Abstract
The role of adrenaline in regulating muscle glycogenolysis and hormone-sensitive lipase (HSL) activity during exercise was examined in six adrenaline-deficient bilaterally adrenalectomised, adrenocortico-hormonal-substituted humans (Adr) and in six healthy control individuals (Con).
Subjects cycled for 45 min at ∼70 % maximal pulmonary O2 uptake (V̇O2,max) followed by 15 min at ∼86 %V̇O2,max either without (−Adr and Con) or with (+Adr) adrenaline infusion that elevated plasma adrenaline levels (45 min, 4.49 ± 0.69 nmol l−1; 60 min, 12.41 ± 1.80 nmol l−1). Muscle samples were obtained at 0, 45 and 60 min of exercise.
In −Adr and Con, muscle glycogen was similar at rest (−Adr, 409 ± 19 mmol (kg dry wt)−1; Con, 453 ± 24 mmol (kg dry wt)−1) and following exercise (−Adr, 237 ± 52 mmol (kg dry wt)−1; Con, 227 ± 50 mmol (kg dry wt)−1). Muscle lactate, glucose-6-phosphate and glucose were similar in −Adr and Con, whereas glycogen phosphorylase (a/a+b× 100%) and HSL (% phosphorylated) activities increased during exercise in Con only. Adrenaline infusion increased activities of phosphorylase and HSL as well as blood lactate concentrations compared with those in −Adr, but did not enhance glycogen breakdown (+Adr, glycogen following exercise: 274 ± 55 mmol (kg dry wt)−1) in contracting muscle.
The present findings demonstrate that during exercise muscle glycogenolysis can occur in the absence of adrenaline, and that adrenaline does not enhance muscle glycogenolysis in exercising adrenalectomised subjects. Although adrenaline increases the glycogen phosphorylase activity it is not essential for glycogen breakdown in contracting muscle. Finally, a novel finding is that the activity of HSL in human muscle is increased in exercising man and this is due, at least partly, to stimulation by adrenaline.
Adrenaline is known to increase glycogen phosphorylase activity via cAMP activation of phosphorylase kinase and this may enhance glycogen breakdown during muscle contraction (Richter et al. 1981b, 1982; Chasiotis & Hultman, 1985). Thus, in adrenodemedullated rats who lack adrenaline, glycogen breakdown is attenuated in contracting skeletal muscle compared with control rats with intact adrenal glands (Richter et al. 1981a; Sonne et al. 1985). Conversely, in adrenodemedullated rats the reduced glycogen breakdown can be corrected by applying adrenaline by infusion to reach plasma concentrations similar to those present in animals with intact adrenaline secretion (Richter et al. 1981b; Arnall et al. 1986; Winder et al. 1987). Furthermore, infusion of adrenaline increases phosphorylase activity and muscle glycogenolysis in perfused contracting rat hindlimb (Richter et al. 1982) and in the running dog (Issekutz, 1985). In exercising humans both enhanced (Spriet et al. 1988; Greenhaff et al. 1991; Febbraio et al. 1998) and unaltered (Kjær et al. 1991; Chesley et al. 1995; Wendling et al. 1996) muscle glycogen breakdown has been seen, when adrenaline concentrations, which were supraphysiological compared with the actual exercise intensity, were established by infusion.
Although the role of adrenaline in regulation of muscle glycogenolysis in exercise has been emphasized, the quantitative importance of adrenaline is, however, debated. Factors like the pre-exercise muscle glycogen content (Richter & Galbo, 1986; Hespel & Richter, 1992; Hargreaves et al. 1995), the availability of plasma glucose and free fatty acids (FFA) (Costill et al. 1977; Hargreaves et al. 1984; Vukovich et al. 1993), Ca2+ release and concentrations of phosphate, ADP, AMP and IMP (Chasiotis et al. 1982; Wendling et al. 1996) have been shown to influence muscle glycogenolysis during exercise. In animal experiments in which adrenergic blocking agents have been used to investigate the role of adrenaline, both a decrease (Gorski & Pietrzyk, 1982; Issekutz, 1985) and an increase in muscle glycogenolysis have been found (Nazar et al. 1972; Juhlin-Dannfelt et al. 1982). In humans both a decrease (Chasiotis & Hultman, 1985) or no change (Galbo et al. 1976) have been reported. Taken together, animal experiments have generally documented an important role for adrenaline in muscle glycogenolysis during exercise, whereas in humans some disagreement exists regarding an effect of adrenaline in concentrations corresponding to the studied exercise condition.
Besides glycogen, triglyceride is an important intramuscular substrate store. Until recently, the enzyme responsible for triglyceride breakdown in muscle was not known. However, evidence has now been produced in rats, that hormone-sensitive lipase (HSL), which regulates lipolysis in adipocytes, is also present in skeletal muscle cells (Langfort et al. 1999). Furthermore, in muscle HSL apparently, just like glycogen phosphorylase, is under dual control by adrenaline and contractions (Langfort et al. 1999, 2000). However, so far no studies are available concerning the existence and regulation of HSL in human muscle.
The aims of the present study, therefore, were to examine the effect of adrenaline on muscle glycogenolysis during exercise, and to determine for the first time the effect of exercise on HSL activity in human skeletal muscle. Our hypothesis was that during exercise adrenaline enhances the activity of muscle glycogen phosphorylase and, in turn, glycogen breakdown, and also enhances muscle HSL activity. A new approach was used: adrenaline-deficient bilaterally adrenalectomised humans were examined during both moderate and high-intensity exercise either without or with an infusion of adrenaline aiming at plasma levels similar to those observed during exercise in normal humans with intact adrenal glands. Results were compared with those obtained in healthy control individuals.
METHODS
Subjects
Six adrenaline-deficient bilaterally adrenalectomised subjects (5 females, 1 male; 45.8 ± 5.8 years, 75.2 ± 8.0 kg, mean ±s.e.m.) volunteered to participate in the experiment. Subjects were in good health and taking adrenocortical replacement medications (hydrocortisone and aldosterone) that were not altered for the purpose of this study. In addition, six normal healthy untrained individuals with intact adrenal glands who were sex- and age-matched (47.0 ± 4.7 years, 69.3 ± 2.4 kg) to the adrenalectomised subjects volunteered to participate in the experiment. The experimental procedures and possible risks of the study were explained to each subject verbally and in writing. All subjects gave their informed, written consent and the experiment was approved by the Ethical Committee of Copenhagen and conformed to the standards set by the Declaration of Helsinki.
Pre-experimental protocol
In order to determine maximum pulmonary oxygen uptake (V̇O2,max) and to establish the relationship between workload and oxygen uptake (V̇O2), all subjects performed an incremental workload test to exhaustion on a modified electromagnetically braked Krogh cycle ergometer (Howlett et al. 1999). Mean V̇O2,max was 1.46 ± 0.19 l min−1 and 2.30 ± 0.31 l min−1 for the adrenalectomised and normal control subjects, respectively. The difference in V̇O2,max potentially indicates that adrenaline is important for V̇O2,max. It follows that adrenalectomised patients cannot be matched to normal subjects by V̇O2,max. For the day preceding each trial the subjects were instructed to abstain from strenuous exercise, tobacco, caffeine and alcohol. The subjects reported to the exercise laboratory in the morning after a 10–12 h overnight fast.
Experimental protocol
The adrenalectomised subjects were studied during two exercise periods, separated by at least 14 days. The exercise trials were performed on a modified cycle ergometer in the semirecumbant position (Howlett et al. 1999). On arrival at the exercise laboratory, the adrenalectomised subjects rested quietly on the cycle ergometer. A catheter was introduced percutaneously into the radial artery for blood sampling and continuous measurement of blood pressure, and a catheter was placed in a forearm vein for infusion of either saline (−Adr) or adrenaline (+Adr) during exercise. Following the rest period, the adrenalectomised subjects performed exercise at 68 ± 1 %V̇O2,max for 45 min, immediately followed by a 15 min bout of exercise at 84 ± 2 %V̇O2,max. From the onset of exercise either isotonic saline or adrenaline was infused continuously (Howlett et al. 1999). In +Adr, an adrenaline solution (2 μg ml−1) was infused at a rate of 0.15 nmol kg−1min−1 for 45 min and the rate was then increased to 0.40 nmol kg−1 min−1 for the final 15 min of exercise. The adrenaline infusion rates were based on expected increases in plasma concentrations as obtained in previous experiments (Kjær et al. 1993). Blood was collected as previously reported (Howlett et al. 1999). Oxygen consumption and heart rate were measured as previously reported (Howlett et al. 1999). Muscle samples were taken at rest, after 45 min of exercise and immediately at the cessation of exercise (< 20 s). Muscle biopsies were obtained from the vastus lateralis portion of the quadriceps muscle under local anaesthesia by the percutaneous needle biopsy technique (Bergström, 1962) modified for suction (Evans et al. 1982). The three samples were obtained from two separate incisions (∼2 cm apart). When a second biopsy was taken through a previously used cutaneous incision the needle was inserted in a new direction to ensure a different sampling site and to avoid the influence of acute trauma on successive biopsy specimens (Costill et al. 1988). Leg selection was random and in the second trial the contralateral leg was biopsied. Muscle samples were frozen in isopentane cooled in dry ice and stored at −80°C for later analysis of muscle glycogen, glucose, glucose-6-phosphate, lactate, glycogen phosphorylase activity and HSL activity.
The sex- and age-matched normal subjects with intact adrenal glands were studied during one exercise period (Con). They exercised at a workload that required 73 ± 3 %V̇O2,max for 45 min, immediately followed by a 15 min bout of exercise at 90 ± 4 %V̇O2,max. The exercise intensity and duration were not significantly different when compared with that of the adrenalectomised subjects.
Analytical techniques
Plasma adrenaline, glucose and lactate were measured (Howlett et al. 1999). Muscle samples were freeze-dried and dissected free of visible connective tissue, fat and blood. Glycogen was measured by a hexokinase method (Lowry & Passonneau, 1972) after acid hydrolysis (Karlsson et al. 1970). Glucose, glucose-6-phosphate and lactate were analysed using standard enzymatic methods (Lowry & Passonneau, 1972). Glycogen phosphorylase activity was assayed in the direction of glycogen synthesis, determined from the incorporation of [14C]glucose-1-phosphate into glycogen (Richter et al. 1982). Total (a+b) phosphorylase activity and phosphorylase a activity were determined with and without AMP in the assay mixture, respectively. The fraction of phosphorylase a is given as a percentage of total activity (a/(a+b) × 100 %). Assays for HSL are described in detail in Langfort et al. (1999). In brief, assays are based on measurements of release of [3H]oleic acid from 1(3)-mono-[3H]oleoyl-2-oleylglycerol, a diacylglycerol analogue not hydrolysable at position 2 and referred to as MOME, and from tri[3H]olein, referred to as TO. Upon phosphorylation by protein kinase A, the activity of adipocyte HSL towards TO increases markedly, whereas the MOME activity does not change significantly. Accordingly, MOME activity was taken as a measure of the total enzyme concentrations while TO activity is a measure of the activated form of HSL and the assay of choice for monitoring changes in the activation of HSL. One unit of enzyme activity is equivalent to 1 μmol of fatty acids released per minute at 37°C. The intra-assay coefficient of variation calculated from eight determinations, each made in triplicate, on a crude muscle supernatant was 2.8 % at 0.71 ± 0.008 mU (mg protein)−1 for TO and 5.6 % at 4.79 ± 0.095 mU (mg protein)−1 for MOME activity (Langfort et al. 1999). By analogy with phosphorylase activity, HSL activity is given as an activity ratio (TO/MOME × 100 %). Results from the adrenalectomised (−Adr and +Adr) subjects were compared using a two-way analysis of variance (ANOVA) for repeated measures (cross-over design); results from the normal (Con) and adrenalectomised (−Adr or +Adr) subjects were compared using a two-way ANOVA for repeated measures (parallel design). The level of significance was set at P < 0.05 in two-tailed testing. Specific differences were determined using the Student-Newman-Keuls post hoc test. All data are reported as means ± standard error of the mean (s.e.m.).
RESULTS
The plasma adrenaline concentrations were similar at rest on the two experimental days in the adrenalectomised subjects (−Adr, 0.04 ± 0.02 nmol l−1; +Adr, 0.03 ± 0.01 nmol l−1), but were ∼10-fold lower than in normal control subjects (0.35 ± 0.03 nmol l−1). During exercise in −Adr, plasma adrenaline did not change from resting levels, whereas in +Adr, infusion of adrenaline resulted in a marked increase (P < 0.05) in plasma arterial levels to 4.49 ± 0.69 and 12.41 ± 1.80 nmol l−1 at 45 and 60 min, respectively. During exercise in Con, plasma adrenaline levels increased (P < 0.05) to 0.90 ± 0.18 nmol l−1 at 45 min and to a peak of 2.20 ± 0.58 nmol l−1 at 60 min. Plasma adrenaline levels were significantly greater in Con than in −Adr, but also significantly lower in Con than in +Adr during exercise.
During exercise in adrenalectomised and control subjects the muscle glycogen content decreased (P < 0.05) (Fig. 1), whereas muscle lactate (Fig. 1), glucose and glucose-6-phosphate (Fig. 2) increased (P < 0.05). No difference was observed between the adrenalectomised (−Adr, +Adr) and control subjects for muscle glycogen, lactate (Fig. 1) and glucose-6-phosphate (Fig. 2). In the adrenalectomised subjects muscle glucose levels increased similarly during moderate exercise, but were significantly greater in +Adr compared with −Adr at 60 min of exercise (P < 0.05). Muscle glucose was similar between Con and −Adr during exercise (Fig. 2). Phosphorylase a (% activity) increased (P < 0.05, main effect) from rest to exercise with infusion of adrenaline (+Adr) and in Con, but not in −Adr (Fig. 3). Neutral lipase activity against TO rose significantly in +Adr and Con, but not in −Adr (Table 1). Neutral lipase activity against MOME did not change in response to exercise in Con or −Adr, but increased in response to adrenaline infusion (P < 0.05; Table 1). The HSL activity ratio (TO/MOME × 100 %) increased (P < 0.05) in both +Adr and Con, but was unchanged during exercise in −Adr (Fig. 3).
Figure 1. Muscle glycogenolysis in adrenalectomised humans.
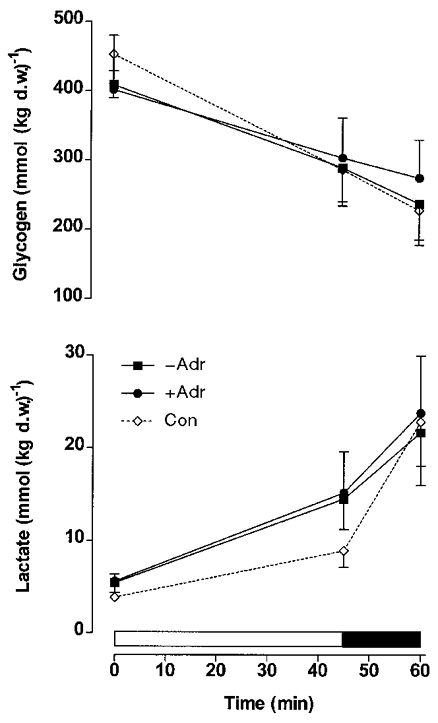
Muscle glycogen and lactate during cycling exercise at ∼70 %V̇O2,max for 45 min followed by 15 min at ∼86 %V̇O2,max in normal control (Con) subjects and in adrenalectomised subjects with saline (−Adr) or adrenaline (+Adr) infusion. Values are means ±s.e.m. (n= 6). d.w., dry weight.
Figure 2. Glucose and glucose-6-phosphate in muscle.
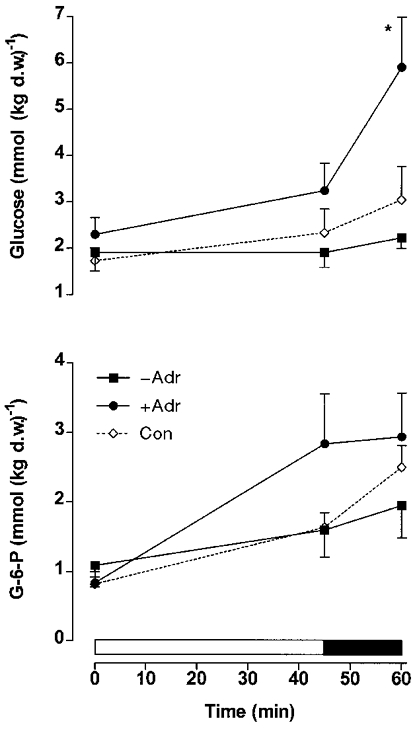
Muscle glucose and glucose-6-phosphate (G-6-P) during cycling exercise at ∼70 %V̇O2,max for 45 min followed by 15 min at ∼86 %V̇O2,max in normal control (Con) subjects and in adrenalectomised subjects with saline (−Adr) or adrenaline (+Adr) infusion. Values are means ±s.e.m. (n= 6). d.w., dry weight. * Significantly different (P < 0.05) from −Adr.
Figure 3. Phosphorylase and hormone-sensitive lipase in exercising adrenalectomised humans.
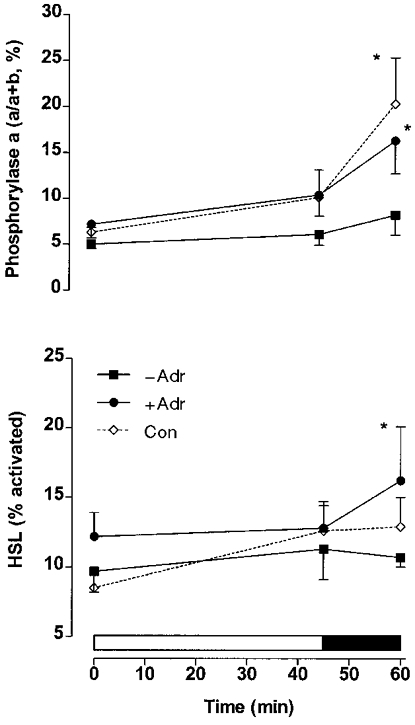
Muscle phosphorylase a and hormone-sensitive lipase (HSL) activity during cycling exercise at ∼70 %V̇O2,max for 45 min followed by 15 min at ∼86 %V̇O2,max in normal control (Con) subjects and in adrenalectomised subjects with saline (−Adr) or adrenaline (+Adr) infusion. Values are means ±s.e.m. (n= 6). * Significantly different (P < 0.05) from −Adr.
Table 1.
Neutral hormonesensitive lipase activity against MOME and TO
| Rest | 45 min | 60 min | |
|---|---|---|---|
| Neutral lipase activity against TO | |||
| (mU (mg protein)−1) | |||
| + Adr | 0.9 ± 0.2 | 1.2 ± 0.2 | 1.7 ± 0.3* |
| −Adr | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.1 |
| Con | 0.7 ± 0.2 | 1.4 ± 0.3* | 1.5 ± 0.3* |
| Neutral lipase activity against MOME | |||
| (mU (mg protein)−1) | |||
| + Adr | 7.8 ± 1.2 | 10.1 ± 1.4* | 11.7 ± 1.6* |
| −Adr | 8.1 ± 1.7 | 7.7 ± 1.2 | 7.7 ± 1.5 |
| Con | 8.2 ± 1.5 | 9.9 ± 1.7 | 10.9 ± 2.1 |
Neutral hormonesensitive lipase activity against 1(3)-mono[3H]oleoyl-2-oleylglycerol (MOME) and tri[3H]olein (TO) measured in skeletal muscle cells obtained from muscle biopsies during 60 min of cycling in normal (Con) and adrenalectomised subjects with saline (−Adr) or adrenaline (+Adr) infusion commencing at the onset of exercise. Values are means ±s.e.m.(n= 6).
Significantly different (P < 0.05) from rest.
Plasma glucose concentrations were similar at rest between Con (5.4 ± 0.3 mmol l−1) and −Adr (5.5 ± 0.3 mmol l−1) (P < 0.05). During exercise, euglycaemia was maintained and plasma glucose was not different between trials in these groups (60 min: Con, 5.2 ± 0.3; −Adr, 5.0 ± 0.2 mmol l−1, P > 0.05). Infusion of adrenaline during exercise in +Adr resulted in an increase in plasma glucose from 5.4 ± 0.3 mmol l−1 (at rest) to 7.1 ± 0.7 mmol l−1 (P < 0.05) and 8.8 ± 0.8 mmol l−1 (P < 0.05) at 45 and 60 min of exercise, respectively. Plasma lactate was similar at rest between adrenalectomised (−Adr, 1.2 ± 0.2 mmol l−1; +Adr, 1.3 ± 0.3 mmol l−1) and control (0.7 ± 0.1 mmol l−1, P < 0.05vs. Adr experiments) subjects. During exercise, plasma lactate increased (P < 0.05) and was similar between Con and −Adr (45 min: 2.9 ± 0.7 vs. 2.9 ± 0.8 mmol l−1), except at 60 min when lactate was higher (P < 0.05) in Con (7.7 ± 1.0 mmol l−1) when compared with −Adr (5.5 ± 0.9 mmol l−1). Compared with −Adr, infusion of adrenaline during exercise in +Adr resulted in an increase (P < 0.05) in plasma lactate to 5.4 ± 1.5 and 7.3 ± 1.7 mmol l−1 at 45 and 60 min of exercise, respectively. Oxygen uptake and heart rate increased with exercise intensity (P < 0.05) in the adrenalectomised subjects, but was similar between −Adr and +Adr (data not shown). The control subjects exercised at a similar relative oxygen uptake (%V̇O2,max), but absolute V̇O2 and heart rate were higher (P < 0.05) in Con compared with both −Adr and +Adr. Mean arterial pressure remained constant throughout exercise and was similar between the adrenalectomised and control subjects (data not shown).
DISCUSSION
The results from the present study of adrenolectomised saline-infused subjects (−Adr) demonstrate that during exercise glycogenolysis can occur despite very low and constant circulating adrenaline levels. The fact that muscle glycogen breakdown during moderate and high-intensity exercise did not differ, whether adrenaline was infused or not in Adr subjects, further indicates that adrenaline is not important for glycogenolysis in these patients. The additional information that, despite marked differences in adrenaline levels, muscle glycogenolysis also did not differ between healthy control subjects (Con) and −Adr subjects, indicates that even in the absence of adrenaline the patients experience a normal glycogenolysis during exercise. We also found that during exercise an increase in glycogen phosphorylase a activity was only seen in experiments in which plasma adrenaline concentrations increased (Con and +Adr) but nevertheless muscle glycogen depletion was identical in those experiments compared with experiments with no adrenaline increase (−Adr). Finally, the study shows for the first time that the activity of hormone-sensitive lipase (HSL) in human muscle increases with exercise. The increase in HSL activity parallelled that of glycogen phosphorylase indicating that during exercise adrenaline indiscriminately, in a feed-forward manner, stimulates the major enzymes regulating muscle glycogen and triglyceride metabolism.
The finding that the exercise-induced rise in glycogenolysis of contracting muscle is maintained, even in the absence of a normal adrenaline response, provides direct support for earlier indirect findings in adrenalectomised individuals, where blood lactate responses were intact even though these subjects had very low levels of adrenaline and no rise in adrenaline during exercise (Barwich et al. 1981; Hoelzer et al. 1986). The findings are also supported by results in healthy individuals with intact adrenal glands in whom adrenaline secretion was diminished by up to 80 % by pharmacological blockade of the coeliac ganglion, and where blood lactate responses during exercise were unaffected by the blockade (Kjær et al. 1993).
In the present study, although muscle phosphorylase activity was elevated by adrenaline infusion in adrenolectomised patients, it was not possible to demonstrate any stimulating effect on glycogen breakdown during exercise (Fig. 1). This indicates that activation of glycogen phosphorylase by phosphorylation is not intimately coupled to glycogen depletion in exercising humans. The same conclusion has been drawn by Chesley et al. (1995) who found that during high-intensity exercise muscle glycogenolysis was unaffected by a doubling of the plasma adrenaline concentration by infusion despite an elevation of the phosphorylase a fraction. Furthermore, adrenaline infusion did not enhance net muscle glycogen breakdown during prolonged aerobic exercise (Wendling et al. 1996). Correspondingly, the rate of glycogen breakdown, as well as lactate release from contracting leg muscle, has been shown to be unaffected by addition of arm cranking to leg cycling exercise which resulted in a 3-fold elevation in the plasma adrenaline concentration (Kjær et al. 1991).
Evidence in favour of a regulating role of adrenaline on muscle glycogenolysis during exercise in man has, however, also been presented. In healthy, young trained subjects, intramuscular glycogen utilisation and carbohydrate oxidation were augmented when plasma adrenaline concentrations were elevated by infusion during submaximal exercise (Febbraio et al. 1998). Differences in state of health, age and training between subjects compared with the present study may explain the diverging results. Infusion of adrenaline has also been shown to enhance muscle glycogenolysis during electrical stimulation of the human thigh muscle (Spriet et al. 1988). However, the fact that adrenaline concentrations were markedly higher than those of the present study makes it more difficult to extrapolate the findings to physiological conditions. In a study by Jansson et al. (1986), infusion of adrenaline into the femoral artery enhanced leg muscle glycogenolysis as shown by an increase in leg lactate release. The adrenaline concentration (∼13 nmol l−1) was comparable to that obtained when adrenaline was infused during intense exercise in the present study, but the exercise intensity was only 54 %V̇O2,max (Jansson et al. 1986). Accordingly, the adrenaline concentrations achieved were supra-physiological when compared with the actual exercise intensity.
In agreement with the idea that supra-physiological concentrations of adrenaline can increase glycogenolysis in humans, it has been demonstrated in exercising individuals that circulating lactate levels increased when supraphysiological adrenaline concentrations were achieved through infusion (Kjær et al. 1993). However, adrenaline-induced increases in blood lactate responses may not reflect an increase in glycogen breakdown in working muscle (Wendling et al. 1996). This is in accordance with our findings in the adrenalectomised subjects, in whom plasma lactate concentrations rose more during exercise with than without adrenaline infusion while glycogen breakdown and lactate concentrations in contracting muscle did not differ between +Adr and −Adr. One possible explanation is that adrenaline stimulated glycogen breakdown and lactate production in other tissues, e.g. non-contracting muscle (Ahlborg 1985). In fact, a recent study combining arterio-venous techniques with stable isotope methodology has shown that during exercise at 65 %V̇O2,max up to 30 % of the total lactate production may originate from tissues other than contracting muscle (Bergman et al. 1999).
The glycogen phosphorylase a activity in muscle increased in the adrenalectomised subjects only when adrenaline was infused during exercise (Fig. 3). However, muscle biopsies were only sampled at 45 and 60 min during exercise, and it is well known that the increase in phosphorylase a activity induced by muscle contraction per se in the absence of any rise in adrenaline lasts only a few minutes (Richter et al. 1982; Connett & Sahlin, 1996). It is interesting to note that muscle glycogen depletion during exercise was identical in experiments in which an increase in phosphorylase a activity was found (Con and +Adr) and in experiments with no increase (−Adr) (Fig. 1). Of course it cannot be completely excluded that, due to methodological limitations in the determination of muscle glycogen concentration, a small difference in glycogenolytic rate nevertheless existed between the mentioned groups. However, contrary to this possibility is the fact that muscle lactate (Fig. 1) and glucose-6-phosphate (Fig. 2) concentrations did not differ between groups. Furthermore, a power analysis revealed that the risk of a type 2 error (and thus failing to demonstrate a true difference between groups) associated with the glycogen data was less than 10 %. The muscle glucose concentration increased more during exercise in adrenaline-infused adrenalectomised patients (+Adr) than in the other groups (−Adr and Con) (Fig. 2), a finding which would be compatible with enhanced glycogenolysis in +Adr patients. However, this difference could be almost completely accounted for by extracellular accumulation of glucose in muscle caused by an exaggerated increase in plasma glucose concentration in +Adr patients. This is so because during 60 min of exercise the mean increase in plasma water glucose concentration was 4.1 and 3.8 mm higher in +Adr compared with −Adr and Con, respectively. Assuming the water content of muscle to be 77 % and extracellular water to make up 17 % of this (Ahlborg et al. 1967), it follows that the increase in muscle glucose per kilogram dry weight resulting from the increase in plasma glucose was 4.1 × 0.17 × 770/230 = 2.3 mmol kg−1 and 3.8 × 0.17 × 770/230 = 2.2 mmol kg−1 higher in +Adr than in −Adr and Con, respectively. The measured increase in muscle glucose was 3.2 and 2.2 mmol kg−1 higher in +Adr compared with −Adr and Con, respectively. The demonstrated increase in plasma glucose concentration primarily reflects the fact that adrenaline impairs glucose clearance (Howlett et al. 1999). As also pointed out by other workers using different experimental protocols (Connett & Sahlin, 1996), activation of glycogen phosphorylase by phosphorylation is not intimately coupled in exercising human muscle. This emphasises the importance of allosteric modulators and phosphorylase availability in the regulation of glycogen phosphorylase activity and, in turn, glycogenolysis (Connett & Sahlin, 1996). Such factors are intimately coupled to workload expressed in relative terms and this was identical between the various groups in our study (Connett & Sahlin, 1996).
In the present study, infusion of adrenaline in adrenalectomised individuals resulted in plasma adrenaline concentrations somewhat higher than in matched healthy controls with intact adrenal glands. Since adrenaline infusion rates were calculated from findings in healthy young individuals (Kjær et al. 1993) it is likely that the rate of adrenaline clearance was lower in the adrenalectomised subjects. As previously discussed (Howlett et al. 1999) this probably reflected the fact that adrenaline clearance decreases with age (Marker et al. 1998). At rest adrenaline infusion influenced metabolic and cardiovascular variables identically in the adrenalectomised and healthy control subjects studied (Howlett et al. 1999). This indicates that adrenalectomised subjects had normal sensitivity to adrenaline, and, accordingly, that hypersensitivity did not account for the normal muscle glycogenolysis during exercise found in these patients. Interestingly, the normal response to adrenaline in adrenalectomised subjects is in contrast to the augmented responses to adrenergic stimulation which develop in spinal cord-injured subjects (Mathias et al. 1976) and in patients with diabetic autonomic neuropathy (Hilsted et al. 1987). Taken together, the studies indicate that impaired nerve function at receptor sites rather that reduced plasma catecholamine levels is responsible for development of adrenergic hypersensitivity.
Hormone-sensitive lipase (HSL) has recently been shown to exist in rat skeletal muscle cells (Langfort et al. 1999). Furthermore, in vitro adrenaline has been shown to cause a prolonged increase in muscle neutral lipase activity, whereas contractions cause a transient increase which was eliminated by anti-HSL antibodies (Langfort et al. 1999, 2000). In the present study, the same assays were used as in the rat studies of these two previous papers, and these results are the first which indicate that in healthy humans HSL activity increases in exercising muscle (Fig. 3). In adrenalectomised adrenaline-infused patients HSL activity increased as in control subjects, whereas no increase was seen in patients without infusion (Fig. 3). These findings indicate that adrenaline was responsible for the HSL activation. Interestingly, HSL and glycogen phosphorylase in muscle were activated in parallel (Fig. 3). Adrenaline also stimulates HSL in adipose tissue and glycogen phosphorylase in muscle. Because adrenaline secretion in exercise is not exclusively stimulated by mechanisms elicited by metabolic demands (Galbo, 1995), it follows that during exercise, at least to some extent, the enzymes controlling mobilisation of the major intra- as well as extramuscular fuel stores are activated indiscriminantly and without accurate coupling to energy needs. This is compatible with the feed-forward concept of metabolic regulation in exercise (Kjær et al. 1987; Vissing et al. 1989; Galbo, 1995).
In summary, adrenalectomised individuals maintain the ability to increase glycogenolysis in exercising skeletal muscle. In these individuals adrenaline increases the glycogen phosphorylase a activity in contracting muscle, but this does not enhance glycogen breakdown. Finally, the activity of HSL in human muscle is increased during exercise and this increase is, at least partly, due to stimulation by adrenaline.
Acknowledgments
The authors would like to thank Mark Hargreaves for valuable discussion, Lisbeth Kall, Regitze Kraunsøe, Inge Rasmussen and Vibeke Staffeldt for their excellent technical assistance, and Erik A Richter and Betina Blomgreen for performing muscle biopsy analysis. This study was supported by grants from the Danish National Research Foundation (J.nr. 504–14), the Danish Medical Research Council (J.nr. 9802636) and the NOVO Foundation.
References
- Ahlborg B, Bergström J, Ekelund LG, Hultman E. Muscle glycogen and muscle electrolytes during prolonged physical exercise. Acta Physiologica Scandinavica. 1967;70:129–142. [Google Scholar]
- Ahlborg G. Mechanism for glycogenolysis in non-exercising human muscle during and after exercise. American Journal of Physiology. 1985;248:E540–545. doi: 10.1152/ajpendo.1985.248.5.E540. [DOI] [PubMed] [Google Scholar]
- Arnall DA, Marker JC, Conlee RK, Winder WW. Effect of infusing epinephrine on liver and muscle glycogenolysis during exercise in rats. American Journal of Physiology. 1986;250:E641–649. doi: 10.1152/ajpendo.1986.250.6.E641. [DOI] [PubMed] [Google Scholar]
- Barwich D, Hägele H, Weiss M, Weicker H. Hormonal and metabolic adjustment in patients with central Cushing’s disease after adrenalectomy. International Journal of Sports Medicine. 1981;2:220–227. [Google Scholar]
- Bergman B, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. Journal of Applied Physiology. 1999;87:1684–1696. doi: 10.1152/jappl.1999.87.5.1684. [DOI] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man. Scandinavian Journal of Clinical and Laboratory Investigation. 1962;14:1–110. doi: 10.1080/00365516209075157. [DOI] [PubMed] [Google Scholar]
- Chasiotis D, Hultman E. The effect of adrenaline infusion on the regulation of glycogenolysis in human muscle during isometric contraction. Acta Physiologica Scandinavica. 1985;123:55–60. doi: 10.1111/j.1748-1716.1985.tb07560.x. [DOI] [PubMed] [Google Scholar]
- Chasiotis D, Sahlin K, Hultman E. Regulation of glycogenolysis in human muscle at rest and during exercise. Journal of Applied Physiology. 1982;52:708–715. doi: 10.1152/jappl.1982.53.3.708. [DOI] [PubMed] [Google Scholar]
- Chesley A, Hultman E, Spriet LL. Effects of epinephrine infusion on muscle glycogenolysis during intense aerobic exercise. American Journal of Physiology. 1995;268:E127–134. doi: 10.1152/ajpendo.1995.268.1.E127. [DOI] [PubMed] [Google Scholar]
- Connett RJ, Sahlin K. Control of glycolysis and glycogen metabolism. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York and Oxford: Oxford University Press; 1996. pp. 870–910. [Google Scholar]
- Costill DL, Coyle E, Dalsky G, Evans W, Fink W, Hoopes D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. Journal of Applied Physiology. 1977;43:695–699. doi: 10.1152/jappl.1977.43.4.695. [DOI] [PubMed] [Google Scholar]
- Costill DL, Pearson DP, Fink WJ. Impaired muscle glycogen storage after muscle biopsy. Journal of Applied Physiology. 1988;64:2245–2248. doi: 10.1152/jappl.1988.64.5.2245. [DOI] [PubMed] [Google Scholar]
- Evans W, Phinney S, Young V. Suction applied to a muscle biopsy maximises sample size. Medicine and Science in Sports and Exercise. 1982;14:101–102. [PubMed] [Google Scholar]
- Febbraio MA, Lambert DL, Starkie RL, Proietto J, Hargreaves M. Effect of epinephrine on muscle glycogenolysis during exercise in trained men. Journal of Applied Physiology. 1998;84:465–470. doi: 10.1152/jappl.1998.84.2.465. [DOI] [PubMed] [Google Scholar]
- Galbo H. Hormonal and Metabolic Adaptation to Exercise. New York: Thieme; 1983. [Google Scholar]
- Galbo H. Integrated endocrine responses and exercise. In: DeGroot LJ, editor. Endocrinology. 3. Philadelphia: W. B. Saunders Company; 1995. pp. 2692–2701. chap. 146. [Google Scholar]
- Galbo H, Holst JJ, Christensen NJ, Hilsted J. Glucagon and plasma catecholamines during beta-receptor blockade in exercising man. Journal of Applied Physiology. 1976;40:855–863. doi: 10.1152/jappl.1976.40.6.855. [DOI] [PubMed] [Google Scholar]
- Gorski J, Pietrzyk K. The effect of beta-adrenergic receptor blockade on intramuscular glycogen mobilization during exercise in the rat. Pflügers Archiv. 1982;48:201–205. doi: 10.1007/BF00422981. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Ren J-M, Söderlund K, Hultman E. Energy metabolism in single human muscle fibers during contractions with and without epinephrine infusion. American Journal of Physiology. 1991;260:E713–718. doi: 10.1152/ajpendo.1991.260.5.E713. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Costill DL, Coggan A, Fink WJ, Nishibata I. Effect of carbohydrate feedings on muscle glycogen utilisation and exercise performance. Medicine and Science in Sports and Exercise. 1984;16:219–222. [PubMed] [Google Scholar]
- Hargreaves M, McConell G, Proietto J. Influence of muscle glycogen on glycogenolysis and glucose uptake during exercise. Journal of Applied Physiology. 1995;78:288–292. doi: 10.1152/jappl.1995.78.1.288. [DOI] [PubMed] [Google Scholar]
- Hespel P, Richter EA. Mechanisms linking glycogen concentration and glycogenolytic rate in perfused contracting rat skeletal muscle. Biochemical Journal. 1992;284:777–780. doi: 10.1042/bj2840777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsted J, Richter EA, Madsbad S, Tronier B, Christensen NJ, Hildebrandt P, Damkjær M, Galbo H. Metabolic and cardiovascular responses to epinephrine in diabetic autonomic neuropathy. New England Journal of Medicine. 1987;284:777–780. doi: 10.1056/NEJM198708133170705. [DOI] [PubMed] [Google Scholar]
- Hoelzer DR, Dalsky GP, Schwartz NS, Clutter WE, Shah SD, Holloszy JO, Cryer PE. Epinephrine is not critical to prevention of hypoglycemia during exercise in humans. American Journal of Physiology. 1986;251:E104–110. doi: 10.1152/ajpendo.1986.251.1.E104. [DOI] [PubMed] [Google Scholar]
- Howlett K, Galbo H, Lorentsen J, Bergeron R, Zimmerman-Belsing T, Bülow J, Feldt-Rasmussen U, Kjær M. Effect of adrenaline on glucose kinetics during exercise in adrenalectomised humans. The Journal of Physiology. 1999;519:911–921. doi: 10.1111/j.1469-7793.1999.0911n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz B., Jr Effect of epinephrine on carbohydrate metabolism in exercising dogs. Metabolism. 1985;34:457–464. doi: 10.1016/0026-0495(85)90212-4. [DOI] [PubMed] [Google Scholar]
- Jansson E, Hjemdahl P, Kaijser L. Epinephrine-induced changes in muscle carbohydrate metabolism during exercise in male subjects. Journal of Applied Physiology. 1986;60:1466–1470. doi: 10.1152/jappl.1986.60.5.1466. [DOI] [PubMed] [Google Scholar]
- Juhlin-Dannfeldt AC, Terblanche SE, Fell RD, Young JC, Holloszy JO. Effect of beta-adrenergic blockade on glycogenolysis during exercise. Journal of Applied Physiology. 1982;53:549–554. doi: 10.1152/jappl.1982.53.3.549. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Diamant B, Saltin B. Muscle metabolites during submaximal and maximal exercise in man. Scandinavian Journal of Clinical and Laboratory Investigation. 1970;26:385–394. doi: 10.3109/00365517009046250. [DOI] [PubMed] [Google Scholar]
- Kjær M, Engfred K, Fernandes A, Secher NH, Galbo H. Regulation of hepatic glucose production during exercise in humans: role of sympathoadrenergic activity. American Journal of Physiology. 1993;265:E275–283. doi: 10.1152/ajpendo.1993.265.2.E275. [DOI] [PubMed] [Google Scholar]
- Kjær M, Kiens B, Hargreaves M, Richter EA. Influence of active muscle mass on glucose homeostasis during exercise in humans. Journal of Applied Physiology. 1991;71:552–557. doi: 10.1152/jappl.1991.71.2.552. [DOI] [PubMed] [Google Scholar]
- Kjær M, Secher NH, Bach FW, Galbo H. Role of motor center activity for hormonal changes and substrate mobilization in humans. American Journal of Physiology. 1987;253:R687–695. doi: 10.1152/ajpregu.1987.253.5.R687. [DOI] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Holm C, Galbo H. Stimulation of hormone-sensitive lipase (HSL) activity by contractions in rat skeletal muscle. Biochemical Journal. 2000. (in the Press) [DOI] [PMC free article] [PubMed]
- Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H. Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochemical Journal. 1999;340:459–465. [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. pp. 199–201. [Google Scholar]
- Marker JC, Clutter WE, Cryer PE. Reduced epinephrine clearance and glycemic sensitivity to epinephrine in older individuals. American Journal of Physiology. 1998;275:E770–776. doi: 10.1152/ajpendo.1998.275.5.E770. [DOI] [PubMed] [Google Scholar]
- Mathias C, Christensen NJ, Corvett JL, Frankl HL, Spalding JMK. Plasma catecholamines during paroxysmal neurogenic hypertension in quadriplegic man. Clinical Research. 1976;39:204–208. doi: 10.1161/01.res.39.2.204. [DOI] [PubMed] [Google Scholar]
- Nazar K, Brezezinska Z, Kowalski W. Mechanism of impaired capacity for prolonged muscular work following beta-blockade in dogs. Pflügers Archiv. 1972;336:72–78. doi: 10.1007/BF00589143. [DOI] [PubMed] [Google Scholar]
- Richter EA, Galbo H. High glycogen levels enhance glycogen breakdown in isolated contracting skeletal muscle. Journal of Applied Physiology. 1986;61:827–831. doi: 10.1152/jappl.1986.61.3.827. [DOI] [PubMed] [Google Scholar]
- Richter EA, Galbo H, Christensen NJ. Control of exercise-induced muscular glycogenolysis by adrenal medullary hormones in rats. Journal of Applied Physiology. 1981a;50:21–26. doi: 10.1152/jappl.1981.50.1.21. [DOI] [PubMed] [Google Scholar]
- Richter EA, Ruderman NB, Gavras H, Belur ER, Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. American Journal of Physiology. 1982;242:E25–32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- Richter EA, Sonne B, Christensen NJ, Galbo H. Role of epinephrine for muscular glycogenolysis and pancreatic hormonal secretion in running rats. American Journal of Physiology. 1981b;240:E526–532. doi: 10.1152/ajpendo.1981.240.5.E526. [DOI] [PubMed] [Google Scholar]
- Sonne B, Mikines KJ, Richter EA, Christensen NJ, Galbo H. Role of liver nerves and adrenal medulla in glucose turnover of running rats. Journal of Applied Physiology. 1985;59:1640–1646. doi: 10.1152/jappl.1985.59.5.1640. [DOI] [PubMed] [Google Scholar]
- Spriet LL, Ren J-M, Hultman E. Epinephrine infusion enhances muscle glycogenolysis during prolonged electrical stimulation. Journal of Applied Physiology. 1988;64:1439–1444. doi: 10.1152/jappl.1988.64.4.1439. [DOI] [PubMed] [Google Scholar]
- Vissing J, Wallace JL, Scheurink JW, Galbo H, Steffens AB. Ventromedial hypothalamic regulation of hormonal and metabolic responses to exercise. American Journal of Physiology. 1989;256:R1019–1026. doi: 10.1152/ajpregu.1989.256.5.R1019. [DOI] [PubMed] [Google Scholar]
- Vukovich MD, Costill DL, Hickey MS, Trappe SW, Cole KJ, Fink WJ. Effect of fat emulsion infusion and fat feeding on muscle glycogen utilization during cycle exercise. Journal of Applied Physiology. 1993;75:1513–1518. doi: 10.1152/jappl.1993.75.4.1513. [DOI] [PubMed] [Google Scholar]
- Wendling PS, Peters SJ, Heigenhauser GJF, Spriet LL. Epinephrine infusion does not enhance net muscle glycogenolysis during prolonged aerobic exercise. Canadian Journal of Applied Physiology. 1996;21:271–284. doi: 10.1139/h96-024. [DOI] [PubMed] [Google Scholar]
- Winder WW, Yang HT, Jaussi AW, Hopkins CR. Epinephrine, glucose, and lactate infusion in exercising adrenodemedullated rats. Journal of Applied Physiology. 1987;62:1442–1447. doi: 10.1152/jappl.1987.62.4.1442. [DOI] [PubMed] [Google Scholar]


