Abstract
The perforated patch whole-cell configuration of the patch-clamp technique was applied to superficial cells in intact mouse pancreatic islets.
Three types of electrical activity were observed corresponding to α-, β- and δ-cells. The δ-cells were electrically active in the presence of glucose but lacked the oscillatory pattern seen in the β-cells. By contrast, the α-cells were electrically silent at high glucose concentrations but action potentials could be elicited by removal of the sugar.
Both α- and β-cells contained transient voltage-activated K+ currents. In the δ-cells, the K+ currents activated above −20 mV and were completely blocked by TEA (20 mm). The α-cells differed from the δ-cells in possessing a TEA-resistant K+ current activating already at −40 mV.
Immunocytochemistry revealed the presence of Kv3.4 channels in δ-cells and TEA-resistant Kv4.3 channels in α-cells. Thus the presence of a transient TEA-resistant current can be used to functionally separate the δ- and α-cells.
A TTX-sensitive Na+ current developed in δ-cells during depolarisations beyond −30 mV and reached a peak amplitude of 350 pA. Steady-state inactivation of this current was half-maximal at −28 mV. The δ-cells were also equipped with a sustained Ca2+ current that activated above −30 mV and reached a peak of 60 pA when measured at 2·6 mm extracellular Ca2+.
A tolbutamide-sensitive KATP channel conductance was observed in δ-cells exposed to glucose-free medium. Addition of tolbutamide (0·1 mm) depolarised the δ-cell and evoked electrical activity. We propose that the KATP channels in δ-cells serve the same function as in the β-cell and couple an elevation of the blood glucose concentration to stimulation of hormone release.
Pancreatic islets contain at least four endocrine cell types. In addition to the insulin-secreting β-cells, which comprise 70 % of the total cell number, the islets also contain somatostatin-secreting δ-cells (5–10 %), glucagon-secreting α-cells (15–20 %) and pancreatic polypeptide-secreting (PP) cells (≤ 2 %). The δ-cells are most numerous in the islets of the tail and body of the pancreas but sparse in the islets of the ventral part of the gland, whereas the PP-cells show the opposite distribution pattern (Bishop & Polak, 1997). Clearly, the complex architecture of the pancreatic islets provides the basis for extensive interplay between the various cell types, either via paracrine mechanisms or via gap junctions.
Both the α- and β-cells are electrically excitable and generate action potentials under conditions associated with stimulation of hormone release (Ashcroft & Rorsman, 1995). Whether this also applies to the δ- and PP-cells is not known but measurements of the cytoplasmic Ca2+ concentration ([Ca2+]i) and patch-clamp measurements suggest they contain both voltage-gated Ca2+ channels and ATP-regulated K+ channels (Berts et al. 1996; Nadal et al. 1999; Liu et al. 1999; Quesada et al. 1999).
We have developed a method that allows patch-clamp measurements of membrane currents in cells within intact pancreatic islets. In previous studies we exploited this novel experimental approach to characterise the electrophysiological properties of the β-cell in situ (Göpel et al. 1999a, b). We have now commenced the electrophysiological characterisation of the other islet cells and demonstrate that both the α- and δ-cells can be distinguished from β-cells in possessing a TTX-sensitive Na+ current that remains activatable at physiological membrane potentials. The α- and β-cells can then be separated by the presence of the selective expression of an A-type K+ current in the glucagon-producing cell. Here we focus on the δ-cells and describe how the various membrane conductances contribute to the generation of the electrical activity in this cell type. The electrophysiological features of the α-cell are considered in the accompanying paper (Göpel et al. 2000).
METHODS
Preparation of pancreatic islets
All experiments were carried out on cells in situ within intact pancreatic islets. NMRI mice were purchased from a commercial breeder (Möllegaard, Ry, Denmark). The experiments described in this and the accompanying paper (Göpel et al. 2000) have been approved by the ethical committee of Lund University. The mice were stunned by a blow to the head and killed by cervical dislocation. Collagenase (0·7 units ml−1) was dissolved in Hank’s buffer and injected into the pancreatic duct immediately after killing the animal and opening of the abdominal cavity. The caudal portion of the pancreas (rich in δ-cells) was then excised and islets were isolated by gentle digestion for 25 min at +37°C. Islets thus obtained were washed extensively in collagenase-free solution and subsequently maintained in short-term tissue culture (< 24 h) in RPMI 1640 containing 5 mm glucose and 10 % (v/v) fetal calf serum and supplemented with 100 μg ml−1 streptomycin and 100 IU ml−1 penicillin (all from Gibco).
Electrophysiology
Electrical activity was recorded from superficial cells in intact pancreatic islets using the perforated patch whole-cell recording mode as described elsewhere (Göpel et al. 1999a). The measurements were performed using an EPC-9 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany) and Pulse (version 8.11) software. Currents were compensated for capacitive transient and linear leak using a P/4 protocol. Patch pipettes were pulled from borosilicate glass (tip resistance, 3–5 MΩ when filled with the pipette solution). The voltage clamp was considered satisfactory when Gseries (series conductance of electrode) exceeded 40 nS (i.e. Rseries ≤ 25 MΩ). We estimate that for the currents shown, the voltage error in the worst cases was below 10 mV, and usually much less.
Solutions
The standard extracellular medium consisted of (mm): 140 NaCl, 3·6 KCl, 2 NaHCO3, 0·5 NaH2PO4, 0·5 MgSO4, 5 Hepes (pH 7·4 with NaOH), 2·6 CaCl2 and (unless otherwise indicated) 10 D-glucose. The pipette solution was composed of (mm): 76 K2SO4, 10 NaCl, 10 KCl, 1 MgCl2 and 5 Hepes (pH 7·35 with KOH). For the recording of inward currents, outward currents were suppressed by the inclusion of TEA-Cl at a concentration of 20 mm in the extracellular medium (NaCl correspondingly reduced to maintain isosmolarity). Voltage-gated Na+ currents were blocked by 0·1 μg ml−1 tetrodotoxin (TTX, Sigma). In one series of experiments, BaCl2 replaced CaCl2 in the extracellular medium and the medium was made devoid of all anions except Cl− to avoid precipitation of Ba2+. Electrical contact with the cell interior was established by addition of 0·24 mg ml−1 of the pore-forming antibiotic amphotericin B to the pipette solution (Rae et al. 1991).
Confocal microscopy
Freshly isolated mouse pancreatic islets were cultured overnight in RPMI 1640 medium and subsequently washed in the standard extracellular solution. All subsequent steps were carried out at 4°C. Islets were fixed with 4 % formaldehyde in PBS overnight and permeabilised with 0·3 % Triton X-100. Non-specific binding was blocked by pretreatment for 2 h with 10 % normal donkey serum (Jackson Immuno Research, West Grove, PA, USA) before incubating with the different primary antibodies for 4–12 h. The primary antibodies were guinea-pig anti-insulin (Eurodiagnostica, Malmö, Sweden), sheep anti-glucagon (Biogenesis Ltd, Poole, UK) and rabbit anti-somatostatin (Dako, Carpinteria, CA, USA). The distribution of the K+ channel proteins Kv1.4, Kv3.4 and Kv4.3 was visualised using rabbit antibodies raised against residues 589–655, 177–195 and 451–467 in the respective rat channel proteins (Alomone, Jerusalem, Israel). After washing with PBS supplemented with 0·3 % Triton X-100, the islets were exposed to the secondary antibodies (Cy5 anti-guinea pig, Cy2 anti-sheep, Texas Red anti-rabbit (all from Jackson Laboratories, West Grove, PA, USA) and FITC anti-rat (Sigma)) for 4–12 h. Islets were finally washed, post-fixed for 30 min in 4 % formaldehyde and mounted in a 1:1 mixture of glycerol and VectaShield (Vector, Burlingame, CA, USA). Immunolabelled islets were imaged on a confocal microscope (Zeiss LSM510, Jena, Germany), using 488 nm (Ar), 543 nm (HeNe) and 633 nm (HeNe) lasers for excitation. Emission was detected at 505–530 nm (green channel; insulin and K+ channels), at 565–615 nm (blue channel; somatostatin), and > 650 nm (red channel; insulin). Crosstalk was minimised by sequentially exciting and scanning each of the channels.
Data analysis
The activation and deactivation parameters of the currents were calculated using Pulsefit software (Heka). Data are presented as mean values ± s.e.m. Statistical significances were evaluated using Student’s t test.
RESULTS
Three types of electrical activity recorded from cells in intact pancreatic islets
Figure 1A shows a confocal image of an intact pancreatic islet. As previously reported (Nadal et al. 1999; Göpel et al. 1999a, b), β-cells (red), δ-cells (green) and α-cells (blue) are all accessible at the surface of the pancreatic islets. The relative abundance of α-, β- and δ-cells in the superficial cell layer in 18 islets from three different mice was 23 ± 3, 62 ± 5 and 15 ± 2 %, respectively. It can be observed that whereas the α- and β-cells exhibit round geometry, the δ-cells exhibit more elongated morphology.
Figure 1. Three types of electrical activity recorded from superficial islet cells.
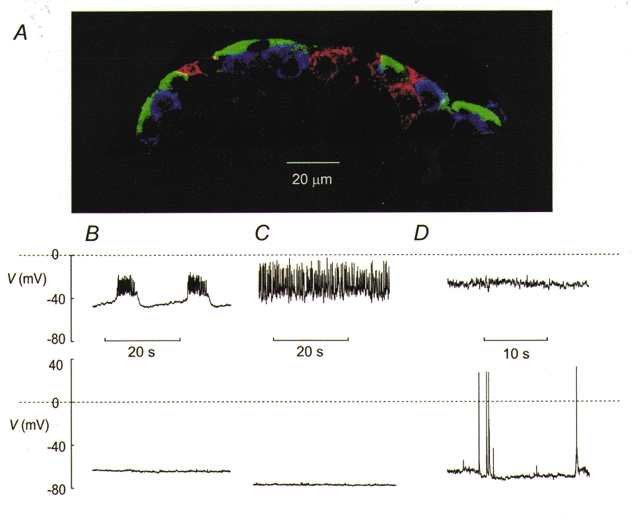
A, immunocytochemistry of mouse pancreatic islet and three types of islet electrical activity. The islet cells were labelled with antibodies against insulin (red), somatostatin (green) and glucagon (blue). B, electrical activity recorded from a β-cell in the presence (top) and absence (bottom) of 10 mm glucose. The β-cell was identified by the characteristic oscillatory electrical activity when exposed to 10 mm glucose (see Göpel et al. 1999a). C, electrical activity from a δ-cell recorded at 10 mm (top) and 1 mm (bottom) glucose. D, depolarised membrane potential in an α-cell exposed to 10 mm glucose (top) and from a different α-cell generating action potentials in the absence of glucose (bottom). The α- and δ-cells were distinguished from the β-cells by the presence of a large Na+ current and were separated by the absence (δ-cells) or presence (α-cells) of a transient A-current during voltage-clamp experiments (see Figs 2–4).
The histology of the islet correlated with the occurrence of three types of electrical activity in the islet. In ∼30 % of the cells, electrical activity in the presence of 10 mm glucose consisted of the characteristic oscillations in membrane potential regarded as a hallmark of the pancreatic β-cell (Fig. 1B). Removal of glucose resulted in membrane repolarisation and the cessation of electrical activity. Cells not exhibiting this type of electrical activity fell into two groups. One group generated action potentials in the presence of 10 mm glucose and repolarised upon glucose removal (Fig. 1C). The action potentials of these cells rarely went above zero. However, some of the non-β-cells were electrically silent in the presence of glucose (Fig. 1D). These cells often had a depolarised membrane potential when exposed to 10 mm glucose but only weak and irregular activity was seen (Fig. 1D). As will be evident in the accompanying paper, this feature need not be indicative of a poor seal or cell rupture (Göpel et al. 2000). Cells with the same electrophysiological properties (see below and Göpel et al. 2000) were sometimes seen to generate spontaneous electrical activity in the absence of glucose consisting of large (80–100 mV) overshooting action potentials starting from a membrane potential of −70 to −60 mV. The observed glucose dependence of the electrical activity and the histology make it likely that the three patterns of action potential firing depicted in Fig. 1B-D were obtained from β-, δ- and α-cells, respectively. We can exclude the contribution of PP-cells to the electrophysiological diversity because our preparations included only the caudal part of the pancreas whereas the PP-cell principally localise in the duodenal part of the gland. We next characterised the voltage-gated membrane conductances that are involved in the generation of the action potentials in α- and δ-cells.
Voltage-gated currents in α- and δ-cells
Figure 2A and B shows the current responses during depolarisations from −70 mV to −10 mV in two different cells. It can be observed that the current responses consist of a spiky inward and a partially inactivating outward current in both cell types. In an attempt to isolate the inward component, 20 mm tetra-ethylammonium (TEA) was included in the extracellular medium to block voltage-gated K+ currents. This abolished the outward component in some cells. However, in other cells application of TEA was less effective and a rapidly activating and inactivating current remained observable in the presence of this broad-spectrum K+ channel blocker (Fig. 2B, lower trace).
Figure 2. Voltage-gated currents in pancreatic α- and δ-cells.
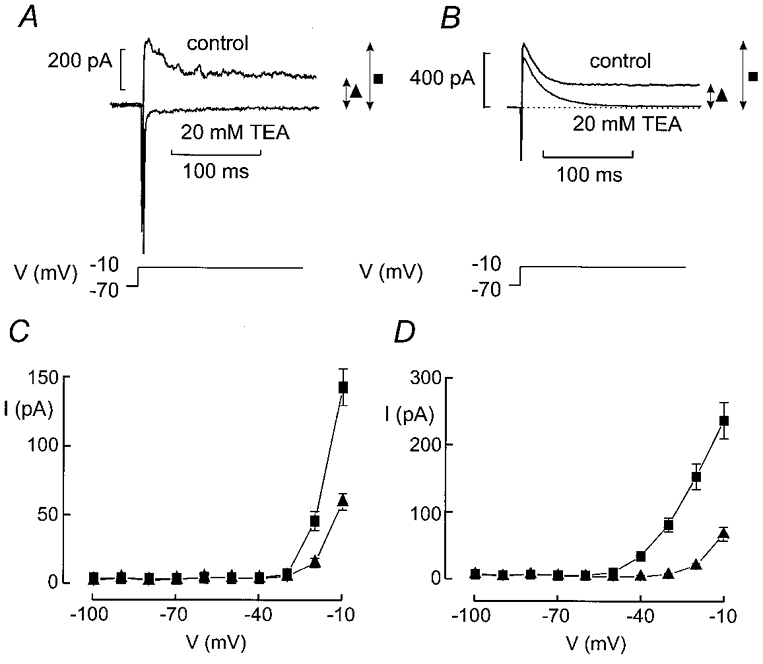
A and B, inward and outward currents elicited by voltage-clamp depolarisations from −70 mV to −10 mV in the absence and presence of 20 mm TEA. Note that whereas the outward currents are abolished in A (revealing the inward currents), a transient TEA-resistant current persists in B. The peak (▪) and sustained K+ currents (▴) were measured as indicated by the arrows. C and D, I-V relationship of the peak (▪) and sustained (▴) K+ current in cells lacking (C) or containing (D) the TEA-resistant outward current. Data are mean values ±s.e.m. of 20 and 15 experiments in C and D, respectively.
Figure 2C and D summarises the current-voltage (I-V) relationships of the peak current (▪) and sustained (▴ measured at the end of the depolarisation) outward current for the TEA-sensitive (C) and partially TEA-resistant (D) cells. The current amplitudes were determined prior to the addition of TEA. In cells containing the TEA-sensitive current, outward currents first became detectable during depolarisations to −20 mV whereas voltage pulses to −50 mV were sufficient in cells with the TEA-resistant component. Once the threshold had been exceeded, stepping to more positive voltages elicited progressively larger transient outward currents in both cell types. In addition, a sustained current component became apparent during depolarisations to voltages above −20 mV. It showed the same voltage dependence in both cell types.
We investigated whether the transient K+ current components exhibit voltage-dependent inactivation. This was investigated by a two-pulse protocol in which the test depolarisation (200 ms to −10 mV) was preceded by 200 ms conditioning pulses to voltages between −100 and −10 mV. Examples of the currents are shown in Fig. 3A and B. The relationship between the relative current amplitude (h∞=I/Imax) and the conditioning voltage (Vm) is summarised in Fig. 3C. The data points can be approximated by the Boltzmann equation:
| (1) |
Figure 3. Two types of steady-state inactivation behaviour of transient K+ current in non-β-cells.
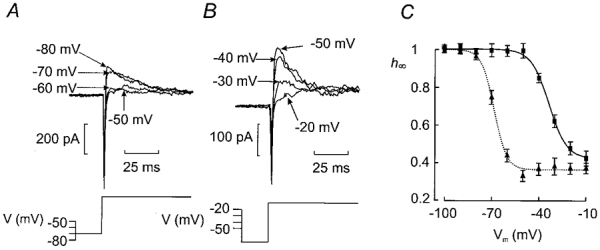
A and B (bottom), prior to a test pulse to −10 mV, the cells were subjected to a 200 ms conditioning pulse to voltages between −100 and −10 mV. The test pulse was preceded by an interval of 20 ms during which the cell was held at −70 mV as indicated schematically by the voltage protocol. Each sequence was repeated at a frequency of 0·4 Hz. A and B (top), the current responses following various conditioning pulses. For clarity, only the current responses following conditioning pulses to membrane potentials between −80 and −50 mV are shown in A and between −50 and −20 mV in B. Note that inactivation occurs at more depolarised voltages in B. C, relationship between conditioning voltage (Vm) and relative current amplitude (h∞ = I/Imax). Two subsets of cells were identified: one with inactivation occurring at negative membrane potentials (▴n = 14) and one with inactivation at more positive voltages (▪; n = 20). The current responses elicited by the depolarisation to −10 mV following a conditioning pulse to −100 mV were taken as Imax. Data are mean values ±s.e.m. The curves were obtained by fitting the Boltzmann equation (eqn (1)) to the data points.
where Vh is the membrane potential at which h∞ is 0·5 and kh is the steepness coefficient. The current amplitude following a conditioning prepulse to −100 mV was taken as the maximum current (Imax). The responses fall in two classes. In 20 out of 34 tested cells (Fig. 3B), current inactivation occurred at fairly positive voltages (Fig. 3C, ▪) and the values of Vh and kh were determined as −34 ± 1 mV and 6 ± 1 mV, respectively. The membrane capacitance of these cells averaged 4·4 ± 0·4 pF. However, in the remaining 14 cells (Fig. 3A), the inactivation was shifted towards more negative voltages (Fig. 3C, ▴) and the values of Vh and kh averaged −68 ± 1 mV and 4 ± 1 mV. The cell capacitance in these cells averaged 5·0 ± 0·3 pF (n = 14). In both groups, ∼30 % of the peak current did not exhibit voltage-dependent inactivation. The membrane capacitance of both subgroups of cells is considerably lower than the 7·4 ± 0·3 pF reported for β-cells in intact pancreatic islets (Göpel et al. 1999a). The current inactivating at more negative voltages is the same as the TEA-resistant current and is seen in cells that are electrically silent in the presence of glucose. This current will be characterised in greater detail in the accompanying paper (Göpel et al. 2000).
Differential expression of Kv3.4 and Kv4.3 in pancreatic α- and δ-cells
Relatively few K+ channel cDNAs give rise to A-type inactivating currents when expressed alone (Conley, 1999a). These include Kv1.4, Kv3.4 and Kv4.1–4.3. To determine whether the two different inactivation behaviours can be attributed to the expression of different Kv channel proteins in islet cells, we applied immunocytochemistry using antibodies raised against Kv1.4, Kv3.4 and Kv4.3 to test for the cell-specific expression of these K+ channels in the islet (Fig. 4A). Kv1.4 was not detected in mouse islets (not shown). Kv3.4 was expressed in both somatostatin-secreting δ-cells and insulin-secreting β-cells but was less prominent (if at all present) in the glucagon-secreting α-cells. In both the β- and δ-cells, Kv3.4 immunoreactivity was confined to the plasma membrane. Kv4.3 was present in the α-cells and exhibited a more diffuse distribution, which was detectable throughout the cytoplasm of the α-cell.
Figure 4. Differential expression of Kv channels in pancreatic islet cells.
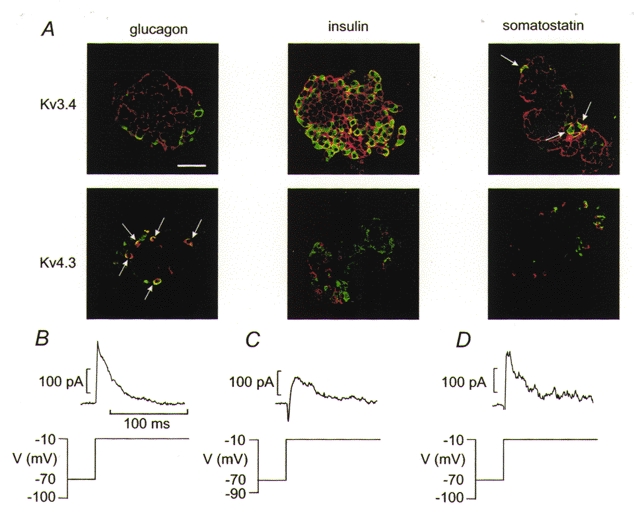
A, imunocytochemical detection of Kv3.4 and Kv4.3 in pancreatic islet cells. In each panel, the presence of hormone (glucagon, insulin or somatostatin as indicated above the images) appears in the green channel and the ion channel proteins (Kv3.4 and Kv4.3 as indicated to the left of the images) are seen in the red channel. Co-localisation of hormone and ion channel is shown in yellow. Note that glucagon-secreting α-cells contain Kv4.3, whereas Kv3.4 is present in the β- and δ-cells. The scale bar is 50 μm and applies to all panels. A few glucagon-containing α-cells positive for Kv4.3 are indicated by arrows. B, inactivating K+ current component in an α-cell. The membrane currents were recorded during depolarisations to −10 mV following 200 ms conditioning pulses to either −100 or −10 mV as indicated (bottom). The resulting difference current is shown (top). C, as in B but a β-cell was used and conditioning pulses were to −90 and −10 mV. D, as in B but a δ-cell was used.
The above data suggest that β- and δ-cells express inactivating K+ currents with the same immunoreactivity. In some β-cells functionally identified by the generation of glucose-induced bursting electrical activity (Fig. 1), an inactivating and TEA-sensitive outward current component, albeit less prominent than in the non-β-cells, was unveiled by subtracting the current responses elicited by a depolarisation to −10 mV following 200 ms conditioning pulses to −90 or −10 mV (Fig. 4C). Half-maximal inactivation (Vh) of this current was observed at −30 ± 3 mV (n = 5). This value is close to the −34 mV observed for the transient current in the δ-cells (Fig. 3C). Given that δ- and β-cells both contain Kv3.4 (see Fig. 4A), it seems reasonable to conclude that the transient TEA-sensitive current observed in non-β-cells is recorded from somatostatin-secreting δ-cells, whereas the current inactivating at negative voltages (complete at −60 mV) is observed in glucagon-secreting α-cells (Fig. 4B). This conclusion is reinforced by the findings that the distribution of Kv4.3 showed no overlap with that of insulin and that the cells positive for Kv4.3 were small, round, situated in the islet periphery and not positive for somatostatin.
Expression of voltage-gated Na+ currents in δ-cells
A large fraction of the superficial cells contain a prominent rapidly activating and inactivating inward current. We next made use of the selective expression of the A-type K+ channel to functionally separate the α- and δ-cells. Figure 5A shows a family of voltage-clamp currents recorded from a δ-cell in the presence of TEA to completely block the outward current. Current responses were small at voltages ≤ −30 mV. Depolarisations to more positive voltages evoked inward currents. The current amplitude increased progressively with the applied voltage up to 0 mV. Figure 5B summarises the I-V relationship of the current recorded from δ-cells. The current reached a maximum around 0 mV where it amounted to 359 ± 43 pA (n = 12). At more positive voltages there was a secondary decline reflecting the decrease in driving force. The extrapolated reversal potential was +74 mV, close to that predicted by the Nernst equation (+69 mV) for a Na+-selective membrane current with the concentration gradients used (extracellular Na+ 143 mm, intracellular Na+ 10 mm).
Figure 5. Characterisation of voltage-gated Na+ currents in pancreatic δ-cells.
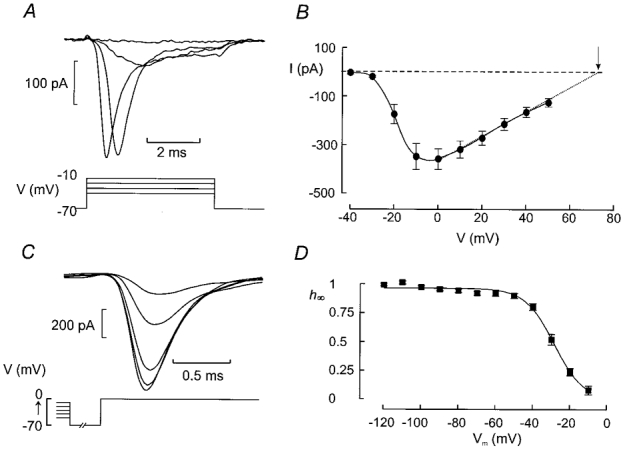
A, Na+ currents observed when the voltage was stepped from −70 mV to potentials between −40 and +50 mV in 10 mV increments (only first 4 pulses shown). The depolarisations were 5 ms long and applied at a frequency of ≈1 Hz. B, I-V relationship of the Na+ current in δ-cells as identified by the absence of an A-current. The dotted line and dashed horizontal line indicate the extrapolated I-V relationship and the zero current level, respectively. The arrow indicates the reversal potential. Data are mean values ±s.e.m. of 12 experiments. C, steady-state inactivation of the Na+ current in pancreatic δ-cells. The cells were subjected to a conditioning pulse (100 ms) to voltages between −120 and −10 mV prior to the 5 ms test pulse which was −10 mV. The cell was held at −70 mV for 1 ms between the conditioning pulse and the test pulse. Current responses shown are those obtained (from bottom to top) following a conditioning pulse to −50, −40, −30, −20 and −10 mV. D, relationship between conditioning voltage (Vm) and relative current amplitude (h∞=I/Imax). The current response elicited by a depolarisation to −10 mV following a conditioning pulse to −120 mV was taken as Imax. Mean values ±s.e.m. of 7 experiments. The curve was obtained by fitting the Boltzmann equation (eqn (1)) to the data points.
Both activation and inactivation of the current accelerated with increasing depolarising commands. The activation and deactivation of the current was described assuming m3h kinetics (Hille, 1992) where m is given by the equation:
| (2) |
and the inactivation of the current follows the expression:
| (3) |
In both equations, t is the time after the onset of the depolarising pulse and τh and τm denote the time constants of inactivation and deactivation, respectively. The activation of the current became progressively faster as the depolarisations went to more positive voltages. In four cells, τm fell from a value of 1·5 ± 0·42 ms at −20 mV to 0·26 ± 0·01 ms at +10 mV. The inactivation of the current accelerated and τh decreased from 0·39 ± 0·06 ms at −10 mV to 0·21 ± 0·03 ms at +20 mV.
Steady-state inactivation of the Na+ current
Voltage-gated Na+ currents characteristically exhibit steady-state inactivation (Hille, 1992). To determine whether this also applies to the Na+ current in somatostatin-secreting cells, we applied a standard two-pulse protocol to δ-cells identified by the absence of a TEA-resistant A-current. A constant depolarisation to −10 mV was thus preceded by 50 ms depolarisations to voltages between −110 and −10 mV. For clarity, only the current elicited by the test depolarisation following conditioning pulses to membrane potentials between −50 and −10 mV are shown in Fig. 5C. The relationship between the voltage during the conditioning pulse (Vm) and the relative peak current amplitude (h∞) is summarised in Fig. 5D. Varying the voltage between −110 and −50 mV had no effect on the amplitude of the current that could subsequently be elicited. However, following conditioning pulses to more positive voltages, the current amplitude became progressively smaller and at −10 mV, the current was fully inactivated. Fitting the Boltzmann equation (eqn (1)) to the data points yielded values of Vh and kh of −28 ± 1 mV (n = 7) and −8 ± 1 mV (n = 7), respectively.
Voltage-gated Ca2+ currents in the δ-cells
Most voltage-gated Na+ currents are highly sensitive to tetrodotoxin (TTX). As shown in Fig. 6A, addition of 0·1 μg ml−1 of TTX inhibited the rapid Na+ current component elicited by a voltage-clamp depolarisation from −70 mV to 0 mV in δ-cells exposed to 20 mm TEA thereby revealing a small TTX-resistant current with slower activation and inactivation kinetics. A family of voltage-gated currents that were evoked by membrane depolarisations from −70 mV to voltages between −40 and 0 mV are shown in Fig. 6B. The I-V relationship of this current is shown in Fig. 6C (□). The current reached a maximum at 0 mV where it amounted to 63 ± 8 pA (n = 5). This is only ∼17 % of the Na+ current amplitude recorded from the same δ-cells. The current undergoes significant inactivation during a 200 ms depolarisation to −10 mV (Fig. 6D). This inactivation disappeared upon equimolar substitution of Ba2+ for Ca2+ (2·6 mm of both ions) as the charge carrier. The I-V relationship recorded with Ba2+ as the permeant ion is displayed in Fig. 6C (▪). With Ba2+ as the charge carrier, inward currents first became detectable during depolarisations to ≥ −30 mV and reached a maximum amplitude of 105 ± 16 (n = 6) at 0 mV. Thus, the δ-cell Ca2+ channels are 1·6-fold more permeable to Ba2+ than to Ca2+. As expected for a Ba2+ current flowing through Ca2+ channels, the current was completely and reversibly inhibited by addition of 5 mm Co2+ to the Ba2+-containing extracellular medium (Fig. 6E).
Figure 6. Ca2+ currents in somatostatin-secreting δ-cells.
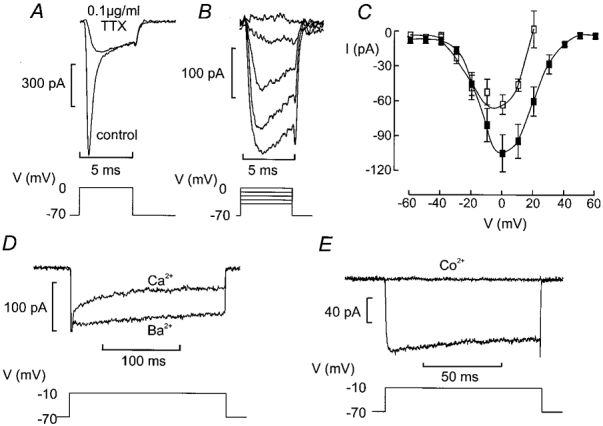
A, current responses elicited by 5 ms depolarisations from −70 mV to 0 mV under control conditions and after addition of 0·1 μg ml−1 of the Na+ channel blocker TTX. B, inward currents elicited by voltage-clamped depolarisation between −40 and 0 mV in the presence of TTX. Pulses were 5 ms long and applied at a frequency of ≈1 Hz. C, I-V relationship of the TTX-resistant current recorded with Ca2+ (□) and Ba2+ (▪) as the charge carrier. In both cases, the concentration of the divalent cation was 2·6 mm. Data are mean values ±s.e.m. of 5 experiments. D, inward currents recorded in the absence of TTX during a 200 ms depolarisation to −10 mV when Ca2+ or Ba2+ was used as the charge carrier (as indicated). The initial truncated transient corresponds to the rapidly inactivating Na+ currents. E, inhibition of Ca2+ currents by Co2+ (5 mm) using Ba2+ as charge carrier recorded in the presence of TTX. All experiments recorded in the presence of 20 mm TEA to block outward K+ currents.
Electrical activity in δ-cells
Figure 7A shows a membrane potential recording from a δ-cell within an intact islet exposed to 10 mm glucose. The cell was identified by the presence of a Na+ current and a transient outward current inactivating at a depolarised membrane potential. The action potentials originated from approximately −50 mV and peaked at −10 mV. Electrical activity was then suppressed by injection of 15 pA of hyperpolarising current resulting in a membrane potential of approximately −70 mV (Fig. 7B). Current pulses of 3–7 pA were then injected to evoke regenerative electrical activity. Injection of 3 pA resulted only in a passive response but currents ≥ 5 pA evoked action potentials. These again originated from −50 mV and did not overshoot.
Figure 7. Regenerative electrical activity in δ-cells.
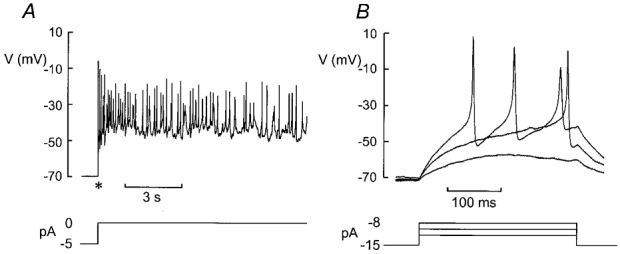
A, electrical activity recorded from a mouse δ-cell (identified by the presence of Na+ current and lack of A-current) in the presence of 10 mm glucose following the release of the voltage clamp (marked by *). B, electrical activity evoked following injection of −15 pA current to repolarise the cell to ≈-70 mV and application of 3, 5 and 7 pA current pulses to evoke action potentials.
δ-cells contain KATP channels
In keeping with earlier reports in dispersed cells, δ-cells in intact islets contain sulphonylurea-sensitive membrane conductances (Berts et al. 1996), presumably corresponding to KATP channels. Figure 8A shows the changes of the membrane potential in a δ-cell exposed to 0 mm glucose before and after addition of 0·1 mm tolbutamide. In a series of four experiments, the membrane potential of the δ-cell in the absence of glucose was −74 ± 5 mV. The membrane conductance under these experimental conditions (estimated by ±10 mV voltage pulses applied from the holding potential) amounted to 2·6 ± 0·7 nS (n = 4). Tolbutamide depolarised the cell by 32 ± 9 mV (n = 4; P < 0·05) and elicited bursts of action potentials. The latter action was associated with a 66 ± 10 % (n = 4; P < 0·01) reduction of the membrane conductance (Fig. 8B). In β-cells from the same preparation, the resting conductance after prolonged (> 15 min) exposure to glucose-free medium (i.e. the same experimental conditions as used for the δ-cells) amounted to 9·0 ± 0·4 nS (n = 6) and fell to 0·82 ± 0·07 nS (P < 0·001) in the presence of 0·1 mm tolbutamide. This was associated with a depolarisation from −82 ± 2 mV to 38 ± 1 mV (n = 6; P < 0·001).
Figure 8. Presence of KATP channels in δ-cells.
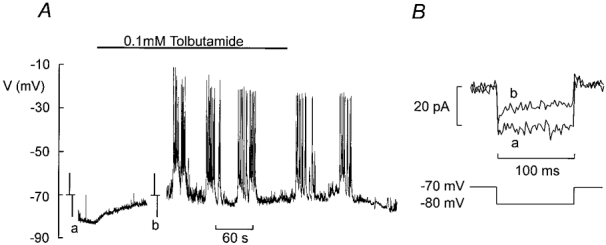
A, electrical activity recorded from a δ-cell (identified by the presence of Na+ current and absence of an A-current) in the absence of glucose before and after addition of 0·1 mm tolbutamide (indicated by horizontal line). At the time points indicated by a and b, the recording was interrupted, the amplifier switched into voltage-clamp mode and ±10 mV pulses were applied from −70 mV to monitor membrane conductance. B, membrane currents evoked by 10 mV hyperpolarising pulses in the absence (a) and presence (b) of 0·1 mm tolbutamide. The current amplitude was measured after the capacitive transients had decayed.
DISCUSSION
We have reported previously that it is possible to perform high-quality patch-clamp experiments on β-cells within intact pancreatic islets (Göpel et al. 1999a, b). We now demonstrate that non-β-cells can be distinguished from the β-cell in being equipped with a voltage-dependent Na+ current and that the α- and δ-cells can be separated by the selective expression of a transient K+ current (A-current) in the glucagon-secreting cell. Here we focus on the electrophysiological properties of the δ-cell and discuss how the various membrane conductances contribute to the electrical activity of the somatostatin-releasing cell.
Both Na+ and Ca2+ channels contribute to the upstroke of the δ-cell action potential
The pancreatic δ-cell is equipped with a large TTX-sensitive Na+ current that activates during depolarisations to voltages ≥ −30 mV and reaches a peak amplitude of −0·35 nA at 0 mV. The δ-cell Na+ current is subject to steady-state potentials > −50 mV and complete at voltages > −10 mV, a voltage range that overlaps that of the δ-cell action potential.
It is of interest that the Na+ currents show extreme variability in the various islet cell types and the values of Vh range from −104 mV in the β-cell (Göpel et al. 1999a), to −47 mV in α-cells (Göpel et al. 2000) and −28 mV in δ-cells. These differences have functional implications and suggest that the Na+ channels contribute little (if at all) to β-cell electrical activity and that Na+ channel activity is unaffected by subthreshold membrane depolarisations in the δ-cell but is more strongly affected in the α-cell. In fact, the different inactivation behaviour of the Na+ current in the various islet cells may account for the divergent effects of the KATP channel blocker tolbutamide on α- and δ-cell electrical activity and hormone secretion (Göpel et al. 2000).
The pancreatic δ-cell is also equipped with a voltage-gated Ca2+ current. Its voltage dependence overlaps that of the Na+ current suggesting that both Na+ and Ca2+ channels contribute to the upstroke of the δ-cell action potential but it is worthy of note that the amplitude of the Na+ current is almost 6-fold larger than that of the Ca2+ current. The fact that electrical activity was observed under conditions associated with stimulation of hormone release (i.e. hyperglycaemia and presence of tolbutamide; Figs 1 and 8) taken together with the fact that Ca2+ channels activate during the action potential is consistent with the notion that somatostatin is released by Ca2+-dependent exocytosis. Although we did not carry out an extensive pharmacological characterisation of the Ca2+ current, both the voltage dependence and kinetics of activation as well as the presence of Ca2+-dependent inactivation and ionic selectivity are consistent with its classification as an L-type Ca2+ current. No evidence was found for the presence of more than one type of Ca2+ current in the δ-cell and the I-V relationship was monophasic.
Presence of inactivating Kv3.4 channels in β- and δ-cells
Immunocytochemistry revealed that the α-cells contain the K+ channel protein Kv4.3, which gives rise to inactivating TEA-resistant K+ currents when expressed in oocytes (Conley, 1999c). Many δ-cells also contain a prominent transient K+ current component, which became observable during depolarisations to membrane potentials above −20 mV. This current differed from that in the α-cells in being completely blocked by TEA at concentrations ≥ 10 mm and inactivating at more depolarised membrane potential (Vh = −34 mV). These properties are not consistent with its classification as an A-current of the classical type (Conley, 1999a). Immunocytochemistry revealed that the somatostatin-secreting δ-cells, whilst not containing Kv4.3, were equipped with Kv3.4. It is pertinent that these K+ channels differ from members of the Kv4 family in being highly sensitive to TEA (Kd < 1 mm) and requiring large depolarisations (-10 mV) to open (Conley, 1999b).
The finding that Kv3.4 was also expressed in β-cells led us to reinvestigate the possible contribution of such channels to the delayed outward K+ current in these cells (see Rorsman & Trube, 1986). By a subtraction protocol we were thus able to identify a transient K+ current component in β-cells with characteristics similar to the transient K+ current in the δ-cells (Fig. 4D). The functional role of this current in β-cell electrical activity remains to be established. However, in both β- and δ-cells, this inactivating K+ component gives rise to a rapidly activating outward current, which will reduce the duration of the action potential.
Comparing the distribution of Kv4.3 and Kv3.4 in the α- and δ-cells revealed interesting differences. Whereas Kv3.4 was almost exclusively confined to the plasma membrane, Kv4.3 showed a more diffuse distribution with much of the immunoreactivity localising to the cytosol. The significance of this observation is obscure but it can be speculated that the fraction of Kv4.3 in the plasma membrane is under dynamic regulation (see Tiffany et al. 2000) and varies depending on, for example, paracrine factors.
KATP channels regulate electrical activity of pancreatic δ-cells
Experiments on identified δ-cells in preparations of dispersed islet cells have previously documented the presence of KATP channels in these cells (Berts et al. 1996). It seems likely that they fulfil the same function in the δ-cell as previously documented in the β-cell: a metabolically regulated K+ conductance to link the metabolic state of the cell to changes of the membrane potential. This concept is fully compatible with the observation that the δ-cells have a negative membrane potential at low glucose concentrations and that application of tolbutamide, like stimulation of glucose, results in both membrane depolarisation and a reduction of the K+ conductance. The presence of KATP channels in the δ-cells and their contribution to the resting potential of the cell probably account for the ability of sulphonylureas to stimulate somatostatin secretion (Ipp et al. 1977; Efendic et al. 1979; Sako et al. 1986). It is worthy of note, however, that the magnitude of the whole-cell KATP conductance in δ-cells is only ∼30 % of that in β-cells (2·7 vs. 9·0 nS).
The electrophysiological properties can be used to distinguish α-, β- and δ-cells
It is clear from the data presented here and in the earlier study (Göpel et al. 1999a) that the electrical properties of mouse islet cells are sufficiently different to allow the safe identification of the α-, β- and δ-cells in intact mouse islets. A cell with a capacitance ≥ 7 pF and no detectable Na+ current when holding at −70 mV is almost certainly a β-cell. It is important to emphasise that this electrophysiological identification is not feasible in rat islets since both α- and β-cells contain voltage-dependent Na+ channels in this species (Hiriart & Matteson, 1988; Gromada et al. 1997). Mouse α- and δ-cells are both equipped with TTX-sensitive Na+ currents but a further separation is possible by the opposite effects of glucose on electrical activity and the presence of an archetypal A-current in α-cells. In the accompanying paper (Göpel et al. 2000) we will exploit these features to perform the first electrophysiological characterisation of mouse α-cells in situ.
Acknowledgments
This study was supported by the Juvenile Diabetes Foundation International, the Knut and Alice Wallenbergs Stiftelse, the Swedish Medical Research Council (grants 8647, 12234 and 13147), the Crafoord Foundation, the Swedish Foundation for Strategic Research, the Swedish Diabetes Association, the Aage and Louise Hansen Foundation, the Novo Nordisk Foundation and the Medical Faculty, Lund University. Dr Kanno’s stay at Lund University was supported by Hirosaki University, Japan.
S. O. Göpel and T. Kanno contributed equally to this study and their names appear in alphabetical order.
References
- Ashcroft FM, Rorsman P. Electrophysiology of pancreatic islet cells. In: Hescheler J, Scherübl H, editors. The Electrophysiology of Neuroendocrine Cells. Boca Raton, FL, USA: CRC Press; 1995. pp. 207–243. [Google Scholar]
- Berts A, Ball A, Dryselius S, Gylfe E, Hellman B. Glucose stimulation of somatostatin-secreting islet cells involves oscillatory Ca2+-signalling. Endocrinology. 1996;137:693–697. doi: 10.1210/endo.137.2.8593819. [DOI] [PubMed] [Google Scholar]
- Bishop AE, Polak JM. The anatomy, organisation and ultrastructure of the islets of Langerhans. In: Pickup J, Williams G, editors. Textbook of Diabetes. Vol. 1. Oxford, UK: Blackwell Science; 1997. pp. 6.1–6.16. [Google Scholar]
- Conley EC. VLG K A-T. In: Conley EC, Brammar WJ, editors. The Ion Channel Facts Book: Voltage-Gated Channels. Vol. 4. San Diego: Academic Press; 1999a. pp. 196–225. [Google Scholar]
- Conley EC. VLG K Kv3-Shaw. In: Conley EC, Brammar WJ, editors. The Ion Channel Facts Book: Voltage-Gated Channels. Vol. 4. San Diego: Academic Press; 1999b. pp. 559–616. [Google Scholar]
- Conley EC. VLG Kv4-Shal. In: Conley EC, Brammar WJ, editors. The Ion Channel Facts Book: Voltage-Gated Channels. Vol. 4. San Diego: Academic Press; 1999c. pp. 617–646. [Google Scholar]
- Efendic S, Enzmann F, Nylén A, Uvnäs-Wallensten K, Luft R. Effect of glucose/sulfonylurea interaction on release of insulin, glucagon, and somatostatin from isolated perfused pancreas. Proceedings of the National Academy of Sciences of the USA. 1979;76:5901–5904. doi: 10.1073/pnas.76.11.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpel S, Kanno T, Barg S, Galvanovskis J, Rorsman P. Voltage-gated and resting membrane currents recorded from B-cells in intact pancreatic islets. The Journal of Physiology. 1999a;521:717–728. doi: 10.1111/j.1469-7793.1999.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpel SO, Kanno T, Barg S, Eliasson L, Galvanovskis J, Renström E, Rorsman P. Activation of Ca2+-dependent K+ channels contributes to rhythmic firing of action potentials in mouse pancreatic β cells. Journal of General Physiology. 1999b;114:759–770. doi: 10.1085/jgp.114.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpel SO, Kanno T, Barg S, Weng X-G, Gromada J, Rorsman P. Regulation of glucagon release in mouse α-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. The Journal of Physiology. 2000;528:509–520. doi: 10.1111/j.1469-7793.2000.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding WG, Barg S, Buschard K, Renström E, Rorsman P. Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. Journal of General Physiology. 1997;110:217–228. doi: 10.1085/jgp.110.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer; 1992. pp. 23–62. [Google Scholar]
- Hiriart M, Matteson DR. Na channels and two types of Ca channels in rat pancreatic β-cells identified with the reverse hemolytic plaque assay. Journal of General Physiology. 1988;91:617–639. doi: 10.1085/jgp.91.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipp E, Dobbs RE, Arimura A, Vale W, Harris V, Unger RH. Release of immunoreactive somatostatin from the pancreas in response to glucose, amino acids, pancreozymin-cholecystokinin, and tolbutamide. Journal of Clinical Investigation. 1977;60:760–765. doi: 10.1172/JCI108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Hellman B, Gylfe E. Ca2+ signaling in mouse pancreatic polypeptide cells. Endocrinology. 1999;140:5524–5529. doi: 10.1210/endo.140.12.7198. [DOI] [PubMed] [Google Scholar]
- Nadal A, Quesada I, Soria B. Homologous and heterologous asynchronicity between identified α, β and δ cells within intact islets of Langerhans in the mouse. The Journal of Physiology. 1999;517:85–93. doi: 10.1111/j.1469-7793.1999.0085z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada I, Nadal A, Soria B. Different effects of tolbutamide and diazoxide in α-, β- and δ-cells within intact islets of Langerhans. Diabetes. 1999;48:2390–2397. doi: 10.2337/diabetes.48.12.2390. [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. Journal of Neuroscience Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Trube G. Calcium and potassium currents in mouse pancreatic β-cells under voltage-clamp conditions. The Journal of Physiology. 1986;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako Y, Wasada T, Umeda F, Ibayashi H. Effect of glibenclamide on pancreatic hormone release from isolated perfused islets of normal and cystamine-treated rats. Metabolism. 1986;35:944–949. doi: 10.1016/0026-0495(86)90059-4. [DOI] [PubMed] [Google Scholar]
- Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K+ channel surface expression and clustering. Journal of Cell Biology. 2000;148:147–158. doi: 10.1083/jcb.148.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]


