Abstract
Retinal ‘on’ bipolar cells possess a metabotropic glutamate receptor (mGluR6) linked to the control of a G-protein and cGMP-activated channels which functions to generate high synaptic amplification of rod signals under dark-adapted conditions.
Desensitization of ‘on’ bipolar cells is initiated by a rise in Ca2+ during background light too weak to adapt rod photoreceptors. Desensitization could also be elicited by raising intracellular Ca2+ above 1 μm.
In order to investigate the mechanism of desensitization, whole-cell current responses to brief flashes and to steps of light were obtained from voltage-clamped ‘on’ bipolar cells in dark-adapted dogfish retinal slices. The inclusion of Ca2+-calmodulin kinase II (CaMKII) inhibitor peptides in the patch pipette solutions not only blocked desensitization of ‘on’ bipolar cells by dim background light and by 50 μm Ca2+, but also increased their flash sensitivity.
The substrate of phosphorylation by CaMKII is the ‘on’ bipolar cell cGMP-activated channels. Desensitization probably results from a reduction in their sensitivity to cGMP and a voltage-dependent decrease in their conductance.
A role for protein kinase C (PKC) in this process was excluded since activating PKC independently of Ca2+ with the phorbol ester PMA failed to induce desensitization of ‘on’ bipolar cells.
Very dim background light in which only one out of three rods would have absorbed a photon doubles the human threshold for detecting a superimposed flash (Rushton, 1965). This desensitization occurs in the retina on the pooling of rod signals in the network of cells postsynaptic to the rods, since it occurs at light intensities too low to reduce rod sensitivity, and has thus been termed ‘network adaptation’ (Dowling & Ripps, 1970). The underlying mechanism remained totally unknown until recently when we showed that desensitization by background light was initiated in ‘on’ bipolar cells in the virtually all-rod dogfish retina by the influx of Ca2+ through their cGMP-activated channels which open with light (Shiells & Falk, 1999a). These channels possess a high permeability to Ca2+ (Yamada et al. 1998; Nawy, 1999b, 2000).
Voltage gain in synaptic transmission from rods to ‘on’ bipolar cells is of the order of 100-fold when fully dark adapted (Ashmore & Falk, 1980). High gain in this system results from the coupling of single metabotropic glutamate receptors (mGluRs) to the control of a large number of cGMP-activated channels and the larger voltage change which results because most of these channels are closed in the dark (Shiells & Falk, 1994). Rod photoreceptors are relatively depolarized in the dark and release glutamate from their synaptic terminals. Glutamate activates the ‘on’ bipolar cell mGluR6 (Nakajima et al. 1993) which is selectively activated by 2-amino-4-phosphonobutyrate (APB) (Shiells et al. 1981). Results from previous electrophysiological studies are consistent with the receptor activating a G-protein and phosphodiesterase (PDE), leading to the hydrolysis of cGMP and thus a reduction in cGMP-activated conductance (Shiells & Falk, 1990, 1992a;Nawy & Jahr, 1990, 1991; de la Villa et al. 1995). Light hyperpolarizes rods, reducing glutamate release, so depolarizing ‘on’ bipolar cell light responses were thought to arise from increases in cGMP-activated conductance. More recently, however, Nawy (1999a) has proposed that the PDE, and hence changes in cGMP, are not directly involved in generating ‘on’ bipolar cell responses to glutamate, since the PDE inhibitor IBMX did not block the responses. Instead, it was proposed that cGMP opens the postsynaptic channels and the G-protein Goα, which has been localized in ‘on’ bipolar cell dendrites (Vardi, 1998), acts directly to close the cGMP-activated channels. However, transducin has also been shown to block rod cGMP-activated channels (Krapivinsky et al. 1989) and, furthermore, IBMX does not block rod light responses (Cervetto & McNaughton, 1986). Just as this evidence does not exclude the cGMP cascade hypothesis in phototransduction, it is also insufficient to exclude the original cGMP cascade hypothesis underlying ‘on’ bipolar cell transduction (Shiells & Falk, 1990; Nawy & Jahr, 1990).
This paper explores the mechanism of desensitization in ‘on’ bipolar cells induced by the rise in Ca2+ with background light, elucidating the biochemical mechanism responsible for desensitization. The terms ‘desensitization’ and ‘light adaptation’ have often been used interchangeably. It is necessary, however, to distinguish between these and define desensitization as the reduction in response to light at all light intensities, whilst light adaptation is a subsequent light- and time-dependent shift in the response to light so as to require higher light intensities. Desensitization is used here in the same sense as the diminished ability of, for example, acetylcholine to open a channel in the presence of a steady background concentration.
Previously, we reported in a brief communication that the protein kinase inhibitor H-7, which has a wide spectrum of action, blocked desensitization of ‘on’ bipolar cells induced by 50 μm Ca2+ and by background light (Shiells & Falk, 1999b). An inhibitor relatively selective for Ca2+-dependent isoforms of protein kinase C (PKC), Gö 6976, whose activity against other kinases is unknown, was similarly effective in blocking desensitization. More recently, highly specific small peptide inhibitors of Ca2+-calmodulin kinase II (CaMKII) have been developed (Ishida et al. 1995). Using these we now show that elevation of free Ca2+ via the patch pipette, or the entry of Ca2+ through the cGMP-activated channels during background light, activates CaMKII. The results suggest that CaMKII phosphorylates the ‘on’ bipolar cell cGMP-activated channels causing them to close, probably by reducing their sensitivity to cGMP. If phosphorylation is blocked, then ‘on’ bipolar cells no longer desensitize with background light or elevation of Ca2+ in the patch pipette solution.
METHODS
Patch-clamp recording from retinal slices
Whole-cell voltage-clamp recordings were obtained from bipolar cells on, or just below, the surface of dark-adapted retinal slices prepared from the retina of the dogfish, Scyliorhinus canicula (Shiells & Falk, 1990). Dogfish were dark adapted overnight and were killed humanely by stunning, decapitation, and then pithing before removal of the eyes under dim red light. The slices were continuously superfused with oxygenated Ringer solution at 16–18°C, and were viewed under infra-red illumination. The Ringer solution contained (mm): NaCl 260, KCl 3, CaCl2 4, NaHCO3 20, MgSO4 0.5, urea 350, D-glucose 10, and Hepes 5, and was buffered to pH 7.7 when bubbled with 95 % O2–5 % CO2. Patch pipettes were coated with a heated mixture of parafilm, mineral oil and wax to improve gigaseal formation, and when filled had resistances of 2–3 MΩ. The patch pipette solution contained (mm): CsCl 260, TEA 20, MgSO4 5, Hepes 10, and urea 350, and was buffered to pH 7.3, to which was added 1 mm ATP and 1 mm GTP just before the experiment. The Ca2+ chelators BAPTA or N,N,N′,N′-tetrakis(2-pyridyl-methyl)ethylenediamine (TPEN), both at 5 mm, were added to the patch pipette solutions for buffering free Ca2+ concentrations. The dissociation constant (KD) of TPEN is 39 μm, which makes it much better than BAPTA (KD= 110 nm) for buffering higher concentrations of free Ca2+. A computer program (MaxChelator) was used to calculate the amount of CaCl2 that we needed to add to yield the required free Ca2+ concentrations (5.03 mm with BAPTA and 1.05 mm with TPEN for 50 μm free Ca2+). Similar desensitization of ‘on’ bipolar cells was obtained using either BAPTA or TPEN to obtain a free Ca2+ concentration of 50 μm in the patch pipette solution. This high Ca2+ concentration was used because 50 μm Ca2+ was effective at desensitizing ‘on’-bipolar cell light responses whilst buffering to 1 μm prevented such desensitization (Shiells & Falk, 1999a). The Ca2+ concentration required for half-maximal activation of CaMKII has been determined to be 4 μm (Kennedy et al. 1983). All chemicals were obtained from Sigma unless otherwise indicated. CaMKII inhibitor peptide (281–309) (Calbiochem), (Ala9)-autocamtide-2 (Calbiochem) and the protein kinase C activator phorbol 12-myristate 13-acetate (PMA) were added to the patch pipette solutions with appropriate free Ca2+ levels. Following gigaseal formation and subsequent rupture of the membrane patch to establish the whole-cell mode, the dark membrane potential was measured under current clamp. Cells were then voltage clamped to their dark potentials (which were corrected for the tip potential) and responses to light were obtained before there was any change in the intracellular media, and after full equilibration with the patch pipette solutions, usually 15–20 min after achieving the whole-cell clamp. Similar equilibration times have been reported in salamander ‘on’ bipolar cells (Lasansky, 1992). Although dogfish ‘on’ bipolar cells have relatively small somas (10 μm), they do possess extensive dendritic fields with up to 1000 rods converging onto a single cell (Ashmore & Falk, 1980), which might account for the long equilibration times. Previous recordings from these cells have demonstrated that as long as ATP and GTP are included in the patch pipette solutions, the dark current and input conductance remain stable, as do the flash responses, which decline only by about 10–15 % over periods of 30–45 min (Shiells & Falk, 1990, 1992c). The addition of low concentrations (20 μm) of cGMP to the patch pipette solutions was found to increase ‘on’ bipolar cell light responses in a similar manner to stimulation of soluble guanylate cyclase by NO (Shiells & Falk, 1992b). Endogenous synthesis of cGMP from GTP therefore seems to be sufficient to maintain light responses over long periods. When Ca2+ levels were buffered to 1 μm in the patch pipette solution, there was no change in dark current or input conductance, and only a slight reduction in flash responses over 15 min of recording (see Fig. 2 in Shiells & Falk, 1999a). Input conductances were measured by applying 1–5 mV voltage command pulses during the recordings and data were rejected if there were any large variations in series resistance (usually 5–10 MΩ).
Figure 2. Desensitization of ‘on’ bipolar cells by 50 μm Ca2+.
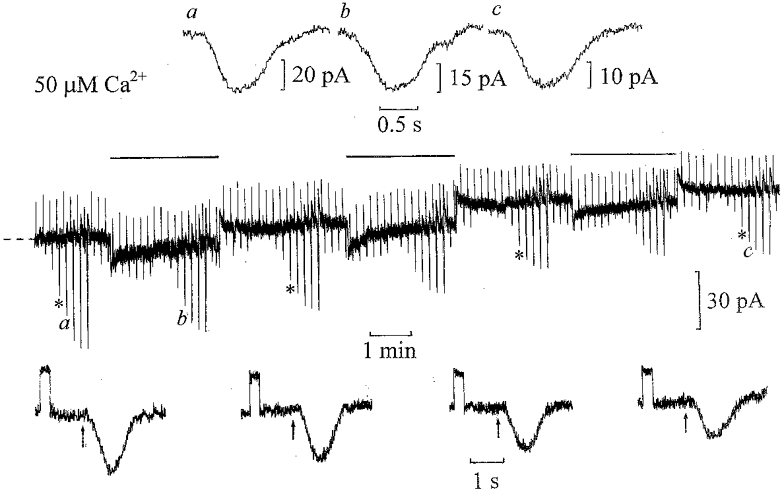
Whole-cell voltage-clamp recording from an ‘on’ bipolar cell with the patch pipette solution containing 50 μm Ca2+ buffered with 5 mm TPEN. The flash intensity was increased by factors of two from 0.125 to 16 Rh** in the dark, and against dim 0.5 Rh** s−1 backgounds. The dim test flash intensity before and during the steps was 0.5 Rh**. The cell was voltage clamped to its dark potential of −39 mV. The current responses to 1 mV voltage commands and responses to 1 Rh** flashes are shown below on expanded time scales at the same gain. On equilibration with 50 μm Ca2+ there was a decrease in input conductance from 25 to 19 nS with an outward shift in dark current of 26 pA. Responses to 2 Rh** flashes shown above the trace (flashes applied at the beginning of each record) were scaled to the same amplitudes in the dark (a) against the background (b) and on equilibration with 50 μm Ca2+ in the dark (c).
Light stimulation
The light absorbed by the rods (rhodopsin molecules isomerized per rod (Rh**)) was estimated from previous ‘on’ bipolar cell measurements in the eyecup (Ashmore & Falk, 1980), because of self-screening by the rods and the variable thickness of the slices (150–250 μm). Half-maximal ‘on’ bipolar cell flash responses were taken to correspond to one Rh**, the half-saturation intensity of the voltage response and the b-wave in eyecup measurements (Shiells & Falk, 1999c). Brief flashes (0.2 ms) were applied using a green light-emitting diode (LED; peak emission wavelength 530 nm) mounted below the preparation. The light was calibrated with a photodiode to increase by factors of two over a range of ten light intensities. For step illumination, another green LED was mounted above the preparation. To test for desensitization, flash intensity-current response relations were determined in the dark, then against dim background light levels just after achieving the whole-cell clamp, and subsequently at intervals up to and on full equilibration with the patch pipette solutions.
RESULTS
Desensitization of ‘on’ bipolar cells by light is blocked by inhibition of CaMKII
The upper trace in Fig. 1 shows a short section of a control recording from an ‘on’ bipolar cell obtained 12 min after achieving whole-cell clamp with no Ca2+ buffer in the patch pipette solution. Under these conditions, when intracellular Ca2+ levels are allowed to rise, desensitizing responses to steps of light can be observed (Shiells & Falk, 1999a). The recording shows initial inward current responses (downward deflections) to a series of light flashes increasing in intensity by factors of two, which allowed us to determine the cell’s light intensity-current response relation in the dark. A dim light, isomerizing 0.5 rhodopsin molecules per rod per second (0.5 Rh** s−1), which is too dim to induce significant desensitization of rods (Fain, 1977), was then turned on (horizontal bar above the trace). This induced a transient inward current rapidly decaying with a time constant of about 5–10 s to a low steady level, as observed previously in the absence of Ca2+ buffering (Shiells & Falk, 1999a; Shiells, 1999). Responses to dim test flashes (0.5 Rh**) were reduced in amplitude after the beginning of the light step. The flash intensity-current response relation was then determined in the presence of the dim background light; responses to dim flashes were reduced by about a factor of two, with a reduction in their maximum responses. On switching off the light there was a transient outward current. The lower trace in Fig. 1 shows a whole-cell recording from another ‘on’ bipolar cell with 10 μm (Ala9)-autocamtide-2 (AIP) added to the patch pipette solution, which contained no Ca2+ buffer. AIP is a highly specific inhibitor of CaMKII, having no inhibitory effect on CaMKIV, PKC or cAMP-dependent protein kinase at 10 μm (Ishida et al. 1995). The same flash protocol was repeated at intervals in the dark and against the same dim backgrounds. As the cell equilibrated with AIP, there was a gradual increase in the dim (0.5 Rh**) test flash responses and input conductance (shown below on expanded time scales), which was accompanied by an inward shift in the dark current. The response to the second background light step, some 8 min after achieving whole-cell clamp, was more sustained, with no desensitization of the dim light responses superimposed on the step and little reduction in their maximum responses. On complete equilibration with the patch pipette solution containing AIP, nearly 14 min after achieving whole-cell clamp, the response to the light step was still more sustained. A slow decay of inward current with a time constant of about a minute was most probably due to a sag in the level of rod hyperpolarization in response to background light (Fain, 1977). Dim flash responses superimposed on the step now showed a slight increase in amplitude compared to those measured in the dark. Similar results were obtained in three ‘on’ bipolar cell recordings with 10 μm AIP in the patch pipette solution. The mean (±s.e.m.) input conductance increased from 13 ± 2 nS to 22 ± 3 nS and was accompanied by mean inward shifts in the dark current of −22 ± 3 pA. An inward shift or increase in dark current accompanied by a increase in input conductance indicates the opening of cGMP-activated channels (Shiells & Falk, 1990). The results show that inhibition of CaMKII blocks the process of desensitization induced by background light, an effect similar to that obtained using the Ca2+ buffer BAPTA in the patch pipette solution to suppress rises in intracellular Ca2+ (Shiells & Falk, 1999a).
Figure 1. Inhibition of CaMKII blocks desensitization by dim background light.
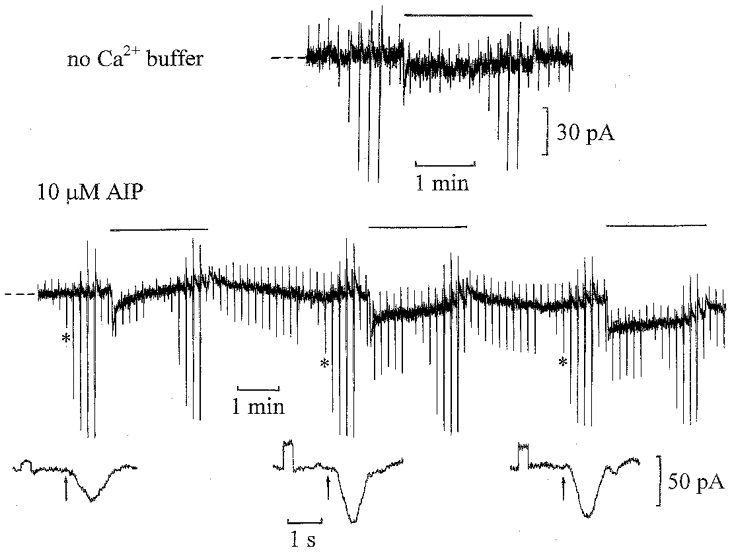
Upper trace: a short section of a whole-cell voltage-clamp recording from an ‘on’ bipolar cell, starting 12 min after achieving whole-cell clamp with no Ca2+ buffer in the patch pipette solution (dark holding potential −38 mV; zero current level indicated here and in subsequent figures by the dashed lines at the beginning of the traces). The flash intensity was increased by factors of two from 0.0625 to 8 Rh** in the dark, resulting in inward current responses (downward deflections), and against dim 0.5 Rh** s−1 backgrounds, indicated here and in subsequent figures by horizontal bars above the traces. The dim test flash intensity before and during the light steps was 0.5 Rh**. The upward deflections are current responses to 1 mV commands and there was a slight decrease in input conductance from 25 to 22 nS during this recording, with no change in the dark current or flash sensitivity. Lower trace: whole-cell voltage-clamp recording from an ‘on’ bipolar cell with no Ca2+ buffer and 10 μm AIP in the patch pipette solution, using the same flash protocol as above. The current responses to 1 mV voltage commands (upward deflections), applied to monitor changes in input conductance, and light responses (marked by asterisks in this and subsequent figures) to 0.5 Rh** flashes are shown below on expanded time scales at the same gain (the timing of the flashes in this and other figures is indicated by the arrows below the traces). The input conductance increased from 10 to 29 nS, and then showed a slight reduction to 26 nS, which was accompanied by an inward shift in the dark current of −19 pA. The cell was voltage clamped to its dark potential of −40 mV.
Desensitization of ‘on’ bipolar cells by elevated Ca2+ is blocked by inhibition of CaMKII
If the desensitizing action of light is mediated by a rise in intracellular Ca2+ which in turn activates CaMKII, then inhibition of CaMKII should also block the effects of raising intracellular Ca2+. Figure 2 illustrates the effect of raising the patch pipette solution to 50 μm free Ca2+ with 5 mm TPEN on the ‘on’ bipolar cell light responses in the dark and against dim 0.5 Rh** s−1 backgrounds. As the Ca2+ diffused into the cell from the patch electrode, the amplitude of the dim (1 Rh**) test flash responses was reduced. The input conductance decreased (shown below on expanded time scales), and was accompanied by an outward shift in the dark current. On applying the background steps before and on equilibration with 50 μm Ca2+ there was further desensitization of the superimposed flash responses. Similar results were obtained in five ‘on’ bipolar cell recordings with the patch pipette solutions buffered to 50 μm Ca2+. The mean ±s.e.m. input conductance decreased from 21 ± 2 nS to 12 ± 3 nS with outward shifts in the dark current of 30 ± 6 pA. The decrease in sensitivity induced by 50 μm Ca2+ in darkness was similar to that induced by background light. The accompanying outward shift in dark current with reduction in input conductance would suggest that Ca2+ acts to close the cGMP-activated channels mediating the ‘on’ bipolar cell light responses. As observed previously (Shiells & Falk, 1999a), there was no significant change in the time course of the light responses in high Ca2+ or in the presence of dim backgrounds (scaled 2 Rh** flash responses shown above in Fig. 2). The time course of these scaled responses was identical, except for the response in the dark on equilibration with 50 μm Ca2+, which was slightly more prolonged.
Figure 3 shows an ‘on’ bipolar cell recording with 20 μm CaMKII inhibitor peptide (281–309) (CaMKIIi) and 50 μm Ca2+ included in the patch pipette solution. The inhibitor is a synthetic peptide containing both the calmodulin binding site and the autophosphorylation site of CaMKII (Waxham & Aronowski, 1993). Just after achieving whole-cell clamp, the flash responses superimposed on the dim background showed some initial desensitization. As the cell equilibrated with CaMKIIi and 50 μm Ca2+, there was a gradual increase in the amplitude of the dim (1 Rh**) test flash response, accompanied by an increase in input conductance (shown below on expanded time scales) and an inward shift in the dark current. These changes are consistent with CaMKIIi blocking the effect of 50 μm Ca2+. Furthermore, dim backgrounds induced progressively more sustained inward current responses, with little reduction in the superimposed test flash responses compared to those in the dark. Similar results were obtained in four ‘on’ bipolar cell recordings under these conditions. The mean ±s.e.m. input conductance increased from 16 ± 2 to 26 ± 4 nS with mean inward shifts in dark current of −31 ± 7 pA. Thus inhibiting CaMKII not only blocks desensitization induced by light, but also that induced by 50 μm Ca2+. In both this recording and that illustrated in Fig. 1 with AIP there was a progressive increase in flash sensitivity on inhibition of CaMKII.
Figure 3. CaMKII inhibition blocks desensitization induced by 50 μm Ca2+.
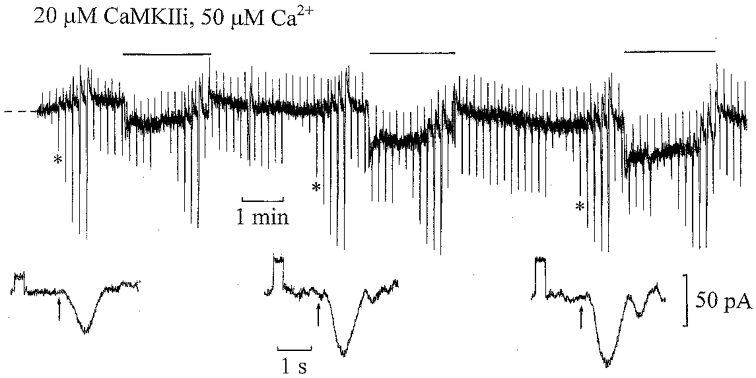
Whole-cell ‘on’ bipolar cell recording with 50 μm Ca2+ and 20 μm CaMKII inhibitor peptide (281–309) (CaMKIIi) in the patch pipette solution. The flash intensities ranged from 0.0625 to 8 Rh** with 0.5 Rh** test flashes applied before and during 0.5 Rh** s−1 steps. The dark holding potential was −37 mV. Current responses to 1 mV voltage commands and responses to 0.5 Rh** flashes are shown below on expanded time scales at the same gain. The input conductance increased from 15 to 45 nS with a small inward shift in the dark current of −15 pA.
The series of flash responses recorded in the dark just after achieving whole-cell clamp (Control) and on equilibration with CaMKIIi and 50 μm Ca2+ are shown in Fig. 4. Over the linear range of the flash responses (up to 0.5 Rh**), there is a pronounced increase in sensitivity on equilibration, whilst the maximum flash responses have similar amplitudes but become more prolonged on inhibition of CaMKII. Prolongation of flash responses is a characteristic of supra-maximal light responses (Ashmore & Falk, 1980). They become more prolonged on CaMKII inhibition because of their increased light sensitivity, suggesting an increased affinity of the channels for cGMP. One possible explanation for this rise in sensitivity, also observed with AIP, is that there is some basal level of CaMKII activity in the dark which is inhibited by the peptides. Some light adaptation may have occurred during preparation of the retinal slices under dim red light, so the rise in sensitivity probably reflects the return to a more dark-adapted condition. Similar results were obtained with AIP and 50 μm Ca2+ in the patch pipette solution. Figure 5 shows a recording from an ‘on’ bipolar cell on equilibration with 10 μm AIP and 50 μm Ca2+. On application of a 0.5 Rh** s−1 background, this cell showed a slight increase in flash sensitivity compared to that in the dark, confirming that inhibition of CaMKII prevents desensitization induced by elevated Ca2+ and by background light. In this and previous recordings with CaMKII inhibitors, there is a slow outward shift in the step response such that the response to light offset was smaller than the response to light onset. This is not due to desensitization of the ‘on’ bipolar cell, since there was an increase in the superimposed flash responses, but rather it is probably due to a sag in the rod voltage response to dim maintained light steps, which becomes more prominent at higher light intensities (Fain, 1977).
Figure 4. Inhibition of CaMKII increases ‘on’ bipolar cell flash sensitivity.
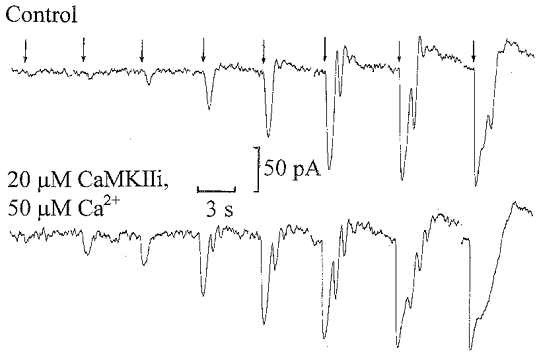
Inward current responses to flashes in the dark ranging from 0.0625 to 8 Rh* recorded just after achieving whole-cell clamp (upper traces, Control) and on equilibration (12 min after achieving whole-cell clamp) with 20 μm CaMKIIi and 50 μm Ca2+ (lower traces) from the recording illustrated in Fig. 3. The timing of the flashes is indicated by the arrows above the records.
Figure 5. CaMKII inhibition by AIP blocks ‘on’ bipolar cell desensitization induced by 50 μm Ca2+.
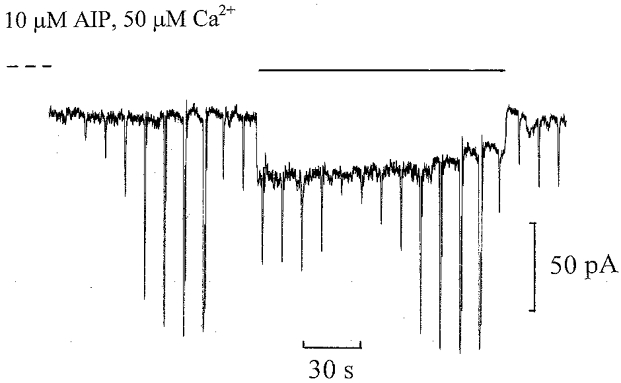
Whole-cell ‘on’ bipolar cell recording equilibrated with 10 μm AIP and 50 μm Ca2+ in the patch pipette solution. The flash intensities ranged from 0.125 to 16 Rh** with a 1 Rh** test flash applied before and during the 0.5 Rh** s−1 background. The dark holding potential was −38 mV. There was an inward shift in dark current of −26 pA.
Flash intensity-current response relations
The upper graph in Fig. 6 shows averaged light-induced inward currents plotted against flash intensity (log2 Rh*/rod) recorded from ‘on’ bipolar cells in the dark and against dim 0.5 Rh** s−1 background light just after achieving whole-cell clamp and on equilibration with 50 μm Ca2+. To illustrate the shift in current induced by the background, the flash responses were measured from the dark current level such that the total light-induced current (background shift in current plus the current response to each flash) is shown. The dashed curve in the presence of the background (Rh** s−1) is shifted upwards relative to that in the dark by the inward current induced by the background. Just after achieving whole-cell clamp, the background desensitized the flash responses, reducing their mean amplitudes compared to those in the dark. The flash responses in the dark and against the background were further reduced (> 50 %) on equilibration with 50 μm Ca2+ (15–20 min after achieving whole-cell clamp). Both background light and elevated Ca2+ reduced the peak total light-induced current.
Figure 6. Flash intensity-total inward current response relations plotted on logarithmic flash intensity axes, showing the current shifts induced by the backgrounds.
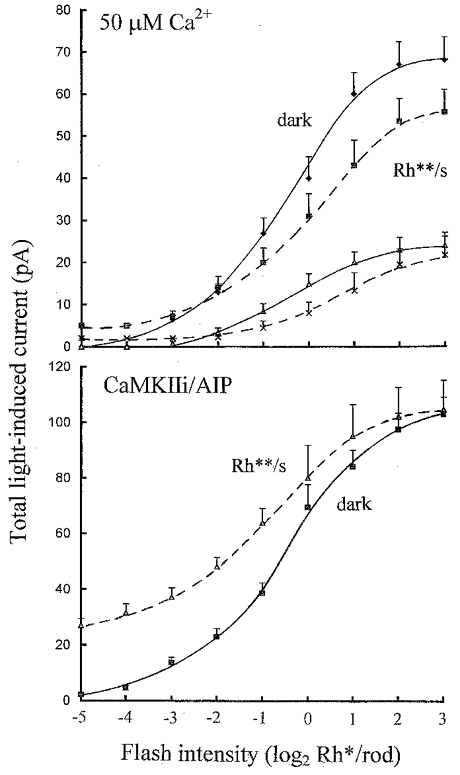
Upper graph: averaged intensity-response relations from 5 ‘on’ bipolar cell recordings in the dark (continuous curves) and against dim 0.5 Rh** s−1 backgrounds (dashed curves), just after achieving whole-cell clamp (diamonds, squares) and on equilibration (triangles, crosses) with 50 μm Ca2+ (error bars show +s.e.m.). Responses were measured from the same dark current level to show the shift in current effected by the backgrounds, i.e. flash responses plus the inward current induced by the light steps. The light intensity axis is shown on a logarithmic scale to base 2 (log2 Rh*/rod). Lower graph: averaged intensity-response relations from 7 ‘on’ bipolar cells, 4 on equilibration with 20 μm CaMKIIi and 50 μm Ca2+ and 3 with 10 μm AIP in the patch pipette solutions in the dark (continuous curve) and against 0.5 Rh** s−1 backgrounds (dashed curve).
The lower graph in Fig. 6 shows averaged flash intensity- current response relations from ‘on’ bipolar cells in the dark and against 0.5 Rh** s−1 background light on equilibration with CaMKIIi or AIP. The shift in current induced by the background was much larger and more sustained than just after achieving whole-cell clamp, or on equilibration with 50 μm Ca2+ (shown above), and there was no desensitization of the superimposed flash responses. There was no reduction in the peak total inward current induced by the background and superimposed flashes compared to that in the dark. These results are very similar to those obtained with BAPTA and no added Ca2+ in the patch pipette solutions to suppress rises in intracellular Ca2+ induced by background light (Shiells & Falk, 1999a).
To derive absolute changes in flash sensitivity from these measurements, flash-induced current responses were plotted against light intensity on linear scales, over the linear response range (up to 0.5 Rh**), disregarding the small shifts in current induced by the background. Figure 7 shows the same data from Fig. 6 plotted in this way. From the slope of the regression lines fitted to these plots (left), the mean flash sensitivity of five ‘on’ bipolar cells was 53 pA Rh**−1 in the dark, which was reduced to 29 pA Rh**−1 by the background. On equilibration with 50 μm Ca2+, the mean flash sensitivity in the dark was reduced to 15 pA Rh**−1. The mean flash sensitivity of seven ‘on’ bipolar cells (right) obtained just after achieving whole-cell clamp in the dark was similar to the previous sample, 51 pA Rh**−1. This almost doubled to 90 pA Rh**−1 in the dark on equilibration with AIP or CaMKIIi, and showed a statistically insignificant reduction to 84 pA Rh**−1 with the 0.5 Rh** s−1 background.
Figure 7. Flash intensity-inward current response relations plotted on linear flash intensity axes, showing absolute changes in flash sensitivity.
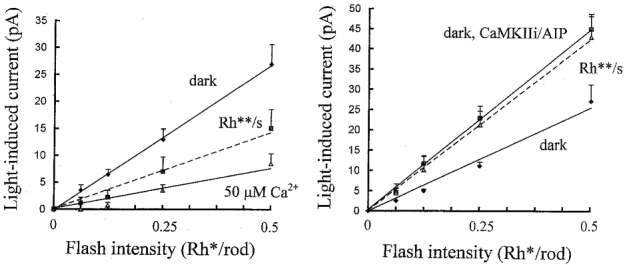
The same data from Fig. 6 was plotted on linear flash intensity axes up to 0.5 Rh**, showing the linear range of light-induced inward currents in the dark and against 0.5 Rh** s−1 backgrounds, without illustrating the shifts in current in response to the backgrounds. Left graph: light-induced current responses measured just after achieving whole-cell clamp (initial) in the dark (diamonds), against 0.5 Rh** s−1 backgrounds (squares) and in the dark on equilibration with 50 μm Ca2+ (triangles). Corresponding flash sensitivities, obtained from the slopes of the linear regression lines, were 53, 29 (Student’s t test, significant difference from dark, P < 0.05) and 15 pA Rh**−1 (t test, significant difference from dark initial, P < 0.01) (n= 5). Right graph: responses measured just after achieving whole-cell clamp (initial) in the dark (diamonds), on equilibration with the CaMKII inhibitor peptides in the dark (squares) and against 0.5 Rh** s−1 backgrounds (triangles) with either 50 μm Ca2+ or no Ca2+ buffer. Corresponding flash sensitivities were 51, 90 (t test, significant difference from initial, P < 0.05) and 84 pA Rh**−1 (not significantly different from the dark on equilibration with CaMKII inhibitors) (n= 7). A correction was applied to the 0.5 Rh** flash responses on equilibration with the CaMKII inhibitors because these were outside the linear response range. These have been corrected for non-linearity using the third-order expansion for a rectangular hyperbola.
CaMKII acts on the cGMP-activated channels
One possible site of CaMKII action could be the ‘on’ bipolar cell cGMP-activated channels. The sensitivity of rod cGMP-activated channels to cGMP has been shown to be reduced by phosphorylation (Gordon et al. 1992). When introduced via the patch pipette with low internal Ca2+, cGMP induces an inward current in ‘on’ bipolar cells due to the opening of cGMP-activated channels (Shiells & Falk, 1990, 1992c; Nawy & Jahr, 1990; de la Villa et al. 1995). To test for a direct effect of CaMKII on the channels, ‘on’ bipolar cell recordings were obtained with a saturating cGMP concentration and 50 μm Ca2+ to fully activate CaMKII. Figure 8 shows the effect of the inclusion of 1 mm IBMX, to block PDE activity (reducing cGMP hydrolysis), and 1 mm cGMP in the 50 μm Ca2+ patch pipette solution on an ‘on’ bipolar cell (upper trace). Instead of the expected inward current induced by cGMP, there was an outward shift in dark current which was accompanied by a decrease in input conductance. Mean outward shifts in dark current of 23 ± 9 pA were observed in three ‘on’ bipolar cells. Conversely, when 10 μm AIP was added to the same patch pipette solution to block CaMKII, the expected effect of cGMP, induction of inward shifts in the dark current and increases in input conductance, was observed (lower trace). Similar results were obtained using 20 μm CaMKIIi instead of AIP. In five cells with AIP (n= 3) or CaMKIIi (n= 2) mean increases in input conductance of 12 ± 2 and 25 ± 1 nS, respectively, were observed, accompanying a mean inward shift in dark current of −74 ± 5 pA on equilibration. Light responses were inhibited by 80 %, but were not completely suppressed by elevating cGMP and IBMX. This could be due to the spread of photocurrent from adjacent non-dialysed ‘on’ bipolar cells, which have been shown to be electrically coupled at their dendrites in other fish retinas (Saito & Kujiroaka, 1988). These results suggest that high Ca2+ activates CaMKII and suppresses the inward current induced by cGMP by phosphorylation of the channels. A voltage-dependent reduction in channel conductance (outward rectification) by high internal Ca2+ has been reported in salamander ‘on’ bipolar cells (Nawy, 2000), and has also been observed in dogfish ‘on’ bipolar cells (R. A. Shiells & G. Falk, in preparation). A reduction in channel affinity for cGMP and a reduction in conductance might account for the outward shifts in dark current with 1 mm cGMP and 50 μm Ca2+.
Figure 8. CaMKII acts on the ‘on’ bipolar cell cGMP-activated channels.
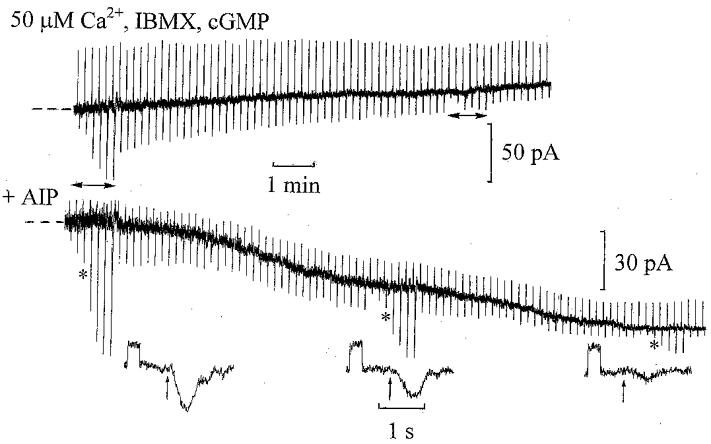
Upper trace: whole-cell recording from an ‘on’ bipolar cell with 1 mm IBMX, 1 mm cGMP and 50 μm Ca2+ in the patch pipette solution. The upward deflections are current responses to 2 mV voltage command pulses applied to monitor changes in input conductance, whilst the downward deflections are inward current responses to 1 Rh** test flashes. At intervals, the intensity-response relation was determined, marked by the horizontal arrows below the trace. The dark holding potential was −29 mV. The input conductance decreased from 29 to 19 nS, with an outward shift in dark current of 21 pA. Lower trace: ‘on’ bipolar cell recording with 10 μm AIP added to the patch pipette solution containing 50 μm Ca2+, 1 mm IBMX and 1 mm cGMP. The dark holding potential was −39 mV. Current responses to 1 mV voltage commands and responses to 0.5 Rh** test flashes are shown below on expanded time scales at the same gain. The input conductance increased from 11 to 16 nS, with an inward shift in the dark current of −54 pA.
Activation of PKC does not induce desensitization
We previously reported that the non-selective protein kinase inhibitor, H-7, and Gö 6976, a nominally selective inhibitor of Ca2+-dependent PKC isoforms, were effective in blocking the desensitization of ‘on’ bipolar cells induced by 50 μm Ca2+ and background light (Shiells & Falk, 1999b). Since the α-isoform of PKC is a marker for the ‘on’ bipolar cell (Suzuki & Kaneko, 1990), a more definitive differentiation between the kinases was to activate PKC independently of Ca2+ with the phorbol ester, PMA. Figure 9A shows a whole-cell recording from an ‘on’ bipolar cell on equilibration with 1 μm PMA with free Ca2+ buffered to 0.5 μm. This resulted in no desensitization of the flash responses, as observed with 50 μm Ca2+. There is a slow outward shift in the current response to the 1 Rh** s−1 step, probably due to the sag in rod hyperpolarization which is more prominent because of the higher light level. The average flash intensity-current response relation in the presence of the background light (Fig. 9B) showed no reduction in the peak total light-induced current compared to that in the dark. In three ‘on’ bipolar cell recordings with PMA, there was no decrease in flash sensitivity or significant change in dark current and input conductance on equilibration with the patch pipette solutions. Concentrations of PMA as high as 10 μm, buffered at 0.5 μm Ca2+, did not mimic the action of background light or 50 μm Ca2+, making a role for PKC in the desensitization of ‘on’ bipolar cells unlikely.
Figure 9. The phorbol ester PMA fails to desensitize ‘on’ bipolar cells.
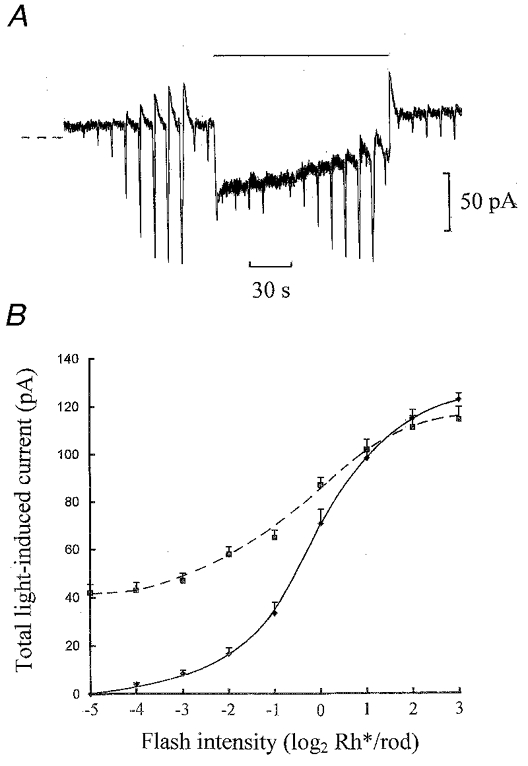
A, whole-cell recording from an ‘on’ bipolar cell on equilibration with 1 μm PMA and free Ca2+ buffered to 0.5 μm in the patch pipette solution. The flash intensities ranged from 0.0625 to 16 Rh** and the test flash intensity was 0.5 Rh** applied before and during a 1 Rh** s−1 light step. The dark holding potential was −39 mV. The sag in the step response is probably due to a pronounced sag in the rod voltage response to the brighter maintained light step. B, averaged light intensity-current response relations from 3 ‘on’ bipolar cells on equilibration with 1 μm PMA and 0.5 μm Ca2+ in the dark (continuous curve) and against 1 Rh** s−1 backgrounds (dashed curve).
DISCUSSION
The results strongly suggest a role for CaMKII in inducing desensitization in ‘on’ bipolar cells by background light. One immunocytochemical study using polyclonal antibodies has localized CaMKII staining in the outer plexiform layer of rat retina (Bronstein et al. 1988). Another study using monoclonal antibodies detected CaMKII immunoreactivity only in amacrine and ganglion cells, with no labelling of bipolar cells or the outer plexiform layer (Ochiishi et al. 1994). It may be that CaMKII in the ‘on’ bipolar cell is a different isoform to the α- and β-isoforms localized in these studies, and it may be restricted to the postsynaptic densities, which could be difficult to detect (Hanson & Schulman, 1992).
The response of an ‘on’ bipolar cell to a dim light step in the absence of Ca2+ buffer in the patch pipette solution consists of an inward current transient which rapidly returns almost to the dark current level during the step. Responses to flashes superimposed on the step were reduced in amplitude compared to those in the dark. When these cells were equilibrated with BAPTA in the patch pipette solution to block rises in Ca2+, their responses to dim light steps became sustained (Shiells & Falk, 1999a; Shiells, 1999), with no reduction of superimposed flash responses. Their step responses also became more sustained with CaMKII inhibitors, or with high concentrations of non-selective protein kinase inhibitors (Shiells & Falk, 1999b) and no desensitization of superimposed flash responses was observed. These results suggest that Ca2+ enters the ‘on’ bipolar cell cGMP-activated channels which open with light, then activates CaMKII which in turn phosphorylates some intracellular target which results in the closing of cGMP-activated channels and reduces subsequent flash sensitivity.
A Ca2+-dependent effect directly on the ‘on’ bipolar cell cGMP-activated channels would account most simply for the observation that elevated Ca2+ depressed flash responses at all light intensities, by a constant factor. Reduction of receptor-G-protein coupling by Ca2+ can be excluded as a principal mechanism of desensitization on the basis that the outward shift in dark current induced by high Ca2+ would have to involve activation of PDE to reduce cGMP, which could only occur if the coupling was increased. This would result in increased, not reduced, flash sensitivity. In both rod photoreceptors (Fain et al. 1989) and olfactory receptors (Zufall et al. 1999), adaptation induced by, respectively, a fall or rise in Ca2+ results in prominent changes in the response time course due to the effects on the intermediate stages, PDE or cyclase activity, of these cascades. The absence of such a change in the time course of the ‘on’ bipolar cell flash response with high Ca2+ or background light suggests that the rise in Ca2+ does not significantly modulate PDE or cyclase activity in this system.
Phosphorylation of rod cGMP-activated channels at both Ser/Thr and tyrosine sites decreases their sensitivity to cGMP (Gordon et al. 1992; Molokanova et al. 1999). A reduction in ‘on’ bipolar cell channel sensitivity to cGMP could account for the suppression of inward current induced by a saturating concentration of cGMP by elevated Ca2+, an effect blocked by the CaMKII inhibitory peptide (281–309) or AIP. The inhibitors of CaMKII not only blocked desensitization by background light and Ca2+ but also increased ‘on’ bipolar cell flash sensitivities. A possible explanation is that there is a pre-existing basal level of CaMKII and phosphatase activity in the dark (perhaps due to some light adaptation) and CaMKII inhibition unmasks dephosphorylation of the channels by phosphatase activity (restoring a more dark-adapted condition). The increase in flash sensitivity, and prolongation of saturating flash responses observed on inhibition of CaMKII, suggests that dephosphorylation of the channels increases their sensitivity to cGMP. An increase in flash sensitivity was not observed with BAPTA with no added Ca2+ in the patch pipette solutions (Shiells & Falk, 1999a), which would block both Ca2+-dependent kinase and phosphatase activity. The precise role of protein phosphatases in recovery from desensitization and the adaptation process remains to be determined. There is some evidence that calcineurin may be expressed in ‘on’ bipolar cells (Nawy, 1999b).
A decrease in the conductance of the channels, however, could also contribute to the reduction in flash sensitivity with high Ca2+. With low internal Ca2+ and when dark adapted, the current-voltage relation of ‘on’ bipolar cell flash responses is linear (Ashmore & Falk, 1980; Shiells & Falk, 1992a) and ‘on’ bipolar cell voltage responses to dim steps of light are sustained. Elevated internal Ca2+ has been recently shown to transform the properties of the channels to an outward rectifier in light-adapted salamander ‘on’ bipolar cells (Nawy, 2000), which would reduce their conductance at more negative potentials. We propose that phosphorylation of the channels underlies the transformation from the dark-adapted linear condition to the light-adapted rectifying condition, and the transition from sustained to transient (or desensitizing) step responses. There is also a direct interaction of the rod channel with Ca2+-calmodulin acting to reduce conductance (Hsu & Molday, 1993). A direct interaction of calmodulin with the ‘on’ bipolar cell cGMP-activated channel would appear to be unlikely, given that Ca2+-induced desensitization could be blocked completely by CaMKII inhibitors.
Nawy (1999a) has proposed that PDE and hence changes in cGMP levels are not directly involved in the action of glutamate on the ‘on’ bipolar cell. At issue, then, is the question of whether the response to light (due to a decrease in glutamate release from rods) is the result of an increased level of cGMP (resulting from PDE inhibition) or to a fall in Goα, resulting in the unblocking of cGMP-activated channels. The profound reduction in ‘on’ bipolar cell light responses with high levels of cGMP would exclude the latter hypothesis. The conclusions of the present paper regarding the mechanism of desensitization by light and by Ca2+ are in no way compromised by the need to resolve the role of Goα and PDE.
Walters et al. (1998) reported that CaMKII might be involved in the regulation of salamander ‘on’ bipolar cell cGMP-activated channels on the basis of results with the inhibitors KN-62 and KN-93. These inhibitors were found to depress the cGMP-activated current which led to the conclusion that Ca2+-stimulated CaMKII phosphorylation increased channel sensitivity to cGMP. This is contrary to our results, which demonstrate that elevating Ca2+ induces desensitization of light responses and blocks responses to cGMP. KN-62 and KN-93 may have exerted a direct blocking action on the ‘on’ bipolar cell cGMP-activated channels, which have distinctly different properties to those of rods (Shiells & Falk, 1992a). Moreover, the experiments were performed in the light, so it is probable that the kinase had been activated and autophosphorylated (Miller et al. 1988) before their recordings had begun. Autophosphorylated CaMKII becomes independent of Ca2+ and calmodulin, and is not inhibited by KN-62 (Tokumitsu et al. 1990).
When fully dark adapted, the gain of the rod-‘on’ bipolar cell pathway is much higher than that of the antagonistic surround contributed by horizontal cells, and there is no surround inhibition either in the ‘on’ bipolar cell (Ashmore & Falk, 1980) or in the ‘on’ ganglion cell (Barlow et al. 1957). Desensitization of the central response by CaMKII activation in the ‘on’ bipolar cell may underly the apparent change in receptive field organization observed in ‘on’ ganglion cell responses with light adaptation, resulting in enhanced contrast sensitivity. With light adaptation, the gain of centre and surround inputs to ‘on’ bipolar cells would become similar due to a reduction in gain, or desensitization, of the central response. ‘Off’ ganglion cells did not show this change in receptive field organization (Barlow & Levick, 1969), consistent with the absence of desensitization in ‘off’ bipolar cells (Shiells & Falk, 1999a) and their lower light sensitivity, which is comparable to horizontal cells (Ashmore & Falk, 1980).
Following activation by Ca2+-calmodulin, CaMKII is known to become autophosphorylated, resulting in a Ca2+- and calmodulin-independent persistently activated state (Miller et al. 1988). This might account for the prolonged reduction in the ‘on’ bipolar cell electroretinogram b-wave or ‘on’ ganglion cell sensitivity following brighter light exposure (Dowling & Ripps, 1970; Enroth-Cugell & Shapley, 1973; Green et al. 1975; Donner et al. 1991; Shiells & Falk, 1999a) and thus in the sensitivity of the rod visual system as a whole (Rushton, 1965; Baylor, 1987), which outlasts the reduction in sensitivity of the rod photoreceptors (Sakmann & Filion, 1972; Thomas & Lamb, 1998). Persistent activation of CaMKII is thought to underly long-term potentiation of synaptic responses in hippocampal neurons, a cellular model of memory formation (Lisman, 1994). It is intriguing that ‘on’ bipolar cells possess the same enzyme, which functions to reduce light sensitivity by phosphorylation of their cGMP-activated channels, providing a mechanism for storing information about the previous history of light exposure.
Acknowledgments
We would like to thank The Wellcome Trust for financial support.
References
- Ashmore JF, Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. The Journal of Physiology. 1980;300:115–150. doi: 10.1113/jphysiol.1980.sp013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, FitzHugh R, Kuffler SW. Change of organization in the receptive fields of the cat’s retina during dark adaptation. The Journal of Physiology. 1957;137:338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. Changes in the maintained discharge with adaptation level in the cat retina. The Journal of Physiology. 1969;202:699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA. Photoreceptor signals and vision. Investigative Ophthalmology and Visual Science. 1987;28:34–49. [PubMed] [Google Scholar]
- Bronstein JM, Wasterlain CG, Bok D, Lasher R, Farber DB. Localization of retinal calmodulin kinase. Experimental Eye Research. 1988;47:391–402. doi: 10.1016/0014-4835(88)90050-4. [DOI] [PubMed] [Google Scholar]
- Cervetto L, McNaughton PA. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. The Journal of Physiology. 1986;370:91–109. doi: 10.1113/jphysiol.1986.sp015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Villa P, Kurahashi T, Kaneko A. L-Glutamate-induced responses and cGMP-activated channels in three subtypes of retinal bipolar cells dissociated from the cat. Journal of Neuroscience. 1995;15:3571–3582. doi: 10.1523/JNEUROSCI.15-05-03571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K, Djupsund K, Reuter T, Vaisanen I. Adaptation to light fluctuations in the frog retina. Neuroscience Research. 1991;15:S175–S184. [PubMed] [Google Scholar]
- Dowling JE, Ripps H. Visual adaptation in the retina of the skate. Journal of General Physiology. 1970;56:491–520. doi: 10.1085/jgp.56.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Shapley RM. Adaptation and dynamics of cat retinal ganglion cells. The Journal of Physiology. 1973;233:271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL. The threshold signal of photoreceptors. In: Barlow HB, Fatt P, editors. Vertebrate Photoreception. London, New York, San Francisco: Academic Press; 1977. pp. 305–323. [Google Scholar]
- Fain GL, Lamb TD, Matthews HR, Murphy RL W. Cytoplasmic calcium concentration as the messenger for light adaptation in salamander rods. The Journal of Physiology. 1989;416:215–243. doi: 10.1113/jphysiol.1989.sp017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Brautigan DL, Zimmerman AL. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992;9:739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- Green DG, Dowling JE, Siegel IM, Ripps H. Retinal mechanisms of visual adaptation in the skate. Journal of General Physiology. 1975;65:483–502. doi: 10.1085/jgp.65.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annual Review of Biochemistry. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- Hsu Y-T, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochemical and Biophysical Research Communications. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Kennedy MB, McGuinness T, Greengard P. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates synapsin I: partial purification and characterization. Journal of Neuroscience. 1983;3:818–830. doi: 10.1523/JNEUROSCI.03-04-00818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky GB, Filatov GN, Filatova EA, Lyubarsky AL, Fesenko EE. Regulation of cGMP-dependent conductance in cytoplasmic membrane of rod outer segments by transducin. FEBS Letters. 1989;247:435–437. doi: 10.1016/0014-5793(89)81386-9. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Properties of depolarizing bipolar cell responses to central illumination in salamander retinal slices. Brain Research. 1992;576:181–196. doi: 10.1016/0006-8993(92)90679-4. [DOI] [PubMed] [Google Scholar]
- Lisman J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends in Neurosciences. 1994;17:404–412. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Miller SG, Patton BL, Kennedy MB. Sequences of autophosphorylation sites in neuronal type II CaM kinase that control Ca2+-independent activity. Neuron. 1988;1:593–604. doi: 10.1016/0896-6273(88)90109-2. [DOI] [PubMed] [Google Scholar]
- Molokanova E, Maddox F, Luetje CW, Kramer RH. Activity-dependent modulation of rod photoreceptor cyclic nucleotide-gated channels mediated by phosphorylation of a specific tyrosine residue. Journal of Neuroscience. 1999;19:4786–4795. doi: 10.1523/JNEUROSCI.19-12-04786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. Journal of Biological Chemistry. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nawy S. The metabotropic receptor mGluR6 may signal through Go, but not phosphodiesterase, in retinal bipolar cells. Journal of Neuroscience. 1999a;19:2938–2944. doi: 10.1523/JNEUROSCI.19-08-02938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. Regulation of the mGluR6 pathway by calcium in retinal bipolar cells. Society for Neuroscience Abstracts. 1999b;25:1431. [Google Scholar]
- Nawy S. Regulation of the on bipolar cell mGluR6 pathway by Ca2+ Journal of Neuroscience. 2000;20:4471–4479. doi: 10.1523/JNEUROSCI.20-12-04471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. cGMP-gated conductance in retinal bipolar cells is suppressed by the photoreceptor transmitter. Neuron. 1991;7:677–683. doi: 10.1016/0896-6273(91)90380-i. [DOI] [PubMed] [Google Scholar]
- Ochiishi T, Terashima T, Sugiura H, Yamauchi T. Immunohistochemical localization of Ca2+/calmodulin-dependent protein kinase II in the rat retina. Brain Research. 1994;634:257–265. doi: 10.1016/0006-8993(94)91928-3. [DOI] [PubMed] [Google Scholar]
- Rushton WAH. Visual adaptation. Proceedings of the Royal Society B. 1965;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- Saito T, Kujiroaka T. Characteristics of bipolar-bipolar coupling in the carp retina. Journal of General Physiology. 1988;91:275–287. doi: 10.1085/jgp.91.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Filion M. Light adaptation of the late receptor potential in the cat retina. In: Arden GB, editor. Advances in Experimental Medicine and Biology, The Visual System. Vol. 24. New York: Plenum Press; 1972. pp. 87–93. [DOI] [PubMed] [Google Scholar]
- Shiells RA. Ca2+ -induced light adaptation in retinal ON-bipolar cells. Keio Journal of Medicine. 1999;48:140–146. doi: 10.2302/kjm.48.140. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proceedings of the Royal Society B. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Properties of the cGMP-activated channel of retinal on-bipolar cells. Proceedings of the Royal Society B. 1992a;247:21–25. doi: 10.1098/rspb.1992.0004. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Retinal on-bipolar cells contain a nitric oxide-sensitive guanylate cyclase. NeuroReport. 1992b;3:845–848. doi: 10.1097/00001756-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. The glutamate receptor linked cGMP cascade of retinal on-bipolar cells is pertussis and cholera toxin-sensitive. Proceedings of the Royal Society B. 1992c;247:17–20. doi: 10.1098/rspb.1992.0003. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Responses of rod bipolar cells isolated from dogfish retinal slices to concentration-jumps of glutamate. Visual Neuroscience. 1994;11:1175–1183. doi: 10.1017/s0952523800006970. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. A rise in intracellular Ca2+ underlies light adaptation in dogfish retinal ‘on’ bipolar cells. The Journal of Physiology. 1999a;514:343–350. doi: 10.1111/j.1469-7793.1999.343ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Ca2+ activation of protein kinase C (PKC) initiates light adaptation in dogfish retinal On-bipolar cells. The Journal of Physiology. 1999b;515.P:34P.. doi: 10.1111/j.1469-7793.1999.343ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Contribution of rod, on-bipolar, and horizontal cell light responses to the ERG of dogfish retina. Visual Neuroscience. 1999c;16:503–511. doi: 10.1017/s0952523899163119. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G, Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981;294:592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Kaneko A. Identification of bipolar cell subtypes by protein kinase C-like immunoreactivity in the goldfish retina. Visual Neuroscience. 1990;5:223–230. doi: 10.1017/s0952523800000298. [DOI] [PubMed] [Google Scholar]
- Thomas MM, Lamb TD. Kinetics of post-bleach recovery of maximal response and amplification constant in human rod photoreceptors. The Journal of Physiology. 1998;504.P:30P. [Google Scholar]
- Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, Hidaka H. KN-62, 1-[N, O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. Journal of Biological Chemistry. 1990;265:4315–4320. [PubMed] [Google Scholar]
- Vardi N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. Journal of Comparative Neurology. 1998;395:43–52. [PubMed] [Google Scholar]
- Walters RJ, Kramer RH, Nawy S. Regulation of cGMP-dependent current in on-bipolar cells by calcium/calmodulin-dependent kinase. Visual Neuroscience. 1998;15:257–261. doi: 10.1017/s0952523898152057. [DOI] [PubMed] [Google Scholar]
- Waxham MN, Aronowski J. Ca2+/calmodulin-dependent protein kinase II is phosphorylated by protein kinase C in vitro. Biochemistry. 1993;32:2923–2930. doi: 10.1021/bi00062a024. [DOI] [PubMed] [Google Scholar]
- Yamada M, Sasa T, Hirasawa H, Shiells RA. Light stimulates an increase in intracellular Ca2+ in carp retinal ON-bipolar cells. The Journal of Physiology. 1998;509.P:66P. [Google Scholar]
- Zufall TL, Ma M, Zufall F. Impaired odor adaptation in olfactory receptor neurons after inhibition of Ca2+/calmodulin kinase II. Journal of Neuroscience. 1999;19(RC19):1–6. doi: 10.1523/JNEUROSCI.19-14-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


