Abstract
The purpose of this study was to quantify the effect of flaxseed (Linum usitatissimum) supplementation on the skin test response of atopic horses. Six horses that displayed a positive skin test for allergy to extract from Culicoides sp. participated in the 42-day, placebo-controlled, double-blind, cross-over trial. Results showed that supplementation with flaxseed for 42 days in our experimental horses reduced the mean skin test response to Culicoides sp. This observation was concurrent with a significant decrease in the long-chain saturated fatty acids; behenic acid (22:0) and lignoceric acid (24:0), in the hair of horses receiving flaxseed. There was also a significant decrease in aspartate aminotransferase, and increase in serum glucose in the treatment animals at specific sampling points. It was concluded that; in this small pilot study, flaxseed was able to reduce the lesional area of the skin test response of atopic horses, alter the fatty acid profile of the hair, reduce inflammation, and did not elicit any negative side-effects in the experimental horses.
Introduction
Recurrent seasonal pruritus, also known as ‘sweet itch’, is a very common dermatological ailment of horses across many parts of North America and Europe. It is characterised by itchy lesions along the ventral midline, dorsal midline, mane, and tail of horses; and is thought to be caused by a type-1 hypersensitivity reaction to the bite of insects from the Culicoides genus (the ‘midges’) (1,2). Affected horses often develop severe skin eruptions due to self-mutilation in an attempt to alleviate the itchiness. The incidence of sweet itch is difficult to conclusively determine, but it is clear that the disease is geographically sensitive, likely as a result of differing entomological profiles. A survey was conducted in British Columbia, and of the 209 horses surveyed in the south-western part of that province, the incidence rate was determined to be 26% (3). British Columbia has an equine population of 113 387 (4). If this survey can be considered representative of the horse population in the province, the incidence rate of 26% translates into 29 480 horses (5).
There is a considerable body of literature detailing investigations into the rationale behind the use of essential fatty acids (EFAs) in the prevention and treatment of recurrent seasonal pruritus in other animals (6,7,8,9). Essential fatty acids can be classified as either “omega-6” or “omega-3”, and each class has a distinct and important biochemical role within the body. These compounds are not inter-convertible in mammals and are important components of all cell membranes. Alpha-linolenic acid (ALA), the parent omega-3 fatty acid, is now widely recognized as an EFA. This is of increasing clinical interest as a precursor to the longer omega-3 chains, in addition to other hormone-like substances involved in many important biological functions in the body. Unlike cellular proteins, which are genetically determined, the EFA composition of cell membranes is exclusively dependent on dietary intake. Also, and perhaps more importantly, the metabolism of omega-3 and omega-6 fatty acids are unique, and they have distinct eicosanoid consequences. Eicosanoids; particularly the series-2 prostaglandins (PGE2, PGI2), thromoboxanes (TXA2), and the inflammatory leukotrienes (LTB4, LTE4), are intimately involved in the inflammatory process and are implicated in the severity of allergic dermatitis (1,10,11). The unique metabolic pathway of ALA suggests that increasing its concentration in the diet may improve the clinical manifestation of the inflammatory dermatitis associated with sweet itch.
Flaxseed is one of the highest natural vegetable sources of ALA (12); and also contains phytoestrogens (lignans), flavonoids, and a complex array of amino acids and minerals (13). Flaxseed is a common additive to many equine diets; often for the purpose of improving skin and hair-coat quality, despite the fact that current research suggests the practice has little academic validity (14). The purpose of this study was to quantify the effect of crushed flaxseed supplementation on the skin test response of atopic horses.
Materials and methods
Flaxseed supplement
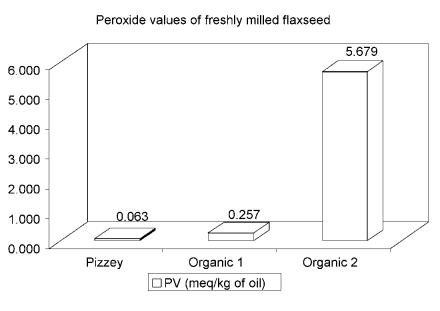
Milled flaxseed was generously donated by Pizzey's Milling and Baking Company. The product was selected for mature, evenly coloured seeds. Evenly coloured seeds are a marker for a highly stable, milled flaxseed product; due to the resulting low peroxide levels (see Figure 1). The product may be stored at room temperature in multi-wall poly-lined paper bags, for fatty acid stability lasting at least 280 d. Guaranteed analysis of the product is described in tables I, II, and III.
Figure 1. Peroxide values of experimental supplement compared with 2 organic flaxseed samples. The experimental supplement (Pizzey) contained 2% dark seeds. The organic 1 contained 7% dark seeds. The organic 2 contained 25% dark seeds.
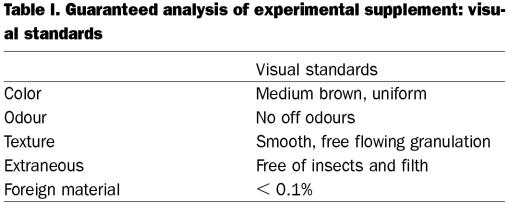
Table I.
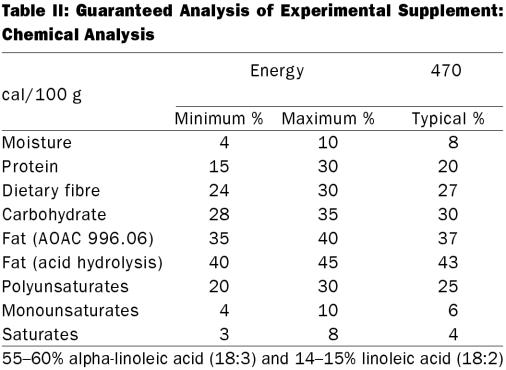
Table II.
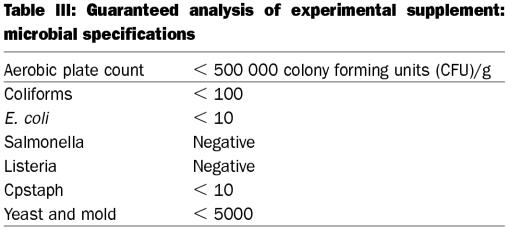
Table III.
Horses — selection criteria
Horses with a history of pruritus were given an intradermal skin test with Culicoides extract (Greer Laboratories, North Carolina, USA), saline (negative control) and histamine (positive control). Reactivity was graded on a scale of 0 to 5 (0 = saline; 5 = histamine), with a score of 2 or more considered a positive test. Six horses displaying a positive test were transported to and housed at the Equine Research Centre for the duration of the 14 wk, double-blind, placebo-controlled, cross-over trial (1 horse had to be removed from the trial due to an unrelated incident).
Horses — supplementation schedule
Horses were housed at the Centre for a minimum of 2 wk, before commencement of sampling. Each horse was randomly allocated to either group A (milled flaxseed) or group B (placebo — bran) (3 horses per group) and received 1 lb supplement/1000 lb body weight (BW)/d of flaxseed (group A) or bran (group B) in their daily ration. Supplementation occurred over a period of 42 d, after which horses received no supplements for 14 d. They were then crossed over and the trial repeated.
Skin testing
On days 0, 21, and 42, horses had a 30 × 15 cm area of hair clipped from the medial or lateral area of the neck (alternating each sampling day). The clipped area was swabbed with alcohol and 3 small pen marks at a distance of approximately 25 mm apart identified the areas that were intradermally injected with Culicoides extract (Greer Laboratories), saline (negative control) and histamine (positive control) (Bioniche Life Sciences, Belleville, Ontario). Reactions were assessed per length and width (mm), and surface temperature (°C) at 20–30 min, 2 h, 4 h, and 18 h post-injection.
Biochemistry and hematology
On the opposite neck, 3 vacutainer blood tubes (1 red-top, 1 lavender-top, 1 green-top) were taken by jugular puncture for analysis of a complete blood count (CBC) and equine serum profile (ESP). Complete blood count was performed on Techincon H*1 (Vitatech Veterinary Research Laboratories, Markham, Ontario). The analysis tested for white blood cells (WBC), red blood cells (RBC), hemoglobin (Hb), hemoglobin concentration (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and absolute and differential neutrophils, lymphocytes, monocytes, eosinophils, basophils, nucleated red blood cells. Equine serum profiles were conducted (Hitachi 911 Biochemical Analyser; Vitatech Veterinary Research Laboratories). The analysis tested for albumin and globulin (A/G) ratio, albumin, alkaline phosphate (alk phos), urea {BUN}, calcium, chloride, cholesterol, CPK, creatinine, glucose, phosphorus, potassium, total protein (Prot {T}), aspartate aminotransferase (AST {SGOT}), sodium, and the sodium and potassium ratio.
Fatty acid analysis
On the opposite side of the neck from the skin testing, all horses were injected sub-cutaneously with 2% lidocaine, and a 6 mm punch-biopsy was preserved in 10% buffered formalin and refrigerated until the termination of sampling. A sample of hair was pulled by the root, from the bottom of the mane, slightly above the withers. The sample was trimmed from the terminal end to a length of approximately 2 inches from the root, and was preserved in 10% buffered formalin, and refrigerated until termination of sampling. One vacutainer of blood (EDTA) was drawn, as described previously, and refrigerated until termination of sampling. Upon completion of the sampling schedule; all skin, hair, and blood samples were shipped on ice to Southern Testing & Research Laboratories Inc. (Wilson, North Carolina, USA), where they underwent fatty acid profile (FAP) analysis. The FAP was conducted by gas chromatography (GC) (Perkin Elmer Autosystem, XL; Perkin Elmer, Norwalk, Connecticut, USA) using GC column RTX-2330; 10% cyanopropylphenyl-90% # biscyanopropyl polysiloxane; 105 m, 0.25 mm # ID, 0.20 μm df (VWR Scientific Products, West Chester, Pennsylvania, USA).
An accurately weighed amount of sample was placed into a fat extraction flask and a known amount of C13 internal standard was added to the flask. The sample underwent fat extraction by a chloroform/methanol method. The fat was extracted into chloroform, which was filtered through sodium sulfate and then dried under vacuum at 40°C. The fat was hydrolyzed with sodium hydroxide to form free fatty acids. The fatty acids were then derived with BF3 and the methyl ester fatty acids were extracted into heptane. The standards and extracts were injected on the GC column to separate the following fatty acids: butyric (C4:0), caprioc (C6:0), caprylic (C8:0), capric (C10:0), undecanoic (C11:0), lauric (C12:0), tridecanoic (C13:0), myristic (C14:0), myristoleic (C14:1), pentadecanoic (C15:0), cis-10-pentadecenoic (C15:1), palmitic (C16:0), palmitoleic (C16:1), heptadecanoic (C17:0), cis-10-heptadecenoic (C17:1), stearic (C18:0), elaidic (C18:n9t), oleic (C18:1n9c), linolelaidic (C18:2n6t), linoleic (C18:2n6c), heneicosanoic (C21:0), cis-11,14-eicosadienoic (C20:2), behenic (C22:0), cis-11,14-eicosatrienoic (C20:3n6), erucic (C22:1n9), cis-11,14,17-eicosatrienoic (C20:3n3), arachidonic (C20:4n6), tricosanoic (C23:0), cis-13,16-docosadienoic (C22:2), lignoceric (C24:0), cis-5,6,11,14,17-eicosapentaenoic (C20:5n3), nervonic (C24:1), and cis-4,7,10,13,16,19-docosahexaenoic (C22:6n3).
Statistical analysis
For all variables, differences between treatments (flaxseed and control) over time (days 0, 21, 42) were analyzed using statistical computer software (Statistical Analysis Systems (SAS), version 8; Cary, North Carolina, USA) using the general linear means (GLM) procedure with the repeated measures statement. Paired t-tests were used to determine if the difference was significant. Skin test results were analyzed by calculating the area of the inflammation (length × width). The repeated measures analysis was used to determine the significance of treatment and time (30 min, 120 min, 480 min, and 1080 min) interaction on each day (days 0, 21, 42). Differences between treatment and control groups at each time period were then determined by using paired difference t-tests and were considered significant if P < 0.05.
Results
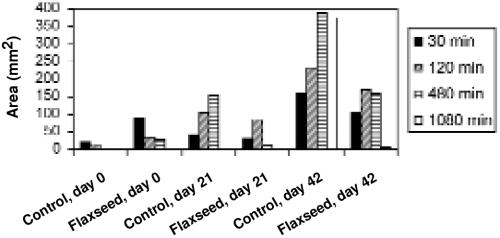
Saline showed no reaction in either the control or flaxseed group at day 0, 21, or 42. Both the culicoides and histamine initiated a reaction of which the length and width were measured (mm). The reaction to culicoides and histamine were significantly different from the reaction to saline (P < 0.05). For the culicoides, there was a significant interaction (P = 0.024) between treatment groups over time on day 42, but not on day 0 or 21. The area of the lesion caused by the culicoides extract was less when the horses were fed flaxseed than when they were on the control diet. The difference in the mean reaction area of the control and flaxseed diet is greatest on day 42 (see Figure 2). Paired t-tests performed on the data at time 30, 120, 480, and 1080 min showed that the mean difference between control and treatment was not significant. This occurred as a result of one experimental subject behaving in an opposite way to all other subjects. The reaction to histamine showed no significant difference between treatment and control groups over time. There were no significant differences between skin surface temperatures of treatment and control horses at any time interval.
Figure 2: Mean area of reaction to Culicoides by skin test. Difference between treatment and control was significant on day 42 (P = 0.024).
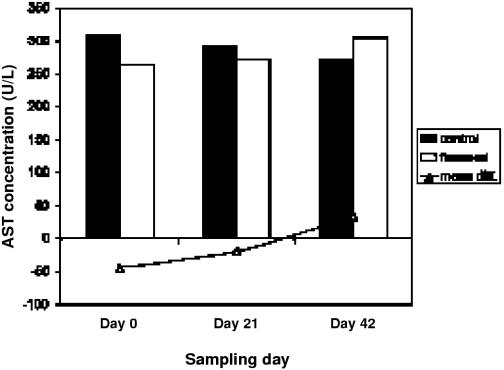
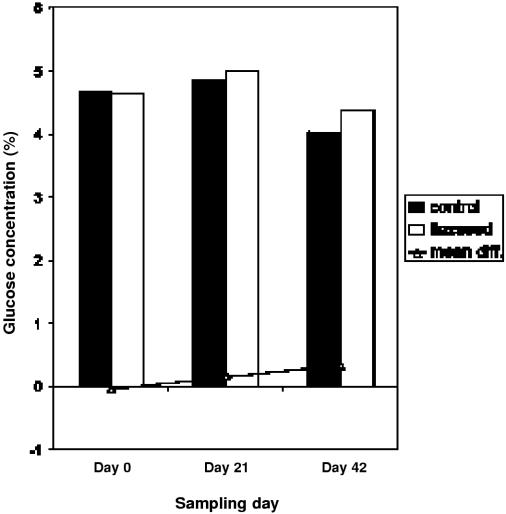
The results of CBC and ESP tests showed no significant difference over time. Results from the paired t-tests showed that mean difference between flaxseed and control was significantly different from 0 for the variables. The overall change in AST between treatment and control groups was not significantly different from 0. However, there was a significantly lower AST level in treatment horses on day 21, P = 0.03 (see Figure 3). On day 42, glucose levels were significantly greater while horses were receiving flaxseed as compared with when they were receiving the control supplement, P = 0.02 (see Figure 4). This increase remained well within the normal physiological range for horses (14), and was not considered to be of clinical significance. Monocytes were significantly different in control and treatment phases at day 0 before supplementation (P = 0.03), but this difference was not significant during any of the other sampling days (data not shown).
Figure 3: Mean concentration of aspartate aminotransferase (AST) in plasma of treatment and control subjects. Difference between treatment and control was significant on day 21 (P = 0.03).
Figure 4. Mean concentration of glucose in plasma of treatment and control subjects. Overall difference between treatment and control from day 0 to day 42 was significant (P = 0.02).
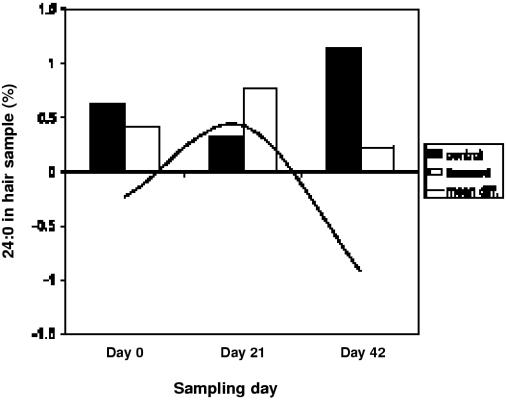
The results of the FAP showed no significant differences between treatment and control on the skin biopsies. Significant differences between treatment and control subjects in the concentrations of erucic acid (22:1n9) and cis-8,11,14-eicosatrienoic acid (20:3n6) in the hair occurred due to measurable levels of these fatty acids in the hair of 1 subject at day 0 and 42, respectively, but levels were undetectable on any other sampling days. There were statistically significant decreases in the levels of behenic acid (C22:0) (P = 0.028) and lignoceric acid (C24:0) (P = 0.015) over time. The paired t-test demonstrated a significant decrease in the levels of lignoceric acid between treatment and control groups on day 42 (P = 0.049) (see Figure 5). Statistical analysis of FAP in the blood samples suggests a significant difference between treatment and control groups for tricosanoic acid (23:0) with a P-value of 0.03. Looking further at these results, the difference occurs because there were measurable levels of this fatty acid in the blood at day 0, but levels were undetectable on days 21 and 42.
Figure 5. Mean concentration of lignoceric acid in hair of treatment and control subjects. Difference between treatment and control was significant on day 42 (P = 0.049).
Discussion
This trial represents a small pilot investigation into the possible benefits of supplementing horses with flaxseed to mitigate the atopic skin response to a common allergen. Results observed in the skin testing procedure provide some evidence that flaxseed has the potential to effectively reduce the allergic response of susceptible horses to Culicoides extract, though our small sample size limits the conclusions that may be drawn from this observation. These results are in contrast with previously published work (15), which reported no effect of feeding a linseed supplement. The main difference in our study is that we used the entire crushed seed, while the Friberg research group (15) used only the extracted oil. The seed contains a number of other phytochemicals and compounds, which may have contributed to the effect observed in the current study. When our experimental subjects were assessed as an average, there was a significant decrease in the area of allergic reaction when horses received the supplemental flaxseed. This significance was not observed when subjects were assessed individually, due to the non-conforming response of 1 subject. It is not known why this animal did not respond similarly to the others. Atopic dermatitis is caused by a number of varying etiologies, and it may be that the flaxseed supplement was not able to affect this particular case because its etiology was unrelated to the mechanism through which flaxseed works. Further studies are needed with a greater sample size to determine whether our observation of significance was based on an experimental anomaly or on actual physiological activity.
The significantly lower AST scores on day 21 in treatment animals suggests that there may be a reduction in inflammatory processes when horses are consuming flaxseed. This is in agreement with the observed average reduction in skin test reactions. With this exception, biochemistry and hematology screens failed to identify any other significant systemic effects of the treatment. There has been some concern over feeding flaxseed to horses, as the plant is a source of cyanogenic glycosides and enzymes (16), which can interact, releasing cyanide. When consumed in sufficient quantities, flax is widely suspected to be potentially toxic, possibly causing cyanide poisoning in animals. For this reason, it is common practice in the field to boil flaxseed for a minimum of one hour, to release the highly volatile cyanide. Cyanide poisoning does not become symptomatic over a long period of time, and there is little difference between toxic and lethal levels of cyanide in the blood (17). Poisoning would be recognized in the blood as changes in the oxygenation status of venous blood, accompanied by rapid, laboured breathing, foaming at the mouth, and dilated pupils. These symptoms were not observed in any of our research subjects. Due to the ability of stomach acid to inactivate the enzymes contained within the seeds, which are required to interact with glycosides in order to form cyanide, it was not expected that signs of toxicity associated with cyanide poisoning would be observed in this study. All changes identified in the biochemistry and hematology profiles remained within the normal physiological range, so statistically significant changes were not considered to be of any clinical significance. This study suggests that feeding flaxseed to horses did not elicit any negative side-effects when consumed at this dosage over a period of 42 d.
The fatty acid analyses showed a significant decrease in the long-chain saturated fatty acids behenic and lignoceric acid in the hair samples of subjects. As the hair of horses is made up predominantly of protein and minerals, it is possible that changes in fatty acid profiles reflect changes in secretions of the sebaceous glands of the skin into the hair. Although there have been no reports in the literature on the fatty acid composition of equine skin as compared with that of sebaceous secretions, it has been reported in other species that there is indeed a significant difference (18). Though we did not see a change in the fatty acid composition of the skin of our subjects, it is possible that changes seen in the hair were a result of changes in sebum secretions. The clinical significance of the decrease in these 2 fatty acids is not clear. However, 1 of the functions of the sebum is to provide a hydrophobic layer on the skin and hair (19) that supports opportunistic and beneficial dermal microflora. An intriguing possibility for the combined effects of altered long-chain saturated fatty acids in the sebum and reduced skin test response to Culicoides, may be deduced from a recent report which detailed immunosuppression caused by UV-B radiation (20). This immunosuppression was thought to be caused by photoisomerization of trans-urocanic acid to cis-urocanic acid. This report showed that many bacterial populations commonly found on the skin are capable of metabolising histidine and trans-urocanic acid to the cis-conformation. Therefore, if these bacterial populations were affected by the altered fatty acid profile of the sebum in the current study, it is possible that the reduced skin test response occurred as a result of immunosuppression caused by the altered stereochemistry of the sebum. It is impossible to positively identify this mechanism within our current data, but the possibility provides intriguing rationale for further study. The results of this trial suggest a possible benefit in using flaxseed to mitigate the skin test response to Culicoides extract of atopic horses. Further studies with a larger sample size are needed to confirm the results observed in this experiment.
Footnotes
Acknowledgments
The authors acknowledge the generous financial support of Pizzey's Milling and Baking Company, the R. Howard Webster Foundation, and the Ontario Ministry of Agriculture, Food and Rural Affairs. Also, our thanks are owed to the owners of Tigull, Fjülnir, Duna, Andi, Hreggur, and Ogri, who generously loaned their Icelandic horses for this trial.
Address all correspondence and reprint requests to Dr. W. O'Neill, Nutraceutical Alliance, 85 Keating St., Guelph, Ontario N1E 7G1; telephone: (519) 829-3541; fax: (519) 767-1081; e-mail: woneill@nutraceuticalalliance.com
Received November 13, 2001. Accepted May 17, 2002.
References
- 1.Rosencrantz WS. Systemic/topical therapy. Veterinary Clinics of North America: Equine Practice 1995;11(1):127–146. [DOI] [PubMed]
- 2.Kleider N, Lees MJ. Culicoides hypersensitivity in the horse: 15 cases in southwestern British Columbia. Can Vet J 1984;25: 26–32. [PMC free article] [PubMed]
- 3.Anderson G, Belton P, Kleider N. The hypersensitivity of horses to culicoides bites in British Columbia. Can Vet J 1988;29:718–723. [PMC free article] [PubMed]
- 4.Evans V. Canadian Horse Industry Research Study. Strategic Equine Marketing, 1998.
- 5.Wright R, Cation J. 1996 Horse Industry Report. Ontario Ministry of Agriculture, Food and Rural Affairs, 1996.
- 6.Baker KP, Quinn PJ. A report on clinical aspects and histopathology of sweet itch. Equine Vet J 1978;10(4):243–248. [DOI] [PubMed]
- 7.Scott DW, Miller WH, Reinhart GA, Mohammed HO, Bagladi MS. Effect of an omega-3/omega-6 fatty acid-containing commercial lamb and rice diet on pruritus in atopic dogs: results of a single-blinded study. Can J Vet Res 1997;61:145–153. [PMC free article] [PubMed]
- 8.Lloyd D, Thomsett LR. Essential fatty acid supplementation in the treatment of canine atopy: a preliminary study. Vet Dermatol 1989;1:41–44.
- 9.Bond R, Lloyd DH, Craig JM. The effects of essential fatty acid supplementation on intradermal test reactivity in atopic dogs: a preliminary study. Vet Dermatol 1993;4(4): 191–197.
- 10.Whelan J, Broughton KS, Kinsella JE. The comparative effects of dietary gamma-linolenic acid and fish oil on 4- and 5-series leukotriene formation in vivo. Lipids 1991;26(2):119–126. [DOI] [PubMed]
- 11.Vaughn DM, Reinhart GA, Swaim SF, et al. Evaluation of effects of dietary n-6 to n-3 fatty acid ratios on leukotriene B synthesis in dog skin and neutrophils. Vet Dermatol 1994;5(4): 163–173. [DOI] [PubMed]
- 12.Cunnane SC, Ganguli S, Menard C, et al. High alpha-linolenic acid flaxseed (Linum usitatissimum): some nutritional properties in humans. Br J Nutr 1993;69:443–453. [DOI] [PubMed]
- 13.Pizzey G. The animal industry as an emerging new market for flaxseed. In: Pearson, W. ed. Complimentary Therapies: A New Vision in Animal Health. Guelph: Equine Research Centre, 1999:9–17.
- 14.Lewis LD. Equine clinical nutrition: feeding and care. Williams and Wilkins, Baltimore, 1995.
- 15.Friberg CA, Logas D. Treatment of culicoides hypersensitive horses with high-dose n-3 fatty acids: a double-blinded crossover study. Vet Dermatol 1999;10:117–122. [DOI] [PubMed]
- 16.Oomah BD, Mazza G, Kenaschuk EO. Cyanogenic compounds in flaxseed. J Agr Food Chem 1992;40:1346–1348.
- 17.Cheville NF. Introduction to Veterinary Pathology. Ames: Iowa State University Press, 1988:443.
- 18.McMaster JD, McEwan Jenkinson D, Noble RC, Elder HY. The lipid composition of bovine sebum and dermis. Br Vet J 1985;141: 34–41. [DOI] [PubMed]
- 19.Nikkari T. Comparative chemistry of sebum. J Invest Dermatol 1974;62:257–267. [DOI] [PubMed]
- 20.Hug DH, Dunkerson DD, Hunter JK. The degradation of L-histidine and trans- and cis-urocanic acid by bacteria from skin and role of bacterial cis-urocanic isomerase. J Photochem Photobiol (B) 1999;50(1):66–73. [DOI] [PubMed]