Abstract
Over the past three decades the changes in sympathoadrenal function that occur with age in healthy adult humans have been systematically studied using a combination of neurochemical, neurophysiological and haemodynamic experimental approaches. The available experimental evidence indicates that tonic whole-body sympathetic nervous system (SNS) activity increases with age. The elevations in SNS activity appear to be region specific, targeting skeletal muscle and the gut, but not obviously the kidney. The SNS tone of the heart is increased, although this appears to be due in part to reduced neuronal reuptake of noradrenaline (norepinephrine). In contrast to SNS activity, tonic adrenaline (epinephrine) secretion from the adrenal medulla is markedly reduced with age. This is not reflected in plasma adrenaline concentrations because of reduced plasma clearance. Despite widely held beliefs to the contrary, sympathoadrenal responsiveness to acute stress is not exaggerated with age in healthy adults. Indeed, adrenaline release in response to acute stress is substantially attenuated in older men. The mechanisms underlying the age-associated increases in SNS activity have not been established, but our preliminary data are consistent with increased subcortical central nervous system (CNS) sympathetic drive. These changes in sympathoadrenal function with advancing age may have a number of important physiological and pathophysiological consequences for human health and disease.
The sympathetic nervous system (SNS) plays a critical role in the maintenance of physiological homeostasis in general, and arterial blood pressure in particular, under basal (resting) conditions and in response to acute stress. Post-ganglionic sympathetic neurons innervating the heart and resistance vessels help control cardiac output, arterial blood pressure and regional vascular conductance, thus ensuring the proper perfusion of vital organs. SNS stimulation of adrenaline (epinephrine) release from the adrenal medulla contributes importantly to the regulation of cardiovascular function as well as energy metabolism. The SNS also has a key role in the regulation of internal body temperature. In addition to these normal physiological interactions, the SNS has been implicated in a number of common clinical disorders including hypertension, congestive heart failure, sudden cardiac death, the insulin resistance (metabolic) syndrome and obesity.
Adult human ageing is associated with a number of important changes in physiological function and regulation to which the SNS may contribute (Rowe & Troen, 1980; Folkow & Svanborg, 1993; Lakatta, 1993; Seals, 1993). Moreover, the incidence of many chronic disease states, including those mentioned above, increases with advancing age (Biermann & Ross, 1977; DeFronzo, 1979; Schoenberger, 1986; Folkow & Svanborg, 1993; Lakatta, 1993). The changes in the sympathoadrenal system that occur with primary ageing in adult humans, and how such changes may impact important physiological and pathophysiological processes, have been systematically investigated by our laboratories and others over the past three decades. This topical review discusses some of the key experimental observations in the area of human ageing and sympathoadrenal function during this period. The review focuses on the results of studies using neurochemical and/or neurophysiological (microneurographical recordings) measures of sympathoadrenal system function. Investigations utilizing measurements derived from spectral analysis of cardiovascular variability are not included because of the difficulty in properly interpreting such results. For additional information on this topic the reader is referred to prior reviews by the authors and others (Rowe & Troen, 1980; Linares & Halter, 1987; Roberts & Tumer, 1987; Esler et al. 1989; Docherty, 1990; Seals, 1993; Seals et al. 1994; Esler, 1995).
Age and the sympathoadrenal system under basal (resting) conditions
SNS. Historically, methods employed to study age-related changes in the SNS in humans have involved measurements of noradrenaline, the primary neurotransmitter released from post-ganglionic sympathetic nerve endings. Initial approaches focused on measuring noradrenaline concentrations in 24 h urine collections and later in plasma obtained from venous or arterial blood samples, the rationale being that elevations in sympathetic nerve firing rates would be manifest as higher concentrations of noradrenaline and vice versa. In general, based on cross-sectional observations, plasma noradrenaline (PNA) concentrations have been reported to increase 10–15 % per decade over the adult age range (Ziegler et al. 1976; Jones et al. 1978; Goldstein et al. 1983). Age-associated elevations in PNA concentrations appear to be more consistently observed and larger when obtained from arterial rather than venous blood samples. Indeed, some studies on rigorously screened, healthy adults have not found significant increases in venous PNA levels with advancing age (Young et al. 1980; Fleg et al. 1985; Taylor et al. 1992a; Ng et al. 1993).
There are well-recognized limitations in using PNA concentrations as a measure of SNS activity (Esler et al. 1979; Folkow et al. 1983). The primary limitation is that such concentrations represent an equilibrium between NA released from sympathetic nerve endings that diffuses into the plasma compartment and the metabolic clearance of that NA (Esler et al. 1979; Folkow et al. 1983). As such, differences in the rate of clearance could confound the interpretation of PNA concentrations as representing SNS activity. Accordingly, isotope dilution-based methods for measuring PNA ‘kinetics’ have been employed to more precisely study the effects of ageing. Specifically, the rate of appearance (spillover) of NA into the plasma compartment has been used as a measure of SNS activity (Esler et al. 1979, 1984). Using this approach, total PNA spillover rates have consistently been found to be elevated in older compared with young adult humans (Rubin et al. 1982; Hoeldtke & Cilmi, 1985; Veith et al. 1986; Schwartz et al. 1987; MacGilchrist et al. 1989; Marker et al. 1994; Poehlman et al. 1995). However, the age-associated elevations generally have not been as great as those observed for PNA concentrations because PNA clearance rates often have been reported to be reduced with ageing (Esler et al. 1981, 1995c; Veith et al. 1986; Morrow et al. 1987; Marker et al. 1994). The latter is thought to be primarily the result of reductions in cardiac output and/or regional blood flows (Veith et al. 1986; Esler et al. 1989), both of which act to reduce clearance (Hasking et al. 1986; Esler et al. 1989; Esler, 1995). The relative contributions of increased appearance and reduced clearance to the elevated PNA concentrations with age differ among studies to date, probably due in large part to differences in sampling site (venous versus arterial) (Veith et al. 1986; Marker et al. 1994). In most cases, the age-related increases in total PNA spillover rates have been greater than the corresponding reductions in clearance (Veith et al. 1986; Schwartz et al. 1987; Marker et al. 1994). This is considered to be much more definitive evidence for an increase in total (net) SNS activity with age than observations based on PNA concentrations alone.
The interpretation of increased total PNA spillover rates as experimental support for age-associated elevations in net whole-body SNS activity, however, must be done with an understanding of the corresponding limitations of this measure. NA release from sympathetic nerve endings is modulated pre-synaptically by adrenergic receptor mechanisms (Langer, 1974). Moreover, 80–90 % of neuronally released NA is taken back up by the sympathetic nerve endings through an active reuptake (Reuptake 1) mechanism (Esler et al. 1990). Thus, changes with age in either or both of these modulatory mechanisms could confound the interpretation of PNA spillover measurements. In this context, there is in vitro evidence for an age-associated decrease in α2 pre-junctional inhibition of peripheral NA release in the rat (Daly et al. 1989; Bucholz & Piper, 1990). This would serve to augment the amount of NA released per unit sympathetic nerve discharge with age and result in an overestimation of SNS activity based on PNA spillover. Although reduced neuronal reuptake of NA has been observed in older adult humans in the heart (Esler et al. 1995b, c) (see below), no age-related differences have been observed systemically (Stromberg et al. 1991). Thus, although there is in vitro evidence supporting impaired peripheral α2-adrenergic modulation of NA release with age, this has not been confirmed in vivo in the intact human. Similarly, neuronal reuptake of NA may be reduced with age in specific organs, but currently there is no compelling support for a significant whole-body effect. Given this, age-related elevations in total PNA spillover can be reasonably viewed as experimental evidence for the concept of a net increase in average SNS activity.
In order to: (1) confirm these findings of increased total PNA spillover as indicating increased central nervous system (CNS) sympathetic outflow with age; and (2) provide insight into the specific regions to which SNS activity is increased with age, direct (intra-neural) recordings of post-ganglionic sympathetic nerve activity to skeletal muscle (MSNA) have been obtained in conscious humans using the microneurographic technique (Sundlof & Wallin, 1978; Wallin & Fagius, 1988). It is widely recognized that central SNS outflow can be regulated in an organ-specific manner (Hasking et al. 1986; Esler et al. 1988, 1990). As such, it is possible that SNS activity could be elevated with age to some tissues but not others.
Several cross-sectional studies have found that both tibial and peroneal MSNA are progressively higher with advancing age (Sundlof & Wallin, 1978; Morlin et al. 1983; Yamada et al. 1989; Iwase et al. 1991; Ebert et al. 1992), and this has been confirmed longitudinally (Fagius & Wallin, 1993). Because MSNA is considered to be a measure of CNS-generated SNS discharge (Wallin & Fagius, 1988), these results support the interpretation of increases in total NA spillover as evidence for elevated central SNS activity with human ageing. The data also suggest that one peripheral target of the increased central sympathetic outflow is limb skeletal muscle. Our more recent investigations on rigorously screened adults have extended these earlier findings by demonstrating that: (1) MSNA increases with age even in healthy, normotensive adults, suggesting a primary effect of physiological ageing (Ng et al. 1993; Jones et al. 1997a, b;Davy et al. 1998a, b;Dinenno et al. 1999) (Fig. 1A); (2) MSNA essentially doubles between the ages of 25 and 65 in these healthy adults (Ng et al. 1993; Jones et al. 1997a, b; Davy et al. 1998a, b; Dinenno et al. 1999) (Fig. 1A and B); (3) this increase in MSNA is observed in both men and women (Ng et al. 1993) (Fig. 1A and B); and (4) these age-associated elevations in MSNA are not always discernable based on venous antecubital PNA concentrations (Ng et al. 1993) (Fig. 1B).
Figure 1. Age-associated increases in muscle sympathetic nerve activity.
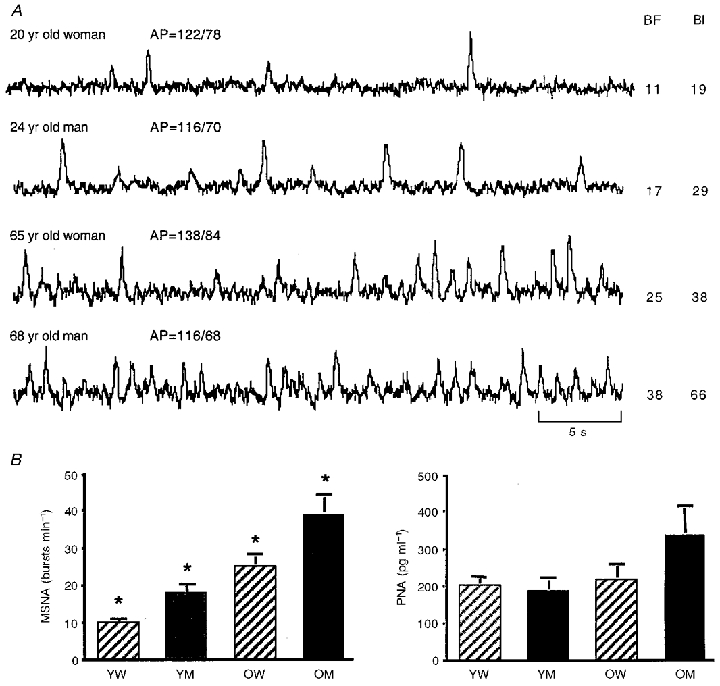
A, integrated peroneal neurograms of muscle sympathetic nerve activity (MSNA) from 4 healthy adult humans under supine resting conditions (top to bottom): young female, young male, older female, older male. MSNA burst frequency (BF; bursts min−1) and burst incidence (BI; bursts (100 heart beats)−1) are higher in the neurograms of the older adults in both sexes. However, the female subjects demonstrate lower MSNA than the males at each age. AP, arterial blood pressure. B, mean ±s.e.m. values for peroneal MSNA in 4 groups of subjects: young women (YW), young men (YM), older women (OW) and older men (OM). MSNA was at least twice as great in the older compared with the young subjects of the same sex. At each age, however, MSNA was significantly lower in the women. These age and sex differences in MSNA were not reflected in the corresponding antecubital venous plasma noradrenaline concentrations. PNA, plasma noradrenaline concentration; *P < 0·05 vs. all other groups.
While these neurophysiological data clearly support the idea of increased SNS activity with age, they provide insight into only a single peripheral tissue – skeletal muscle. The microneurographic technique cannot be used to measure SNS activity to internal organs. Accordingly, to gain further insight into other regions to which SNS activity may be elevated with age (and, thus, contribute to the increase in total PNA spillover), we next performed a series of experiments in which PNA spillover was determined for selective internal organs including the heart, gut and kidneys (Esler et al. 1995a, b, c; Mazzeo et al. 1997). Cardiac PNA spillover rate was found to be almost twice as great in healthy older compared with young men (Esler et al. 1995c); however, this appeared to represent not only increased SNS activity to the heart, but also diminished neuronal NA reuptake (Fig. 2). Hepatomesenteric PNA spillover rates also were found to increase with age by 50 % in healthy men (Mazzeo et al. 1997) (Fig. 2). As neuronal reuptake does not influence hepatomesenteric PNA spillover to the same extent as in the heart (Esler et al. 1990), these findings are consistent with elevations in SNS activity. No significant differences were observed for renal NA spillover rates with age (Esler et al. 1995c).
Figure 2. Influence of ageing on noradrenaline (NA) kinetics in the heart and hepatomesenteric circulation, and on secretion of adrenaline.
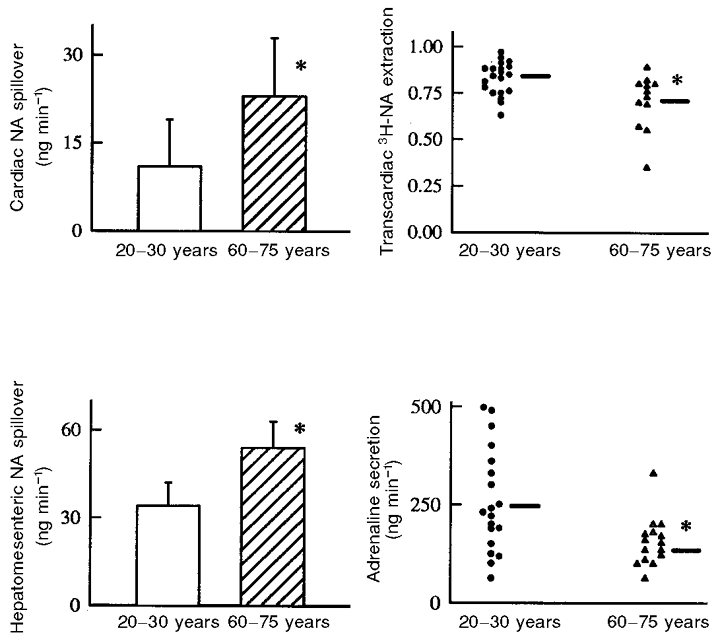
Cardiac noradrenaline spillover was higher in older men (upper left panel). This appeared to be due in part to reduced neuronal reuptake of noradrenaline, evident in reduced transcardiac extraction of plasma tritiated noradrenaline (upper right panel); removal of noradrenaline from plasma by the heart is largely by uptake into the cardiac sympathetic nerves. Noradrenaline spillover into the hepatomesenteric circulation was also higher in the older men (lower left panel), but unlike in the heart, this was most probably due exclusively to increased sympathetic nerve firing rates, as plasma tritiated noradrenaline extraction across the gut and liver (not shown) was normal. In contrast to the augmentation of sympathetic tone in the heart and hepatomesenteric circulation, adrenaline secretion rates were reduced in the older men (lower right panel). Mean +s.e.m. values are indicated in the histograms. *P < 0·05 vs. young men.
Taken together, the evidence to date supports the view that primary human ageing is associated with a net activation of the SNS (Fig. 3). PNA concentrations are elevated due to a combination of augmented PNA spillover from sympathetic nerve endings and reduced metabolic clearance of NA. Skeletal muscle is a major target of the increased central SNS activity as well as the gut. Sympathetic tone is elevated in the heart with age in humans, apparently due to both reduced neuronal reuptake of NA and increased cardiac sympathetic nerve discharge. Finally, at present there is no compelling evidence that SNS activity to the kidney is elevated in healthy ageing.
Figure 3. Regional changes in the sympathoadrenal system with primary (healthy) human ageing.
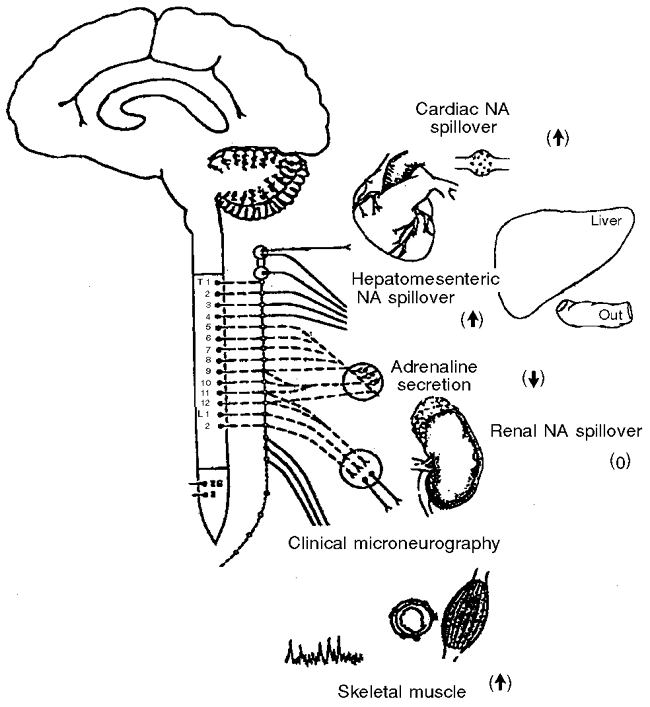
Augmentation of sympathetic activity, evident in increased noradrenaline (NA) spillover rates or sympathetic nerve firing measured with microneurography, occurs with ageing in the sympathetic outflows of the heart, hepatomesenteric circulation and skeletal muscle vasculature. In contrast, ageing produced no obvious change in sympathetic nervous activity in the kidney, and a reduction in adrenaline secretion by the adrenal medulla. Symbols in parentheses indicate an increase, decrease or no change in activity.
Adrenaline release from the adrenal medulla
Historically, plasma concentrations of adrenaline have been used to determine possible effects of ageing on adrenaline secretion from the adrenal medulla. Generally investigations to date have found that plasma adrenaline concentrations either become slightly lower or do not change across the adult age range (Franco-Morselli et al. 1977; Weidman et al. 1978). As is the case with PNA concentrations, however, the interpretation of plasma adrenaline levels as a measure of secretion from the adrenal medulla is not straightforward given the possibility of age-related changes in clearance. To address this, we employed tracer methodology to study plasma adrenaline kinetics (Esler et al. 1995a). We found that adrenaline secretion from the adrenal medulla was 40 % lower in older compared with young healthy men (Fig. 2). This difference was not reflected in the corresponding arterial plasma adrenaline concentrations, which were not significantly different with age, because plasma clearance was 20 % lower in the older men. In the same investigation, we also examined the possibility that adrenaline is released from the heart, perhaps acting to augment cardiac NA release via stimulation of pre-junctional β-adrenergic receptors. Adrenaline was released from the heart only in the older men, despite the fact that their adrenaline secretion from the adrenal medulla was reduced.
In summary (Fig. 3), in contrast to the increase in SNS activity, adrenaline secretion from the adrenal medulla is markedly reduced with advancing age under resting conditions in healthy humans. The lower secretion in older humans is not apparent from plasma concentrations, which do not change significantly with age, because of a reduction in the rate of clearance of adrenaline from the circulation. Finally, adrenaline is released from the heart at rest in older humans. It is not known if this contributes mechanistically to the aforementioned age-associated increases in cardiac NA spillover via pre-junctional β-adrenergic stimulation.
Mechanisms underlying age-associated changes in the sympathoadrenal system
SNS. Two primary mechanisms have been hypothesized to explain age-related increases in peripheral SNS activity under resting conditions: (1) reduced tonic baroreflex inhibition of ‘normal’ central SNS outflow; and (2) a primary increase in CNS-generated sympathetic nerve discharge.
Both arterial and cardiopulmonary baroreflexes tonically inhibit central SNS outflow in humans (Mancia & Mark, 1983; Mark & Mancia, 1983). Thus one possibility is that this tonic inhibition lessens with advancing age, allowing progressively greater levels of SNS activity to peripheral tissues (Rowe & Troen, 1980). The experimental support for this hypothesis was based largely on results of studies: (1) in humans showing age-related reductions in cardiovagal baroreflex sensitivity (Gribbin et al. 1971; Lindblad, 1977; Cleroux et al. 1989; Ebert et al. 1992); (2) in humans using PNA concentrations as a measure of SNS activity during baroreflex perturbations (Shimada et al. 1985); or (3) in animals in which arterial and/or cardiopulmonary baroreflex control of renal SNS activity was shown to be reduced in senescent animals (Hajduczok et al. 1991a, b). However, in a series of studies performed in healthy humans (Davy et al. 1998a, b; Tanaka et al. 1999), we found that baroreflex control of MSNA was not obviously reduced in older compared with young adults. Other investigators earlier had reported similar findings (Ebert et al. 1992; Matsukawa et al. 1996). In fact, our results indicated that at least one expression of baroreflex control of MSNA (i.e. responses to graded hypovolemia) actually was augmented in older adults (Davy et al. 1998a). There is an age-associated reduction in baroreflex-evoked peripheral vasconstriction (Cleroux et al. 1989; Davy et al. 1998a), but this appears to be due to a decrease in peripheral vascular responsiveness to sympathetic stimulation rather than an inability to evoke the necessary adjustments in SNS activity (Davy et al. 1998a).
The results of earlier studies in young and senescent beagles (Hajduczok et al. 1991a, b) suggested that the marked elevation in basal peripheral SNS activity in the older animals could not be completely explained by a reduction in tonic baroreflex inhibition. Rather, it was postulated that a primary increase in CNS-generated sympathetic outflow also must contribute. Accordingly, we have addressed the potential involvement of this mechanism in preliminary studies measuring brain NA turnover (M. D. Esler & D. R. Seals, unpublished data). In several clinical contexts, most notably cardiac failure and essential hypertension, Esler and colleagues (Ferrier et al. 1993; Lambert et al. 1994, 1995) have demonstrated the possible importance of projections of noradrenergic neurons to the forebrain in generating elevated levels of peripheral SNS activity. Indeed, even in healthy young men in which SNS activity varies only within a narrow normal range, there is a strong and positive relation between NA turnover in the subcortical areas of the brain and peripheral SNS activity (Lambert et al. 1998). Our preliminary findings indicate that subcortical NA turnover under resting conditions is at least 2-fold higher in healthy older compared with young men (317 ± 50 vs. 107 ± 18 ng min−1). This elevation in forebrain NA turnover was positively and significantly related to corresponding age-associated elevations in cardiac NA spillover, which was increased with age. In contrast, cortical NA turnover did not vary with age.
The exact mechanism responsible for the apparent marked increase in subcortical brain NA turnover with age is unclear. In this context, it should be noted that clonidine administration, a central α2-adrenergic receptor agonist that augments pre-junctional inhibition of NA release, evoked similar dose-dependent reductions in PNA concentrations and total PNA spillover rates in young and older men (Featherstone et al. 1987). These results do not support reduced central α2-adrenergic pre-junctional inhibition of NA release as a primary mechanism involved in age-associated increases in forebrain NA turnover. The evidence also is against a reduction in brain neuronal NA reuptake contributing to the increased subcortical NA turnover with age. Reduced neuronal NA disproportionately increases 3-methoxy-4-hydroxy phenylglycol (MHPG) overflow and reduces dihydroxyphenylglycol (DHPG) overflow (Eisenhofer et al. 1991), which was not evident with ageing in our study.
Finally, it is possible that some as yet unidentified humoral signal with either peripheral afferent or direct CNS sympathoexcitatory effects may increase with advancing age and provide a tonic stimulus for the elevation in SNS activity with age. At present, however, there is no compelling experimental support for any such circulating signal.
In summary, the available data do not support the concept that impairments in tonic baroreflex inhibition of central sympathetic outflow play a major role in age-associated increases in peripheral SNS activity in humans. Rather, our preliminary data are consistent with the possibility that elevations in total and/or organ-specific SNS activity may be due, at least in part, to increased activity of noradrenergic neurons in subcortical areas of the brain known to modulate medullary pre-ganglionic sympathetic discharge.
Adrenaline secretion from the adrenal medulla
The mechanism(s) contributing to the reductions in adrenaline secretion from the adrenal medulla with advancing age have not been investigated to date. Possibilities include age-associated: (1) reductions in pre-ganglionic nerve activity to the adrenal medulla; (2) reductions in adrenaline secretion in response to equivalent (or even greater) levels of pre-ganglionic nerve activity; and (3) reduction in adrenaline synthesis and storage in the adrenal medulla.
Age and sympathoadrenal adjustments to acute stress
For purposes of the present review, we define acute stress as a stimulus that requires rapid and, in some cases, marked adjustments in the sympathoadrenal system in order to maintain homeostasis. Over the past 20–30 years there has been a widely held and much emphasized view that even healthy ageing is associated with augmented sympathoadrenal responsiveness to acute stress (Rowe & Troen, 1980). This belief appears to be based largely on early studies showing greater increases in venous PNA concentrations in response to a variety of acute laboratory stressors (Palmer et al. 1978; Young et al. 1980; Barnes et al. 1982; Sowers et al. 1983). These studies had some limitations that could alter, perhaps fundamentally, the interpretation of their results. The first and most obvious are those related to PNA concentrations as a measure of changes in SNS activity with age. For example, reduced neuronal reuptake or systemic plasma clearance of NA, both of which have been reported to occur with age (Esler et al. 1981, 1995c; Veith et al. 1986; Morrow et al. 1987; Marker et al. 1994), would result in greater PNA concentrations in response to a particular stress-evoked increase in SNS activity. Second, in some cases the exact level of stress used was not documented, leaving open the possibility that the older adults may have been subjected to a greater sympathoexcitatory stimulus. Third, the SNS adjustments to stress may be influenced by several factors (disease, obesity, physical activity, gender) that were not always controlled; thus, it is not possible to isolate the effects of the ageing process. Finally, in some of our preliminary studies in this area (Taylor et al. 1991, 1992b; Davy et al. 1995), using well-controlled experimental conditions, we were unable to confirm greater increases in antecubital venous PNA concentrations in response to various forms of laboratory stress in healthy older adults.
Accordingly, we performed a series of investigations aimed at determining if primary ageing is associated with augmented sympathoadrenal adjustments to acute stress (Ng et al. 1994, 1995; Esler et al. 1995a, b; Davy et al. 1997; Mazzeo et al. 1997). An important goal of these studies was to gain insight into possible age-related differences in regional SNS responses as well as adrenaline secretion from the adrenal medulla. Subject characteristics and stress stimuli were carefully controlled in order to experimentally isolate, as much as is possible, the effects of ageing per se. Because changes in sympathoadrenal responsiveness with age could be stimulus specific, we employed several different types of stress including isometric and dynamic exercise, orthostasis, cognitive challenge, local cold stimulation and hypoxia; each of these stimuli produces sympathoexcitation via different afferent and/or CNS pathways.
In general, we found that the absolute increases in measures of net whole-body SNS activity (i.e. arterial PNA concentrations and total PNA spillover) in response to these stressors were not different in young and older healthy adults (Esler et al. 1995a, b; Mazzeo et al. 1997) (middle panel, Fig. 4). With regard to regional SNS activity, the absolute unit increases in MSNA (Ng et al. 1994, 1995; Davy et al. 1997) and hepatomesenteric PNA spillover (Mazzeo et al. 1997) were similar in young and older subjects. In fact, the relative (percentage) increases in these measures of SNS activity actually were smaller in the older adults because of their elevated baseline (resting) levels. In contrast, the increases in cardiac PNA spillover were consistently greater, in some cases markedly so, in older compared with younger men in response to a variety of acute stressors (Esler et al. 1995b) (bottom panel, Fig. 4). As was the case at rest, lower neuronal reuptake of NA appeared to contribute significantly to the greater increases in cardiac PNA spillover rates in response to acute stress with age (Esler et al. 1995b).
Figure 4. The influence of ageing on increases in (top to bottom) adrenaline secretion and the spillover of noradrenaline to plasma from the whole body and from the heart during the application of laboratory stress.
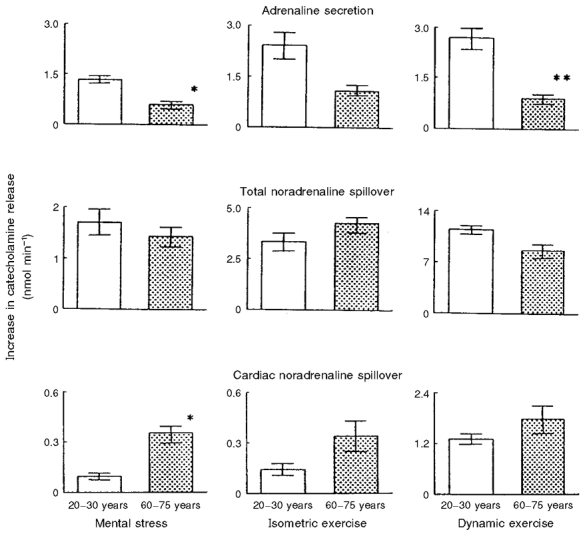
The increases in total noradrenaline spillover rates with the stimuli were not affected by ageing. Contrasting effects of ageing were observed for stimulation of adrenaline secretion (lower in older men) and cardiac noradrenaline spillover (higher in older men). Reprinted (from Esler et al. 1995a) with permission. *P < 0·05 and **P < 0·01 indicate significance of differences between younger and older men. Mean ±s.e.m. values are shown.
We also measured the magnitude of increase in adrenaline secretion from the adrenal medulla in response to several types of stress using isotope dilution methodology (Esler et al. 1995a). Unlike the SNS responses, the absolute stress-evoked increases in adrenaline secretion were markedly attenuated with age (top panel, Fig. 4). Specifically, the augmentation in adrenaline release in response to stress in the older men was only 33–44 % of that observed in the young controls.
In summary, in striking contrast to the prevailing view, the results of our systematic investigations overwhelmingly support the concept that primary human ageing is not associated with exaggerated sympathoadrenal responsiveness to acute stress. The increase in cardiac PNA spillover with stress does appear to be augmented in older adults, but this may be due largely to faulty neuronal uptake of NA. Importantly, the ability of the adrenal medulla to secrete adrenaline in response to stress is markedly impaired even in healthy older adults.
Conclusions
In conclusion, experimental data from our laboratories and others support the view that chronic (basal) SNS activity increases with advancing age in healthy adult humans (Fig. 3). The elevations in SNS activity appear to be region specific, targeting skeletal muscle and the gut, but not obviously the kidney. The SNS tone of the heart is increased, although this appears to be due at least in part to reduced neuronal NA reuptake. In contrast to SNS activity, basal adrenaline secretion from the adrenal medulla is markedly reduced with primary ageing in humans. This is not reflected in plasma adrenaline concentrations because of reduced plasma clearance.
The mechanisms underlying these age-associated changes in sympathoadrenal function have not been established. Our preliminary results suggest that the increase in basal peripheral SNS activity with age is associated with elevated forebrain noradrenergic activity. These data are consistent with the hypothesis that increased CNS sympathetic drive may be a key mechanism involved. In contrast to studies in experimental animals, currently there is little or no evidence in humans to support a role for reduced baroreflex inhibition in the increases in SNS activity with age. The mechanism(s) underlying blunted adrenaline secretion from the adrenal medulla remain to be investigated.
Finally, despite widely held beliefs to the contrary, the results of our systematic investigations demonstrate that sympathoadrenal responsiveness to acute stress is not exaggerated with age, at least in healthy adults. Indeed, as observed under resting conditions, adrenaline release in response to acute stress is substantially attenuated in older men.
Acknowledgments
The work from the laboratory of Professor Seals cited in this review was supported by National Institutes of Health award AG06537. Professor Seals wishes to acknowledge the important contributions of trainees and/or colleagues Robin Callister, Kevin Davy, Frank Dinenno, Pamela Parker Jones, Mary Beth Monroe, Alexander Ng, Mary Jo Reiling, Hirofumi Tanaka and J. Andrew Taylor. The work from the laboratory of Professor Esler cited in this review was supported in part by an institute grant from the National Health and Medical Research Council of Australia to the Baker Medical Research Institute, and in part by National Institutes of Health award AG06537 (USA). Professor Esler wishes to acknowledge the important contributions of his colleagues David Kaye, Gavin Lambert, Gary Jennings, Mario Vaz, Claudia Ferrier and Graeme Eisenhofer.
References
- Barnes R, Raskind M, Gumbrecht G, Halter J. The effects of age on the plasma catecholamine response to mental stress in man. Journal of Clinical Endocrinology and Metabolism. 1982;54:64–69. doi: 10.1210/jcem-54-1-64. [DOI] [PubMed] [Google Scholar]
- Biermann EL, Ross R. Aging and atherosclerosis. Atherosclerosis Reviews. 1977;2:79–111. [Google Scholar]
- Bucholz J, Piper SD. Effect of age on prejunctional modulation of norepinephrine release. Journal of Pharmacology and Experimental Therapeutics. 1990;252:159–164. [PubMed] [Google Scholar]
- Cleroux J, Giannattasio C, Bolla G, Cuspidi C, Grassi G, Mazzola C, Sampieri L, Seravalle G, Valsecchi M, Mancia G. Decreased cardiopulmonary reflexes with aging in normotensive humans. American Journal of Physiology. 1989;257:H961–968. doi: 10.1152/ajpheart.1989.257.3.H961. [DOI] [PubMed] [Google Scholar]
- Daly RN, Goldberg PB, Roberts J. The effect of age on presynaptic alpha 2 adrenoceptor autoregulation of norepinephrine release. Journals of Gerontology. 1989;44:B59–66. doi: 10.1093/geronj/44.3.b59. [DOI] [PubMed] [Google Scholar]
- Davy K, Johnson D, Seals D. Cardiovascular, plasma norepinephrine, and thermal adjustments to prolonged exercise in young and older healthy humans. Clinical Physiology. 1995;15:169–181. doi: 10.1111/j.1475-097x.1995.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Davy KP, Jones PP, Seals DR. Influence of age on the sympathetic neural adjustments to alterations in systemic oxygen levels in humans. American Journal of Physiology. 1997;273:R690–695. doi: 10.1152/ajpregu.1997.273.2.R690. [DOI] [PubMed] [Google Scholar]
- Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension. 1998a;32:298–304. doi: 10.1161/01.hyp.32.2.298. [DOI] [PubMed] [Google Scholar]
- Davy KP, Tanaka H, Andros EA, Gerber JG, Seals DR. Influence of age on arterial baroreflex inhibition of sympathetic nerve activity in healthy adult humans. American Journal of Physiology. 1998b;275:H1768–1772. doi: 10.1152/ajpheart.1998.275.5.H1768. [DOI] [PubMed] [Google Scholar]
- Defronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979;28:1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Docherty JR. Cardiovascular responses in aging: a review. Pharmacological Reviews. 1990;42:103–125. [PubMed] [Google Scholar]
- Ebert T, Morgan B, Barney J, Denahan T, Smith J. Effects of aging on baroreflex regulation of sympathetic activity in humans. American Journal of Physiology. 1992;263:H798–803. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Smolich JJ, Cox HS, Esler MD. Neuronal reuptake of norepinephrine and production of dihydroxyphenylglycol by cardiac sympathetic nerves in the anesthetized dog. Circulation. 1991;84:1354–1363. doi: 10.1161/01.cir.84.3.1354. [DOI] [PubMed] [Google Scholar]
- Esler M. The Sympathetic Nervous System and Catecholamine Release and Plasma Clearance in Normal Blood Pressure Control, in Aging and in Hypertension. 2. New York: Raven Press; 1995. [Google Scholar]
- Esler M, Jackman G, Bobik A. Determination of norepinephrine apparent release rate and clearance in humans. Life Sciences. 1979;25:1461–1470. doi: 10.1016/0024-3205(79)90371-0. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiological Reviews. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Leonard P, Sacharias N, Burke F, Johns J, Blombery P. Contribution of individual organs to total noradrenaline release in humans. Acta Physiologica Scandinavica. 1984;suppl. 527:11–16. [PubMed] [Google Scholar]
- Esler M, Kaye D, Thompson J, Jennings G, Cox H, Turner A, Lambert G, Seals D. Effects of aging on epinephrine secretion, and on regional release of epinephrine from the human heart. Journal of Clinical Endocrinology and Metabolism. 1995a;80:435–442. doi: 10.1210/jcem.80.2.7852502. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert G, Jennings G. The influence of aging on catecholamine metabolism. In: Amery A, Staessen J, editors. Handbook of Hypertension, Hypertension in the Elderly. Vol. 12. Amsterdam: Elsevier Science Publishers; 1989. pp. 85–98. [Google Scholar]
- Esler M, Skews H, Leonard P, Jackman G, Bobik A, Korner P. Age-dependence of noradrenaline kinetics in normal subjects. Clinical Science. 1981;60:217–219. doi: 10.1042/cs0600217. [DOI] [PubMed] [Google Scholar]
- Esler MD, Thompson JM, Turner AG, Kaye DM, Jennings GL, Cox HS, Lambert GW, Seals DR. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation. 1995b;91:351–358. doi: 10.1161/01.cir.91.2.351. [DOI] [PubMed] [Google Scholar]
- Esler MD, Turner AG, Kaye DM, Thompson JM, Kingwell BA, Morris M, Lambert GW, Jennings GL, Seals DR. Aging effects on human sympathetic neuronal function. American Journal of Physiology. 1995c;268:R278–285. doi: 10.1152/ajpregu.1995.268.1.R278. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clinical Autonomic Research. 1993;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- Featherstone JA, Veith RC, Flatness D, Murburg MM, Villacres EC, Halter JB. Age and alpha-2 adrenergic regulation of plasma norepinephrine kinetics in humans. Journals of Gerontology. 1987;42:271–276. doi: 10.1093/geronj/42.3.271. [DOI] [PubMed] [Google Scholar]
- Ferrier C, Jennings G, Eisenhofer G, Lambert G, Cox H, Kalff V, Kelly M, Esler M. Evidence for increased noradrenaline release from subcortical brain regions in essential hypertension. Journal of Hypertension. 1993;11:1217–1227. [PubMed] [Google Scholar]
- Fleg JL, Tzankoff SP, Lakatta EG. Age-related augmentation of plasma catacholamines during dynamic exercise in healthy males. Journal of Applied Physiology. 1985;59:1033–1039. doi: 10.1152/jappl.1985.59.4.1033. [DOI] [PubMed] [Google Scholar]
- Folkow B, Dibona GF, Hjemdahl P, Toren PH, Wallin BG. Measurements of plasma norepinephrine concentrations in human primary hypertension. Hypertension. 1983;5:399–403. doi: 10.1161/01.hyp.5.4.399. [DOI] [PubMed] [Google Scholar]
- Folkow B, Svanborg A. Physiology of cardiovascular aging. Physiological Reviews. 1993;73:725–764. doi: 10.1152/physrev.1993.73.4.725. [DOI] [PubMed] [Google Scholar]
- Franco-Morselli R, Elghozi JL, Joly E, Di giuilio S, Meyer P. Increased plasma adrenaline concentrations in benign essential hypertension. British Medical Journal. 1977;2:1251–1254. doi: 10.1136/bmj.2.6097.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Lake CF, Chernow B, Zeigler MG, Coleman MD, Taylor AA, Mitchell JR, Kopin KJ, Keiser HR. Age-dependence of hypertensive-normotensive differences in plasma norepinephrine. Hypertension. 1983;5:100–104. doi: 10.1161/01.hyp.5.1.100. [DOI] [PubMed] [Google Scholar]
- Gribbin BT, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circulation Research. 1971;29:424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- Hajduczok G, Chapleau M, Abboud F. Increase in sympathetic activity with age II. Role of impairment of cardiopulmonary baroreflexes. American Journal of Physiology. 1991a;260:H1121–1127. doi: 10.1152/ajpheart.1991.260.4.H1121. [DOI] [PubMed] [Google Scholar]
- Hajduczok G, Chapleau M, Johnson S, Abboud F. Increase in sympathetic activity with age I. Role of impairment of arterial baroreflexes. American Journal of Physiology. 1991b;260:H1113–1120. doi: 10.1152/ajpheart.1991.260.4.H1113. [DOI] [PubMed] [Google Scholar]
- Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–621. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- Hoeldtke RD, Cilmi KM. Effects of aging on catecholamine metabolism. Journal of Clinical Endocrinology and Metabolism. 1985;60:479–484. doi: 10.1210/jcem-60-3-479. [DOI] [PubMed] [Google Scholar]
- Iwase S, Mano T, Watanabe T, Saito M, Kobayashi F. Age-related changes of sympathetic outflow to muscles in humans. Journals of Gerontology. 1991;46:M1–5. doi: 10.1093/geronj/46.1.m1. [DOI] [PubMed] [Google Scholar]
- Jones D, Hamilton C, Reid J. Plasma noradrenaline, age, and blood pressure: a population study. Clinical Journal of Molecular Medicine. 1978;55:73–75. doi: 10.1042/cs055073s. [DOI] [PubMed] [Google Scholar]
- Jones PP, Davy KP, Alexander S, Seals DR. Age-related increase in muscle sympathetic nerve activity is associated with abdominal adiposity. American Journal of Physiology. 1997a;272:E976–980. doi: 10.1152/ajpendo.1997.272.6.E976. [DOI] [PubMed] [Google Scholar]
- Jones PP, Davy KP, Seals DR. Relations of total and abdominal adiposity to muscle sympathetic nerve activity in healthy older males. International Journal of Obesity. 1997b;21:1053–1057. doi: 10.1038/sj.ijo.0800515. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiological Reviews. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- Lambert G, Ferrier C, Kaye D, Kalff V, Kelly M, Cox H, Turner A, Jennings G, Esler M. Monoaminergic neuronal activity in subcortical brain regions in essential hypertension. Blood Pressure. 1994;3:55–66. doi: 10.3109/08037059409101522. [DOI] [PubMed] [Google Scholar]
- Lambert G, Kaye D, Lefkovits J, Jennings G, Turner A, Cox H, Esler M. Central nervous system monoamine neurotransmitter turnover and its association with sympathetic nervous activity in treated heart failure patients. Circulation. 1995;92:1813–1818. doi: 10.1161/01.cir.92.7.1813. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Kaye DM, Thompson JM, Turner AG, Cox HS, Vaz M, Jennings GL, Wallin BG, Esler MD. Internal jugular venous spillover of noradrenaline and metabolites and their association with sympathetic nervous activity. Acta Physiologica Scandinavica. 1998;163:155–163. doi: 10.1046/j.1365-201X.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- Langer SZ. Presynaptic regulation of catecholamine release. Biochemistry and Pharmacology. 1974;23:1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- Linares OA, Halter JB. Sympathochromaffin system activity in the elderly. Journal of the American Geriatrics Society. 1987;35:448–453. doi: 10.1111/j.1532-5415.1987.tb04667.x. [DOI] [PubMed] [Google Scholar]
- Lindblad LE. Influence of age on sensitivity and effector mechanisms of the carotid baroreflex. Acta Physiologica Scandinavica. 1977;101:43–49. doi: 10.1111/j.1748-1716.1977.tb05981.x. [DOI] [PubMed] [Google Scholar]
- Macgilchrist AJ, Hawksby C, Howes LG, Reid JL. Rise in plasma noradrenaline with age results from an increase in spillover rate. Gerontology. 1989;35:7–13. doi: 10.1159/000212994. [DOI] [PubMed] [Google Scholar]
- Mancia G, Mark AL. Arterial baroreflexes in humans. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System. Vol. 3. Bethesda, MD, USA: American Physiological Society; 1983. pp. 755–794. [Google Scholar]
- Mark A, Mancia G. Cardiopulmonary baroreflexes in humans. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, Peripheral Circulation and Organ Blood Flow. Vol. 3. Bethesda, MD, USA: American Physiological Society; 1983. pp. 795–813. [Google Scholar]
- Marker J, Cryer P, Clutter W. Simplified measurement of norepinephrine kinetics: application to studies of aging and exercise training. American Journal of Physiology. 1994;267:E380–387. doi: 10.1152/ajpendo.1994.267.3.E380. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Sugiyama Y, Mano T. Age-related changes in baroreflex control of heart rate and sympathetic nerve activity in healthy humans. Journal of the Autonomic Nervous System. 1996;60:209–212. doi: 10.1016/0165-1838(96)00057-4. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Rajkumar C, Jennings G, Esler M. Norepinephrine spillover at rest and during submaximal exercise in young and old subjects. Journal of Applied Physiology. 1997;82:1869–1874. doi: 10.1152/jappl.1997.82.6.1869. [DOI] [PubMed] [Google Scholar]
- Morlin C, Wallin BG, Eriksson BM. Muscle sympathetic activity and plasma noradrenaline in normotensive and hypertensive man. Acta Physiologica Scandinavica. 1983;118:117–121. doi: 10.1111/j.1748-1716.1983.tb07315.x. [DOI] [PubMed] [Google Scholar]
- Morrow LA, Linares OA, Hill TJ, Sanfield JA, Supiano MA, Rosens SG, Halter JB. Age differences in the plasma clearance mechanisms for epinephrine and norepinephrine in humans. Journal of Clinical Endocrinology and Metabolism. 1987;65:508–511. doi: 10.1210/jcem-65-3-508. [DOI] [PubMed] [Google Scholar]
- Ng A, Johnson D, Callister R, Seals D. Muscle sympathetic nerve activity during postural change in healthy young and older adults. Clinical Autonomic Research. 1995;5:57–60. doi: 10.1007/BF01845500. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. American Journal of Physiology. 1994;267:H344–353. doi: 10.1152/ajpheart.1994.267.1.H344. [DOI] [PubMed] [Google Scholar]
- Palmer GJ, Zeigler MG, Lake CR. Response of norepinephrine and blood pressure to stress increases with age. Journals of Gerontology. 1978;33:482–487. doi: 10.1093/geronj/33.4.482. [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Gardner AW, Goran MI, Arciero PJ, Toth MJ, Ades PA, Calles-Escandon J. Sympathetic nervous system activity, body fatness, and body fat distribution in younger and older males. Journal of Applied Physiology. 1995;78:802–806. doi: 10.1152/jappl.1995.78.3.802. [DOI] [PubMed] [Google Scholar]
- Roberts J, Tumer N. Age-related changes in autonomic function of catecholamines. Reviews in Biological Research in Aging. 1987;3:257–298. [Google Scholar]
- Rowe JW, Troen BR. Sympathetic nervous system and aging in man. Endocrine Reviews. 1980;1:167–179. doi: 10.1210/edrv-1-2-167. [DOI] [PubMed] [Google Scholar]
- Rubin PC, Scott PJW, Mclean K, Reid JL. Noradrenaline release and clearance in relation to age and blood pressure in man. European Journal of Clinical Investigation. 1982;12:121–125. doi: 10.1111/j.1365-2362.1982.tb00948.x. [DOI] [PubMed] [Google Scholar]
- Schoenberger JA. Epidemiology of systolic and diastolic systemic blood pressure elevation in the elderly. American Journal of Cardiology. 1986;57:45–51C. doi: 10.1016/0002-9149(86)91026-x. [DOI] [PubMed] [Google Scholar]
- Schwartz R, Jaeger L, Veith R. The importance of body composition to the increase in plasma norepinephrine appearance rate in elderly men. Journals of Gerontology. 1987;42:546–551. doi: 10.1093/geronj/42.5.546. [DOI] [PubMed] [Google Scholar]
- Seals DR. Influence of aging on autonomic-circulatory control in humans at rest and during exercise. In: Gisolfi CV, Lamb DR, editors. Perspectives in Exercise Science and Sports Medicine: Exercise, Heat and Thermoregulation. Vol. 6. Dubuque, Iowa: Brown & Benchmark; 1993. pp. 257–304. [Google Scholar]
- Seals DR, Taylor JA, Ng AV, Esler MD. Exercise and aging: autonomic control of the circulation. Medicine and Science in Sports and Exercise. 1994;26:568–576. [PubMed] [Google Scholar]
- Shimada K, Kitazumi T, Sadakane N, Ogura H, Ozawa T. Age-related changes of baroreflex function, plasma norepinephrine, and blood pressure. Hypertension. 1985;7:113–117. doi: 10.1161/01.hyp.7.1.113. [DOI] [PubMed] [Google Scholar]
- Sowers JR, Rubenstein LZ, Stern N. Plasma norepinephrine responses to posture and isometric exercise increase with age in the absence of obesity. Journals of Gerontology. 1983;38:315–317. doi: 10.1093/geronj/38.3.315. [DOI] [PubMed] [Google Scholar]
- Stromberg JS, Linares OA, Supiano MA, Smith MJ, Foster AH, Halter JB. Effect of desipramine on norepinephrine metabolism in humans: interaction with aging. American Journal of Physiology. 1991;261:R1484–1490. doi: 10.1152/ajpregu.1991.261.6.R1484. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. The Journal of Physiology. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Davy KP, Seals DR. Cardiopulmonary baroreflex inhibition of sympathetic nerve activity is preserved with age in healthy humans. The Journal of Physiology. 1999;515:249–254. doi: 10.1111/j.1469-7793.1999.249ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Hand GA, Johnson DG, Seals DR. Sympathoadrenal-circulatory regulation during sustained isometric exercise in young and older men. American Journal of Physiology. 1991;261:R1061–1069. doi: 10.1152/ajpregu.1991.261.5.R1061. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Hand GA, Johnson DG, Seals DR. Augmented forearm vasoconstriction during dynamic exercise in healthy older men. Circulation. 1992a;86:1789–1799. doi: 10.1161/01.cir.86.6.1789. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Hand GA, Johnson DG, Seals DR. Sympatho-circulatory regulation of arterial pressure during orthostatic stress in young and older men. American Journal of Physiology. 1992b;263:R1147–1155. doi: 10.1152/ajpregu.1992.263.5.R1147. [DOI] [PubMed] [Google Scholar]
- Veith R, Featherstone J, Linares O, Halter J. Age differences in plasma norepinephrine kinetics in humans. Journals of Gerontology. 1986;41:319–324. doi: 10.1093/geronj/41.3.319. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Annual Review of Physiology. 1988;50:565–576. doi: 10.1146/annurev.ph.50.030188.003025. [DOI] [PubMed] [Google Scholar]
- Weidman P, Beretta-Piccoli C, Ziegler W, Keusch G, Gluck Z, Reubi F. Age versus urinary sodium for judging renin, aldosterone and catecholamine levels: studies in normal subjects and patients with essential hypertension. Kidney International. 1978;14:619–628. doi: 10.1038/ki.1978.171. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Miyajima E, Tochikubo O, Matsukawa T, Ishii M. Age-related changes in muscle sympathetic nerve activity in essential hypertension. Hypertension. 1989;13:870–877. doi: 10.1161/01.hyp.13.6.870. [DOI] [PubMed] [Google Scholar]
- Young J, Rowe J, Pallotta J, Sparrow D, Landsberg L. Enhanced plasma norepinephrine response to upright posture and oral glucose administration in elderly human subjects. Metabolism. 1980;29:532–539. doi: 10.1016/0026-0495(80)90078-5. [DOI] [PubMed] [Google Scholar]
- Ziegler MG, Lake CR, Kopin IJ. Plasma noradrenaline increases with age. Nature. 1976;261:333–335. doi: 10.1038/261333a0. [DOI] [PubMed] [Google Scholar]


