Abstract
To determine the activity characteristics displayed by different subpopulations of neurons in a single intrinsic cardiac ganglionated plexus, the behaviour and co-ordination of activity generated by neurons in two loci of the right atrial ganglionated plexus (RAGP) were evaluated in 16 anaesthetized dogs during basal states as well as in response to increasing inputs from ventricular sensory neurites.
These sub-populations of right atrial neurons received afferent inputs from sensory neurites in both ventricles that were responsive to local mechanical stimuli and the nitric oxide donor nitroprusside. Neurons in at least one RAGP locus were activated by epicardial application of veratridine, bradykinin, the β1-adrenoceptor agonist prenaterol or glutamate. Epicardial application of angiotensin II, the selective β2-adrenoceptor agonist terbutaline and selective α-adrenoceptor agonists elicited inconsistent neuronal responses.
The activity generated by both populations of atrial neurons studied over 5 min periods during basal states displayed periodic coupled behaviour (cross-correlation coefficients of activities that reached, on average, 0·88 ± 0·03; range 0·71–1) for 15–30 s periods of time. These periods of coupled activity occurred every 30–50 s during basal states, as well as when neuronal activity was enhanced by chemical activation of their ventricular sensory inputs.
These results indicate that neurons throughout one intrinsic cardiac ganglionated plexus receive inputs from mechano- and chemosensory neurites located in both ventricles. That such neurons respond to multiple chemical stimuli, including those liberated from adjacent adrenergic efferent nerve terminals, indicates the complexity of the integrative processing of information that occurs within the intrinsic cardiac nervous system.
It is proposed that the interdependent activity displayed by populations of neurons in different regions of one intrinsic cardiac ganglionated plexus, responding as they do to multiple cardiac sensory inputs, forms the basis for integrated regional cardiac control.
The classical view of the autonomic nervous system presumes that its intrinsic cardiac component represents a parasympathetic efferent neuronal relay station, in which medullary neurons synapse with parasympathetic efferent postganglionic neurons. In such a concept, the latter neurons project to end effectors on the heart with little or no integrative capabilities occurring therein (Blomquist et al. 1987). Similarly, intrathoracic extracardiac sympathetic ganglia have been thought to act solely as efferent relay stations for sympathetic efferent projections to the heart (Skok, 1973). However, recent anatomical and functional data indicate the presence of the multiple neuronal subtypes within the intrinsic cardiac nervous system (Gagliardi et al. 1988; Ardell, 1994; Yuan et al. 1994; Edwards et al. 1995; Horackova & Armour, 1995). We have proposed that afferent, local circuit, as well as efferent cholinergic and adrenergic neurons are present in these ganglia and that these neurons function as part of a hierarchy of intrathoracic and central neuronal feedback loops involved in regulating regional cardiodynamics on a beat-to-beat basis (Armour et al. 1998).
The intrinsic cardiac nervous system consists of multiple aggregates of neurons and associated neural interconnections, localized to discrete atrial and ventricular regions (Yuan et al. 1994). Among these distinct ganglionated plexuses, preferential control of specific cardiac functions has been identified. For example, right atrial ganglionated plexus (RAGP) neurons have been associated with primary, but not exclusive, control of sinoatrial nodal function and inferior vena cava-inferior atrial ganglionated plexus neurons primarily, but not exclusively, with control of atrioventricular nodal function (Ardell, 1994). One population of intrinsic cardiac neurons, the parasympathetic postganglionic ones (Blomquist et al. 1987), receives direct inputs from medullary parasympathetic preganglionic neurons (Armour & Hopkins, 1984; Gagliardi et al. 1988). Another population of adrenergic efferent neurons (Butler et al. 1986) receives inputs from more centrally located neurons in intrathoracic ganglia and the spinal cord (Gagliardi et al. 1988; Armour & Hopkins, 1990). That ventricular sensory neurites continue to influence the activity generated by neurons on the heart following chronic decentralization of the intrinsic cardiac nervous system has been interpreted as indicating that the somata of afferent neurons are located within the intrinsic cardiac nervous system (Ardell et al. 1991), some of which project axons to central neurons (Yuan et al. 1993). This latter concept has received anatomical confirmation (Cheng et al. 1997). Functional data also indicate that the intrinsic cardiac nervous system contains local circuit neurons interconnecting intrinsic cardiac afferent with efferent neurons (Gagliardi et al. 1988; Armour & Hopkins, 1990).
We have proposed that neural control of cardiac function resides in the network of nested feedback loops made up of the intrinsic cardiac nervous system, extracardiac intrathoracic autonomic ganglia, the spinal cord and brainstem (Armour et al. 1998). Within this hierarchy, the intrinsic cardiac nervous system functions as a distributive processor at the level of the target organ. It has been proposed that the redundancy of function and non-coupled behaviour displayed by neurons in intrathoracic extracardiac and intrinsic cardiac ganglia minimizes the dependency for such control on a single population of peripheral autonomic neurons (Armour et al. 1998). In this regard, we have proposed that network interactions occurring within the intrinsic cardiac nervous system integrate parasympathetic and sympathetic efferent inputs with afferent feedback to modify cardiac rate and regional contractile force throughout each cardiac cycle (Gagliardi et al. 1988; Armour & Hopkins, 1990; Ardell, 1994).
Consistent coherence of activity generated by differing populations of neurons is indicative of principal and direct synaptic interconnections between them or, conversely, the sharing by such neurons of common inputs. Such relationships have been identified among medullary and spinal cord sympathetic efferent preganglionic neurons (Gebber et al. 1990), as well as among different populations of sympathetic efferent preganglionic neurons (Kocsis, 1994; Kocsis et al. 1999). We hypothesize that different populations of neurons, distributed spatially within the intrinsic cardiac nervous system, respond to cardiac perturbations in a co-ordinate fashion. If neurons in one part of this neuronal network respond to inputs from a single region of the heart, such as the mechanosensory neurites associated with a right ventricular ventral papillary muscle, then the potential for imbalance within the different populations of neurons regulating various cardiac regions might occur, and thus its neurons would display little coherence of activity. In other words, relatively low levels of specific inputs on a spatial scale to the intrinsic cardiac nervous system would result in low coherence among its various neuronal components (Kember et al. 2000). On the other hand, excessive input to this spatially distributed nervous system would destabilize it (Kember et al. 2000), leading to cardiac arrhythmia formation, etc. (Yuan et al. 1993). Thus the present experiments were devised to determine if different populations of neurons contained within a single intrinsic cardiac ganglionated plexus exhibit coherence of activity or act as independent entities.
We sought to determine how adjacent populations of intrinsic cardiac neurons respond to specific, localized ventricular mechanical or chemical stimuli. In this manner we characterized not only some of the putative inputs to neurons located in different regions of one intrinsic cardiac ganglionated plexus, but also the degree to which such populations of neurons interact when responding to alterations in the cardiac milieu to exert preferential control over regional cardiac function.
METHODS
Animal preparation
Adult mongrel dogs (n = 16) of either sex, weighing between 17 and 22 kg, were used in this study. All experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, National Academy Press, Washington DC (1996), and were approved by the institutional animal care and use committee of Dalhousie University.
General methods
Canines were tranquilized with sodium pentothal (15–20 mg kg−1i.v.) and then anaesthetized with sodium pentothal (5 mg kg−1i.v. to effect every 5–10 min for the duration of the surgical procedures). Following anaesthesia induction, each animal was intubated. Positive-pressure ventilation was initiated and maintained with a Bird Mark 7A ventilator using a gas mixture of 95 % O2 and 5 % CO2. After the surgery was completed, anaesthesia was changed to α-chloralose, which was first administered as a bolus (25–50 mg kg−1i.v.) and then as repeat doses (25 mg kg−1i.v.) every hour or less throughout the experiments, as required. When neuronal activity was recorded, spontaneous activity was suppressed for 5–10 min following bolus injections of α-chloralose due to the neuronal depressor effects of this agent. Therefore, at least 10 min were allowed to elapse following such injections before recordings proceeded. Noxious stimuli were applied to a paw occasionally throughout the experiments in order to ascertain the adequacy of the anaesthesia by assessing alterations in heart rate or blood pressure and a lack of withdrawal by a limb when pinched.
A bilateral thoracotomy was made in the fourth intercostal space. Umbilical tape was placed around the descending thoracic aorta so that this vessel could be partially occluded later in the experiments. A lead II ECG was recorded. Left atrial chamber, left ventricular chamber and aortic pressures were measured using Bentley Trantec model 800 transducers connected to a PE50 catheter placed in the left atrial chamber, or a Cordis no. 7 pig-tail catheter inserted into the left ventricular chamber via a femoral artery, or a Cordis no. 6 catheter inserted into the ascending aorta via the other femoral artery. Miniature solid-state pressure transducers (Konigsberg Instruments, Pasadena, CA, USA; model P190, 5 mm diameter, 1·5 mm thick) were inserted in the right ventricular conus and the left ventricular ventral wall to record regional intramyocardial pressures. These sensing devices were employed because intraventricular pressure is not a sensitive index for detecting inotropic changes induced in some ventricular regions by autonomic efferent neurons. All data, including neuronal activity, were recorded on an Astro-Med Inc. model MT 9500 eight-channel rectilinear recorder (Astro-Med Inc., West Warwick, RI, USA).
Recording neuronal activity
The activity generated by neurons in two different loci of the ventral right atrial ganglionated plexus was studied in each animal. For recording neuronal activity, two tungsten microelectrodes (Frederick Haer & Co., model ME 25-10-2) were inserted into separate loci in the fat on the ventral surface of the canine right atrium, which contains the ventral component of the right atrial ganglionated plexus (Yuan et al. 1994). Each recording microelectrode had a shank diameter of 250 μm, an exposed tapered tip of 1 μm and impedance of 9–11 MΩ at 1000 Hz. A circular ring of heavy-gauge wire was placed gently on the surface of the epicardial fat overlying the ventral surface of the right atrium to minimize epicardial motion. This fat was explored with the tungsten microelectrodes mounted on separate micromanipulators at depths ranging from the surface of the fat to regions adjacent to cardiac musculature, as has been described previously (Gagliardi et al. 1988). Proximity to cardiac musculature was indicated by increases in the amplitude of the ECG artifact. Each indifferent electrode was attached to the adjacent pericardium. Individual action potentials so derived were differentially amplified by Princeton Applied Research model 113 amplifiers that had bandpass filters set at 300 Hz to 10 kHz and amplification ranges of ×100–500. The output of these devices, further amplified (×50–200) and filtered (bandwidth 100 Hz to 2 kHz) by means of optically isolated amplifiers (Applied Microelectronics Institute, Halifax, Nova Scotia, Canada), was led to a Nicolet model 207 oscilloscope and to a Grass AM8 Audio Monitor. The caudal region of this ganglionated plexus was searched in particular, as that is where most spontaneously active neurons can be identified (Gagliardi et al. 1988).
Electrical activity recorded by these methods is generated by the somata and/or dendrites of neurons adjacent to the electrode tip, whether neurons are located in the central nervous system (Gebber et al. 1990) or peripheral autonomic ganglia (Armour & Janes, 1988; Gagliardi et al. 1988; Armour et al. 1994). When such a recording electrode penetrates axons adjacent to a small mediastinal ganglion along the course of a nerve adjacent to the heart (Armour & Janes, 1988) or nerves associated with nodose (Armour et al. 1994) or dorsal root (Huang et al. 1995) ganglia, barely detectable action potentials (signal-to-noise ratios below 2:1) are discerned. The same holds true with respect to results obtained when such an electrode penetrates an intrinsic cardiac nerve (Armour & Hopkins, 1990). Thus this method of recording does not permit the identification of activity generated by individual axons of passage. In accordance with this, when a similar recording electrode penetrates an intrathoracic extracardiac (Armour et al. 1998) or nodose (Armour et al. 1994) ganglion, individual action potentials with similar configurations and signal-to-noise ratios greater than 3:1 can be identified for hours. Action potentials are generated after a fixed latency by a small population of intrinsic cardiac neuronal somata/dendrites, as identified by these same techniques, when electrical stimuli are delivered (0·1 Hz) to axons in intrathoracic extracardiac vagal or sympathetic nerves (Gagliardi et al. 1988; Armour & Hopkins, 1990). As most identified neurons in intrinsic cardiac ganglia are local circuit ones, electrical stimuli applied to efferent axons in the vagi, subclavian ansae or cardiopulmonary nerves induce action potentials from the vast majority of identified neurons after varied latencies, indicative of multiple synapses (Gagliardi et al. 1988; Armour & Hopkins, 1990). For these reasons, the collision technique of neuronal identification is not appropriate for this preparation.
Interventions
Two separate loci within the right atrial ganglionated plexus were identified from which action potentials with signal-to-noise ratios greater than 3:1 were recorded. Individual units were identified by the amplitude and configuration of their action potentials. Gently touching various ventricular epicardial loci with a saline-soaked cotton swab activated identified neurons. Once the extent of the ventricular epicardial region(s) that initiated neuronal responses when touched was determined, chemicals known to modify ventricular sensory neurites associated with cardiac afferent neurons (Thompson et al. 2000) were applied to these epicardial loci for 60–100 s using 1 cm × 1 cm gauze squares soaked with 0·5 ml of each respective chemical. This was done to determine whether identified neurons received cardiac chemosensory inputs. Sensory fields were washed for 30 s with normal saline (∼2 ml s−1) after each chemical was removed, at least 5 min being allowed to elapse before the next intervention.
The following agents, obtained from Sigma (USA) and BDH (Toronto, Ontario, Canada), were applied to epicardial sensory fields in pharmacological doses dissolved in physiological Tyrode solution. The agents investigated were: (1) the sodium channel modifying agent veratridine (7·5 μm); (2) the peptide bradykinin (1 μm); (3) the nitric oxide donor nitroprusside (50 μm); (4) the excitatory amino acid glutamate (100 μm); (5) the β1-adrenoceptor agonist prenaterol (10 μm); (6) the β2-adrenoceptor agonist terbutaline (10 μm); (7) the α1-adrenoceptor agonist phenylephrine (10 μm); (8) the α2-adrenoceptor agonist clonidine (10 μm); and (9) angiotensin II (10 μm). The order of their application varied between experiments. When neuronal responses were elicited, the same agents were reapplied to the same epicardial locus at least twice to verify the reproducibility of the induced responses. Gauze squares soaked with room temperature normal saline were also applied to identified epicardial sensory fields to determine whether neuronal responses elicited by epicardial chemical application were due to vehicle effects or the mechanical effects elicited by gauze squares. To determine if altered cardiovascular states affected neuronal activity, the descending thoracic aorta was then partially occluded for about 5 s.
Data acquisition and analysis
The activity generated by neurons in two different loci within the ventral right atrial ganglionated plexus was recorded simultaneously along with a lead II ECG, left atrial and left ventricular chamber pressures, right and left ventricular intramyocardial pressures and aortic pressure using an Astro-Med Inc. model MT 9500 eight-channel rectilinear recorder. Data were stored on VHS tape (T120 Scotch, 3M Canada Inc., London, Ontario, Canada) using a VCR recorder (A.R. Vetter, Co. Model 820, Rebersburg, PA, USA) for later analysis. Cardiac indices derived over 30 s periods were analysed before and during peak responses elicited by each intervention. The means (± 1 s.e.m.) of cardiac indices were calculated from these data. Spontaneous fluctuations of cardiodynamics were minimal during control periods, heart rate varying less than 5 beats min−1 and systolic pressure fluctuating less than 5 mmHg. Thresholds for classifying cardiovascular changes were chosen to be greater than these ranges.
Fluctuations in the amplitude of action potentials generated by a unit varied by 20–80 μV over several minutes, depending on the site; action potentials retained their same configurations over time. Action potentials recorded from a given locus with the same configuration were considered to be generated by a single unit. Action potentials generated at a given locus were counted for 30 s periods in order to establish average activity immediately prior to and during maximal responses elicited by each intervention. Within a given site, multiple units (2–5) were identified by means of a computer program that recorded the heights and configurations of individual action potentials. The threshold for neuronal activity changes was taken as a change of more than 20 % from baseline values. Data are expressed as means ± s.e.m. One-way ANOVA and Student's paired t test with Bonferroni correction for multiple tests were used for statistical analysis. A significance value of P < 0·01 was used for these determinations.
The coupling of activity generated by the two populations of identified neurons was analysed continuously over time as they received constantly changing inputs (Kember et al. 2000). In order to accomplish this, the activity generated by neurons in two different loci of the one intrinsic cardiac ganglionated plexus was digitized continuously at sampling frequencies of 4000 Hz over 5 min periods of time during control states. This was done by window discriminating action potentials recorded at each site, i.e. values below a threshold were zeroed and those above the assigned window value were truncated to have unit value. Then the instantaneous activity level generated by individual units was computed simultaneously as a centred moving average over 2 s periods. The activity generated by the two different populations of identified neurons were cross-correlated over 5 min time periods using sliding windows of 10 s duration (Armour et al. 1998). This sliding window approach permitted the determination of a continuous cross-correlation coefficient analysis of the activities generated by the two populations of identified neurons, with absent correlation being set to zero. Due to the fact that chemical as opposed to mechanical stimuli induced consistent, long-term responses during their application, the activity changes induced by epicardial application of veratridine underwent cross-correlation analysis as well. Continuous analysis of heart rate and peak left ventricular systolic pressure was performed over the same time periods in order to compare alterations in activity generated by different populations of right atrial neurons with concomitant changes in monitored cardiac indices.
RESULTS
Spontaneous activity generated by neurons in two different loci of one intrinsic cardiac ganglionated plexus
Employing these extracellular recording techniques, action potentials with signal-to-noise ratios greater than 3:1 were consistently recorded from two disparate sites within the right atrial ganglionated plexus of each dog. The two sites from which spontaneous action potentials could be recorded were located in the middle and caudal pole of the right atrial ganglionated plexus. Action potentials generated by, on average, three (range 2–5) spontaneously active neurons, as identified by their action potential heights and configurations, were recorded from each locus. During control states, the activity displayed by these two populations averaged 9·3 ± 3·1 to 29·1 ± 7·2 impulses per minute (Table 1 and Table 2). In 15 of the 16 animals, neurons in both sites displayed bursting of activity during control states that occurred every 10–30 s (Fig 1 and Fig 2). Their cyclic behaviour was, for the most part, maintained when neuronal activity was enhanced by epicardial stimuli (Fig 3 and Fig 4). In a few instances epicardial chemical stimuli suppressed neuronal activity at one site and thus reduced or eliminated such cyclical behaviour (Fig. 5).
Table 1.
Neuronal activity responses elicited by veratridine, bradykinin, glutamate, nitroprusside, prenaterol, touch and partial occlusion of the thoracic aorta (aortic occlusion)
| Intervention (n = 16 dogs) | HR (beats min−1) | LAP (mmHg) | RV IMP (mmHg) | LV IMP (mmHg) | LVP (mmHg) | Activity at site 1 (i.p.m.) | Activity at site 2 (i.p.m.) |
|---|---|---|---|---|---|---|---|
| Control | 123 ± 6 | 10 ± 1 | 29 ± 3 | 85 ± 6 | 127 ± 9 | 28.5 ± 5.4 | 21.9 ± 4.4 |
| Veratridine | 123 ± 6 | 10 ± 1 | 29 ± 3 | 87 ± 7 | 130 ± 6 | 52.8 ± 16.4 | 41.6 ± 9.5* |
| Control | 124 ± 6 | 10 ± 1 | 29 ± 3 | 86 ± 6 | 128 ± 5 | 29.1 ± 7.2 | 12.6 ± 2.4 |
| Bradykinin | 123 ± 6 | 10 ± 1 | 29 ± 3 | 87 ± 6 | 128 ± 5 | 42.5 ± 9.8 | 32.3 ± 7.4* |
| Control | 120 ± 6 | 9 ± 1 | 28 ± 2 | 81 ± 4 | 125 ± 5 | 16.7 ± 3.3 | 14.9 ± 4.7 |
| Glutamate | 122 ± 7 | 10 ± 1 | 28 ± 2 | 82 ± 4 | 128 ± 5 | 31.1 ± 5.7* | 19.9 ± 5.3 |
| Control | 121 ± 6 | 10 ± 1 | 27 ± 2 | 84 ± 5 | 127 ± 5 | 22.1 ± 4.8 | 14.8 ± 4.1 |
| Nitroprusside | 122 ± 7 | 10 ± 1 | 29 ± 2 | 87 ± 6 | 130 ± 5 | 39.1 ± 8.3* | 25.8 ± 7.1* |
| Control | 122 ± 5 | 9 ± 1 | 27 ± 2 | 84 ± 5 | 127 ± 5 | 22.7 ± 5.1 | 17.6 ± 3.9 |
| Prenaterol | 122 ± 6 | 9 ± 1 | 27 ± 2 | 84 ± 5 | 127 ± 5 | 37.6 ± 7.8* | 28.9 ± 6.9 |
| Control | 123 ± 6 | 10 ± 1 | 29 ± 3 | 85 ± 6 | 128 ± 5 | 19.9 ± 3.4 | 21.2 ± 4.0 |
| Touch | 123 ± 6 | 10 ± 1 | 29 ± 3 | 88 ± 7 | 133 ± 7 | 51.3 ± 7.9* | 52.4 ± 13.0* |
| Control | 128 ± 7 | 9 ± 1 | 29 ± 3 | 83 ± 4 | 104 ± 6 | 25.1 ± 4.3 | 22.5 ± 4.7 |
| Aorticn occlusion | 129 ± 7 | 9 ± 1 | 30 ± 2 | 102 ± 6* | 138 ± 8* | 32.5 ± 5.7 | 39.1 ± 5.2* |
Each intervention listed activated multiple neurons in at least one right atrial locus in every animal tested. Activity was generated by, on average, three units at each site (sites 1 and 2, expressed as impulses per minute, i.p.m.). Right atrial ganglionated plexus site 2 was located caudal to site 1. Heart rate (HR), left atrial systolic pressure (LAP), right (RV IMP) and left (LV IMP) ventricular intramyocardial systolic pressures, and left ventricular chamber systolic pressure (LVP) were unaffected by each intervention; aortic occlusion was the exception.
P < 0.01 control versus intervention.
Table 2.
Effects of the other chemicals tested
| Intervention | HR (beats min−1) | LAP (mmHg) | RV IMP (mmHg) | LV IMP (mmHg) | LVP (mmHg) | Activity at site 1 (i.p.m.) | Activity at site 2 (i.p.m.) |
|---|---|---|---|---|---|---|---|
| Control (n = 13) | 117 ± 7 | 10 ± 1 | 27 ± 3 | 87 ± 7 | 125 ± 8 | 17.7 ± 4.0 | 16.2 ± 4.5 |
| Angiotensin II | 119 ± 7 | 10 ± 1 | 27 ± 3 | 87 ± 7 | 127 ± 7 | 53.0 ± 16.1* | 30.1 ± 7.4* |
| Control (n = 7) | 122 ± 5 | 10 ± 1 | 29 ± 3 | 86 ± 7 | 128 ± 5 | 20.2 ± 7.8 | 22.3 ± 4.0 |
| Phenylephrine | 122 ± 5 | 10 ± 1 | 29 ± 3 | 85 ± 6 | 127 ± 6 | 12.2 ± 5.4 | 11.8 ± 2.1* |
| Control (n = 12) | 119 ± 7 | 10 ± 1 | 28 ± 3 | 88 ± 7 | 129 ± 6 | 9.3 ± 3.1 | 10.6 ± 3.5 |
| Clonidine | 121 ± 6 | 10 ± 1 | 28 ± 2 | 89 ± 7 | 127 ± 6 | 18.4 ± 5.6* | 19.0 ± 5.4* |
| Contro (n = 11) | 122 ± 5 | 10 ± 1 | 29 ± 3 | 86 ± 7 | 128 ± 5 | 24.6 ± 7.8 | 24.9 ± 4.4 |
| Terbutaline | 122 ± 5 | 10 ± 1 | 29 ± 3 | 89 ± 7 | 129 ± 6 | 43.5 ± 10.0* | 36.5 ± 5.2 |
Neurons in many, but not all animals (tabulated as n) were modified by epicardial application of angiotensin II, phenylephrine, clonidine or terbutaline. Monitored cardiac variables were unaffected by these interventions. (Abbreviations are the same as in Table 1.)
Figure 1. The activity generated by two different populations of neurons in the right atrial ganglionated plexus recorded during control states.
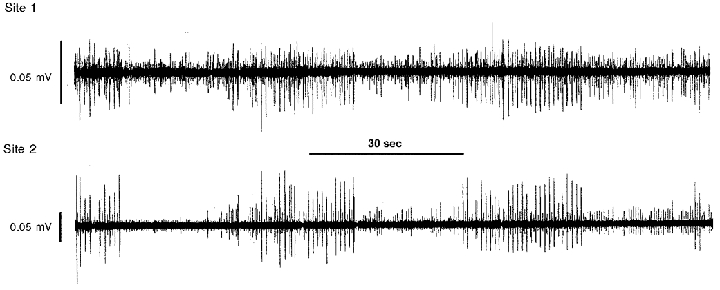
The maximum activity generated by two populations of right atrial neurons (5 units identified at site 1; 4 units identified at site 2, as shown by differing action potential heights) occurred approximately every 40 s. Both neuronal populations displayed bursts of activity that were in-phase with one another.
Figure 2. Comparisons of activity generated by two populations of right atrial neurons during control states recorded over time.
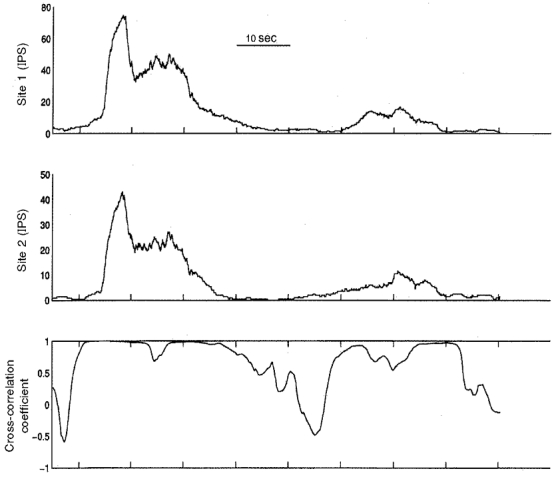
Ongoing activity generated by neurons in two regions of the right atrial ganglionated plexus (sites 1 and 2, upper two panels) of one animal was found to be cyclical in nature during control states; maximum activity was generated by the two populations at almost the same time. The activities generated by the two populations displayed coherence (as determined by continuous cross-correlation of their activities; lowest panel) off and on over time, particularly when bursts of activity occurred. The coherence of the activities generated by these populations of neurons reached unity on three occasions during this 90 s analysis period. IPS, impulses per second.
Figure 3. Activity generated by two different populations of neurons recorded before and during epicardial application of veratridine.
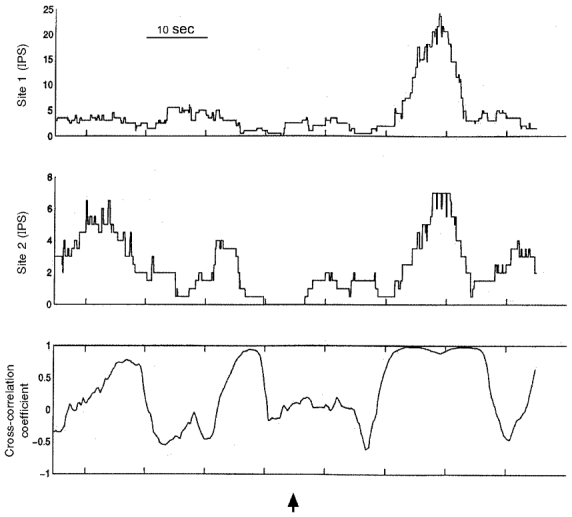
The activity generated by both populations of neurons (particularly that generated by neurons at site 1) increased when veratridine was applied topically to the right ventricular sinus epicardium (commencing at arrow). The coherence of activity generated by these two populations of neurons reached unity during the bursting of activity so elicited. IPS, impulses per second.
Figure 4. An example of chemical stimuli modifying neuronal activity and recorded cardiac variables.
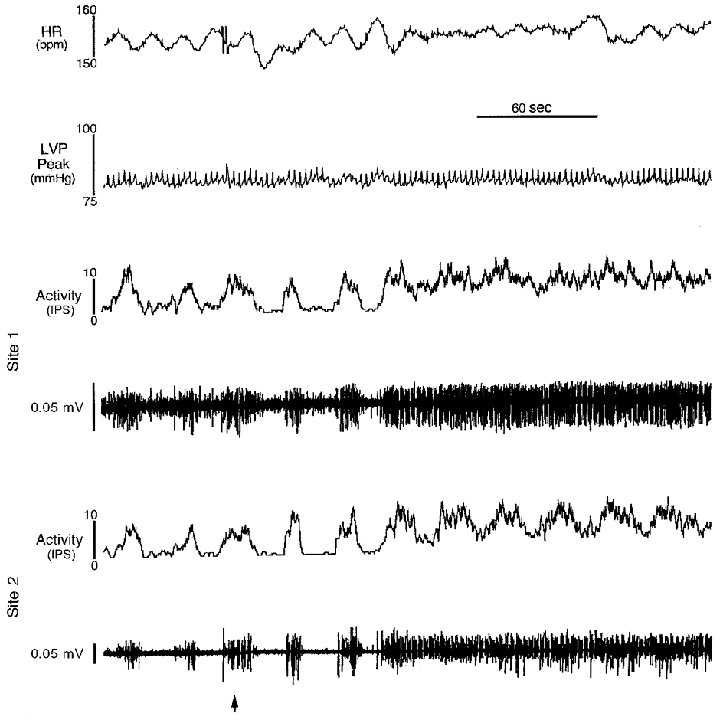
Heart rate (HR), left ventricular chamber systolic pressure (LVP peak) and neuronal activities (Activity) were recorded in two populations of right atrial neurons before and during application of veratridine (commencing at arrow) to an epicardial locus on the right ventricular sinus. Although average heart rate (as determined by a lead II ECG) remained similar during this intervention, its variability decreased as neuronal activity became enhanced. Veratridine increased overall activity generated by neurons at site 1, while reducing its bursting-type discharge. At site 2, the bursting-type discharge was maintained, while overall activity increased. HR, average ongoing heart rate; LVP peak, ongoing peak left ventricular chamber systolic pressure.
Figure 5. Cardiovascular and neuronal activities elicited by bradykinin.
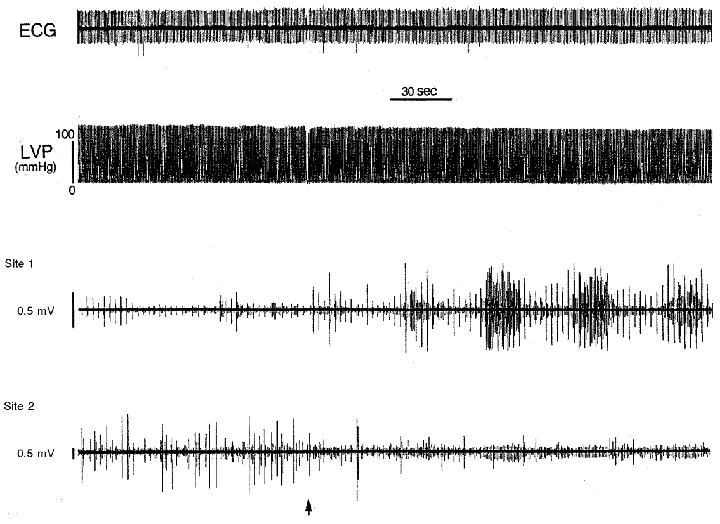
When bradykinin was applied to an epicardial locus on the right ventricular sinus (commencing at arrow), heart rate (as determined by a lead II ECG) and left ventricular chamber pressure (LVP) remained unaffected. The activity generated by multiple neurons at one site (1) increased, while that of the other population (site 2) decreased. Note that the activity generated by neurons at site 1 displayed cyclic activity every 45 s once the stimulus was applied.
The coupling of activity generated by the two populations of investigated right atrial neurons fluctuated continuously over time during control states. Most importantly, the duration of coupled behaviour displayed by both populations of neurons was sustained over many cardiac cycles. The peak correlation coefficient of activities generated by the two populations of neurons in the 16 animals during control states ranged between 0·71 and 1·0 (average of 0·88 ± 0·02). Cycles of maximum coherent behaviour, lasting for up to 20 cardiac cycles, occurred every 15–30 s (Fig. 2, bottom left).
Epicardial mechanical stimuli
Local epicardial touch enhanced the activity generated by both populations of neurons (Table 1). Mechanical stimuli induced neuronal responses that had a rapid onset. No detectable changes in monitored cardiac indices occurred during focal epicardial application of mechanical stimuli. Mechanically induced neuronal responses waned rapidly after the mechanical stimulus was removed. Epicardial sites that affected neuronal activity when touched were located in the right ventricular conus or sinus (cranial 2/3) or the cranial aspects of the ventral and lateral surfaces of the left ventricle. Increasing ascending aortic pressure by partially occluding the aorta increased left ventricular wall and chamber systolic pressures, as well as aortic root pressure. This intervention also enhanced the activity generated by one of the populations of identified neurons (Table 1).
Epicardial chemical stimuli
The epicardial application of tested chemicals enhanced the activity generated by neurons in one or both investigated loci in the right atrial ganglionated plexus in each dog (Figs 3–5). Chemically induced responses took time to develop and lasted, on average, 2·1 min after removal of the chemical before they returned to baseline values. Monitored cardiovascular variables did not change during epicardial application of chemicals (Tables 1 and 2), presumably owing to the fact that small quantities of chemicals were utilized to minimize any possibility that the chemical would enter the circulation in sufficient quantities to affect distant tissues.
Chemosensory inputs to identified neurons were multimodal in nature, more neurons responding to nitroprusside than to any other chemical tested (Table 1). The ion modifying agent veratridine (Figs 3 and 4), the peptide bradykinin (Fig. 5), the excitatory amino acid glutamate and the β1-adrenoceptor agonist prenaterol, when applied individually to epicardial loci, consistently activated neurons in at least one locus (Table 1). Fewer neurons responded to epicardial application of angiotensin II, the β2-adrenoceptor agonist terbutaline or the α2-adrenoceptor agonist clonidine (Table 2). The α1-adrenoceptor agonist phenylephrine was the only pharmacological agent that, when applied to epicardial loci, decreased neuronal activity (Table 2). Veratridine-induced increases in neuronal activity were sometimes accompanied by reductions in heart rate variability (Fig. 4). Reapplication of chemicals to previously responsive epicardial loci induced similar neuronal responses. Although in most cases epicardial application of a chemical enhanced the activity generated by both populations of neurons (Fig. 3), in a few instances the activity generated by one population was enhanced while that of the other was suppressed (Fig. 5). Epicardial application of gauze squares soaked with room temperature normal saline elicited no neuronal responses.
When the correlation coefficient of activity generated by the different populations of right atrial neurons studied was evaluated during epicardial application of veratridine, coupling of activity occurred periodically that was similar in nature to the coupled behaviour identified in control states. The average peak correlation coefficient of activities generated between the two populations of neurons during epicardial application of veratridine reached 0·90 ± 0·03 (range 0·75–1·0). Such coupling of activity displayed by the two populations of neurons lasted for up to 20 cardiac cycles (Fig. 3).
DISCUSSION
The results of the present investigation indicate that neurons located in a single aggregate of the intrinsic cardiac nervous system, the right atrial ganglionated plexus, frequently display activity responses that are qualitatively similar. Many of the neurons within one intrinsic cardiac ganglionated plexus displayed tightly coupled behaviour for a number of cardiac cycles at a time, coupled activity occurring every 15–30 s even during heightened sensory inputs from ventricular sensory neurites (Figs 2 and 3). Such coupling of activity presumably represents the basis for co-ordinating control over regional cardiac function by populations of neurons in one cardiac locus. In this regard, the right atrial ganglionated plexus has been associated with primary, but not exclusive, control of sinoatrial nodal function (Ardell, 1994). Thus increased activity generated by neurons within the right atrial ganglionated plexus would be expected to alter heart rate variability (Fig. 4).
It is generally thought that neurons in peripheral autonomic ganglia do not play a major role in information processing (Skok, 1973; Malpas, 1998). However, recent evidence repudiates that view in as much as various populations of neurons in extracardiac and intrinsic cardiac intrathoracic ganglia are responsive to regional cardiodynamic changes (Armour et al. 1998). These responses are reflective of afferent feedback that is manifest through distinct intracardiac and intrathoracic feedback loops, which in turn are influenced by interactions with spinal cord and brainstem feedback loops (Gagliardi et al. 1988; Armour & Hopkins, 1990). While these nested feedback loops act in an overall co-ordinated fashion, the activity generated by neurons in intrinsic cardiac and intrathoracic extracardiac ganglia exhibits non-coupled behaviour even when they are mutually entrained to cardiac events by cardiovascular afferent feedback (Armour et al. 1998). Thus data are accumulating that indicate differential control is exerted by different populations of neurons contained within intrathoracic extracardiac and intrinsic cardiac ganglia, and that these populations interact on a beat-to-beat basis in a relatively independent fashion (Armour et al. 1998).
The intrinsic cardiac nervous system contains afferent neurons that interact with local circuit neurons to influence parasympathetic and sympathetic efferent postganglionic neurons innervating specific cardiac regions (Ardell, 1994; Horackova & Armour, 1995). Data derived from this study indicate that neurons within a single intrinsic cardiac ganglionated plexus display tightly coupled behaviour much of the time (Fig. 2), reflective of interdependent interactions among such neurons. The majority of these neurons received inputs from mechanical and chemical sensory neurites distributed throughout the ventricles (Table 1). That a nitric oxide donor, for instance, can modify the sensory inputs to the intrinsic cardiac nervous system may have implications with respect to how nitrates affect cardiac control when administered therapeutically.
Neurons in at least one identified locus studied in each ganglionated plexus were affected by epicardial application of the peptide bradykinin, the excitatory amino acid glutamate or the β1-adrenoceptor agonist prenaterol (Table 1). Fewer intrinsic cardiac neurons were affected by epicardial application of the ion channel modifying agent veratridine, angiotensin II, α-adrenoceptor agonists or a β2-adrenoceptor agonist (Table 2). Thus intrinsic cardiac neurons receive graded sensory inputs that are polymodal in nature. That inhibitory responses were elicited by epicardial application of phenylephrine is in keeping with the fact that this α-adrenoceptor agonist not only excites sensory neurites associated with cardiac afferent neurons, but also suppresses some of them (Thompson et al. 2000). That ventricular sensory inputs to populations of intrinsic cardiac neurons are sensitive to adrenoceptor agents liberated by adjacent sympathetic efferent postganglionic axons indicates the presence of feedback control at the level of the target organ.
The co-ordination of autonomic outflows from different populations of neurons in one intrinsic cardiac ganglionated plexus depends to a large extent upon the sharing of inputs from such cardiac sensory neurites. That populations of neurons distributed throughout an intrinsic cardiac ganglionated plexus frequently display transient (15–30 s), repetitive short-term (millisecond) neuronal interactions during control states (Fig. 2), and that such constantly fluctuating coupling of neuronal behaviour persists during enhancement of their ventricular sensory inputs (Fig. 3), indicates the dynamic nature of such coupling.
Cardiac neurons in intrathoracic extracardiac and intrinsic cardiac ganglia generate low-frequency rhythms that are frequently out of phase with one another. That neurons in intrathoracic extracardiac ganglia usually do not generate activity correlating with that generated by intrinsic cardiac neurons may relate to their varied sensory inputs (Armour et al. 1998). Presumably the relatively slow patterns of activity generated by neurons in extracardiac and intrinsic cardiac ganglia act to stabilize overall co-ordination of regional cardiac function over time. That they can differ in their pattern generation indicates that control does not depend solely on one population of intrathoracic neurons (Armour et al. 1998). With respect to differing populations of neurons in one region of the heart, that many neurons displayed not only short-term coupled behaviour but also low-frequency rhythms indicates not only that they share common inputs, but also that many neurons in one ganglionated plexus exert concomitant control over varied cardiac regions. Presumably some of this coupled behaviour depends on inputs from extracardiac neurons (Armour et al. 1998).
It is concluded that the coherence of activity generated by distinct populations of neurons within one intrinsic cardiac ganglionated plexus determines the integrative control expressed by such neurons over specific cardiac function. Unlike interactions among neurons in intrathoracic extracardiac versus intrinsic cardiac ganglia, neurons distributed in a single intrinsic cardiac ganglionated plexus display interdependence of function much of the time. That activation of regional cardiac afferent neurons influences multiple local circuit neurons within a single intrinsic cardiac ganglionated plexus suggests the presence of local feedback mechanisms at the level of the target organ, another variable that will have to be taken into account when assessing neurocardiological function. It is proposed that the interdependent activity displayed by different populations of neurons in one region of the heart, modulated not only by extracardiac sensory inputs but also by local afferent neuronal feedback, forms the basis for regional cardiac control.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Richard Livingston. This work was supported by the Medical Research Council of Canada (MA-10122), the Nova Scotia Heart and Stroke Foundation, the NIH (grant no. HL58140) and the American Heart Association.
References
- Ardell JL. Anatomy and function of mammalian intrinsic cardiac neurons. In: Armour JA, Ardell JL, editors. Neurocardiology. New York: Oxford University Press; 1994. pp. 95–114. [Google Scholar]
- Ardell JL, Butler CK, Smith F, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. American Journal of Physiology. 1991;260:H713–721. doi: 10.1152/ajpheart.1991.260.3.H713. [DOI] [PubMed] [Google Scholar]
- Armour JA, Collier K, Kember G, Ardell JL. Differential selectivity of cardiac neurons in separate intrathoracic ganglia. American Journal of Physiology. 1998;274:R939–949. doi: 10.1152/ajpregu.1998.274.4.R939. [DOI] [PubMed] [Google Scholar]
- Armour JA, Hopkins DA. Anatomy of extrinsic efferent autonomic nerves and ganglia innervating the mammalian heart. In: Randall WC, editor. Nervous Control of Cardiovascular Function. New York: Oxford University Press; 1984. pp. 20–45. [Google Scholar]
- Armour JA, Hopkins DA. Activity of in vivo canine ventricular neurons. American Journal of Physiology. 1990;258:H326–336. doi: 10.1152/ajpheart.1990.258.2.H326. [DOI] [PubMed] [Google Scholar]
- Armour JA, Huang MH, Pelleg A, Sylven C. Responsiveness of in situ canine nodose ganglion cardiac afferent neurons to epicardial mechanoreceptor and/or chemoreceptor stimuli. Cardiovascular Research. 1994;28:1218–1225. doi: 10.1093/cvr/28.8.1218. [DOI] [PubMed] [Google Scholar]
- Armour JA, Janes RD. Neuronal activity recorded extracellularly from in situ mediastinal ganglia. Canadian The Journal of Physiology and Pharmacology. 1988;66:119–127. doi: 10.1139/y88-022. [DOI] [PubMed] [Google Scholar]
- Blomquist TM, Priola DV, Romero AM. Source of intrinsic innervation of canine ventricles: a functional study. American Journal of Physiology. 1987;252:H638–644. doi: 10.1152/ajpheart.1987.252.3.H638. [DOI] [PubMed] [Google Scholar]
- Butler CK, Smith FM, Cardinal R, Murphy DA, Hopkins DA, Armour JA. Cardiac responses to electrical stimulation of discrete loci in canine atrial or ventricular ganglionated plexi. American Journal of Physiology. 1990;259:H1365–1373. doi: 10.1152/ajpheart.1990.259.5.H1365. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, Doyle FJ. Vagal afferent innervation of the atria of the rat heart reconstructed with confocal microscopy. Journal of Comparative Neurology. 1997;381:1–17. doi: 10.1002/(sici)1096-9861(19970428)381:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GDS, Klemm MM, Steele PA. Different types of ganglion cell in the cardiac plexus of guinea-pigs. The Journal of Physiology. 1995;486:453–471. doi: 10.1113/jphysiol.1995.sp020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi M, Randall WC, Bieger D, Wurster RD, Hopkins DA, Armour JA. Activity of in vivo canine cardiac plexus neurons. American Journal of Physiology. 1988;255:H789–800. doi: 10.1152/ajpheart.1988.255.4.H789. [DOI] [PubMed] [Google Scholar]
- Gebber GL, Barnes SM, Kocsis BK. Coherence of medullary unit activity and sympathetic nerve discharge. American Journal of Physiology. 1990;259:R561–571. doi: 10.1152/ajpregu.1990.259.3.R561. [DOI] [PubMed] [Google Scholar]
- Horackova M, Armour JA. Role of peripheral autonomic neurons in maintaining adequate cardiac function. Cardiovascular Research. 1995;30:326–335. doi: 10.1016/0008-6363(95)00105-0. [DOI] [PubMed] [Google Scholar]
- Huang MH, Sylven C, Horackova M, Armour JA. Ventricular sensory neurons in canine dorsal root ganglia: effects of adenosine and substance P. American Journal of Physiology. 1995;269:R318–324. doi: 10.1152/ajpregu.1995.269.2.R318. [DOI] [PubMed] [Google Scholar]
- Kember GC, Fenton GA, Collier K, Armour JA. Aperiodic stochastic resonance in a hysteretic population of cardiac neurons. Physical Reviews E. 2000;61:1816–1824. doi: 10.1103/physreve.61.1816. [DOI] [PubMed] [Google Scholar]
- Kocsis B. Basis for differential coupling between rhythmic discharges of sympathetic efferent nerves. American Journal of Physiology. 1994;267:R1008–1019. doi: 10.1152/ajpregu.1994.267.4.R1008. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Karlsson T, Wallin BG. Cardiac- and noncardiac-related coherence between sympathetic drives to muscles of different human limbs. American Journal of Physiology. 1999;276:R1608–1616. doi: 10.1152/ajpregu.1999.276.6.R1608. [DOI] [PubMed] [Google Scholar]
- Malpas SC. The rhythmicity of sympathetic nerve activity. Progress in Neurobiology. 1998;56:65–96. doi: 10.1016/s0301-0082(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Skok VI. Physiology of Autonomic Ganglia. Tokyo: I. Shoin Ltd; 1973. [Google Scholar]
- Thompson GW, Horackova M, Armour JA. Chemotransduction properties of nodose ganglion cardiac afferent neurons in guinea-pigs. American Journal of Physiology — Regulatory, Integrative and Comparative Physiology. 2000;279:R433–439. doi: 10.1152/ajpregu.2000.279.2.R433. [DOI] [PubMed] [Google Scholar]
- Yuan B-X, Ardell JL, Hopkins DA, Armour JA. Gross and microscopic anatomy of canine intrinsic cardiac neurons. Anatomical Record. 1994;239:75–87. doi: 10.1002/ar.1092390109. [DOI] [PubMed] [Google Scholar]
- Yuan B-X, Hopkins DA, Ardell JL, Armour JA. Differential cardiac responses induced by nicotinic sensitive canine intrinsic atrial and ventricular neurons. Cardiovascular Research. 1993;27:760–769. doi: 10.1093/cvr/27.5.760. [DOI] [PubMed] [Google Scholar]


