Abstract
Hyaluronan (HA), an anionic polysaccharide of synovial fluid, attenuates fluid loss from joints as joint pressure is raised (‘outflow buffering’). The buffering is thought to depend on the expanded molecular domain of the polymer, which causes reflection by synovial extracellular matrix, leading to flow-dependent concentration polarization. We therefore assessed the effects of polysaccharides of differing average molecular volume and charge.
Trans-synovial fluid drainage(Q̇s) was measured at controlled joint fluid pressure (Pj) in knees of anaesthetized rabbits. The joints were infused with polydisperse HA of weight-average mass 2100 kDa (4 mg ml−1, n = 17), with polydisperse neutral dextran of similar average mass (2000 kDa; n = 7) or with Ringer solution vehicle (n = 2). The role of polymer charge was assessed by infusions of neutral or sulphated dextran of average molecular mass 500 kDa (n = 6).
When HA was present, Q̇s increased little with pressure, forming a virtual plateau of ∼4 μl min−1 from 10 to 25 cmH2O. Neutral dextran 2000 failed to replicate this effect. Instead, Q̇s increased steeply with Pj, reaching eight times the HA value by 20 cmH2O (P = 0.0001, ANOVA). Dextran 2000 reduced flows in comparison with Ringer solution.
Analysis of the aspirated joint fluid showed that 31 ± 0.07 % (s.e.m.) of dextran 2000 in the filtrand was reflected by synovium, compared with ≥ 79 % for HA. The viscometric molecular radius of the dextran, ∼31 nm, was smaller than that of HA (101–181 nm), as was its osmotic pressure.
Anionic dextran 500 failed to buffer fluid drainage, but it reduced fluid escape and synovial conductance dQ̇s/dPj more than neutral dextran 500 (P < 0.0001, ANOVA). The anionic charge increased the molecular volume and viscosity of dextran 500.
The results support the hypothesis that polymer molecular volume influences its reflection by interstitial matrix and outflow buffering. Polymer charge influences flow through an effect on viscosity and possibly electrostatic interactions with negatively charged interstitial matrix.
Hyaluronan is a polydisperse, linear, unbranched anionic polysaccharide with an average mass of 2000–7000 kDa and a concentration of 2–4 mg ml−1 in human and rabbit synovial fluids. The chain comprises alternating N-acetyl-D-glucosamine and D-glucuronic acid residues joined by β(1–4) and β(1–3) linkages, respectively (Fraser & Laurent, 1996). Hyaluronan contributes to joint lubrication (Mabuchi et al. 1994), influences synoviocyte cell biology (Strachan et al. 1990) and has a major effect on fluid kinetics. Regarding this last effect, synovial fluid hyaluronan greatly attenuates the escape of fluid from joints through the interstitial spaces in the synovial lining, producing a virtual plateau in the pressure-flow relation (McDonald & Levick, 1995). Hyaluronan achieves this effect by creating a graded opposition to outflow that increases with joint pressure, a process referred to here as ‘outflow buffering’. We have previously suggested that outflow buffering is due primarily to the expanded, highly hydrated molecular domain of hyaluronan. The domain is so large as to cause a substantial fraction of the hyaluronan molecules in the filtrand (the fluid filtering out of the joint cavity across the synovial lining) to be reflected by synovial extracellular matrix (Scott et al. 1998), as it is by other relatively coarse filters (Parker & Winlove, 1984; Barry et al. 1996; Fraser & Laurent, 1996). Ultrafiltration theory predicts that the resulting concentration polarization at the unstirred tissue-fluid interface will raise the local osmotic pressure and thus create a dynamic, graded opposition to outflow (Coleman et al. 1999). The physiological importance of the process lies in preventing excessive loss of synovial fluid from the joint cavity during a period of sustained flexion.
A key feature of the above hypothesis is that outflow buffering should depend on molecular size. Dependence on average molecular mass has been confirmed experimentally using sonicated hyaluronan molecules of reduced chain length; the buffering of drainage was lost when average molecular mass was reduced to 300 kDa, which corresponds to a radius of gyration (Rg) of 33 nm (Coleman et al. 2000). Although we suggested that molecular domain volume rather than mass itself was likely to be the critical molecular parameter, the two parameters are linked and changed in parallel in the above investigation.
The primary aim of the present study was to test the hypothesis that the hydrated domain volume rather than molecular mass is a critical molecular parameter for outflow buffering. To this end we compared the ability of hyaluronan and dextran of similar average molecular mass, ∼2000 kDa, to buffer trans-synovial outflow. Dextran 2000, a product of Leuconostoc mesenteroides, is a polydisperse α-polyglucosan, i.e. a glucose polysaccharide formed primarily by α (1–6) glycosidic bonds. Although it has a similar average molecular mass to rabbit hyaluronan, dextran 2000 differs fundamentally in chain structure due to multiple branch points formed by non-α (1–6) links at ∼9–20 monosaccharide intervals (Wales et al. 1953; Senti et al. 1955). As a result of the extensive branching, dextran 2000 has a smaller, less highly hydrated molecular domain than hyaluronan (Ogston & Woods, 1954; Granath, 1958; Bohrer et al. 1979).
Dextrans also differ from hyaluronan in electrical charge. Hyaluronan is a polyanion at physiological pH due to an ionized carboxyl group on each disaccharide, whereas native dextrans are neutral (Lanken et al. 1985). A modified, anionic (sulphated) form of dextran is available, however, of average molecular mass 500 kDa. To assess the role of polysaccharide negative charge in outflow buffering, trans-synovial flows were compared in the presence of sulphated dextran 500 and neutral dextran 500. We have shown previously that hyaluronan of ∼530 kDa average molecular mass produces outflow buffering and quasi-plateau formation (McDonald & Levick, 1995; Coleman et al. 2000).
METHODS
Physiological methods in vivo
Fluid drainage from the cavity of rabbit knees (trans-synovial flow, Q̇s) was measured at controlled, incremental intra-articular fluid pressures (Pj) in the presence of 4 mg ml−1 neutral dextran 2000 (mass 2000 kDa) (n = 6 joints) or 4 mg ml−1 hyaluronan of molecular mass 2100 kDa (n = 17 joints), a concentration occurring in normal rabbit synovial fluid (Price et al. 1996). The hyaluronan experiments were a subset of those reported by Coleman et al. (1999). In four of the six rabbits receiving dextran 2000, the contralateral knee was infused with hyaluronan solution, and in the other two rabbits the contralateral knee was infused with Ringer solution. A seventh experiment with dextran 2000 was carried out at a higher concentration, 10 mg ml−1.
In a separate series of six animals, sulphated dextran 500 at 4 mg ml−1 was studied in one knee, and neutral dextran 500 at 4 mg ml−1 in the opposite knee.
Measurement of intra-articular pressure and trans-synovial drainage rate
New Zealand White rabbits weighing 2–3 kg were anaesthetized with 30 mg kg−1 sodium pentobarbitone plus 500 mg kg−1 urethane i.v. and tracheostomized. Anaesthesia of sufficient depth to abolish the corneal blink reflex was maintained by 15 mg sodium pentobarbitone plus 250 mg urethane i.v. every 30 min. The infusion and recording systems were described by Coleman et al. (1999). Briefly, an intra-articular cannula was connected to a water-calibrated differential pressure transducer to measure joint fluid pressure (Pj). A second intra-articular cannula was connected to an infusion reservoir, the height of which controlled Pj. Flow from the reservoir into the joint cavity, Q̇in, was recorded by a photoelectric drop counter. Sustained absorption rates became measurable at ∼2–3 cmH2O. From this point, Pj was increased in steps of ∼2–4 cmH2O by raising the infusion reservoir at 30–60 min intervals, at which times the flows are in a steady state. Net trans-synovial drainage rate, Q̇s, was calculated from Q̇in at the end of each period by subtracting the known volumetric creep of the cavity walls, i.e. the slight increase in joint volume with time caused by viscous creep of the cavity walls at constant pressure, as described by Coleman et al. (1999). Experiments continued to ∼24 cmH2O, which corresponds to a taut arthritic effusion. Procedures conformed to UK legislation and animals were killed by an overdose of i.v. sodium pentobarbitone at the end of the experiment.
Measurement of polysaccharide reflection by the synovial lining
At the end of three experiments with dextran 2000 the intra-articular fluid was mixed by a series of flexion-extension cycles and samples aspirated from the joint cavity and infusion line. Polysaccharide concentrations were determined by high performance liquid chromatography (HPLC; see below). The dextran 2000 reflected fraction, i.e. (mass in filtrand — mass in filtrate)/mass in filtrand, was calculated from the increase in the intra-articular concentration and cumulative fluid drainage during the experiment as described by Scott et al. (1998), who used this approach to determine hyaluronan reflected fractions.
Biochemical and biophysical methods
Materials
Rooster comb hyaluronan and dextrans (Sigma, UK) were dissolved in Baxter Ringer solution (147 mM Na+, 4 mM K+, 2 mM Ca2+, 156 mM Cl−, pH 7.2; Baxter Healthcare Ltd, Thetford, Norfolk, UK) and adjusted to pH 7.4 with drops of NaOH solution. Rooster hyaluronan had an average molecular mass of 2.1 × 106 Da by size-exclusion gel chromatography and its polydispersity index, i.e. the ratio of weight-average molecular mass Mw to number-average molecular mass Mn (0.97 × 106 Da), was 2.16 (Coleman et al. 1999). The nominal weight-average molecular masses for the commercial dextrans were 2000 kDa, 464 kDa (neutral dextran 500) and 500 kDa (sulphated dextran 500). The dextran polydispersity index is reported to be 1.25–1.50 (Mariani et al. 1955; Gribbon & Hardingham, 1998; Gribbon et al. 1999). Our estimate for dextran 2000 (Lot 96H1191) was 1.56, with Mn calculated from the measured osmotic first virial coefficient (0.0197 cmH2O l g−1) and Mw as specified by the supplier.
Polysaccharide analysis by HPLC
Dextran concentrations were determined by HPLC (Waters Ltd, Watford, UK) using the same column as for hyaluronan, namely a size exclusion TosoHaas TSK G6000 PWXL column (Anachem Ltd, Luton, UK). The column was calibrated with known concentrations of dextran, the absorbance of which was measured at 206 nm. The retention time of dextran 2000 in the exclusion column was considerably longer than that for rooster hyaluronan, despite their nearly identical molecular masses, indicating a substantial difference in average molecular volume (see Results).
Viscometric studies of molecular domain volume, radius Rg and critical concentration for molecular overlap
The relative viscosities (ηr) of dextran solutions over the range 1–10 mg ml−1 were measured in an Ostwald viscometer at 23–26°C with Ringer solution as the reference fluid. Intrinsic viscosity [η] was determined by linear extrapolation of a plot of the logarithm of reduced viscosity, log ((ηr − 1)/C), versus concentration C to zero concentration. Intrinsic viscosity is the space occupied by a gram of solute at infinite dilution, and is thus a function of the average molecular domain volume (Flory, 1971; Boyd & Phillips, 1993).
The space occupied by a flexible polymer chain in solution is conventionally characterized by an equivalent sphere with a radius of gyration Rg; Rg is the root mean square of segment distances from the molecular centre of gravity (Boyd & Phillips, 1993). An average Rg for a polydisperse polymer can be estimated from [η] and molecular mass M using the relation Rg3 = [η] M/8.84NA, where NA is Avogadro's number (Flory, 1971; Boyd & Phillips, 1993). An average Rg for dextrans has also been determined by light scattering (Senti et al. 1955; Granath, 1958).
The volume domains of adjacent polymer molecules are so large that they overlap at a ‘critical concentration’ C *; above this, the entire body of the solution is spanned by a quasi-infinite network of polymer chains. C* was defined by de Gennes (1979) as the concentration at which the number of chain segments (monomers) per unit volume of solution equals the number of chain segments n per unit volume of molecular domain at extreme dilution. Thus C* equals n/(4/3)πRg3. Alternatively, the onset of physically significant interactions between adjacent molecules can be determined experimentally, because it causes a sharp steepening of the relation between the logarithms of specific viscosity and concentration (Morris et al. 1980; DeSmedt et al. 1993). The concentration at which the steepening occurs, C**, is often higher than C* in practice. In the present study the measured viscosities of dextran solutions of 1–10 mg ml−1 were used to evaluate C**. Results for rooster comb hyaluronan C* and C** have been published previously (Scott et al. 2000).
Osmometry
The osmotic pressures of 4–20 mg ml−1 solutions of dextran 2000, neutral dextran 500 and sulphated dextran 500 in phosphate-buffered saline at pH 7.4 were measured using an Amicon PM30 membrane (Amicon, Lexington, USA) of nominal exclusion 30 kDa. The electronic osmometer and results for hyaluronan have been described previously (McDonald & Levick, 1995; Coleman et al. 1999).
Statistical methods
To enable the comparison of flows at identical pressures (since Pj varied a little between experiments), flows were interpolated to standard pressures at 2.5 cmH2O intervals by linear interpolation between the two bounding measurements. Flow-pressure sets were compared by two-way analysis of variance (ANOVA), with repeated measures and Bonferroni's post hoc test as appropriate. Slopes were fitted by linear regression analysis. Two regression slopes were compared by analysis of covariance (ANCOVA) as implemented in Graphpad Prism (Zar, 1984). Comparison of a regression slope and null hypothesis slope (e.g. unity, Fig. 2) was made by calculation of Student's t (Bailey, 1959). P < 0.05 was accepted as a significant difference. Means and slopes are followed by their standard errors throughout.
Figure 2. Paired measurements of the interstitial drainage of Ringer solution and 4 mg ml−1 dextran 2000 solution at equal pressure in contralateral joints of the same two animals.
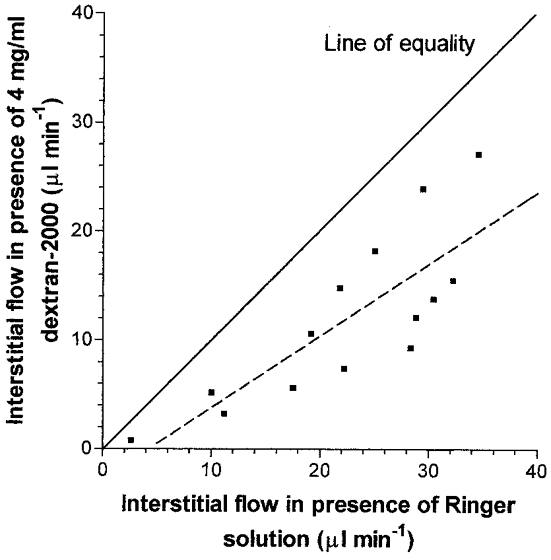
Pairs of measurements were made at the same pressure over the range 2–20 cmH2O. The slope of the dashed regression line, 0.66 ± 0.14, is significantly less than 1, which represents the line of equality (P < 0.05, Student's t test).
RESULTS
Effect of dextran 2000 compared with hyaluronan
Figure 1 compares the pressure-flow relation for the interstitial drainage pathway in the presence of 4 mg ml−1 dextran 2000 and 4 mg ml−1 hyaluronan. It is clear from these results that polysaccharide structure had a substantial effect on fluid drainage (P < 0.0001, two-way ANOVA). Differences in drainage rate at individual pressures were significant at ≥ 7.5 cmH2O (P < 0.05, Bonferroni's test). By 20 cmH2O the mean interstitial flow in the presence of dextran was 8.0 times greater than that in the presence of hyaluronan (P < 0.001, Bonferroni's test).
Figure 1. Effect of dextran 2000 and rooster comb hyaluronan on interstitial fluid dynamics.
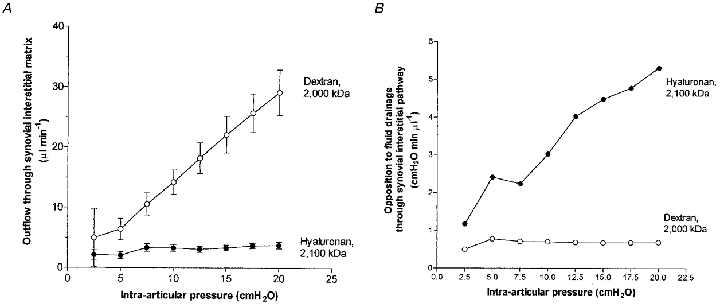
A, comparison of the effect of dextran 2000 solution (4 mg ml−1, ○, n = 6) and rooster comb hyaluronan solution (4 mg ml−1, •, n = 17) on rate of fluid drainage through the synovial interstitial pathway at controlled joint pressures (means ± s.e.m.). B, effect on opposition to flow through the interstitial pathway, calculated as (mean Pj)/(mean Qs) at each pressure.
Interstitial flow in the presence of intra-articular hyaluronan attained a virtual plateau of ∼4 μl min−1 above 7.5 cmH2O. Dextran 2000 failed to reproduce this action of hyaluronan. Instead, drainage rate increased markedly throughout the pressure range. In two joints the slope of the relation steepened at 7.5–12.5 cmH2O, and in four it did not. At pressures corresponding to the hyaluronan plateau, i.e. ≥ 7.5 cmH2O, the response of drainage to pressure elevation in the presence of dextran 2000 was 1.50 ± 0.02 μl min−1 cmH2O−1 (regression slope ± standard error for pooled results from six joints; correlation r2 = 0.999). The dextran slope was 39 times steeper than the slope for hyaluronan, which was 0.04 ± 0.02 μl min−1 cmH2O−1 over the same pressure range by regression analysis of pooled results from 17 joints. The difference in slope was significant (P < 0.0001, ANCOVA).
In one experiment the concentration of infused dextran 2000 was raised to 10 mg ml−1 and the pressure range extended to 50 cmH2O. The pressure-flow relation showed no tendency towards convexity or a plateau, even at the higher concentration and pressures.
The opposition to fluid drainage, calculated as the intra-articular pressure required to drive unit trans-synovial flow, i.e. Pj/Q̇s, is of interest because it depends on the effective osmotic pressure across the membrane and the interstitial fluid viscosity as well as the intrinsic membrane resistance (Coleman et al. 1999). For dextran 2000 the mean opposition to outflow was relatively insensitive to pressure, falling slightly from 0.78 cmH2O min μl−1 at 5 cmH2O to 0.69 cmH2O min μl−1 at 20 cmH2O. For hyaluronan, however, the mean opposition was very sensitive to pressure and increased 4.5-fold from 1.17 cmH2O min μl−1 at the lowest pressure to 5.31 cmH2O min μl−1 by 20 cmH2O. We refer to this change as the ‘outflow buffering’ effect of hyaluronan (see Introduction).
Effect of dextran 2000 compared with Ringer solution
The steep, often concave (steepening) relation between the trans-synovial interstitial flow of Ringer solution and the driving pressure is well known (Edlund, 1949; for review, see Levick et al. 1999). In the two joints infused with Ringer solution in the present paired study, the drainage rate again increased steeply with pressure and averaged 33 μl min−1 at 20 cmH2O. In the paired, contralateral knees containing dextran 2000 the flow increased less steeply with pressure and averaged 21 μl min−1 at 20 cmH2O. As shown in Fig. 2, dextran 2000 reduced the interstitial flow at matched pressures in all 14 measurements (P < 0.001, Student's paired t test) and the plot of dextran flow versus Ringer solution flow had a slope of 0.66 ± 0.14.
Reflection of dextran 2000 by synovial interstitium
Analysis of fluid taken from the joint cavity and infusion line at the end of three experiments showed that the dextran concentration in the joint cavity increased in all three cases, from 4.0 to 5.6, 6.0 and 7.0 mg ml−1, respectively (mean 6.2 ± 0.4 mg ml−1). There was little change in the mean retention times of the chromatograms (infusate 7.97 ± 0.02 min, aspirate 8.01 ± 0.02 min). From the increase in concentration and cumulative fluid drainage, the reflected fraction for dextran 2000 was calculated to be 0.31 ± 0.07 (see Methods).
Effect of neutral versus negatively charged dextran 500 on interstitial flow
Even when its molecular mass is reduced to ∼530 kDa, the polyanion hyaluronan causes outflow buffering, with a quasi-plateau of 8–10 μl min−1 (Fig. 3). To test whether the addition of fixed anionic sites to dextran 500 might cause it to reproduce the action of hyaluronan, Q̇s-Pj relations were determined in six animals where one joint received 4.0 mg ml−1 sulphated dextran 500 and the contralateral joint received neutral dextran 500. The anionic dextran 500 failed, however, to generate buffering of outflow. The interstitial drainage rate increased steeply with pressure throughout the pressure range in the presence of anionic dextran (Fig. 3).
Figure 3. Effect of anionic versus neutral polysaccharide on interstitial flow (means ± s.e.m.).
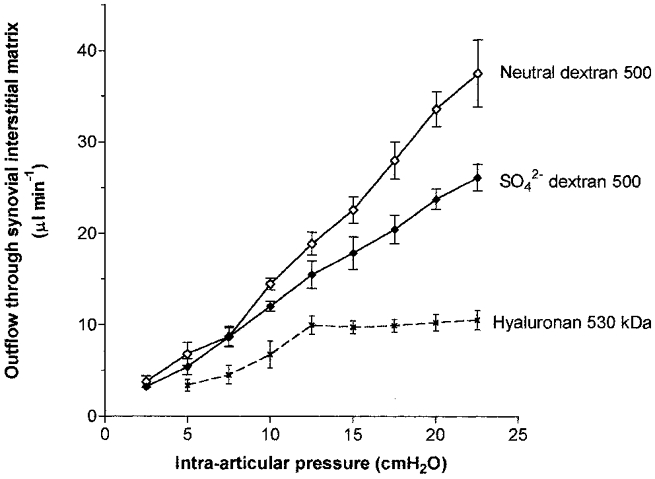
The results for neutral dextran 500 (◊) and sulphated dextran 500 (♦) from the present study can be compared with those for hyaluronan of mass 530 kDa (×) from Coleman et al. (2000). All three solutes were infused at 4 mg ml−1.
The anionic dextran 500 reduced interstitial drainage rate to a greater extent, however, than neutral dextran 500. For example, the drainage rate with sulphated dextran at 22.5 cmH2O averaged 26.2 ± 1.4 μl min−1 in contrast to 37.6 ± 3.7 μl min−1 with neutral dextran 500. The depression of flow by polymer charge was significant (P < 0.0001, n = 6, two-way ANOVA) and differences at individual pressures reached significance at > 15 cmH2O (P < 0.01, Bonferroni's test). The anionic charge reduced the slope of the relation, dQs/dPj, by 33 %, from 1.75 ± 0.07 μl min−1 cmH2O−1 with neutral dextran 500 to 1.17 ± 0.02 μl min−1 cmH2O−1 with sulphated dextran 500 (regression analyses of pooled results from six pairs of joints). The difference in slope was significant (P < 0.0001, ANCOVA). Moreover, the reduction in slope associated with the negative charge was greater than could be accounted for by the difference in the viscosity of the dextran solutions (see below).
An unpaired comparison can be made between the effect of neutral dextran 2000 (n = 6 joints) and neutral dextran 500 (n = 6 joints). Although the drainage rates at high pressures were a little lower in animals infused with neutral dextran 2000 than in those infused with neutral dextran 500, the overall effect of dextran molecular mass did not reach statistical significance in these unpaired experiments (P = 0.23, two-way ANOVA).
Viscosity and effective molecular size of dextrans compared with hyaluronans of similar mass
Figure 4 contrasts the viscous properties of dextrans and hyaluronan. The relative viscosity of 4 mg ml−1 dextran 2000 was 1.4, in contrast to 65 for 4 mg ml−1 rooster comb hyaluronan at a shear rate of 70 s−1. The intrinsic viscosity of dextran 2000 was 76 ml g−1 (95 % confidence interval, 69–83 ml g−1), which is similar to values of 72–74 ml g−1 reported by Ingleman & Halling (1950) and Senti et al. (1955). The intrinsic viscosity of dextran 2000 was much smaller than that of the rooster hyaluronan, namely 2976 ml g−1 (95 % confidence interval, 2503–3537 ml g−1), despite the close similarity of the molecular masses. Viscometry thus confirmed that dextran 2000 occupies a smaller average molecular domain than hyaluronan. Given the almost identical molecular masses, the cube root of [η] indicates that the average radius of the dextran domain is ∼0.3 times that of the hyaluronan. This is in agreement with the average radii of gyration, namely 31 nm for dextran 2000 as determined here by viscometry (see Methods), or ∼34 nm by light scattering (Senti et al. 1955; Granath, 1958), in comparison with 101–181 nm for rooster hyaluronan (Coleman et al. 1999).
Figure 4. Viscous and osmotic properties of the neutral and anionic polysaccharides infused into joints.
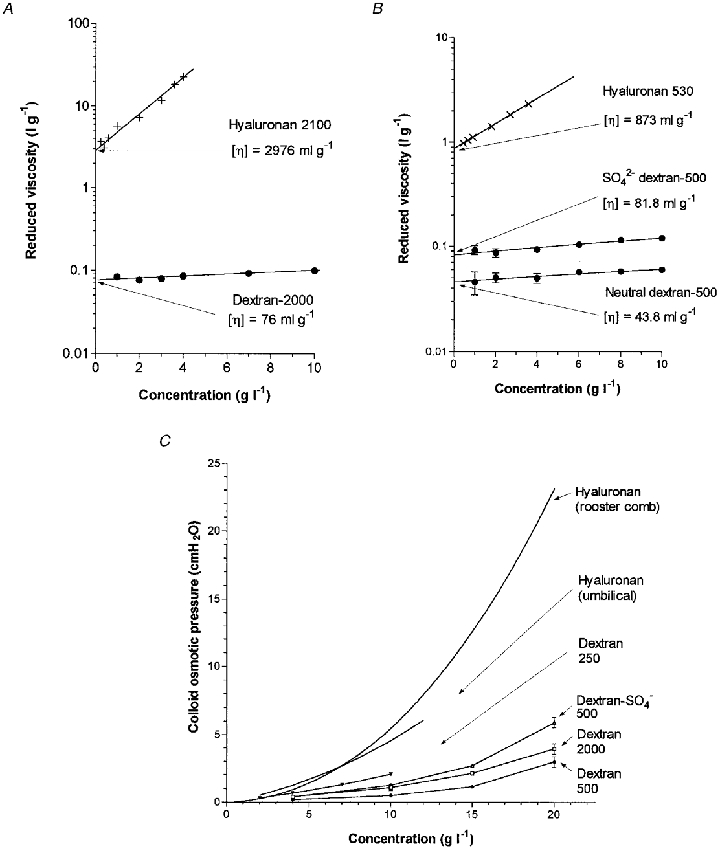
A, reduced viscosities, i.e. (relative viscosity − 1)/concentration, as a function of concentration for dextran 2000. Intrinsic viscosity [η] is given by the intersection at zero concentration (linear regression analysis). Results for rooster hyaluronan of similar molecular mass from Coleman et al. (1999) are reproduced for comparison. B, comparison of viscosities of sulphated and neutral dextran 500. Results for shortened hyaluronan chains of similar molecular mass from Coleman et al. (2000) are included for comparison. C, osmotic pressures of neutral dextran 2000, neutral dextran 500 and sulphated dextran 500 in phosphate-buffered saline at room temperature. Curves for neutral dextran 250 (Mariani et al. 1955), rooster hyaluronan (Coleman et al. 1999) and umbilical hyaluronan (McDonald & Levick, 1995) are also shown.
The HPLC chromatograms (Fig. 5) provided additional evidence for a substantial difference in polymer domain size. The exclusion column retention time for dextran 2000 was 7.95 ± 0.01 min (n = 22), which is substantially longer than for rooster hyaluronan, 7.24 ± 0.01 min (n = 60, P < 0.001, Student's unpaired t test). The chromatogram distributions were similar for the two solutes, indicating that their degrees of polydispersity did not differ substantially, in agreement with their polydispersity indices (see Materials).
Figure 5. Exclusion-column chromatograms of rooster comb hyaluronan and dextran 2000 at 206 nm.
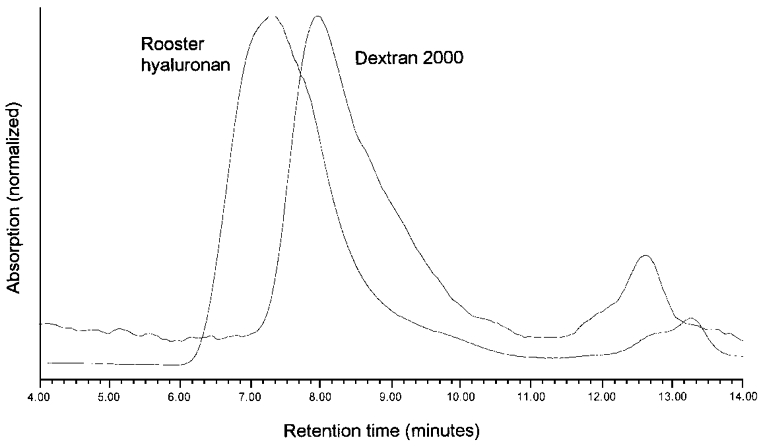
Peak heights have been normalized. The peak retention times were 7.28 and 7.92 min, respectively. The peak profiles show no major difference in polydispersity.
The viscometric results also showed that the sulphation of dextran substantially increases its molecular volume (Fig. 4B). The relative viscosity of 4 mg ml−1 of sulphated dextran 500, 1.373 ± 0.009 (n = 5), was 15 % bigger than that of neutral dextran 500, 1.199 ± 0.020 (n = 5). The intrinsic viscosity of sulphated dextran 500, 81.8 ml g−1 (95 % confidence interval, 74.1–90.6 ml g−1, n = 5), was almost twice that of neutral dextran 500, 43.8 ml g−1 (95 % confidence interval, 36.8–52.0 ml g−1, n = 5). The latter is close to a value of 45 ml g−1 reported by Senti et al. (1955). Rg for neutral dextran 500 is 18–19 nm by light scattering (Senti et al. 1955) and 16 nm by calculation from the present intrinsic viscosity (see Methods). Rg for sulphated dextran 500 was 20 nm as calculated from intrinsic viscosity.
Critical overlap concentration C*
The relation between the logarithm of specific viscosity and the logarithm of concentration was linear for dextrans over the range 1–10 mg ml−1, and lacked the sharp upturn that indicates the onset of intermolecular coupling. This upturn occurs at ∼1 mg ml−1 with rooster hyaluronan solution (Scott et al. 2000). In keeping with the linearity of the dextran plot, the concentration at which dextran 2000 domains first touch, calculated as the reciprocal of the measured intrinsic viscosity, is 13 mg ml−1, while de Gennes' C* is still higher, namely 20 mg ml−1 for Rg = 34 nm (see Methods). Thus the dextran solutions were in the dilute regime, in which adjacent molecular domains do not overlap, while rooster hyaluronan at the same concentration was in the semi-dilute regime, where adjacent molecular domains overlap. The overlap status of the polymer appears to influence the degree of reflection (Scott et al. 1998), which in turn influences the degree of concentration polarization (Coleman et al. 1999; Scott et al. 2000); see Discussion.
Osmotic pressure of dextran solutions
The osmotic pressures of the infused solutions of dextran 2000, dextran 500 and sulphated dextran 500 (4 mg ml−1) were very low, namely 0.4, 0.2 and 0.4 cmH2O, respectively. The osmotic pressures of rooster hyaluronan and umbilical hyaluronan at 4 mg ml−1 are also low, namely 1.0 and 1.2 cmH2O, respectively (McDonald & Levick, 1995; Coleman et al. 1999). Both dextran and hyaluronan show non-linear increases in osmotic pressure with concentration, but the hyaluronan curves were considerably steeper than the dextran curves; the osmotic pressure of rooster hyaluronan reached 23.5 cmH2O at 20 mg ml−1 whereas the osmotic pressures of dextran 2000, dextran 500 and sulphated dextran 500 at 20 mg ml−1 were only 4.0, 3.0 and 5.9 cmH2O, respectively (Fig. 4C).
DISCUSSION
The principal physiological findings were that dextran, whether neutral or anionic, failed to generate outflow buffering, whereas hyaluronan of similar average molecular mass buffered interstitial flow strongly, and that anionic dextran reduced interstitial flow to a greater degree than neutral dextran of similar molecular mass. These findings are discussed in turn.
Role of molecular domain volume in buffering interstitial flow
Taken in conjunction with previous experiments using hyaluronans of different chain lengths (Coleman et al. 2000), the results show that a polysaccharide mass of ≥ 500 kDa is a necessary but not sufficient condition for the buffering of flow through synovial interstitium. To reiterate, ‘buffering’ is defined as the generation of an opposition to flow that increases with pressure. The results in vitro showed that there are substantial differences in average molecular domain volume and osmotic pressure (but not polydispersity) between hyaluronan and dextran, and these probably underlie the different effects on interstitial flow, as follows.
The small size of the dextran 2000 molecular domain relative to hyaluronan, confirmed here by viscometry and the exclusion column, is due primarily to chain branching and secondarily to chain electroneutrality. Theory predicts that, due to their smaller domain volume, branched chains will permeate small voids more freely than flexible linear chains of equal molecular mass (Casassa, 1967). In keeping with this, only 31 % of dextran 2000 molecules in the filtrand were reflected, whereas 79–95 % of hyaluronan molecules of similar molecular mass are reflected (Scott et al. 1998). The low fractional reflection of dextran 2000 by the synovial interstitial matrix will weaken the process of local concentration polarization that is thought to underlie the buffering of outflow (Coleman et al. 1999). Indeed, the failure of dextran 2000 to buffer outflow is similar to the failure of hyaluronan fragments of reduced domain size. The radius of gyration of dextran 2000, namely 31–34 nm, and the reflected fraction of 31 % are almost identical to those of 300 kDa hyaluronan (33 nm radius, 33 % reflected fraction), which likewise fails to buffer interstitial flow (Coleman et al. 2000). It is concluded, therefore, that polymer domain volume rather than molecular mass per se is the primary factor governing translational interactions between a polymer and the synovial interstitium, and hence the ability to buffer outflow.
In addition, differences in chain flexibility and domain shape may contribute to the different effects of dextran and hyaluronan in vivo. The dextran chain is highly flexible, with an estimated distance of ≤ 1 nm between flexion sites (Granath, 1958), whereas the hyaluronan chain is greatly stiffened by hydrogen bonds (Gribbon et al. 1999) and has a segment persistence length of 4 nm (Cleland, 1977). The shape of the hyaluronan domain is approximately spherical, while that of dextran is a rod-like elongated ellipsoid at small molecular masses and increasingly spherical as molecular mass increases (Granath, 1958); its axial ratio is ≤ 4.5 at 530 kDa (Ogston & Woods, 1954). Both flexibility and asymmetry facilitate the permeation of a polymer through narrow pores (Preston & Snowdon, 1973; Munch et al. 1979). For example, dextrans of 9–33 kDa permeate the glomerular basement membrane more easily than Ficoll, an almost spherical polymer of sucrose and epichlorohydrin, for the same Stokes-Einstein radius (Bohrer et al. 1979). Analogous findings have been reported for artificial porous systems (Laurent et al. 1975; Schultz et al. 1979).
If the osmotic polarization hypothesis is correct, the difference in osmotic pressure curves for dextran and hyaluronan must be a further factor influencing their buffering ability. Because osmotic pressure increases very steeply with hyaluronan concentration, a moderate concentration polarization of hyaluronan generates osmotic pressures that approach intra-articular pressure in magnitude. A far greater degree of concentration polarization is necessary to raise dextran osmotic pressure to a comparable level (Fig. 4C).
Effective interstitial pore size in relation to polymer radius
Polymer reflection and concentration polarization depend not only on the polymer properties but also on the porosity of the interstitial matrix. The strand-like interstitial matrix elements, namely the glycosaminoglycan chains, glycoproteins and microfibrils, transect the void volume (water space) and create narrow spaces that partially reflect the solute. The spaces are probably irregular, but it is of interest to assess their characteristic scale. A simple approach is to ask ‘If the matrix spaces were replaced by uniform cylindrical pores perforating an otherwise impermeable membrane, what pore radius would produce a similar reflection?’
The formalism for the reflection of a solid spherical solute of Einstein hydrodynamic radius Re by a cylindrical pore is given by Curry (1984). The radius of a solid sphere whose hydrodynamic frictional property equals that of the larger, hydrated domain of a polymer is given by Einstein's viscosity relation Re3 = [η] M/2.5 NA (4π/3) (Munch et al. 1979). Re is 29 nm for dextran of 2000 kDa. Substitution of Re= 29 nm and reflection coefficient σ ≥ 0.31 into the pore equations 5.22 and 5.80 of Curry (1984) gives an equivalent cylindrical pore radius of ≤ 87 nm. We have previously estimated an equivalent pore radius of ≤ 41–70 nm from hyaluronan reflection data in the dilute regime (Coleman et al. 1999). It is emphasized, however, that the true morphology of the interstitial voids is far more complex than that of a cylindrical pore.
As noted earlier, flexible polymers can permeate pores of smaller radius than their own unconstrained radius by partial uncoiling and reptation, i.e. a snake-like, thermodynamic wriggling motion (Munch et al. 1979). Also, reflected fractions underestimate σ at low flows. For these reasons the above estimates merely indicate the upper limit of the equivalent interstitial pore radius.
A different form of scale estimate can be derived from the hydraulic permeability of synovial matrix. The ‘mean hydraulic radius’ of the synovial matrix, namely the ratio of matrix void volume to fibre surface area, is 15–45 nm (Levick, 1987; Levick et al. 1999). The relation between this and the solute-reflecting radius derived above is a complex issue and depends on the choice of pore model.
Reduction of interstitial flow by neutral dextrans
Although dextran 2000 did not buffer fluid escape, i.e. there was no quasi-plateau of flow and no increase in opposition with pressure, it nevertheless attenuated the interstitial flow (Fig. 2). This may be due to two effects. First, the bulk-phase viscosity of the dextran solution was 40 % greater than that of Ringer solution, and may have been higher still at the tissue interface due to partial reflection and a moderate concentration polarization. A relative viscosity of 1.4 reduces the fluidity (i.e. 1/viscosity), and hence the bulk flow, to 71 % of control. This is not significantly different from the observed reduction to 66 % of control. This near agreement may be partly fortuitous, however, because partial steric exclusion within the interstitial matrix attenuates the effect of a macromolecule on fluidity (Levick, 1994; Levick & McDonald, 1994). Second, the dextran permeating the matrix will exert a small osmotic pressure around the numerous synovial capillaries, which, being fenestrated, have a high endothelial conductance (Knight & Levick, 1985; Levick & Smaje, 1987). Although perivascular, osmotic enhancement of microvascular filtration reduces the net trans-synovial drainage, the experimental results of McDonald & Levick (1993) and a related model (Levick, 1994) show that this effect is likely to be small in the present experiments, due to the low osmotic pressures of the dextran 2000 solution.
The above effects, coupled with the substantial biological variability of rabbit synovium and a very weak dextran concentration polarization effect, may explain why the pressure-flow relation in 4 of 6 joints showed no steepening with pressure, unlike saline-infused joints (Edlund, 1949).
Further reduction of interstitial flow by polymer charge
The addition of fixed negative charges to dextran 500 caused a substantial fall in interstitial flow and a 33 % reduction in slope dQ̇s/dPj when compared with neutral dextran 500 (Fig. 3). Several mechanisms may be involved. First, the negative charge increased the polymer domain volume, as shown by a rise in intrinsic viscosity from 44 to 82 ml g−1 upon sulphation. Although Bohrer et al. (1979) suggested that sulphation has little effect on the viscosity of dextrans of size 9–33 kDa, we observed a substantial increase with dextran 500, perhaps due to the greater freedom of motion of the longer chains. An increase in domain volume and hence viscosity will reduce interstitial flow. Second, the presence of fixed charges increased the osmotic pressure of sulphated dextran, presumably through the Gibbs-Donan effect on counter-ion distribution. Nevertheless, sulphated dextran osmotic pressures remained much smaller than hyaluronan osmotic pressures. Third, whereas the negative charge reduced the fluidity by 12.7 %, it reduced the regression slope dQ̇s/dPj by 32.8 %, which is more than twice the reduction attributable to the altered fluidity (difference significant; P < 0.01, Student's t test). The extra reduction may arise from the electrostatic repulsion between the negative charges on the dissolved polymer and the fixed negative charges on the stationary matrix glycosaminoglycans (Maroudas, 1975; Deen et al. 1980; Grodzinsky, 1983; Parker, 1986; Lent et al. 1987). Reduced mobility of the charged dextran relative to the flowing solvent may create additional hydraulic drag, and hence slow the interstitial flow.
To summarize, although a high molecular mass is necessary for the buffering action of a dissolved polymer on interstitial flow, it is not a sufficient condition per se; polymer conformation (domain volume and shape) and charge are also important. The relatively small effect of high molecular weight dextrans on interstitial flow provides further support for the osmotic polarization hypothesis for the action of hyaluronan.
Acknowledgments
We are grateful for the help of Ms Sharmila Sabaratnam with some of the viscometric results. The study was supported by Wellcome Trust grant 039033/Z/93.
References
- Bailey NTJ. Statistical Methods in Biology. London: Hodder & Stoughton; 1959. [Google Scholar]
- Barry SI, Gowman LM, Ethier CR. Obtaining the concentration-dependent diffusion coefficient from ultrafiltration experiments: application to hyaluronate. Biopolymers. 1996;39:1–11. [Google Scholar]
- Bohrer MP, Deen WM, Robertson CR, Troy JL, Brenner B. Influence of molecular configuration on the passage of macromolecules across the glomerular capillary wall. Journal of General Physiology. 1979;74:583–593. doi: 10.1085/jgp.74.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd RH, Phillips PJ. The Science of Polymer Molecules. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Casassa EF. Equilibrium distribution of flexible polymer chains between a macroscopic solution phase and small voids. Polymer Letters (Journal of Polymer Science Part B) 1967;5:773–778. [Google Scholar]
- Cleland RL. The persistence length of hyaluronic acid; an estimate from small-angle X-ray scattering and intrinsic viscosity. Archives of Biochemistry and Biophysics. 1977;180:57–68. doi: 10.1016/0003-9861(77)90008-x. [DOI] [PubMed] [Google Scholar]
- Coleman PJ, Scott D, Mason RM, Levick JR. Characterization of the effect of high molecular weight hyaluronan on trans-synovial flow in rabbit knees. The Journal of Physiology. 1999;514:265–282. doi: 10.1111/j.1469-7793.1999.265af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PJ, Scott D, Mason RM, Levick JR. Role of hyaluronan chain length in the buffering of trans-synovial interstitial flow. The Journal of Physiology. 2000;526:425–434. doi: 10.1111/j.1469-7793.2000.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry FE. Mechanics and thermodynamics of transcapillary exchange. In: Renkin EM, Michel CC, editors. Handbook of Physiology, The Cardiovascular System, The Microcirculation. IV. Bethesda, MD USA: American Physiological Society; 1984. pp. 309–374. section 2. [Google Scholar]
- Deen WM, Satvat B, Jamieson JM. Theoretical model for glomerular filtration of charged solutes. American Journal of Physiology. 1980;238:F126–136. doi: 10.1152/ajprenal.1980.238.2.F126. [DOI] [PubMed] [Google Scholar]
- De gennes P-G. Scaling Concepts in Polymer Physics. Ithaca and London: Cornell University Press; 1979. [Google Scholar]
- Desmedt SC, Dekeyser P, Ribitsch V, Lauwers A, Demeester J. Viscoelastic and transient network properties of hyaluronic acid as a function of concentration. Biorheology. 1993;30:31–41. [PubMed] [Google Scholar]
- Edlund T. Studies on the absorption of colloids and fluid from rabbit knee joints. Acta Physiologica Scandinavica. 1949;18(suppl. 62):1–108. [Google Scholar]
- Flory PJ. Principles of Polymer Chemistry. Ithaca, New York: Cornell University Press; 1971. [Google Scholar]
- Fraser JRE, Laurent TC. Hyaluronan. In: Comper WD, editor. Extracellular Matrix, Molecular Components and Interactions. Vol. 2. Amsterdam: Harwood Academic Publishers; 1996. pp. 141–199. [Google Scholar]
- Granath KA. Solution properties of branched dextrans. Journal of Colloidal Science. 1958;13:308–328. [Google Scholar]
- Gribbon P, Hardingham TE. Macromolecular diffusion of biological polymers measured by confocal fluorescence recovery after photobleaching. Biophysical Journal. 1998;75:1032–1039. doi: 10.1016/S0006-3495(98)77592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribbon P, Heng BC, Hardingham TE. The molecular basis of the solution properties of hyaluronan investigated by confocal fluorescence recovery after photobleaching. Biophysical Journal. 1999;77:2210–2216. doi: 10.1016/S0006-3495(99)77061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinsky AJ. Electromechanical and physicochemical properties of connective tissue. CRC Critical Reviews in Biomedical Engineering. 1983;9:133–199. [PubMed] [Google Scholar]
- Ingleman B, Halling MS. Some physico-chemical experiments on fractions of dextrans. Arkiv för Kemi. 1950;1:61–80. [Google Scholar]
- Knight AD, Levick JR. Effects of fluid pressure on the hydraulic conductance of interstitium and fenestrated endothelium in the rabbit knee. The Journal of Physiology. 1985;360:311–332. doi: 10.1113/jphysiol.1985.sp015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanken PN, Hansen-Flaschen JH, Sampson PM, Pietra G, Haselton FR, Fishman AP. Passage of uncharged dextrans from blood to lung lymph in awake sheep. Journal of Applied Physiology. 1985;59:580–591. doi: 10.1152/jappl.1985.59.2.580. [DOI] [PubMed] [Google Scholar]
- Laurent TC, Preston BN, Pertoft H, Gustafsson B, Mccabe M. Diffusion of linear polymers in hyaluronate solutions. European Journal of Biochemistry. 1975;53:129–136. [Google Scholar]
- Lent PLEM, Berg WB, Schalkwijk J, Putte LBA, Berselaar L. The impact of protein size and charge on its retention in articular cartilage. Journal of Rheumatology. 1987;14:798–805. [PubMed] [Google Scholar]
- Levick JR. Flow through interstitium and other fibrous matrices. Quarterly Journal of Experimental Physiology. 1987;72:409–438. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- Levick JR. An analysis of the interaction between extravascular plasma protein, interstitial flow and capillary filtration; application to synovium. Microvascular Research. 1994;47:90–125. doi: 10.1006/mvre.1994.1007. [DOI] [PubMed] [Google Scholar]
- Levick JR, Mcdonald JN. Viscous and osmotically mediated changes of interstitial flow induced by extravascular albumin in synovium. Microvascular Research. 1994;47:68–89. doi: 10.1006/mvre.1994.1006. [DOI] [PubMed] [Google Scholar]
- Levick JR, Mason RM, Coleman PJ, Scott D. Physiology of Synovial Fluid and Trans-synovial Flow. In: Archer CW, Benjamin M, Caterson B, Ralphs JR, editors. Biology of the Synovial Joint. Amsterdam: Harwood Academic Publishers; 1999. pp. 235–252. [Google Scholar]
- Levick JR, Smaje LH. An analysis of the permeability of a fenestra. Microvascular Research. 1987;33:233–256. doi: 10.1016/0026-2862(87)90020-3. [DOI] [PubMed] [Google Scholar]
- Mabuchi K, Tsukamoto Y, Obara T, Yamaguchi T. The effect of additive hyaluronic acid on animal joints with experimentally reduced lubricating ability. Journal of Biomedical Materials Research. 1994;28:865–870. doi: 10.1002/jbm.820280805. [DOI] [PubMed] [Google Scholar]
- Mcdonald JN, Levick JR. Effect of extravascular plasma protein on pressure-flow relations across synovium in anaesthetized rabbits. The Journal of Physiology. 1993;465:539–559. doi: 10.1113/jphysiol.1993.sp019692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald JN, Levick JR. Effect of intra-articular hyaluronan on pressure-flow relation across synovium in anaesthetized rabbits. The Journal of Physiology. 1995;485:179–193. doi: 10.1113/jphysiol.1995.sp020722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani E, Ciferri A, Maraghini M. Osmotic measurements on dilute dextran solutions. Journal of Polymer Science. 1955;18:303–304. [Google Scholar]
- Maroudas A. Fluid transport in cartilage. Annals of the Rheumatic Diseases. 1975;34(suppl. 2):77–81. [PubMed] [Google Scholar]
- Morris ER, Rees RA, Welsh EJ. Conformation and dynamic interactions in hyaluronate solutions. Journal of Molecular Biology. 1980;138:383–400. doi: 10.1016/0022-2836(80)90294-6. [DOI] [PubMed] [Google Scholar]
- Munch WD, Zestar LP, Anderson JL. Rejection of polyelectrolytes from microporous membranes. Journal of Membrane Science. 1979;5:77–102. [Google Scholar]
- Ogston AG, Woods EF. The sedimentation of some fractions of degraded dextrans. Transactions of the Faraday Society. 1954;50:635–643. [Google Scholar]
- Parker JC. Transvascular clearance and distribution of charged macromolecules in ANTU lung injury. Journal of Applied Physiology. 1986;60:1221–1229. doi: 10.1152/jappl.1986.60.4.1221. [DOI] [PubMed] [Google Scholar]
- Parker KH, Winlove CP. The macromolecular basis of the hydraulic conductivity of the arterial wall. Biorheology. 1984;21:181–196. doi: 10.3233/bir-1984-211-221. [DOI] [PubMed] [Google Scholar]
- Preston BN, Snowden JM. Diffusion properties of model extracellular systems. In: Kulonen E, Pikkarainen J, editors. Biology of the Fibroblast. London: Academic Press; 1973. pp. 215–225. [Google Scholar]
- Price FM, Levick JR, Mason RM. Glycosaminoglycan concentration in synovium and other tissues of rabbit knee in relation to hydraulic resistance. The Journal of Physiology. 1996;495:803–820. doi: 10.1113/jphysiol.1996.sp021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JS, Valentine R, Choi CY. Reflection coefficients of homopore membranes: effect of molecular size and configuration. Journal of General Physiology. 1979;73:49–60. doi: 10.1085/jgp.73.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Coleman PJ, Mason RM, Levick JR. Direct evidence for the partial reflection of hyaluronan molecules by the lining of joints during trans-synovial flow. The Journal of Physiology. 1998;508:619–623. doi: 10.1111/j.1469-7793.1998.619bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Coleman PJ, Mason RM, Levick JR. Concentration-dependence of interstitial flow buffering by hyaluronan in synovial joints. Microvascular Research. 2000;59:345–353. doi: 10.1006/mvre.1999.2231. [DOI] [PubMed] [Google Scholar]
- Senti FR, Hellman NN, Ludwig NH, Babcock GE, Tobin R, Glass CA, Lamberts BL. Viscosity, sedimentation and light scattering properties of fractions of acid-hydrolysed dextran. Journal of Polymer Science. 1955;17:527–546. [Google Scholar]
- Strachan RK, Smith P, Gardner DL. Hyaluronate in rheumatology and orthopaedics; is there a role? Annals of the Rheumatic Diseases. 1990;49:949–952. doi: 10.1136/ard.49.11.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales M, Marshall PA, Weissberg SG. Intrinsic viscosity-molecular weight relationships for dextran. Journal of Polymer Science. 1953;10:229–240. [Google Scholar]
- Zar J. Biostatistical Analysis. 2. New York: Prentice-Hall; 1984. [Google Scholar]


