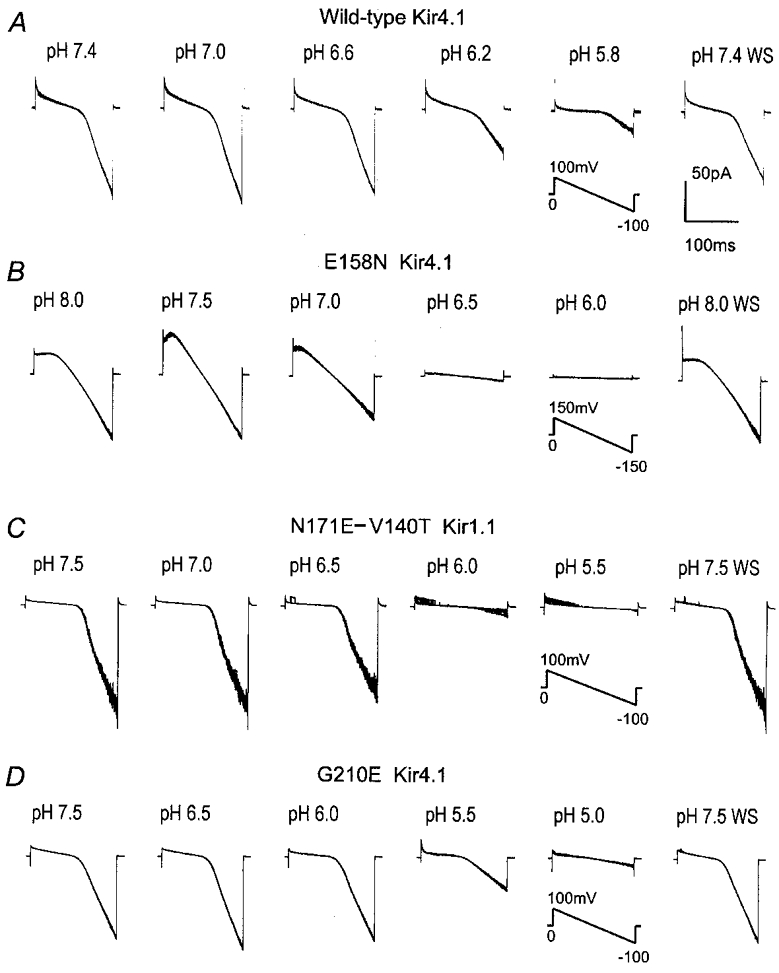
Macroscopic currents were recorded from inside-out patches with symmetrical K+ concentration (150 mm) on both sides of the patch membranes. Ramp command potentials were applied to the patches as indicated in each group. Exposure of the internal membranes to solutions with various pH values produced graded inhibitions of the inward rectifying currents of WT Kir4.1 (A), E158N Kir4.1 mutant (B), N171E-V140T Kir1.1 mutant (C) and G210E Kir4.1 mutant (D). Note the different pH levels studied for each mutant, and that 8 superimposed traces are displayed in each panel. WS, washout.
Figure 6. The pHi and CO2 sensitivities of Kir1.1, Kir4.1 and their mutants.
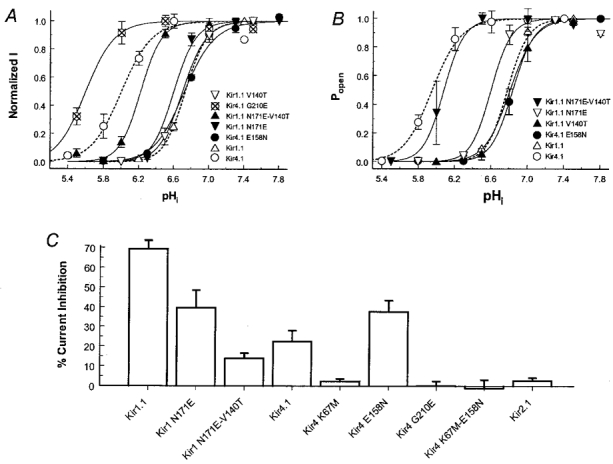
A, concentration-dependent inhibition of Kir1.1, Kir4.1 and their mutants. Macroscopic Kir currents were recorded in inside-out patches using symmetrical concentrations of K+ (150 mm) on both sides of the patch membranes. Exposure of the internal membranes to solutions of acidic pH produced a fast and reversible inhibition of inward rectifying currents. The amplitude of these Kir currents is expressed as a function of intracellular pH (pHi) using the Hill equation: y= 1/{1 + (pKa/x)nH}, where y is the normalized current amplitude, pKa is the midpoint pH value for channel inhibition, x is pHi, and nH is the Hill coefficient. The pKa and nH here are 6.00 and 2.3 for the WT Kir4.1 (n= 6), and 6.73 and 3.7 for the WT Kir1.1 (n= 6). See Table 1 for pKa and nH values of other mutants. Data are presented as means ±s.e.m.B, pH dependence of Popen. Single channel activity was studied in inside-out patches and expressed as the open-state probability (Popen). At pH 5.5–7.5, the relationship of Popen to pHi can be expressed with the Hill equation (dashed lines, Kir1.1 and Kir4.1; continuous lines, their mutants). The pKa and nH values are listed in Table 1. C, CO2 sensitivity was examined in whole-cell oocytes using two-electrode voltage clamp. Currents were recorded before, during and after 15 % CO2 exposure. The duration of CO2 exposure was 6 min for Kir1.1 and its mutants, and 8 min for Kir4.1 and its mutants. Percentage inhibitions of the inward rectifying currents are shown.
Table 1.
Proton sensitivity of Kir1.1, Kir4.1 and their mutants
| Name | pKa | nH | n |
|---|---|---|---|
| Kir1.1 | 6.73 | 3.7 | 6 |
| Kir4.1 | 6.00 | 2.3 | 6 |
| Kir1.1, N171E | 6.60 | 3.7 | 4 |
| Kir1.1, V140T | 6.70 | 3.3 | 5 |
| Kir1.1, N171E-V140T | 6.22 | 3.5 | 7 |
| Kir4.1, E158N | 6.72 | 2.6 | 5 |
| Kir4.1, G210E | 5.60 | 2.3 | 6 |
| Popen Kir1.1 | 6.78 | 4.0 | 3 |
| Popen Kir4.1 | 5.96 | 2.5 | 7 |
| Popen Kir1.1, N171E | 6.60 | 4.0 | 2 |
| Popen Kir1.1, V140T | 6.84 | 3.9 | 2 |
| Popen Kir1.1, N171E-V140T | 6.08 | 3.7 | 2 |
| Popen Kir4.1, E158N | 6.81 | 3.3 | 2 |
Macroscopic currents and single channel activity (Popen) were studied in inside-out patches when the intracellular side of membranes was exposed to solutions with various pH levels. They were inhibited in a concentrationdependent manner by low pH.The inhibitions were described using the Hill equation with pKa and nH shown in the table (n= number of patches).
When the E158N Kir4.1 mutant was expressed in Xenopus oocytes, we found that the mutant channel had a higher CO2 sensitivity than its wild-type. Exposure to 15 % CO2 caused an inhibition of the mutant channel by 37.6 ± 5.6 % (n= 6), which was significantly larger than that of the WT Kir4.1 (P < 0.05, n= 6) (Fig. 6C). This enhanced CO2 sensitivity was due to the rightward shift of the titration curve, as shown in our studies using inside-out patches (Figs 5B and 6A). The E158N Kir4.1 mutant had a pKa value (6.72) almost identical to that of Kir1.1 (pKa 6.73).
The inhibition of the E158N mutant was caused by a strong suppression of the channel open-state probability (Popen) without any significant change in single channel conductance (35.7 ± 0.8 pS at pH 7.5 versus 36.0 ± 1.0 pS at pH 6.5, P > 0.05, n= 7), and the inhibition of the macroscopic currents can be fully explained with the Popen suppression (Fig 6B and Fig 7). Similarly, the N171E mutant did not show any change in its unitary conductance with low pH (27.7 ± 1.6 pS at pH 7.5 versus 27.7 ± 0.9 pS at pH 6.5, P > 0.05, n= 7).
Figure 7. Concentration-dependent inhibition of single channel activity by acidic pH.
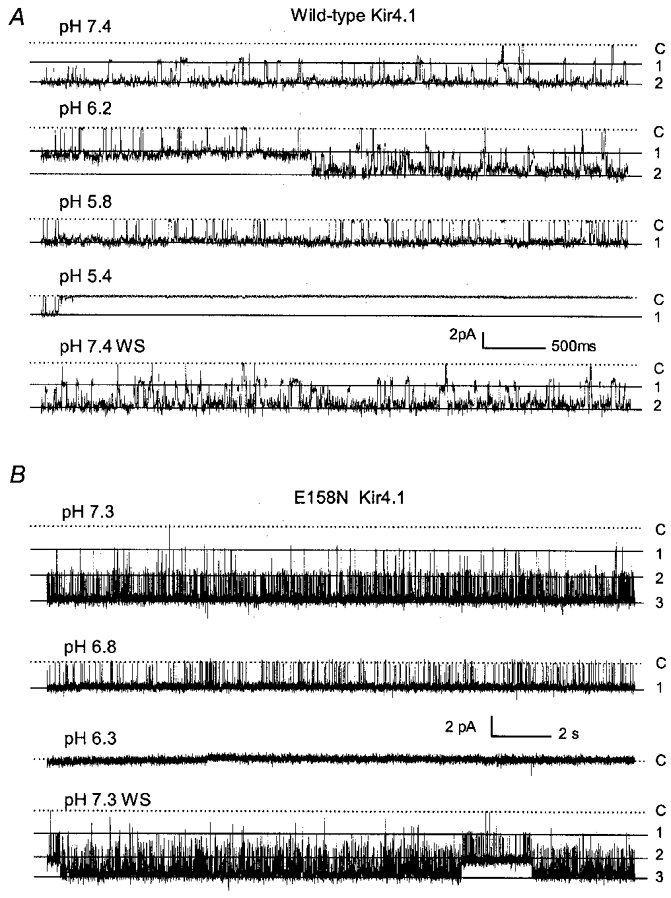
A, single channel currents were recorded from an inside-out patch obtained from a Kir4.1-injected oocyte under the same conditions as in Fig. 3. At a membrane potential (Vm) of −120 mV, two active channels were seen at pHi 7.4, both of which had a slope conductance of 22 pS and high baseline single channel activity (Popen= 0.896). Decreases in pHi caused a graded inhibition of the single channel activity (Popen= 0.694 at pH 6.2, and 0.433 at pH 5.8). These currents were almost completely inhibited at pHi 5.4 (Popen= 0.005). Channel activity resumed after washout (Popen= 0.865). Labels on the right: C, closure; 1, the first opening; 2, the second opening. B, single channel currents of the E158N Kir4.1 mutant were recorded from another inside-out patch. At a Vm of −80 mV, three active channels were seen at pHi 7.3, all of which had the same slope conductance of 37 pS (top, Popen= 0.876). Only one channel was seen when pHi dropped to 6.8 (Popen= 0.295). These currents were abolished at pHi 6.3 (Popen= 0.001). Channel activity recovered after washout (Popen= 0.859, pH 7.3).
We also tested the pH sensitivity of the reverse mutation (N171E) in Kir1.1. This mutant showed a modest decrease in CO2 and pH sensitivities with a pKa of 6.60, only 0.12 pH units lower than that of the WT Kir1.1 (Fig. 6A and C). Clearly, other residue(s) in Kir1.1 also contribute to its high pH sensitivity. To test this possibility, we studied Val140 in Kir1.1. Mutations of Val140 plus Asn171 (N171E-V140T) caused a large decrease in CO2 sensitivity (Fig. 6C) and a leftward shift in the titration curve by 0.6 pH units (pKa 6.08), leading to a channel that had a pH sensitivity closer to that of WT Kir4.1 than Kir1.1 (Figs 5C and 6A). The single V140T mutation did not affect the channel sensitivity to pHi (Fig. 6A and B). These results therefore indicate that the site Glu158 is a major contributor to the difference in pH sensitivity between Kir4.1 and Kir1.1, although additional residues such as Val140 are also required.
Two other residues were also studied in their proton sensitivity. One of these, Lys67 in Kir4.1, is known to control pH sensitivity in an all-or-none manner (Schulte et al. 1999; Xu et al. 2000). This was also the case in K67M-E158N, which showed no sensitivity to 15 % CO2 (−1.1 ± 4.2, n= 4) (Fig. 6C). The other was Gly210 in Kir4.1. The G210E mutation caused a decrease in CO2 and pH sensitivities (pKa 5.60, nH 2.3) (Figs 5D, 6A and C).
DISCUSSION
In these studies, we have shown that a site that was previously known as an inward rectification controller is critical in the channel unitary conductance and proton sensitivity of both Kir1.1 and Kir4.1. Thus, a residue necessary for rectification, conductance and proton sensitivity is demonstrated in our experiments.
Previous studies have indicated that inward rectification in Kir channels is mainly determined by two negatively charged residues with one in M2 and the other in the C-terminus (Fakler et al. 1994; Lu & MacKinnon, 1994; Stanfield et al. 1994; Wible et al. 1994; Taglialatela et al. 1995; Yang et al. 1995). Consistent with these observations, our data have shown that the E158N mutation converts Kir4.1 to a weaker rectifier, and the N171E mutation renders Kir1.1 a stronger inward rectification. Therefore, this particular site is the molecular determinant for the distinct rectification in Kir1.1 and Kir4.1. These observations, however, do not exclude the possibility that additional rectification sites exist in Kir4 channel, as a salmon weakly inward rectifying K+ channel (sWIRK) shows Kir1.1-like weak rectification with both rectification controlling sites in the M2 region and C-terminus identical to those of Kir4.1 (Kubo et al. 1996). This plus the fact that the strength of inward rectification in Kir4.1 is not as strong as in Kir2 channels (Bond et al. 1994; Yang et al. 1995) indicates that residues in addition to Glu158 also play a role. We have attempted to identify these sites by looking at Gly210. Our results indicate that Gly210 is not one of them, because the G210E mutant shows inward rectification similar to the WT Kir4.1. Since the N171E Kir1.1 mutant shows a strong rectification, the additional rectification controller(s) may be those residues found in Kir1.1 but not Kir4.1. We have thus studied several sites in the pore-forming sequences. Whereas Val140 is not involved, the lack of expression of functional channels in other mutations obscures the illustration of the potential rectification controller(s).
Single channel conductance is believed to reflect the channel pore size and the efficiency of ion conduction through the channels. In voltage-gated K+ channels whose channel pore was composed of S5 and S6 transmembrane domains and an H5 loop, many sites within or around these domains have been shown to determine the conductance (Taglialatela et al. 1994; Shieh & Kirsch, 1994). In Kir channels, certain residues in the N-terminus (Choe et al. 1997), C-terminus (Yang et al. 1995) and even the extracellular linkers (Repunte et al. 1999) are also important for the single channel conductance. Since amino acid sequences in the M1, M2 and H5 domains are highly homologous between Kir1.1 and Kir4.1, these channels are optimal to study the determinant residues for single channel conductance. Indeed, our data have shown that Asn171 in Kir1.1 (Glu158 in Kir4.1) is one of them. By swapping amino acids (glutamate versus asparagine) at this site, we have largely switched the conductance between Kir1.1 and Kir4.1. Although these experiments suggest that glutamate is related to a smaller conductance, the size of the residue seems more critical than its charge. Wible et al. (1994) have shown that mutation of this residue to a smaller, negatively charged aspartate (N171D) does not affect the conductance. Thus we believe that the residue at this site may control the single channel conductance by affecting the physical size of the conductive pore, although ions binding to residues lining inner walls of the channels are also important in controlling the conductance in other channels (Spassova & Lu, 1998, 1999).
It may be worth noting that we have found that Val140 is another residue involved in single channel conductance, which is almost doubled in the V140T Kir1.1 mutant. This seems to contradict our original notion as threonine is seen at this site in the small-conductance Kir4.1. What causes this discrepancy is unclear. It is clear, however, that the single channel conductance is determined by more than one residue. Indeed, we have observed a significant attenuation of the conductance when Val140 is jointly mutated with Asn171. These data thereby suggest a close interaction of residues in the pore-forming sequences and further support the role of Glu158 in reducing single channel conductance of Kir4.1.
Kir1.1 and Kir4.1 are CO2 and pH sensitive although their CO2 and pH sensitivities are clearly different. A parallel study in this group has shown that an N-terminal residue (Val66 in Kir1.1) and a few C-terminal histidines are some of the important contributors to the distinct pH sensitivity in this Kir channel (Xu et al. 2000a). In the present study, we have shown that Glu158 is also important in Kir4.1 channel sensitivity to intracellular protons. Replacing it with the corresponding residue in Kir1.1 leads to a Kir4.1 mutant that has a pH sensitivity almost identical to that of the WT Kir1.1. Reverse mutation of this residue plus the Val140 in Kir1.1 results in a pH sensitivity in the mutant Kir1.1 more like that of WT Kir4.1. These results strongly suggest that this site is also critical for proton sensing in both Kir4.1 and Kir1.1 channels.
It is known that a lysine residue near to the M1 region is a critical player of the pH sensitivity in both Kir1.1 (Lys80, Fakler et al. 1996) and Kir4.1 (Lys67, Xu et al. 2000b). Mutations of this residue completely eliminate channel sensitivity to intracellular protons (Fakler et al. 1996; Xu et al. 2000b). This is further supported by our current studies showing that double mutations (K67M-E158N) have no additional effect on the pH sensitivity of the K67M Kir4.1 mutant channels. Lys80, as well as several other recently demonstrated residues responsible for pH sensitivity in Kir1.1, indicates that proton sensing requires multiple sites in the channel proteins (Fakler et al. 1996; Choe et al. 1997; Schulte et al. 1999; Chanchevalap et al. 2000). Glu158 found in our current studies seems to be another contributor to the pH sensitivity in Kir4.1. It is possible that Glu158 becomes titratable at pH ∼6.0 by its interaction with other pore-lining residue(s) leading to a change in its pKa (pKa in free amino acid, 4.07), as previously demonstrated in a cyclic nucleotide-gated channel (Morrill & MacKinnon, 1999). Titration of Glu158, however, appears to cause a leftward shift of the titration curve, reducing the channel sensitivity to intracellular protons. Instead of glutamate, there is a non-titratable asparagine at this site in Kir1.1, so that the ROMK channels as well as the E158N Kir4.1 mutant show higher pH sensitivity than the WT Kir4.1. Supporting this titration theory are also our previous observations showing that the unitary conductance increases with low pH, which may be a result of the loss of negative charge at the glutamate residue following protonation (Yang & Jiang, 1999). However, such a change in conductance is not seen in the N171E Kir1.1 mutant. Therefore, whether this glutamate indeed can be protonated requires further investigations. Alternatively, there is a possibility that this residue is involved in channel gating processes by interacting with other proton-sensing residues. In any case, our results may provide useful information about a critical residue in the M2 pore-forming domain contributing to the Kir gating by intracellular protons.
In these studies, we have found that the second rectification-controlling site is also related to channel sensitivity to intracellular protons, as mutation of Gly210 to glutamate markedly reduces the pH sensitivity of Kir4.1. The consistent effect of these two rectification controllers in reducing pH sensitivity demonstrates another common function of these two sites, in addition to rectification and pore formation (Doyle et al. 1998; Kubo et al. 1998; Lu et al. 1999a, b).
The discovery of a multifunctional site is not only an interesting finding but also implies its potential function in cellular physiology. Glu158 in Kir4.1 corresponds to N171 in Kir1.1, D172 in Kir2.1 and N160 in Kir6. This site is responsible for inward rectification, ion selectivity and perhaps permeation properties in Kir2.1 (Yang et al. 1995; Abrams et al. 1996). In Kir6.2, it also affects the ATP sensitivity (Shyng et al. 1997). These, as well as our current findings, indicate that Glu158 is a convergent site of multiple biophysical properties in several Kir channels. Genetic variation in such a critical residue can bring about a brand-new Kir channel that has rectification, conductance and pH sensitivity drastically different from its parental channel. Considering the diverse cellular demands for channel properties, such a genetic variation may satisfy a number of functional needs of the cells. Thus, the demonstration of the multifunctional site is also helpful in understanding the diversity of Kir channels and their potential physiological functions in the cell.
Acknowledgments
This work was supported by the NIH (RO1 HL58410), the Grant-in-Aid Award (9950528 N) from the American Heart Association, and the Career Investigator Award from the American Lung Association. We would like to thank Dr Steven Hebert and Dr John Adelman for their generosity in sharing with us the Kir1.1 and Kir4.1 cDNAs.
H. Xu and Z. Yang contributed equally to these studies.
References
- Abrams CJ, Davies NW, Shelton PA, Stanfield PR. The role of a single aspartate residue in ionic selectivity and block of a murine inward rectifier K+ channel Kir2.1. The Journal of Physiology. 1996;493:643–649. doi: 10.1113/jphysiol.1996.sp021411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CT, Pessia M, Xia XM, Lagrutta A, Kavanaugh MP, Adelman JP. Cloning and expression of a family of inward rectifier potassium channels. Receptors and Channels. 1994;2:183–191. [PubMed] [Google Scholar]
- Bredt DS, Wang TL, Cohen NA, Guggino WB, Snyder SH. Cloning and expression of two brain-specific inwardly rectifying potassium channels. Proceedings of the National Academy of Sciences of the USA. 1995;92:6753–6757. doi: 10.1073/pnas.92.15.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanchevalap S, Yang Z, Cui N, Qu Z, Zhu G, Liu C, Giwa LR, Abdulkadir L, Jiang C. Involvment of histidine residues in CO2 and pH sensing of ROMK1 channel. Journal of Biological Chemistry. 2000;275:7811–7817. doi: 10.1074/jbc.275.11.7811. [DOI] [PubMed] [Google Scholar]
- Choe H, Zhou H, Palmer LG, Sackin H. A conserved cytoplasmic region of ROMK modulates pH sensitivity, conductance, and gating. American Journal of Physiology. 1997;273:F516–529. doi: 10.1152/ajprenal.1997.273.4.F516. [DOI] [PubMed] [Google Scholar]
- Doi T, Fakler B, Schultz JH, Schulte U, Brandle U, Weidemann S, Zenner HP, Lang F, Ruppersberg JP. Extracellular K+ and intracellular pH allosterically regulate renal K(ir)1.1 channels. Journal of Biological Chemistry. 1996;271:17261–17266. doi: 10.1074/jbc.271.29.17261. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Fakler B, Brandle U, Bond C, Glowatzki E, Konig C, Adelman JP, Zenner HP, Ruppersberg JP. A structural determinant of differential sensitivity of cloned inward rectifier K+ channels to intracellular spermine. FEBS Letters. 1994;356:199–203. doi: 10.1016/0014-5793(94)01258-x. [DOI] [PubMed] [Google Scholar]
- Fakler B, Schultz JH, Yang J, Schulte U, Brandle U, Zenner HP, Jan LY, Ruppersberg JP. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO Journal. 1996;15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Miyashita T, Kubokawa K. A weakly inward rectifying potassium channel of the salmon brain. Glutamate 179 in the second transmembrane domain is insufficient for strong rectification. Journal of Biological Chemistry. 1996;271:15729–15735. doi: 10.1074/jbc.271.26.15729. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Yoshimichi M, Heinemann SH. Probing pore topology and conformational changes of Kir2.1 potassium channels by cysteine scanning mutagenesis. FEBS Letters. 1998;435:69–73. doi: 10.1016/s0014-5793(98)01038-2. [DOI] [PubMed] [Google Scholar]
- Lu Z, MacKinnon R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 1994;371:243–246. doi: 10.1038/371243a0. [DOI] [PubMed] [Google Scholar]
- Lu T, Nguyen B, Zhang X, Yang J. Architecture of a K+ channel inner pore revealed by stoichiometric covalent modification. Neuron. 1999a;22:571–580. doi: 10.1016/s0896-6273(00)80711-4. [DOI] [PubMed] [Google Scholar]
- Lu T, Zhu YG, Yang J. Cytoplasmic amino and carboxyl domains form a wide intracellular vestibule in an inwardly rectifying potassium channel. Proceedings of the National Academy of Sciences of the USA. 1999b;96:9926–9931. doi: 10.1073/pnas.96.17.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill JA, MacKinnon R. Isolation of a single carboxyl-carboxylate proton binding site in the pore of a cyclic nucleotide-gated channel. Journal of General Physiology. 1999;114:71–83. doi: 10.1085/jgp.114.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annual Review of Physiology. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Oishi K, Omori K, Ohyama H, Shingu K, Matsuda H. Neutralization of aspartate residues in the murine inwardly rectifying K+ channel IRK1 affects the substate behaviour in Mg2+ block. The Journal of Physiology. 1998;510:675–683. doi: 10.1111/j.1469-7793.1998.675bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte VP, Nakamura H, Fujita A, Horio Y, Findlay I, Pott L, Kurachi Y. Extracellular links in Kir subunits control the unitary conductance of SUR/Kir6.0 ion channels. EMBO Journal. 1999;18:3317–3324. doi: 10.1093/emboj/18.12.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte U, Hahn H, Konrad M, Jeck N, Derst C, Wild K, Weidemann S, Ruppersberg JP, Fakler B, Ludwig J. pH gating of ROMK (Kir1.1) channels: control by an Arg-Lys-Arg triad disrupted in antenatal Bartter syndrome. Proceedings of the National Academy of Sciences of the USA. 1999;96:15298–15303. doi: 10.1073/pnas.96.26.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh CC, Kirsch GE. Mutational analysis of ion conduction and drug binding sites in the inner mouth of voltage-gated K+ channels. Biophysical Journal. 1994;67:2316–2325. doi: 10.1016/S0006-3495(94)80718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh RC, Chang JC, Kuo CC. K+ binding sites and interactions between permeating K+ ions at the external pore mouth of an inward rectifier K+ channel (Kir2.1) Journal of Biological Chemistry. 1999;274:17424–17430. doi: 10.1074/jbc.274.25.17424. [DOI] [PubMed] [Google Scholar]
- Shyng S, Ferrigni T, Nichols CG. Control of rectification and gating of cloned KATP channels by the Kir6.2 subunit. Journal of General Physiology. 1997;110:141–153. doi: 10.1085/jgp.110.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova M, Lu Z. Coupled ion movement underlies rectification in an inward-rectifier K+ channel. Journal of General Physiology. 1998;112:211–221. doi: 10.1085/jgp.112.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova M, Lu Z. Tuning the voltage dependence of tetraethylammonium block with permeant ions in an inward-rectifier K+ channel. Journal of General Physiology. 1999;114:415–426. doi: 10.1085/jgp.114.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR, Davies NW, Shelton PA, Sutcliffe MJ, Khan IA, Brammar WJ, Conley EC. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. The Journal of Physiology. 1994;478:1–6. doi: 10.1113/jphysiol.1994.sp020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela M, Champagne MS, Drewe JA, Brown AM. Comparison of H5, S6, and H5-S6 exchanges on pore properties of voltage-dependent K+ channels. Journal of Biological Chemistry. 1994;269:13867–13873. [PubMed] [Google Scholar]
- Taglialatela M, Ficker E, Wible BA, Brown AM. C-terminus determinants for Mg2+ and polyamine block of the inward rectifier K+ channel IRK1. EMBO Journal. 1995;14:5532–5541. doi: 10.1002/j.1460-2075.1995.tb00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible BA, Taglialatela M, Ficker E, Brown AM. Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature. 1994;371:246–249. doi: 10.1038/371246a0. [DOI] [PubMed] [Google Scholar]
- Xu H, Yang Z, Cui N, Giwa L, Abdulkadir L, Patel M, Sharma P, Shen W, Jiang C. Molecular determinants for the distinct pH sensitivity of Kir1.1 and Kir4.1. American Journal of Physiology. 2000a doi: 10.1152/ajpcell.2000.279.5.C1464. (in the Press) [DOI] [PubMed] [Google Scholar]
- Xu H, Yang Z, Cui N, Qu Z, Jiang C. Modulation of Kir4.1 and Kir5.1 by hypercapnia and intracellular acidosis. The Journal of Physiology. 2000b;524:725–735. doi: 10.1111/j.1469-7793.2000.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Jan YN, Jan LY. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 1995;5:1047–1054. doi: 10.1016/0896-6273(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Yang Z, Jiang C. Opposite effects of pH on open-state probability and single channel conductance of Kir4.1 channels. The Journal of Physiology. 1999;520:921–927. doi: 10.1111/j.1469-7793.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu GY, Chanchevalap S, Cui NR, Jiang C. Effects of intra- and extracellular acidification on single channel Kir2.3 currents. The Journal of Physiology. 1999;516:699–710. doi: 10.1111/j.1469-7793.1999.0699u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu GY, Chanchevalap S, Liu C, Xu HX, Jiang C. CO2 inhibits specific inward rectifier K+ channels by decreases in intra- and extracellular pH. Journal of Cellular Physiology. 2000;183:53–64. doi: 10.1002/(SICI)1097-4652(200004)183:1<53::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]


