Abstract
It has been suggested that the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel may utilize a novel gating mechanism in which open and closed states are not in thermodynamic equilibrium. This suggestion is based on the assumption that energy of ATP hydrolysis drives the gating cycle.
We demonstrate that CFTR channel gating occurs in the absence of ATP hydrolysis and hence does not depend on an input of free energy from this source. The binding of ATP or structurally related analogues that are poorly or non-hydrolysable is sufficient to induce opening. Closing occurs on dissociation of these ligands or the hydrolysis products of those that can be cleaved.
Not only can channel opening occur without ATP hydrolysis but the temperature dependence of the open probability (Po) is reversed, i.e. Po increases as temperature is lowered whereas under hydrolytic conditions, Po increases as temperature is elevated. This indicates that there are different rate-limiting steps in the alternate gating pathways (hydrolytic and non-hydrolytic).
These observations demonstrate that phosphorylated CFTR behaves as a conventional ligand-gated channel employing cytoplasmic ATP as a readily available cytoplasmic ligand; under physiological conditions ligand hydrolysis provides efficient reversibility of channel opening.
CFTR shares structural homology with the ABC transporter family of proteins that hydrolyse ATP to provide free energy to transport chemical compounds across the cell membrane against a chemical gradient. In contrast, CFTR functions as an ion channel and allows the movement of Cl− ions down an electrochemical gradient (Sheppard & Welsh, 1999). No input of free energy is required to accomplish this ion translocation; however, CFTR can hydrolyse ATP as do other ABC proteins although at a lower rate. Indeed the rate of hydrolysis appears to be of the same order as that of channel gating (Li et al. 1996). Even prior to the assay of CFTR ATPase activity, it was postulated that the energy of hydrolysis may drive the channel gating transitions (Baukrowitz et al. 1994; Hwang et al. 1994; Gadsby & Nairn, 1999). However, there is no direct evidence that free energy of ATP hydrolysis is a driving force for CFTR ion channel gating. Two experimental facts are taken as evidence that hydrolysis is necessary for gating. First, the non-hydrolysable ATP analogue AMP-PNP is unable to open the CFTR ion channel (Carson & Welsh, 1993). Second, Mg2+ is a necessary cofactor for channel opening (Anderson et al. 1991; Dousmanis et al. 1996). However, analysis of the published data suggests that such a straightforward interpretation may not apply. Quinton & Reddy (1992) first observed CFTR activation by 5 mm AMP-PNP in the presence of 0.5 mm ATP. However, when purified and free of ATP the non-hydrolysable AMP-PNP does not open the channel at 36°C (Carson & Welsh, 1993) and indeed is without any detectable impact on CFTR at that temperature (Schultz et al. 1995). On the other hand, however, when present together with ATP at room temperature (22°C) AMP-PNP does interact with CFTR and locks the channel in the open state (Gunderson & Kopito, 1994; Hwang et al. 1994; Baukrowitz et al. 1994). Mathews et al. (1998) subsequently demonstrated that this effect is temperature sensitive and does not occur above 32°C. Weinreich et al. (1999) more recently provided evidence of competitive inhibition by AMP-PNP of the action of ATP at both nucleotide binding domains at room temperature. Neither they nor Hwang et al. (1994) earlier detected channel opening by 0.5 mm AMP-PNP alone at room temperature. However, the possible influence of higher concentrations of AMP-PNP alone at lower temperatures has not been tested.
With respect to the divalent cation requirement, Anderson et al. (1991) reported that in Mg2+-free buffer ATP failed to activate CFTR conductance. Later similar experiments were analysed at the single channel level in planar lipid bilayers (Gunderson & Kopito, 1995). It was shown that at a free Mg2+ concentration of approximately 125 nm (25 μm total Mg, 1 mm EDTA, 1 mm ATP) channel gating still occurred, albeit with modified kinetics. Schultz et al. (1996) later confirmed this result in patch-clamp experiments. They controlled free Mg2+ very carefully and found that the CFTR ion channel is gated at a free Mg2+ concentration of about 1 μm indicating that Mg2+ is not an absolute requirement for channel activity and thereby supporting the argument that ATP hydrolysis is also not required.
In this paper we compare the CFTR ion channel gating process supported by MgATP with that supported by ATP alone and present direct evidence that poorly hydrolysable and non-hydrolysable nucleoside triphosphate analogues can gate CFTR ion channels. These findings confirm that nucleoside triphosphate hydrolysis is not absolutely required for CFTR ion channel gating and hence that ATP hydrolysis is not a driving force for the gating. Rather, as we have argued previously, channel opening is dependent upon an entropy decrease brought about by the binding of a nucleoside triphosphate (Aleksandrov & Riordan, 1998). Closing may occur either on dissociation of ATP in the divalent ion-free situation, of the non-hydrolysable analogue, or on hydrolysis and dissociation of the product in the presence of divalent cations. Under physiological conditions the hydrolytic pathway may increase gating efficiency in at least two ways. First, the CFTR.MgATP transition state structure may stabilize the open state more effectively than CFTR.ATP; second, hydrolysis provides effective reversibility by terminating the MgATP-containing transition state.
METHODS
Membrane vesicles and single channel measurements
Microsomal vesicles from Chinese hamster ovary (CHO) cells stably expressing CFTR (Chang et al. 1993) were isolated as described previously (Aleksandrov & Riordan, 1998). The vesicle pellet was resuspended in buffer (250 mm sucrose, 10 mm Hepes, pH 7.2, 5 mm MgCl2) to yield a total protein concentration of about 1–3 mg ml−1. Vesicles of uniform diameter of ∼1 μm were obtained after brief bath sonication (three 20 s periods). The relative amount of CFTR protein was estimated by immunoblotting. The membrane vesicles were stored at −80°C until used. Membrane vesicles were thawed and phosphorylated with 100 units ml−1 PKA catalytic subunit and 0.5 mm Na2ATP for 20 min at room temperature directly before use. PKA was washed out by repeated (3 times) sedimentation and resuspension in 5 × volume of Mg2+-free buffer containing 250 mm sucrose, 10 mm Hepes, pH 7.2, 150 mm KCl. This diluted Na2ATP to ∼2 nm in the vesicle stock solution and to ∼0.04 nm in the ‘cis’ compartment of the bilayer chamber. Vesicles were sonicated for 20 s to restore uniform size after the final sedimentation-resuspension cycle. Single channel measurements were also made as described previously (Aleksandrov & Riordan, 1998).
Estimation of Mg2+ and MgATP concentrations
EqCal Software (Biosoft, Cambridge, UK) was used to calculate equilibrium concentrations of species in fluoride-containing buffer. For the initial concentrations (300 mm NaCl, 100 mm NaF, 300 μm Na2ATP, 100 μm MgCl2 and 20 mm Hepes/KOH, pH 7.2) the calculated concentrations of species were as follows: [ATP4-]= 240 μm, [HATP3-]= 60 μm, [Mg2+]= 74 pM, [MgATP2-]= 0.28 nm. The excess of the total Mg appeared in the form of [MgF2] (≈ 100 μm) because of a high fluoride concentration and a high equilibrium constant (logK = 8.1; Smith & Martel, 1976).
AMP-PNP treatment
To remove contaminating ATP in AMP-PNP, a 200 mm stock solution was treated with 100 units ml−1 hexokinase, 100 mm dextrose and 210 mm MgCl2 at 30°C for 20 min. The enzyme was removed by ultrafiltration (Amicon 30) to yield an ATP-free stock of 200 mm MgAMP-PNP. The effectiveness of this treatment was verified by addition of 0.15 μmγ-[32P]-ATP to this stock prior to hexokinase treatment and thin layer chromatography and electronic autoradiography after treatment.
8SE construct
The 8SE construct was made based on the 4SE mutant CFTR construct containing S660E, S737E, S795E and S813E (Chang et al. 1993). The Dra III fragment containing 4SE was amplified by polymerase chain reaction (PCR) and an oligonucleotide primer to introduce the S700E mutation into the fragment. This PCR product was used as a template for further amplification to introduce the S686E, S712E and S768E mutations. The Dra III fragment in the pNUT-4SE-CFTR was replaced by the Dra III fragment containing S660E/S686E/S700E/S712E/S737E/S768E/S795E/S813E (8SE). The sequence of the final 8SE construct was verified after insertion into the pNUT-4SE-CFTR plasmid.
Materials
Phospholipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine were from Avanti Polar Lipids (Birmingham, AL, USA). The PKA catalytic subunit was from Promega (Madison, WI, USA). Hexokinase was from Roche Molecular Biochemicals (Indianapolis, IN, USA). All other chemicals were from Sigma (St Louis, MO, USA).
RESULTS
CFTR gating in the presence and absence of Mg2+ ions
The concentration of MgATP can be set with either the divalent cation or the free nucleotide in excess. We have compared the ability of a fixed ATP concentration to gate the CFTR channel when Mg2+ is in excess, and hence the concentration of MgATP is approximately equivalent to that of total ATP, with that in the presence of a strong chelator and no added Mg2+. Although CFTR channel activity has been reported at ATP concentrations as low as 1 μm (Gunderson & Kopito, 1994) we did not readily detect CFTR channels below 50–100 μm with Mg2+ present in excess (2 mm). Even in this concentration range sufficient numbers of single channel openings for numerical analysis were not obtained during the typical single channel lifetime of ∼15 min. However, at 300 μm ATP, activity was robust (Fig. 1). Under these conditions where [ATP]total≈[MgATP], open probabilities increased with increasing temperature, i.e. Po≈ 0.16 at 25°C, 0.26 at 30°C and 0.38 at 35°C due primarily to decreased mean closed times. A similar relationship is seen at higher MgATP concentrations (Aleksandrov & Riordan, 1998).
Figure 1. Single CFTR channels gated by ATP with excess Mg2+ ions.
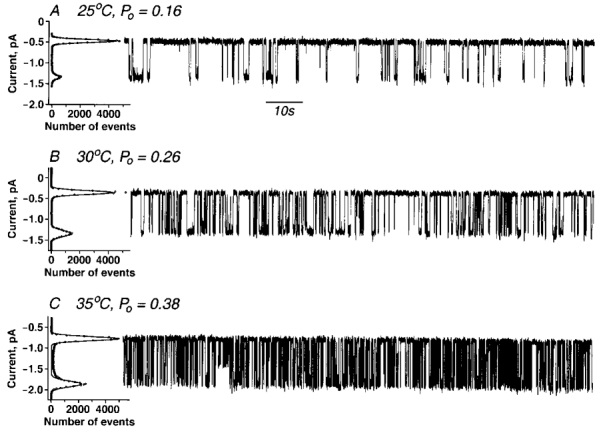
Wild-type CFTR ion channels were recorded in symmetrical solutions: 300 mm NaCl, 2 mm MgCl2, 1 mm EGTA, 20 mm Hepes/KOH, pH 7.2. Each trace is of 2 min duration. Po values were calculated from all points histograms which are shown at the left of each trace. Prephosphorylated membrane vesicles and 300 μm Na2ATP were added to the ‘cis’ compartment only. Transmembrane potential difference Vm=−75 mV. A, +25 °C, Po= 0.16± 0.04 (n= 4). B, +30 °C, Po= 0.26± 0.03 (n= 7). C, +35 °C, Po= 0.38± 0.03 (n= 5).
To evaluate the ability of this same total ATP concentration (300 μm) to support gating in the virtual absence of Mg2+ a high concentration of fluoride was added as chelator. The MgF2 complex is formed with a binding constant comparable to that of MgEDTA (Smith & Martel, 1976). As a consequence, in the solution used (300 mm NaCl, 100 mm NaF, 300 μm ATP, 20 mm Hepes KOH, pH 7.2, 100 μm MgCl2) the [Mg2+]free is ≈ 0.1 nm and [MgATP]≈ 0.3 nm. Traces of CFTR channel activity with this solution are shown in Fig. 2A–C. The open probabilities are approximately 0.85 at 25°C, 0.52 at 30°C and 0.25 at 35°C. Thus the Po value at 25°C was considerably greater in these Mg2+-free conditions than when Mg2+ was present in excess. Moreover with the Mg2+-free buffer, Po decreases with increasing temperature. Although the high F− concentration used to chelate Mg2+ was previously shown to stimulate CFTR channel activity (Berger et al. 1998), we found that EDTA had the same influence as fluoride (Fig. 2D). The inverse temperature dependence observed using ATP alone compared with MgATP explains the earlier results of Schultz et al. (1995) in patch-clamp experiments who observed increased Po values as temperature was lowered. They had employed a solution containing 10 mm F−, 0.3 mm ATP and 2 mm MgCl2 in which case more than 99 % of total ATP was present as free nucleotide. The opposite temperature relationships observed with gating supported by MgATP and free ATP indicate there are different rate-limiting steps in the processes under the two experimental conditions. As enzymatic hydrolysis of ATP is not known to occur independently of a divalent cation these observations suggest that CFTR channel gating can occur by other than an energy-driven gating cycle.
Figure 2. ATP-gated CFTR channels in Mg2+-free buffer.
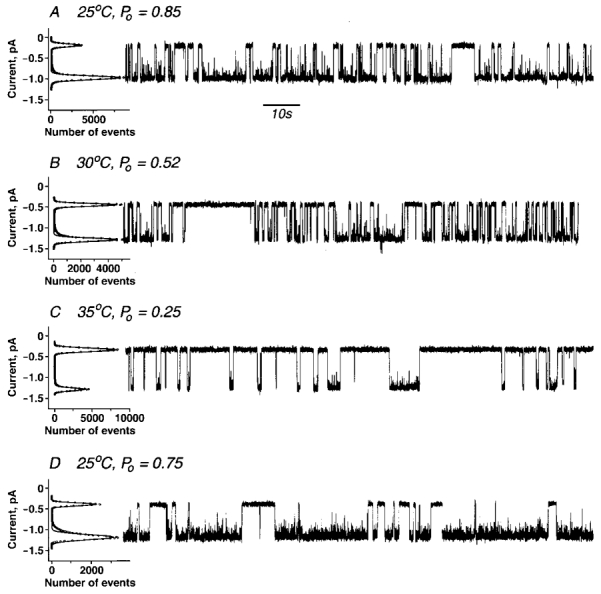
In A-C, wild-type CFTR ion channels were recorded in symmetrical solutions containing: 300 mm NaCl, 100 mm NaF, 20 mm Hepes/KOH, pH 7.2. In D, 100 mm NaF was replaced with 2 mm EDTA. Other experimental details are as in Fig. 1. A, +25 °C, Po= 0.83± 0.07 (n= 4). B, +30 °C, Po= 0.51± 0.04 (n= 6). C, +35 °C, Po= 0.25± 0.04 (n= 3).
CFTR gating by ATPγS and AMP-PNP
If ATP hydrolysis were essential for CFTR gating ATPγS should be much less effective than ATP. On the other hand, ATPγS has the most similar structure to ATP of any available analogues and hence would be expected to have a similar affinity for binding sites. We tested its ability to support CFTR gating under the same extremely low Mg2+ conditions used above with ATP. Figure 3 demonstrates that at 25°C ATPγS strongly supports channel gating. However, as the temperature was elevated, the ability of ATPγS to gate the channel decreased dramatically and was nearly undetectable at temperatures above 30°C. Again these observations are in agreement with the conclusion of Schultz et al. (1995) that CFTR does not bind ATPγS at 36°C. The fact that this poorly hydrolysable nucleotide is more supportive of gating under conditions that are even less favourable for hydrolysis, i.e. lower divalent cation concentration and temperature, further supports the argument that gating can occur without hydrolysis.
Figure 3. CFTR channels gated by ATPγS and AMP-PNP.
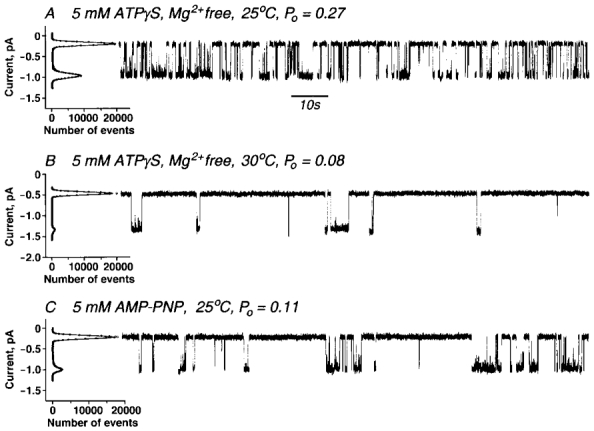
Wild-type CFTR ion channels were recorded in symmetrical solutions: 300 mm NaCl, 100 mm NaF, 20 mm Hepes/KOH, pH 7.2. Transmembrane potential difference Vm=−75 mV. Traces shown are with 5 mm ATPγS at +25 °C, Po= 0.27± 0.04 (n= 5) (A); 5 mm ATPγS at +30 °C, Po= 0.08± 0.04 (n= 5) (B); and 5 mm AMP-PNP at +25 °C, Po= 0.11± 0.05 (n= 3) (C).
Additional evidence of a non-hydrolytic gating pathway was obtained using non-hydrolysable AMP-PNP (Fig. 3C). The compound was first treated with hexokinase to remove traces of contaminating ATP. As with ATP in the absence of Mg2+and ATPγS, activity was inversely proportional to temperature and recordings could not be consistently obtained at 30°C or higher. Even at 25°C channel appearance was much decreased from that with ATP or ATPγS without Mg2+. Nevertheless, the ability of high concentrations of AMP-PNP to cause the channel to open and close at low but not at higher temperatures is consistent with all previous observations using this compound. While Quinton & Reddy (1992) had found that a mixture of the analogue and ATP could generate CFTR currents at 36°C, Carson & Welsh (1993) clearly showed that the channel was not opened by pure AMP-PNP alone at this temperature. Its interaction with CFTR, which causes the channel to be locked open in the presence of ATP, also does not occur at temperatures above 32°C (Mathews et al. 1998).
Non-hydrolytic gating of constitutively active CFTR channels
In all of the above experiments CFTR had been prephosphorylated with PKA. Although the kinase and its substrate, ATP, were washed out prior to adding an analogue to support gating, the presence of a very small amount of ATP carried over from the phosphorylation reaction was possible. Therefore we employed a CFTR variant that does not require phosphorylation by PKA for activity. Partial constitutive activity is exhibited when serine residues at eight of the dibasic PKA phosphorylation sites in the R-domain are replaced by acidic amino acids (Rich et al. 1993). Typical CFTR channels with increasing activity at higher temperatures in the presence of 5 mm MgATP are observed when glutamates are present at these sites (8SE; Fig. 4A–C). This made it possible to test the influence of hexokinase-treated AMP-PNP on CFTR that had not been prephosphorylated and hence never exposed to ATP. Figure 4D shows that like the phosphorylated wild-type the unphosphorylated 8SE variant was gated by AMP-PNP at 25°C. Thus without exposure of CFTR to a hydrolysable ATPase substrate at any stage, channel gating does occur.
Figure 4. Gating of 8SE CFTR channels by ATP and AMP-PNP.
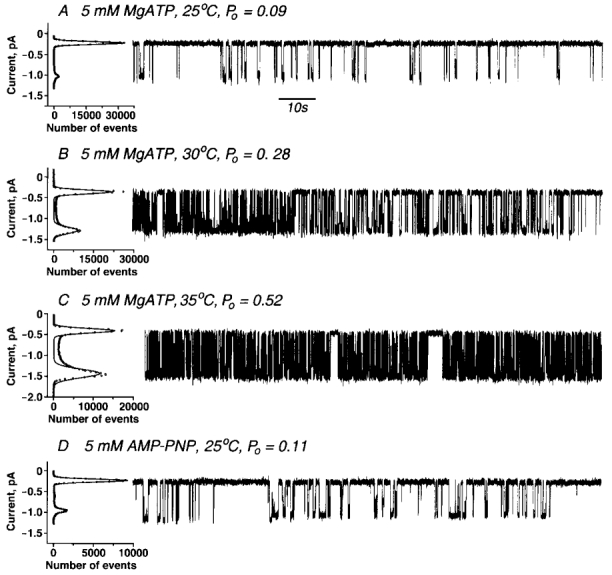
8SE mutant CFTR channels were recorded in symmetrical solutions: 300 mm NaCl, 5 mm MgCl2, 1 mm EGTA, 20 mm Hepes/KOH, pH 7.2. Membrane vesicles were not phosphorylated. Transmembrane potential difference Vm=−75 mV. Traces shown are with 5 mm MgATP at +25 °C, Po= 0.09± 0.03 (n= 5) (A); 5 mm MgATP at +30 °C, Po= 0.28± 0.04 (n= 8) (B); 5 mm MgATP at +35 °C, Po= 0.52± 0.04 (n= 4) (C); and 5 mm MgAMP-PNP at +25 °C in symmetrical solutions: 300 mm NaCl, 100 mm NaF, 10 mm Hepes/KOH, pH 7.2. Po= 0.11± 0.04 (n= 3) (D).
DISCUSSION
Because of its sequence similarity with members of the ABC transporter superfamily, there was initially skepticism that the CFTR gene codes for an ion channel (Hyde et al. 1990). When convincing evidence was obtained that this was the case, there were suggestions that the energy of ATP hydrolysis at the nucleotide binding domains might drive CFTR gating transitions (for review see Sheppard & Welsh, 1999) as it does the translocation of solute by the transporters. The utilisation of the energy stored in a chemical bond, however, is not known to be used to drive the gating transitions of any other ion channel. Rather these are thermally driven processes in which an electric field (voltage gated) or ligand binding (ligand gated) determines the distribution between states. We showed previously that the open and closed states of the CFTR channel are in thermal equilibrium and that closing has an energy of activation (Ea) equivalent to diffusion in water, precluding the possibility that it is an energy-driven process (Aleksandrov & Riordan, 1998). The opening transition, however, has a high Ea that in theory could come from ATP hydrolysis. Nevertheless, direct measurements of the activation enthalpy for channel gating indicated that a change in entropy (or molecular configuration) was sufficient for channel opening. This could be brought about by ATP operating as a ligand whose binding induces a structural transformation in the CFTR molecule. This may occur by an induced-fit process in which the ligand affinity for the binding site improves step by step as the fit improves until an optimal bound configuration coincident with the channel open state is achieved.
This structural reorganisation can then be relaxed either by ligand dissociation or hydrolysis followed by dissociation of the hydrolysis products depending on the experimental conditions. The experiments reported in this paper confirm that the binding of ATP or an analogue under conditions where hydrolysis does not occur is sufficient to open the channel. As might be expected, this non-hydrolytic opening is more effective at lower temperatures where binding stability is higher. In contrast, gating increases with increasing temperatures under conditions where hydrolysis occurs. In this case, hydrolysis, which is accelerated at higher temperatures, provides for the more rapid dissociation of products than of ATP in the absence of hydrolysis and in this way more rapidly enables closing and the initiation of a new cycle. Under physiological conditions the hydrolytic pathway will be predominant but the existence of the non-hydrolytic pathway demonstrates that hydrolysis is not essential to gating and hence that the transitions are not driven by the energy of ATP hydrolysis. Instead ATP serves as a readily available cytoplasmic ligand whose binding is structurally coupled to channel opening which is efficiently made reversible by its hydrolysis. It should be emphasized that the major point of this study is not that the CFTR channel normally functions in the absence of ATP hydrolysis but that the mechanism of gating is not obligatorily dependent on hydrolysis.
Acknowledgments
This work was supported by the NIDDK of the NIH (DK51619).
References
- Aleksandrov AA, Riordan JR. Regulation of CFTR ion channel gating by MgATP. FEBS Letters. 1998;431:97–101. doi: 10.1016/s0014-5793(98)00713-3. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Hwang T-C, Nairn AC, Gadsby DC. Coupling of CFTR channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. doi: 10.1016/0896-6273(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Berger HA, Travis SM, Welsh MJ. Fluoride stimulates cystic fibrosis transmembrane conductance regulator Cl− channel activity. American Journal of Physiology. 1998;274:L305–312. doi: 10.1152/ajplung.1998.274.3.L305. [DOI] [PubMed] [Google Scholar]
- Carson MR, Welsh MJ. 5-Adenylylimidodiphosphate does not activate CFTR chloride channels in cell-free patches of membrane. American Journal of Physiology. 1993;265:L27–32. doi: 10.1152/ajplung.1993.265.1.L27. [DOI] [PubMed] [Google Scholar]
- Chang X-B, Tabcharani JA, Hou Y-X, Jensen TJ, Kartner N, Alon N, Hanrahan JW, Riordan JR. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all ten PKA consensus phosphorylation sites. Journal of Biological Chemistry. 1993;268:11304–11311. [PubMed] [Google Scholar]
- Dousmanis AG, Nairn AC, Gadsby DC. [Mg2+] governs CFTR Cl− channel opening and closing rates, confirming hydrolysis of two ATP molecules per gating cycle. Biophysical Journal. 1996;70:A127. [Google Scholar]
- Gadsby DC, Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiological Reviews. 1999;79:S77–107. doi: 10.1152/physrev.1999.79.1.S77. [DOI] [PubMed] [Google Scholar]
- Gunderson KL, Kopito RR. Effects of pyrophosphate and nucleotide analogs suggest a role for ATP hydrolysis in Cystic Fibrosis Transmembrane Regulator channel gating. Journal of Biological Chemistry. 1994;269:19349–19353. [PubMed] [Google Scholar]
- Gunderson KL, Kopito RR. Conformational states of CFTR associated with channel gating: the role of ATP binding and hydrolysis. Cell. 1995;82:231–239. doi: 10.1016/0092-8674(95)90310-0. [DOI] [PubMed] [Google Scholar]
- Hwang T-C, Nagel G, Nairn AC, Gadsby DC. Regulation of the gating of cystic fibrosis transmembrane conductance regulator Cl channels by phosphorylation and ATP hydrolysis. Proceedings of the National Academy of Sciences of the USA. 1994;91:4698–4702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S-C, Emsley C, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, Gallagher MP, Gill DR, Hubbard RE, Higgins CF. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Li C, Ramjeesingh M, Wang W, Garami E, Hewryk M, Lee D, Rommens JM, Galley K, Bear CE. ATPase activity of the Cystic Fibrosis Transmembrane Conductance regulator. Journal of Biological Chemistry. 1996;271:28463–28468. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- Mathews CR, Tabcharani JA, Hanrahan JW. The CFTR chloride channel: nucleotide interections and temperature-dependent gating. Journal of Membrane Biology. 1998;163:55–66. doi: 10.1007/s002329900370. [DOI] [PubMed] [Google Scholar]
- Quinton PM, Reddy MM. Control of CFTR chloride conductance by ATP levels through non-hydrolytic binding. Nature. 1992;360:79–81. doi: 10.1038/360079a0. [DOI] [PubMed] [Google Scholar]
- Rich DP, Berger HA, Cheng SH, Travis SM, Saxena M, Smith AE, Welsh MJ. Regulation of the Cystic Fibrosis Transmembrane Conductance Regulator Cl− channel by negative charge in the R-domain. Journal of Biological Chemistry. 1993;268:20259–20267. [PubMed] [Google Scholar]
- Schultz BD, Bridges RJ, Frizzell RA. Lack of conventional ATPase properties in CFTR chloride channel gating. Journal of Membrane Biology. 1996;151:63–75. doi: 10.1007/s002329900058. [DOI] [PubMed] [Google Scholar]
- Schultz BD, Venglarik CJ, Bridges RJ, Frizzell RA. Regulation of CFTR Cl− channel gating by ADP and ATP analogues. Journal of General Physiology. 1995;105:329–361. doi: 10.1085/jgp.105.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiological Reviews. 1999;79:S23–45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Smith RM, Martell AE. Critical Stability Constants, Inorganic Complexes. Vol. 4. New York: Plenum Press; 1976. p. 96. [Google Scholar]
- Weinreich F, Riordan JR, Nagel G. Dual effect of ADP and adenylylimidodiphosphate on CFTR channel kinetics show binding to two different nucleotide binding sites. Journal of General Physiology. 1999;114:55–70. doi: 10.1085/jgp.114.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]


