Abstract
Glucocorticoid administration to women at risk of preterm delivery to accelerate fetal lung maturation has become standard practice. Antenatal glucocorticoids decrease the incidence of intraventricular haemorrhage as well as accelerating fetal lung maturation. Little is known regarding side effects on fetal cerebral function. Cortisol and synthetic glucocorticoids such as betamethasone increase fetal blood pressure and femoral vascular resistance in sheep.
We determined the effects of antenatal glucocorticoid administration on cerebral blood flow (CBF) in fetal sheep. Vehicle (n = 8) or betamethasone (n = 8) was infused over 48 h via the jugular vein of chronically instrumented fetal sheep at 128 days gestation (term 146 days). The betamethasone infusion rate was that previously shown to produce fetal plasma betamethasone concentrations similar to human umbilical vein concentrations during antenatal glucocorticoid therapy.
Regional CBF was measured in 10 brain regions, using coloured microspheres, before and 24 and 48 h after onset of treatment, and during hypercapnic challenges performed before and 48 h after onset of betamethasone exposure. Betamethasone exposure decreased CBF in all brain regions measured except the hippocampus after 24 h of infusion (P < 0·05). The CBF decrease was most pronounced in the thalamus and hindbrain (45–50 % decrease) and least pronounced in the cortical regions (35–40 % decrease). It was mediated by an increase in cerebral vascular resistance (CVR, P < 0·05) and led to a decrease in oxygen delivery to subcortical and hindbrain structures of 30–40 %, to 8·6 ± 1·1 ml (100 g)−1 min−1, and 40–45 %, to 11·0 ± 1·6 ml 100 g−1 min−1, respectively (P < 0·05).
After 48 h of betamethasone treatment, the reduction in CBF was diminished to about 25–30 %, but was still significant in comparison to vehicle-treated fetuses in all brain regions except three of the five measured cortical regions (P < 0·05). CVR and oxygen delivery were unchanged in comparison to values at 24 h of treatment. The CBF increase in response to hypercapnia was diminished (P < 0·05).
These observations demonstrate for the first time that glucocorticoids exert major vasoconstrictor effects on fetal CBF. This mechanism may protect the fetus against intraventricular haemorrhage both at rest and when the fetus is challenged. Betamethasone exposure decreased the hypercapnia-induced increase in CBF (P < 0·05) due to decreased cerebral vasodilatation (P < 0.05).
A recent National Institutes of Health consensus conference recommended antenatal glucocorticoid administration to women at risk of preterm delivery to accelerate fetal lung maturation and to avoid bronchopulmonary dysplasia and decrease the incidence of intraventricular haemorrhage (Crowley et al. 1990; Ballard & Ballard, 1995; Elimian et al. 1999). However, little is known about the cerebrovascular side effects of exposure to glucocorticoids during fetal development.
Adrenalectomy accompanied by decreased glucocorticoid production results in increased hippocampal and cortical blood flow in adult rats (Endo et al. 1994). Betamethasone has direct vasoconstrictor effects on peripheral femoral resistance vessels in late gestation fetal sheep (Derks et al. 1997; Anwar et al. 1999). In artifically ventilated premature newborns, cerebral blood flow (CBF) was estimated indirectly after dexamethasone treatment by measuring blood flow velocities in carotid or large cerebral arteries using ultrasound Doppler flowmetry. Ohlsson et al. (1994) found a decrease or no change in the blood flow velocity of the middle cerebral artery and Cabanas et al. (1997) an increase in the end-diastolic blood flow velocity in the internal carotid and the anterior cerebral arteries accompanied by an increase in arterial blood pressure following dexamethasone administration to newborn human infants. As the resistance index was lowered they suggested that the effect of glucocorticoids was to produce an increase in CBF. The only study we have been able to find that evaluates the effects of glucocorticoids in the human fetus did not show any change in the blood flow velocity waveforms and the pulsatility index in the large fetal arteries, including the large cerebral arteries, after dexamethasone treatment (Cohlen et al. 1996). There are several limitations to these studies. First, Doppler flowmetry cannot be used to determine global CBF or regional CBF directly. Second, the velocity results were variable. Finally, studies in artifically ventilated premature babies do not reveal the rules by which the fetus lives. We therefore aimed to evaluate the effects of antenatal glucocorticoid treatment on regional CBF under controlled conditions in a well-established animal model.
We hypothesised that glucocorticoid administration to the fetal sheep will decrease regional CBF. We infused betamethasone directly into the fetal jugular vein at a rate which produces fetal plasma betamethasone concentrations similar to those measured in human umbilical cord plasma at Caesarean section within 24 h of maternal glucocorticoid treatment (Derks et al. 1997). Betamethasone was administered directly to the fetus to avoid potential influences due to differences in transplacental passage of glucocorticoids between the ovine and primate placentae. Since blood vessels of the fetal sheep brain are responsive to an increase in arterial PCo2 (Pa,CO2) as early as 60 days of gestational age (dGA) (Habgood et al. 1991) and near-term fetal sheep respond to hypercapnia in a way that is qualitatively similar to that in the adult, though the response is less pronounced (Purves & James, 1969; Rosenberg et al. 1982; Ashwal et al. 1984), we measured the effects of betamethasone on a hypercapnic challenge to the fetus. Regional CBF was measured in 10 brain regions using coloured microspheres before and 24 and 48 h after onset of treatment and during hypercapnic challenges that were performed before onset and after 48 h of betamethasone exposure.
METHODS
Surgical instrumentation
All procedures were approved by the Cornell University Animal Use and Care Committee procedures and the animal welfare commission of the Thuringian state government for animal research. Sixteen Rambouillet-Colombia ewes bred on a single occasion and of known gestational age were acclimated to the animal facilities for at least 5 days before surgery and kept in rooms with controlled light-dark cycles (14 h light-10 h dark: lights off at 21.00 h and lights on at 07.00 h). Surgery was performed under halothane general anaesthesia at 120 ± 1 dGA (mean ± s.e.m.) using techniques previously described in detail (Nathanielsz et al. 1980). Briefly, ewes were instrumented with catheters inserted into the carotid artery for blood sampling and into the jugular vein for post-operative administration of antibiotics. A maternal tracheal catheter was placed to administer CO2 (Gleed et al. 1986) in the hypercapnia study. Fetuses were instrumented with polyvinyl catheters (Tygon; 0·1 mm i.d., 0·18 mm o.d.) inserted into the axillary artery and the jugular and pedal veins, with the tips of the catheters advanced to lie in the ascending aorta and the superior and inferior vena cava, respectively. Another catheter was placed in the amniotic cavity to record the amniotic pressure in order to permit correction of the fetal arterial blood pressure. Stainless-steel screw electrodes were implanted bilaterally on the fetal parietal skull to record fetal electrocorticogram and above the orbit on each side to monitor the electrooculogram. Stainless-steel wire electrodes were implanted into the fetus to record electrocardiogram (ECG), fetal breathing movements and tonic nuchal muscle activity and on the surface of the uterine body to record myometrial activity.
Animals were allowed at least 5 days of post-operative recovery before the start of any observations. During this time all ewes received a daily dose of 1 g ampicillin (AMP-Equine; SmithKline Beecham) i.v. and 1 g ampicillin into the amniotic cavity. Ewes received phenylbutazone orally (Equiphene paste, Luitpold Pharmaceuticals) 0·5 g twice daily for 3 days to provide post-operative analgesia. All catheters were maintained patent via a continuous infusion of heparin at 12·5 i.u. ml−1 in 0·9 % NaCl solution delivered at 0·5 ml h−1.
Experimental protocol
Animal preparations were randomly subdivided into either a vehicle-treated group (0·9 % NaCl solution, n = 8) or a betamethasone-treated group (n = 8). All experiments were started at 126 ± 1 dGA with a 48 h control period followed by a 48 h experimental period (Fig. 1). Sleep states, blood pressure and ECG were monitored continuously during the control and experimental periods. To check fetal health, fetal and maternal arterial blood samples were taken daily at 09.00 h throughout the study for measurement of blood gases, haemoglobin concentration and oxygen saturation using a blood gas analyser (ABL600, Radiometer, Copenhagen, Denmark; measurements corrected to 39°C) and a Haemoximeter (OSM2, Radiometer, Copenhagen, Denmark).
Figure 1. Time line of study.
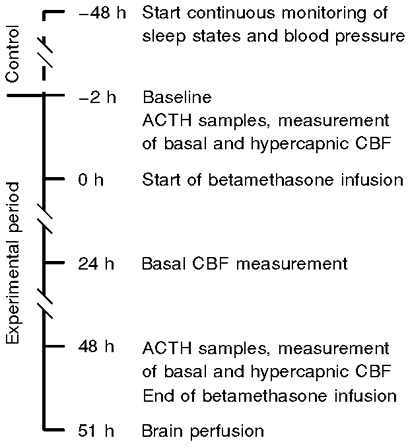
The experimental period started at 09.00 h to prevent possible effects of variations due to normal intrinsic 24 h rhythms. CBF was first measured under baseline conditions to obtain a control value followed by a second measurement during hypercapnia. Hypercapnia was achieved by infusion of about 13 l min−1 CO2 to the maternal trachea. Carbon dioxide flow was adjusted as a result of repeated fetal Pa,CO2 measurements to attain fetal Pa,CO2 values of approximately 65–0 mmHg. The Pa,CO2 was maintained at this level, and CBF was measured when the fetus entered the next quiet sleep state, about 30–45 min after onset of hypercapnic challenge. The hypercapnia was terminated immediately after the blood flow measurement. Blood gas values obtained immediately before and after coloured microsphere injection were averaged to obtain the value during CBF measurement.
Following hypercapnia, betamethasone (Celestone Soluspan, Schering) or vehicle infusion was started into the fetal jugular vein at a rate of 1 ml h−1 (10 μg betamethasone h−1) and maintained over the next 48 h. CBF measurements were repeated at 24 and 48 h after the start of the betamethasone infusion. Subsequent to the 48 h CBF measurement, a second hypercapnic challenge was performed. At the conclusion of the experiment the ewes were anaesthetised with 4 % halothane and the fetuses delivered by Caesarean section. Fetuses were killed by exsanguination while under halothane anaesthesia. To provide good histology for future studies the fetal brain was perfused via the left carotid artery with a phosphate-buffered solution containing 2 % paraformaldehyde and 0·5 % glutaraldehyde after a 5 min rinse with heparinised physiological saline. Ewes were killed by intravenous injection of pentobarbital sodium solution (Fatal-Plus, Vortech Pharmaceuticals). Fetal catheter position was checked and the fetal brain removed.
Samples for measurement of fetal plasma adrenocorticotrophic hormone (ACTH) concentrations were taken into plastic centrifuge tubes on the first experimental day and 48 h later immediately before conducting the experimental procedures to determine basal and hypercapnic CBF, i.e. before and 48 h after the start of vehicle or betamethasone infusion (Fig. 1). All tubes were placed on ice and immediately centrifuged at 1200 g for 5 min in a refrigerated (4°C) centrifuge. Plasma was removed and stored at −20°C until hormones were assayed. Fetal plasma ACTH levels were measured by radioimmunoassay (RIA) as described previously (Unno et al. 1997). The intraassay coefficient of variation was 6·8 %.
CBF measurement
CBF (ml (100 g)−1 min−1) was measured by coloured microspheres using the reference sample method. Sterile polystyrene microspheres of 15 μm diameter, surface-coated with one of five dyes in saline and 0·01 % Tween 80, were purchased from Dye-Trak, Triton Technology. In a previous study we found an excellent correlation (r = 0·995–0·999) between flow rates estimated by coloured and radio-labelled microspheres, even in organs with low perfusion, in tissue samples containing 400–750 microspheres (Walter et al. 1997). Before injection, microspheres were vortexed and sonicated for at least 10 min and drawn up into a sterile syringe immediately before injection. All CBF measurements were made during quiet sleep. CBF in quiet sleep is not as variable as in REM sleep, probably as a result of the reduced cortical activation that occurs during quiet sleep (Abrams et al. 1988). Quiet sleep was characterised by a high-voltage, slow-frequency electrocorticogram pattern, absence of sustained, rapid eye movements, and occasional nuchal tone. In addition, we avoided microsphere injection during myometrial contractures which are known to be associated with a fall in fetal Pa,O2, changes in electrocortical activity and sleep state (Nathanielsz et al. 1980). Approximately one million microspheres were injected into the fetal inferior vena cava 2 min after the onset of quiet sleep and flushed over a 20 s period. The number of injected coloured microspheres was sufficient to ensure an adequate number of microspheres per tissue sample (> 400) to meet the requirement of a systematic error of about 10 % at the 95th confidence level (Buckberg et al. 1971). Using this number of coloured microspheres, we did not observe cardiovascular side effects. Beginning 15–25 s before the injection of the coloured microspheres, a reference blood sample of 4 ml was withdrawn over 2 min from the ascending aorta into a heparinised glass syringe at a rate of 2 ml min−1 with a syringe pump (model 944, Harvard Apparatus). The amount of blood withdrawn for each administration of the coloured microspheres for the reference flows (4 ml) and hormone analyses (3 ml) was replaced by maternal arterial blood.
After necropsy, brain tissue samples weighing 0·5–2·0 g were dissected in a standardised manner from 10 discrete regions of one randomly chosen hemisphere including cortical structures (samples taken from the frontal, parietotemporal, parietooccipital, median and occipital lobe), subcortical structures (samples taken from the caudate putamen, thalamus, hippocampus and mesencephalon) and brainstem structures (samples taken from the pons and medulla). Reference blood and tissue samples were digested in 4 M KOH containing 4 % Tween 80 and microspheres were filtered through a membrane with 10 μm pores. The dyes were recovered from the coloured microspheres by adding 150 μl N,N-dimethylformamide and their concentrations were determined by spectrophotometry to quantify the microspheres, as previously described in detail (Walter et al. 1997). Absolute flows were calculated using the formula:
The total cortical flow was calculated as the mean of the five cortical flows measured, the flow to the hindbrain as the mean of the flows to the mesencephalon, pons and medulla and the total CBF as the mean of the flows to all regions measured. Cerebral vascular resistance (CVR) was calculated as mean arterial blood pressure divided by CBF. Oxygen delivery (ml (100 g)−1 min−1) to the different brain regions was calculated using the equation:
Oxygen content (ml dl−1) in ascending aorta blood was calculated as:
where [Hb] is the haemaglobin concentration (g dl−1) and 1·34 is the constant for haemoglobin carrying capacity (ml O2 g−1).
Data acquisition
Fetal arterial blood pressure and amniotic pressure were measured continuously using calibrated pressure transducers (Cobe, Lakewood, CO, USA) connected to the fetal axillary and amniotic catheters. ECG, electrocorticogram, nuchal electromyogram, fetal breathing movements and uterine electromyogram were amplified using a data acquisition system previously described in detail (Unno et al. 1999). All biophysical parameters monitored were digitised using a 16 channel A/D board (DT 2801F, Data Translation, Marlborough, MA, USA), continuously stored on a hard disk of a PC and, additionally, recorded on a multichannel chart recorder (TA11, Gould).
Statistical analysis
Differences in CBF, CVR, oxygen delivery, hormone levels, mean arterial blood pressure, heart rate and blood gases at different time points within the experimental groups were tested for significance by Wilcoxon's sign rank test. The Mann-Whitney rank sum test was used for comparison between the experimental groups. All results are given as means ± s.e.m.P values < 0·05 were considered to be significant.
RESULTS
Physiological parameters and hormonal effects
All animals studied were healthy throughout the protocol and did not show any signs of onset of labour, as indicated by the myometrial electromyogram which remained in the contracture pattern throughout the experiment. There were no significant differences in fetal weights between the vehicle- (3·69 ± 0·27 kg) and betamethasone-treated (3·11 ± 0·28 kg) groups. Arterial blood gases did not change throughout the baseline periods of the experiment other than during the hypercapnic challenge and did not differ between the vehicle- and the betamethasone-treated groups (Table 1). Vehicle infusion did not change the fetal arterial plasma ACTH (19·21 ± 4·77 vs. 21·10 ± 7·64 pg ml−1) concentration. Betamethasone infusion induced a significant decrease in fetal ACTH, from 20·21 ± 2·3 to 12·62 ± 0·98 pg ml−1 (P < 0·05).
Table 1.
Physiological variables during betamethasone infusion
| pH | PCO2 (mmHg) | PO (mmHg) | O2 ( %) | Hb (g dl−1) | ||
|---|---|---|---|---|---|---|
| Vehicle | Baseline | 7.37 ± 0.01 | 47.1 ± 2.4 | 22.3 ± 0.6 | 58 ± 5 | 10.2 ± 1.0 |
| 24 h infusion | 7.33 ± 0.03 | 47.4 ± 1.1 | 22.1 ± 1.2 | 57 ± 3 | 9.9 ± 0.7 | |
| 48 h infusion | 7.36 ± 0.01 | 46.6 ± 2.3 | 22.0 ± 1.3 | 55 ± 4 | 10.0 ± 0.3 | |
| Betamethasone | Baseline | 7.36 ± 0.01 | 47.7 ± 0.8 | 21.7 ± 1.0 | 59 ± 2 | 10.2 ± 0.4 |
| 24 h infusion | 7.36 ± 0.01 | 48.3 ± 0.5 | 21.3 ± 0.7 | 58 ± 2 | 11.4 ± 0.4 | |
| 48 h infusion | 7.38 ± 0.01 | 46.7 ± 0.9 | 22.2 ± 1.3 | 57 ± 3 | 11.2 ± 0.5 |
Physiological variables of vehicleand betamethasone-treated fetuses before and 24 and 48 h after onset of treatment.
Baseline fetal arterial blood pressure was not statistically different between the groups. Vehicle infusion over 48 h did not change the fetal mean arterial blood pressure (53 ± 3 vs. 52 ± 3 mmHg). Betamethasone infusion led to a marked increase in mean arterial blood pressure from 53 ± 1 to 66 ± 3 mmHg (P < 0·05). The increase in fetal mean arterial blood pressure occurred within the first 6 h after the onset of betamethasone infusion and remained elevated at a constant level over the infusion period (Fig. 2).
Figure 2. Fetal mean arterial blood pressure (MABP) before and during betamethasone infusion.
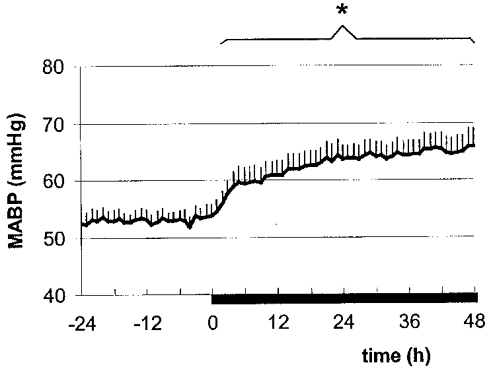
The bar indicates the period of betamethasone infusion. Values are means ± s.e.m., n = 8; * P < 0·05. MABP was averaged during quiet and REM sleep to show an overall increase.
Effects of betamethasone on CBF
Under baseline conditions, blood flows to the hindbrain structures, the mesencephalon, pons and medulla, were about 70 % higher than to the cerebral cortex (Fig. 3). Associated with the heterogeneous blood flow distribution, CVR was highest in the cerebral cortex and lowest in the hindbrain, with about 50–60 % of the resistance of the vasculature being present in the cerebral cortex (Fig. 4). The high blood flows to the hindbrain resulted in about a 60–70 % higher oxygen delivery to the mesencephalon and 80–90 % higher oxygen delivery to the pons and medulla (Fig. 5) compared to the cerebral cortex.
Figure 3. Regional CBF in vehicle- and betamethasone-treated fetuses at baseline before and 24 and 48 h after the start of infusion.
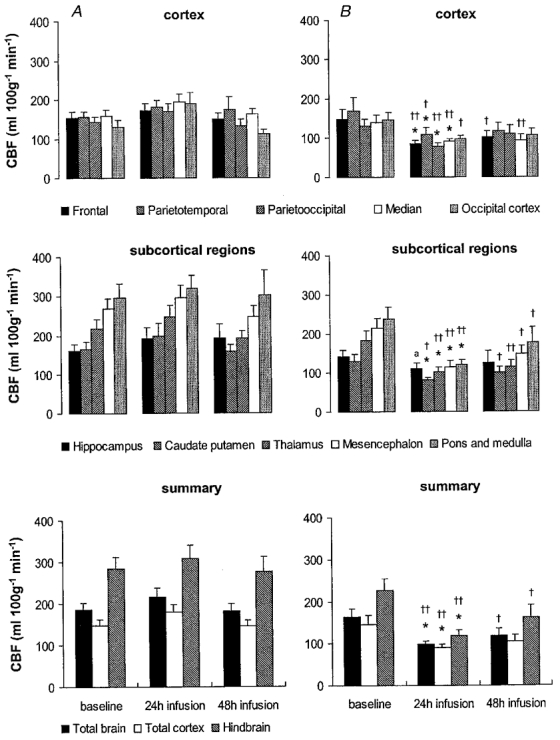
Following baseline measurements during vehicle infusion in all animals, vehicle (A) or betamethasone (B) was infused continuously. Values are means ± s.e.m., n = 8 for both groups; *P < 0·05 in comparison to baseline values; †† P < 0·01, †P < 0·05, aP < 0·07 in comparison to vehicle-treated animals.
Figure 4. Cerebral vascular resistance (CVR) in vehicle- and betamethasone-treated fetuses at baseline before and 24 and 48 h after the start of infusion.
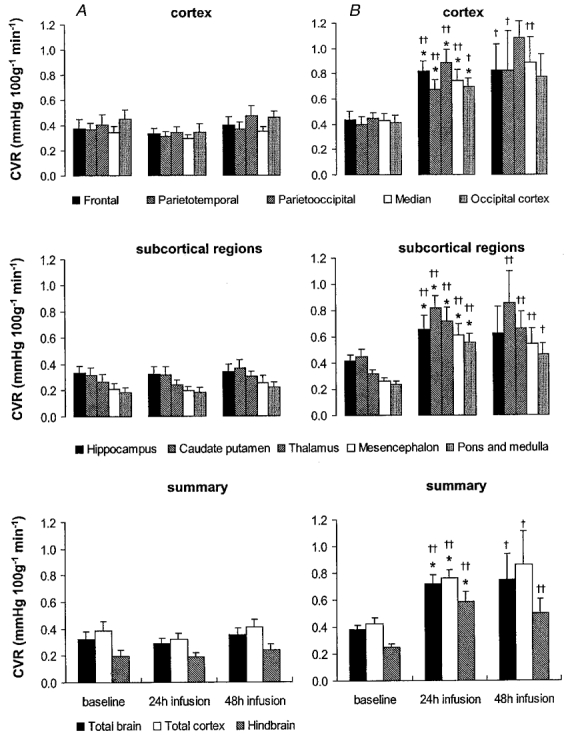
Following baseline measurements during vehicle infusion in all animals, vehicle (A) or betamethasone (B) was infused continuously. Values are means ± s.e.m., n = 8 for both groups; * P < 0·05 in comparison to baseline values, †† P < 0·01, † P < 0·05 in comparison to vehicle-treated animals.
Figure 5. Oxygen delivery in vehicle- and betamethasone-treated fetuses at baseline before and 24 and 48 h after the start of infusion.
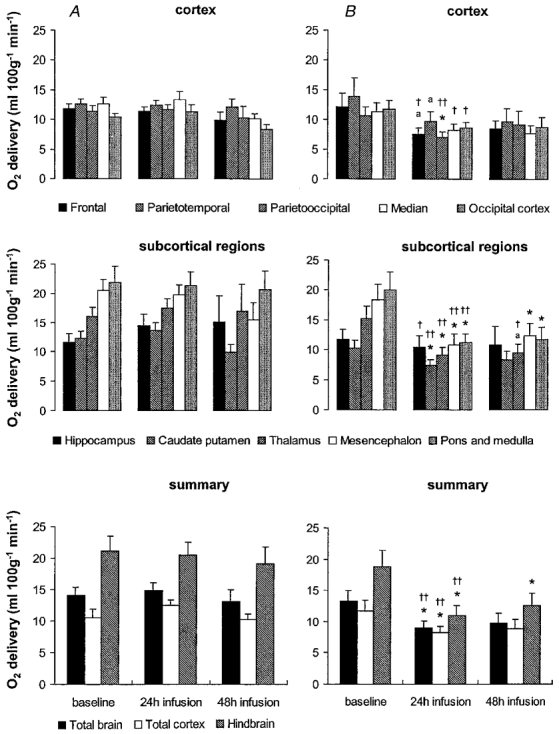
Following baseline measurements during vehicle infusion in all animals, vehicle (A) or betamethasone (B) was infused continuously. Values are means ± s.e.m., n = 8 for both groups; * P < 0·05, aP < 0·07 in comparison to baseline values, †† P < 0·01, † P < 0·05 in comparison to vehicle-treated animals.
Vehicle-infused fetuses did not show any significant changes of CBF, CVR or oxygen delivery over the 48 h infusion period. Betamethasone infusion decreased the CBF in comparison to baseline levels in all brain regions except the hippocampus after 24 h of treatment (P < 0·05, Fig. 3). The CBF decrease was most pronounced in the thalamus and hindbrain (45–50 % decrease) and least pronounced in the cortical regions (35–40 % decrease, P < 0·05, Fig. 3). Flow decreased about 20 % in the hippocampus but this decrease did not reach significance at the 0·05 level. At this time, CBF to all brain regions except the hippocampus was also significantly decreased in comparison to the vehicle-treated animals (P < 0·01, except for the caudate putamen, parietotemporal and occipital cortex when P < 0·05, Fig. 3). The decrease in regional CBF was mediated by an increase in CVR in all brain regions investigated in comparison to baseline values (P < 0·05, Fig. 4). The increase in CVR ranged from about 80 % in the cortical regions to 130 % in the hindbrain structures. In addition, CVR was increased in all areas of the brain in comparison to vehicle-treated animals (P < 0·05, Fig. 4).
The betamethasone-induced decrease in regional CBF resulted in a significant decrease in oxygen delivery of 30–40 % to 8·6 ± 1·1 ml (100 g)−1 min−1 and 40–45 % to 11·0 ± 1·6 ml (100 g)−1 min−1 to the subcortical and hindbrain regions, respectively, which are the regions most affected by the CBF decrease (P < 0·05, Fig. 5). The decrease in oxygen delivery was less pronounced in the cortex. Significance at the P < 0·05 level in comparison to baseline values was only reached in the parietooccipital cortex. The calculated oxygen delivery to the total cortex decreased by 35 % to 8·3 ± 0·9 ml (100 g)−1 min−1(P < 0·05, Fig. 5). In keeping with the absence of a significant CBF decrease in the hippocampus there was no decrease in oxygen delivery compared to baseline in the hippocampus. In comparison to the vehicle-treated animals, however, oxygen delivery was decreased in the hippocampus, and in all cortical regions except the parietotemporal cortex (P < 0·05), as well as in the subcortical and hindbrain regions (P < 0·01, Fig. 5). The regional decreases of oxygen delivery led to a significant reduction in the overall oxygen delivery to the total brain by 30–35 % to 9·0 ± 1·1 ml (100 g)−1 min−1 (P < 0·05).
Forty-eight hours after onset of betamethasone exposure the reduction in total CBF had diminished to about 25–30 %, by which time CBF was not significantly different from baseline values (Fig. 3). However, significant differences in regional CBF between the vehicle- and the betamethasone-treated fetuses were still observed in all brain regions except the parietotemporal, parietooccipital and occipital cortex (P < 0·05, Fig. 3). In keeping with the CBF values, CVR was not significantly increased in comparison to baseline but a significant elevation still existed in all brain regions except the hippocampus, parietooccipital and occipital cortex in comparison to the vehicle-treated animals (P < 0·05 or 0·01 depending on the brain region, Fig. 4). The slight increase in CBF led to a normalisation of oxygen delivery except in hindbrain structures (P < 0·05, Fig. 5).
Effects of betamethasone infusion on cerebral vasodilatation induced by hypercapnia
Carbon dioxide infusion increased the Pa,CO2 in fetal arterial blood by about 20 mmHg (Table 2). The Pa,CO2 increase was not significantly different between the two CO2 exposures within a group (i.e. before and after vehicle or betamethasone administration) or between groups. The increase in fetal Pa,CO2 was accompanied by a decrease in pH (P < 0·05, Table 2) and an increase in Pa,O2 in the fetal arterial blood (P < 0·05, Table 2). However, O2 saturation did not change significantly. The pH and Pa,O2 changes were not significantly different before and after vehicle or betamethasone infusion or between the two animal groups.
Table 2.
Physiological variables during hypercapnia
| Before treatment | 48 h after treatment | ||||
|---|---|---|---|---|---|
| Baseline | Hypercapnia | Baseline | Hypercapnia | ||
| pH | Vehicle | 7.37 ± 0.01 | 7.23 ± 0.02* | 7.36 ± 0.01 | 7.23 ± 0.01* |
| Betamethasone | 7.36 ± 0.01 | 7.22 ± 0.02* | 7.38 ± 0.01 | 7.24 ± 0.01* | |
| Pa,CO2 (mmHg) | Vehicle | 47.1 ± 2.4 | 67.7 ± 2.0* | 46.6 ± 2.3 | 65.5 ± 2.8* |
| Betamethasone | 47.7 ± 0.8 | 68.8 ± 2.8* | 46.7 ± 0.9 | 69.5 ± 2.8* | |
| Pa,O2 (mmHg) | Vehicle | 22.3 ± 0.6 | 28.4 ± 1.3* | 22.0 ± 1.3 | 28.7 ± 1.1* |
| Betamethasone | 21.7 ± 1.0 | 26.9 ± 1.1* | 22.2 ± 1.3 | 28.9 ± 2.0* | |
| O2 ( %) | Vehicle | 57.8 ± 5.3 | 61.4 ± 2.9 | 54.6 ± 3.6 | 61.8 ± 3.0 |
| Betamethasone | 59.1 ± 2.2 | 61.3 ± 1.4 | 57.4 ± 3.2 | 60.1 ± 3.9 | |
Arterial blood gases and pH values of vehicle- and betamethoasone-treated fetuses during hypercapnia challenge before and 48 h after treatment
(P > 0.05 in comparison with baseline).
Under baseline conditions, i.e. before vehicle or betamethasone treatment, hypercapnia induced a significant increase in total CBF (P < 0·05, Fig. 6) that was not different between the two experimental groups. The rise in regional CBF occurred in all brain regions but was most marked in the subcortical regions and in the hindbrain, in which CBF rose 40–60 and 70–80 %, respectively. The increase in CBF was mediated by a decrease in CVR ranging from about 30 % in the cortical regions to 40 % in the hindbrain (Fig. 7). There was no difference in the decrease in CVR between the two groups. The hypercapnia-induced rise in CBF led to an increase in oxygen delivery to all brain regions which was not different between both experimental groups (P < 0·05, Fig. 8).
Figure 6. Regional CBF during hypercapnia before and 48 h after vehicle (A) or betamethasone (B) treatment.
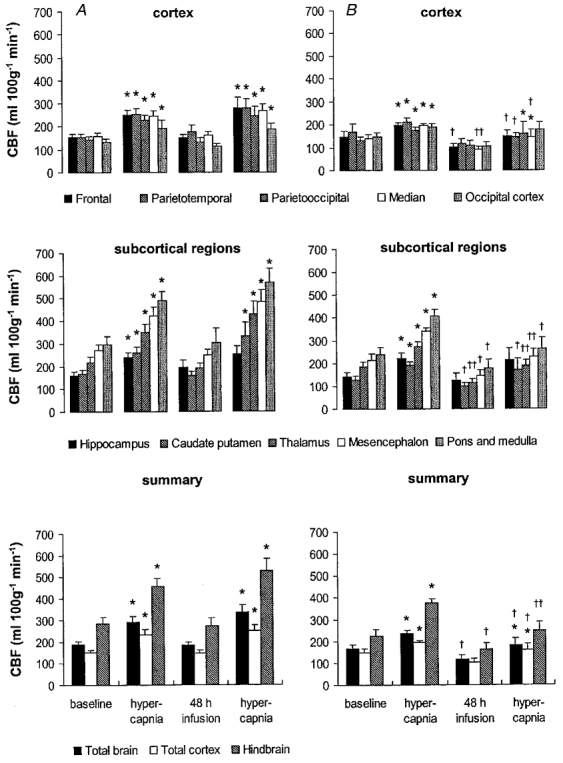
Values are means ± s.e.m., n = 8 for both groups; *P < 0·05 in comparison to baseline values immediately before hypercapnia, ††P < 0·01, †P < 0·05 in comparison to vehicle-treated animals.
Figure 7. Cerebral vascular resistance (CVR) during hypercapnia before and 48 h after vehicle (A) or betamethasone (B) treatment.
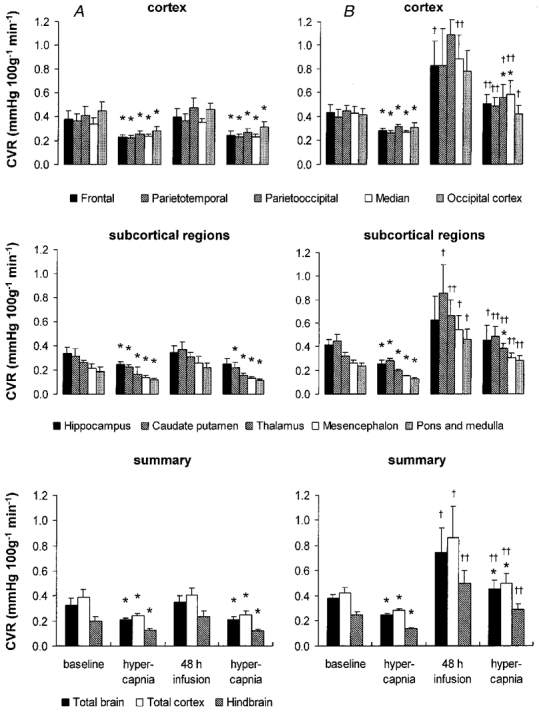
Values are means ± s.e.m., n = 8 for both groups; *P < 0·05 in comparison to baseline values immediately before hypercapnia, ††P < 0·01, †P < 0·05 in comparison to vehicle-treated animals.
Figure 8. Oxygen delivery during hypercapnia before and 48 h after vehicle (A) or betamethasone (B) treatment.
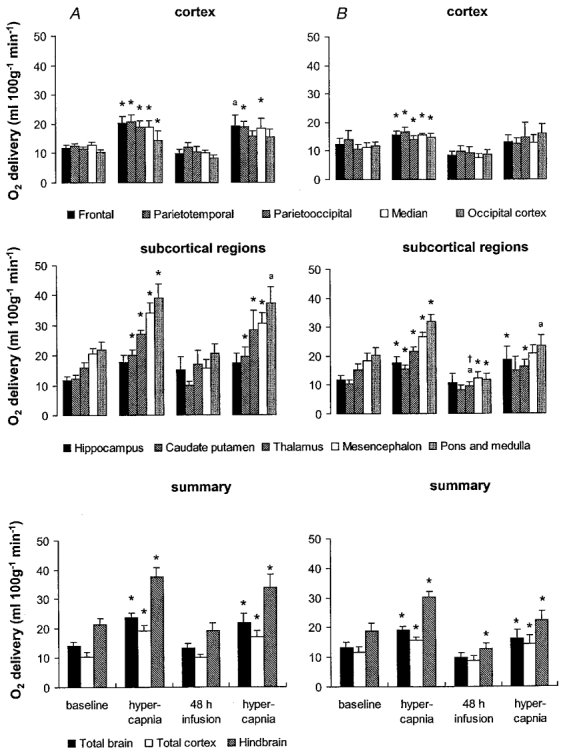
Values are means ± s.e.m., n = 8 for both groups; *P < 0·05, aP < 0·07 in comparison to baseline values immediately before hypercapnia, †P < 0·05 in comparison to vehicle-treated animals.
The regional CBF responses to hypercapnia were not different after 48 h of vehicle treatment from the responses to hypercapnia before treatment. The increase in CBF and decrease in CVR due to hypercapnia were significant in all brain regions (P < 0·05, Figs 6 and 7) except the hippocampus. The amounts of oxygen delivered to all brain regions during hypercapnia before and after vehicle treatment also did not differ significantly. In contrast to the CBF increase and CVR decrease, significance was not reached at the P < 0·05 level for the increase in oxygen delivery in three of five cortical regions and in the pons and medulla (Fig. 8).
The CBF of the betamethasone-treated fetuses showed a diminished response to hypercapnia after 48 h of exposure. A significant CBF increase could be shown only for two of the five measured cortical regions, and for the calculated flows of the total cortex and the total brain (P < 0·05, Fig. 6). Moreover, regional CBF to all brain regions measured except the hippocampus and two of the cortical regions was significantly lower in the betamethasone-treated fetuses than in the vehicle-treated fetuses during hypercapnia (P < 0·05, except for the thalamus, mesencephalon and hindbrain when P < 0·01, Fig. 6). The diminished CBF response to hypercapnia was due to a significant decrease in cerebral vasodilatation in response to hypercapnia. A decrease in CVR was visible in only two of the five measured cortical regions, in the thalamus and for the calculated flows of the total cortex and the total brain (P < 0·05, Fig. 7). As indicated by the changes in CBF, the diminished vasodilatory capacity of the cerebral vessels after 48 h of betamethasone exposure was clearly shown by the significant increase in CVR in all brain regions in comparison to the vehicle-treated animals (P < 0·01, except for the the hippocampus and the parietooccipital and occipital cortex when P < 0·05, Fig. 7). The diminished vasodilatory capacity of the cerebral vessels led to a slightly lower ability to increase oxygen delivery. This is shown by the absence of a significant increase in oxygen delivery during hypercapnia after betamethasone exposure in the cortical regions, the caudate putamen and the mesencephalon (Fig. 8). Significant differences in comparison to the vehicle-treated animals could not be demonstrated. In the light of our observation of significant differences in CBF and CVR but not oxygen delivery during hypercapnia after vehicle and betamethasone treatment (Figs 6–8) we conclude that the betamethasone-induced reduction in the vasodilatory capacity during hypercapnia did not lead to a major decrease in oxygen delivery.
DISCUSSION
This is the first study to evaluate the effects of glucocorticoids on regional CBF in the fetus of any species. We took several precautions to ensure that the information we obtained was directly related to the impact of glucocorticoids on fetal function. To avoid the confounding effects of varied fetal activity states, we always measured CBF during quiet sleep. Experiments were performed at the same time of day to avoid any potential influence of any 24 h rhythms on CBF (Endo et al. 1989). In addition, we avoided microsphere injection during uterine contractures which are known to be associated with a fall in fetal Pa,O2 and changes in electrocortical activity and sleep state (Nathanielsz et al. 1980). Betamethasone at doses resembling those to which the human fetus is exposed during antenatal glucocorticoid therapy significantly reduced CBF within 24 h in all brain regions investigated except the hippocampus. The CBF reduction was mediated by an increase in CVR and led to a decrease in oxygen delivery primarily in the subcortical and hindbrain structures.
The amount of betamethasone infused was sufficient to suppress fetal ACTH secretion. It has previously been shown that after adrenalectomy in adult rats hippocampal blood flow increased by about 50 % and prefrontal cortical blood flow by about 20 % (Endo et al. 1994). In this study CBF was measured by H2 clearance, and therefore a more detailed subdivision of regional blood flows was not available. The same group of investigators showed that long-term glucocorticoid treatment to adult rats over 12 weeks led to a decrease in hippocampal blood flow accompanied by severe histological damage in the CA1 and CA3 region (Endo et al. 1997). In another study in neonatal rats treated with a single dose of 0·1 mg dexamethasone kg−1i.p. 24 h before hypoxia, CBF was similar in vehicle- and dexamethasone-treated animals under hypoxic conditions (Tuor et al. 1993).
Betamethasone has a direct vasoconstrictor effect on femoral resistance vessels in fetal sheep (Derks et al. 1997). Femoral arteries from betamethasone-treated fetuses are more sensitive to depolarising potassium and less sensitive to the vasodilators bradykinin and forskolin than resistance vessels from control fetuses (Anwar et al. 1999). These changes will contribute to an increase in peripheral vascular resistance and a rise in mean arterial blood pressure (Derks et al. 1997). In the present study we observed a similar effect of increased vasoconstriction in the cerebral vascular bed which would contribute to the observed decrease in basal CBF.
Betamethasone has a specific affinity for type II glucocorticoid receptors which are present in most brain regions (Ahima et al. 1991; Cintra et al. 1994). Type II receptors have also been demonstrated in the brains of fetal sheep of the same gestational age as those we used in our study (Rose et al. 1985; Yang et al. 1990). However, interactions between type II and type I receptors which occur predominantly in the hippocampus and hypothalamus (Ahima et al. 1991) or specific betamethasone-independent type I receptor-mediated effects may contribute to the less pronounced response of hippocampal flow to betamethasone exposure observed in the present study.
The reduction in CBF led to a decrease in oxygen delivery in the subcortical and hindbrain structures 24 and 48 h after the onset of betamethasone exposure. However, oxygen delivery to the parietotemporal cortex, the region with the lowest oxygen delivery, was 7·0 ± 0·9 ml (100 g)−1 min−1, which was still well above the threshold of 3 ml (100 g)−1 min−1 below which neuronal cell damage occurs in fetal sheep of the same gestational age as we used in our study (Berger et al. 1996).
We have shown that, regardless of the mechanism, the dose of betamethasone used alters complex neuronal activity during REM sleep (M. Schwab, K. Schmidt, M. Roedel, K. Schmidt, T. Müller, H. Schubert, M. A. Anwar and P. W. Nathanielsz, unpublished observations) and disturbs the neuronal cytoskeleton by alteration of microtubule-associated proteins (M. Schwab, I. Antonow-Schlorke, B. Kühn, T. Müller, H. Schubert, B. Walter, U. Sliwka and P. W. Nathanielsz, unpublished observations). Though the impaired CBF does not induce irreversible neuronal damage, it probably prevents the neurons from completely meeting the metabolic demands of cortical activation during REM sleep. In addition, alteration of microtubule-associated proteins is known to occur as an early intracellular structural event in response to ischaemic challenges in neonatal rat brains (Malinak & Silverstein, 1996; Ota et al. 1997). However, a correlation between the decrease in regional CBF and a loss of immunoreactivity of the microtubule-associated proteins could not be shown for all brain regions investigated. Moreover, oxygen delivery was not significantly decreased in the cerebral cortex where a loss of microtubule-associated protein immunoreactivity occurred as well. Thus, it is likely that the neurotoxic effects of betamethasone rather than a metabolic insufficiency contribute to the alteration of the microtubule-associated proteins.
Saturation of the glucocorticoid receptors followed by downregulation and desensitisation may play a role in the diminution of blood flow 48 h after onset of betamethasone exposure. The fetal plasma betamethasone concentration during infusions similar to those used in our study has been shown to be about 10 ng ml−1 (Derks et al. 1997). In humans, glucocorticoid receptors are fully occupied at physiological cortisol concentrations of about 50–60 ng ml−1 (McEwen et al. 1987). However, synthetic glucocorticoids have a higher type II receptor affinity compared to cortisol (Yang et al. 1990).
Several factors including nitric oxide, adenosine and activation of potassium channels have been implicated in the dilatation of the cerebral vasculature that accompanies hypercapnia (review in Faraci & Heistad, 1998). The increase in CBF during hypercapnia in our study was lower than that reported previously in near-term fetal sheep (Purves & James, 1969; Ashwal et al. 1984). In those studies, however, Pa,O2 was kept constant (Purves & James, 1969) or the Pa,CO2 values were corrected to control values of O2 content (Ashwal et al. 1984). The response of the cerebral circulation to hypercapnia in the present study is therefore likely to be reduced by the coincident rise in Pa,O2 because the fetal cerebral circulation is very sensitive to oxygenation. We deliberately allowed the concomitant increase in Pa,O2 since one of the neurotoxic actions of glucocorticoids is probably to increase the susceptibility of neurons to metabolic insults by impairment of their energy metabolism (Sapolsky, 1986). The heterogeneous pattern of regional CBF, with high CBF values and a high responsiveness to hypercapnia in the hindbrain and lower values in the cerebral cortex, are in accordance with results of previous studies in the near-term fetal lamb (Ashwal et al. 1984; Szymonowicz et al. 1988).
In our study, cerebral vasodilatation due to hypercapnia was attenuated by betamethasone treatment. Inhibition of prostaglandin synthesis by the prostaglandin synthase (PGHS) inhibitor indomethacin reduces CBF in normocapnic adult rats by 50 % and reduces the CBF increase induced by hypercapnia by 80 % (Dahlgren et al. 1981). Glucocorticoids inhibit stimulated but not basal prostaglandin synthesis by inhibition of PGHS-2 (reviews in Masferrer & Seibert, 1994; GoppeltStruebe, 1997). Thus, the vasodilator response of pial arterioles to CO2 in newborn piglets was diminished by about 50 % after dexamethasone treatment due to an attenuated PGE2 synthesis (Wagerle et al. 1991). PGHS-2 is expressed constitutively in the developing brain and cerebral vessels (Peri et al. 1995; Leffler, 1997) and cortisol treatment over 5 days decreases PGHS-2 expression in the cortex and hippocampus of the fetal sheep brain (Figueroa et al. 1999). Therefore decreased prostaglandin synthesis could explain the effect of glucocorticoids on basal CBF which we have observed. Our study does not permit evaluation of glucocorticoid-induced changes in local prostaglandin production within the brain. Therefore an inhibitory effect of betamethasone on prostaglandin synthesis cannot be excluded. Further studies are needed to evaluate the potential role of betamethasone-induced inhibition of prostaglandin synthesis within the brain in the effects of betamethasone treatment on regional CBF reported here and on the brain's capacity for autoregulatory adjustments in CVR. It remains to be determined whether the slight decrease in oxygen delivery associated with the CBF decrease might have adverse effects during hypoxic challenges.
In conclusion, the decrease in CBF shown in the present study proves the need for careful assessment of the requirement for antenatal glucocorticoid treatment in each individual pregnancy. Glucocorticoids administered antenatally have a beneficial effect in protecting the fetus and newborn from intraventricular haemorrhage (Crowley et al. 1990; Ballard & Ballard, 1995; Elimian et al. 1999). However, changes in critical homeostatic functions may alter the capacity of neurons to survive adverse conditions such as hypoxic-ischaemic events.
One of the major goals of this study was to address the hypothesis that glucocorticoid administration decreases CBF at rest and also in the face of challenges that cause vasodilatation and potentially increase the risk of haemorrhage. Our data support this protective hypothesis. Neonatal morbidity secondary to confounding perinatal conditions is reduced after maternal glucocorticoid administration (Crowley et al. 1990), probably due to an improved cardiovascular response at birth, as shown in fetal sheep (Stein et al. 1993). It is critically important to weigh the adverse effects of glucocorticoids against their clear life-saving benefits. Further studies are required to determine the physiological and clinical consequences of these findings.
Acknowledgments
We are grateful to Dr Xiu-Ying Ding for expert surgical assistance, Dr R. A. Wentworth and Doug Kliewer for the help with the data acquisition system, Stella Vincent for performing the hormone assays, Ute Jaeger for processing the coloured microspheres and Karen Moore for the help with the manuscript. This work was supported by the Deutsche Akademie der Naturforscher Leopoldina, Boehringer Ingelheim Fonds and NIH grant HD 21840.
References
- Abrams RA, Hutchison AA, Jay TM, Sokoloff L, Kennedy C. Local cerebral glucose utilization non-selectively elevated in rapid eye movement sleep of the fetus. Brain Research. 1988;468:65–70. doi: 10.1016/0165-3806(88)90008-9. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Krozowski Z, Harlan RE. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. Journal of Comperative Neurology. 1991;313:522–538. doi: 10.1002/cne.903130312. [DOI] [PubMed] [Google Scholar]
- Anwar MA, Schwab M, Poston L, Nathanielsz PW. Betamethasone mediated vascular dysfunction and changes in hematological profile in the ovine fetus. American Journal of Physiology. 1999;276:H1137–1143. doi: 10.1152/ajpheart.1999.276.4.H1137. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Dale PS, Longo LD. Regional cerebral blood flow: studies in the fetal lamb during hypoxia, hypercapnia, acidosis, and hypotension. Pediatric Research. 1984;18:1309–1316. doi: 10.1203/00006450-198412000-00018. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. American Journal of Obstetrics and Gynecology. 1995;173:254–262. doi: 10.1016/0002-9378(95)90210-4. [DOI] [PubMed] [Google Scholar]
- Berger R, Lehmann T, Karcher J, Schachenmayr W, Jensen A. Relation between cerebral oxygen delivery and neuronal cell damage in fetal sheep near term. Reproduction, Fertility and Development. 1996;8:317–321. doi: 10.1071/rd9960317. [DOI] [PubMed] [Google Scholar]
- Buckberg GD, Luck JC, Payne DB, Hoffman JI, Archie JP, Fixler DE. Some sources of error in measuring regional blood flow with radioactive microspheres. Journal of Applied Physiology. 1971;31:598–604. doi: 10.1152/jappl.1971.31.4.598. [DOI] [PubMed] [Google Scholar]
- Cabanas F, Pellicer A, Garcia alix A, Quero J, Stiris TA. Effect of dexamethasone therapy on cerebral and ocular blood flow velocity in premature infants studied by colour Doppler flow imaging. European Journal of Pediatrics. 1997;156:41–46. doi: 10.1007/s004310050550. [DOI] [PubMed] [Google Scholar]
- Cintra A, Zoli M, Rosen L, Agnati LF, Okret S, Wikstrom AC, Gustaffsson AJ, Fuxe K. Mapping and computer assisted morphometry and microdensitometry of glucucorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system. Neuroscience. 1994;62:843–897. doi: 10.1016/0306-4522(94)90481-2. [DOI] [PubMed] [Google Scholar]
- Cohlen BJ, Stigter RH, Derks JB, Mulder EJ, Visser GH. Absence of significant hemodynamic changes in the fetus following maternal betamethasone administration. Ultrasound in Obstetrics and Gynecology. 1996;8:252–255. doi: 10.1046/j.1469-0705.1996.08040252.x. [DOI] [PubMed] [Google Scholar]
- Crowley P, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. British Journal of Obstetrics and Gynaecology. 1990;97:11–25. doi: 10.1111/j.1471-0528.1990.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Dahlgren N, Nilsson B, Sakabe T, Siesjo BK. The effect of indomethacin on cerebral blood flow and oxygen consumption in the rat at normal and increased carbon dioxide tensions. Acta Physiologica Scandinavica. 1981;111:475–485. doi: 10.1111/j.1748-1716.1981.tb06766.x. [DOI] [PubMed] [Google Scholar]
- Derks JB, Giussani DA, Jenkins SL, Wentworth RA, Visser GH, Padbury JF, Nathanielsz PW. A comparative study of cardiovascular, endocrine and behavioural effects of betamethasone and dexamethasone administration to fetal sheep. The Journal of Physiology. 1997;499:217–226. doi: 10.1113/jphysiol.1997.sp021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elimian A, Verma U, Canterino J, Shah J, Visintainer P, Tejani N. Effectiveness of antenatal steroids in obstetric subgroups. Obstetrics and Gynecology. 1999;93:174–179. doi: 10.1016/s0029-7844(98)00400-1. [DOI] [PubMed] [Google Scholar]
- Endo Y, Jinnai K, Endo M, Fujita K, Kimura F. Diurnal variation of cerebral blood flow in rat hippocampus. Stroke. 1989;21:1464–1469. doi: 10.1161/01.str.21.10.1464. [DOI] [PubMed] [Google Scholar]
- Endo Y, Nishimura JI, Kimura F. Adrenalectomy increases local cerebral blood flow in the rat hippocampus. Pflügers Archiv. 1994;426:183–188. doi: 10.1007/BF00374770. [DOI] [PubMed] [Google Scholar]
- Endo Y, Nishimura JI, Kobayashi S, Kimura F. Long-term glucocorticoid treatments decrease local cerebral blood flow in the rat hippocampus, in association with histological damage. Neuroscience. 1997;79:745–752. doi: 10.1016/s0306-4522(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiological Reviews. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- Figueroa JP, Zhang J, Green J, Rose JC, Massmann GA. Regional differences on the effects of chronic cortisol administration on PGHS-2 in fetal sheep bain. Journal of Gynecological Investigation. 1999;6(suppl.):181A. [Google Scholar]
- Gleed RD, Poore ER, Figueroa JP, Nathanielsz PW. Modification of maternal and fetal oxygenation using tracheal gas infusion. American Journal of Obstetrics and Gynecology. 1986;155:429–435. doi: 10.1016/0002-9378(86)90846-x. [DOI] [PubMed] [Google Scholar]
- Goppeltstruebe M. Molecular mechanisms involved in the regulation of prostaglandin biosynthesis by glucocorticoids. Biochemistry and Pharmacolology. 1997;53:1389–1395. doi: 10.1016/s0006-2952(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Habgood MD, Jones SE, Maroni JM, Pickard JD, Richards HK, Saunders NR. Cerebral blood flow in the anaesthetized immature sheep fetus and the response to hypercapnia. Experimental Physiology. 1991;76:495–505. doi: 10.1113/expphysiol.1991.sp003515. [DOI] [PubMed] [Google Scholar]
- Leffler CW. Prostanoids: Intrinsic modulators of cerebral circulation. News in Physiological Sciences. 1997;12:72–77. [Google Scholar]
- Mcewen BS, Chao H, Spencer R, Brinton R, Macisaac L, Harrelson A. Corticosteroid receptors in brain: relationship of receptors to effects in stress and aging. Annals of the New York Academy of Sciences. 1987;512:394–401. doi: 10.1111/j.1749-6632.1987.tb24975.x. [DOI] [PubMed] [Google Scholar]
- Malinak C, Silverstein FS. Hypoxic-ischemic injury acutely disrupts microtubule-associated protein 2 immunostaining in neonatal rat brain. Biology of the Neonate. 1996;69:257–267. doi: 10.1159/000244319. [DOI] [PubMed] [Google Scholar]
- Masferrer JL, Seibert K. Regulation of prostaglandin synthesis by glucocorticoids. Receptor. 1994;4:25–30. [PubMed] [Google Scholar]
- Nathanielsz PW, Bailey A, Poore ER, Thorburn GD, Harding R. The relationship between myometrial activity and sleep state and breathing in fetal sheep throughout the last third of gestation. American Journal of Obstetrics and Gynecology. 1980;138:653–659. doi: 10.1016/0002-9378(80)90083-6. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Bottu J, Govan J, Ryan ML, Myhr T, Fong K. The effect of dexamethasone on time averaged mean velocity in the middle cerebral artery in very low birthweight infants. European Journal of Pediatrics. 1994;153:363–366. doi: 10.1007/BF01956420. [DOI] [PubMed] [Google Scholar]
- Ota A, Ikeda T, Ikenoue T, Toshimori K. Sequence of neuronal responses assessed by immunohistochemistry in the newborn rat brain after hypoxia-ischemia. American Journal of Obstetrics and Gynecology. 1997;177:519–526. doi: 10.1016/s0002-9378(97)70139-x. [DOI] [PubMed] [Google Scholar]
- Peri KG, Hardy P, Li DY, Varma DR, Chemtob S. Prostaglandin G/H synthase-2 is a major contributor of brain prostaglandins in the newborn. Journal of Biological Chemistry. 1995;270:24615–24620. doi: 10.1074/jbc.270.41.24615. [DOI] [PubMed] [Google Scholar]
- Purves MJ, James IM. Observations on the control of cerebral blood flow in the sheep fetus and newborn lamb. Circulation Research. 1969;25:651–667. doi: 10.1161/01.res.25.6.651. [DOI] [PubMed] [Google Scholar]
- Rose JC, Kute TE, Winkler L. Glucocorticoid receptors in sheep brain tissues during development. American Journal of Physiology. 1985;249:E345–349. doi: 10.1152/ajpendo.1985.249.4.E345. [DOI] [PubMed] [Google Scholar]
- Rosenberg AA, Jones MD, Jr, Traystman RJ, Simmons MA, Molteni RA. Response of cerebral blood flow to changes in PCO2 in fetal, newborn, and adult sheep. American Journal of Physiology. 1982;242:H862–866. doi: 10.1152/ajpheart.1982.242.5.H862. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoid toxicity in the hippocampus: reversal by supplementation with brain fuels. Journal of Neuroscience. 1986;6:2240–2244. doi: 10.1523/JNEUROSCI.06-08-02240.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein HM, Oyama K, Martinez A, Chappell BA, Buhl E, Blount L, Padbury JF. Effects of corticosteroids in preterm sheep on adaptation and sympathoadrenal mechanisms at birth. American Journal of Physiology. 1993;264:E763–769. doi: 10.1152/ajpendo.1993.264.5.E763. [DOI] [PubMed] [Google Scholar]
- Szymonowicz W, Walker L, Cussen AM, Cannata J, Yu VY. Developmental changes in regional cerebral blood flow in fetal and newborn lambs. American Journal of Physiology. 1988;254:H52–58. doi: 10.1152/ajpheart.1988.254.1.H52. [DOI] [PubMed] [Google Scholar]
- Tuor UI, Simone CS, Barks JD, Post M. Dexamethasone prevents cerebral infarction without affecting cerebral blood flow in neonatal rats. Stroke. 1993;24:452–457. doi: 10.1161/01.str.24.3.452. [DOI] [PubMed] [Google Scholar]
- Unno N, Giussani DA, Man WKH, Hing A, Ding X-Y, Collins JH, Nathanielsz PW. Changes in ACTH and cortisol responsiveness following repeated partial umbilical cord occlusions in the late gestation ovine fetus. Endocrinology. 1997;138:259–263. doi: 10.1210/endo.138.1.4880. [DOI] [PubMed] [Google Scholar]
- Unno N, Wong CH, Jenkins SL, Wentworth RA, Ding X-Y, Li C, Robertson SS, Smotherman WP, Nathanielsz PW. Blood pressure and heart rate in the ovine fetus: Ontogenic changes and the effects of fetal adrenalectomy. American Journal of Physiology. 1999;276:H248–256. doi: 10.1152/ajpheart.1999.276.1.H248. [DOI] [PubMed] [Google Scholar]
- Yang K, Jones SA, Challis JR. Changes in glucocorticoid receptor number in the hypothalamus and pituitary of the sheep fetus with gestational age and after adrenocorticotropin treatment. Endocrinology. 1990;126:11–17. doi: 10.1210/endo-126-1-11. [DOI] [PubMed] [Google Scholar]
- Wagerle L, Degiulio PA, Mishra OP, Delivoria papadopoulous M. Effect of dexamethasone on cerebral prostanoid formation and pial arteriolar reactivity to CO2 in newborn pigs. American Journal of Physiology. 1991;260:H1313–1318. doi: 10.1152/ajpheart.1991.260.4.H1313. [DOI] [PubMed] [Google Scholar]
- Walter B, Bauer R, Gaser E, Zwiener U. Validation of the multiple colored microsphere technique for regional blood flow measurements in newborn piglets. Basic Research in Cardiology. 1997;92:191–200. doi: 10.1007/BF00788636. [DOI] [PubMed] [Google Scholar]


