Abstract
In this study we examined whether the rostral ventrolateral medulla (RVLM) maintains resting sympathetic vasomotor tone and activates sympathetic nerve activity (SNA) after the depletion of bulbospinal C1 adrenergic neurones.
Bulbospinal C1 cells were destroyed (≈84% loss) by bilateral microinjections (spinal segments T2-T3) of an anti-dopamine-β-hydroxylase antibody conjugated to the ribosomal toxin saporin (anti-DβH-SAP).
Extracellular recording and juxtacellular labelling of bulbospinal barosensitive neurones in the RVLM revealed that treatment with anti-DβH-SAP spared the lightly myelinated neurones with no tyrosine hydroxylase immunoreactivity.
In rats treated with anti-DβH-SAP, inhibition of RVLM neurones by bilateral microinjection of muscimol eliminated splanchnic SNA and produced the same degree of hypotension as in control rats.
Following treatment with anti-DβH-SAP the sympathoexcitatory (splanchnic nerve) and pressor responses to electrical stimulation of the RVLM were reduced.
Treatment with anti-DβH-SAP also eliminated the majority of A5 noradrenergic neurones. However, rats with selective lesion of A5 cells by microinjection of 6-hydroxydopamine into the pons showed no deficits to stimulation of the RVLM.
In summary, the loss of 84% of bulbospinal adrenergic neurones does not alter the ability of RVLM to maintain SNA and arterial pressure at rest in anaesthetized rats, but this loss reduces the sympathoexcitatory and pressor responses evoked by RVLM stimulation. The data suggest sympathoexcitatory roles for both the C1 cells and non-C1 cells of the RVLM and further suggest the C1 cells are critical for the full expression of sympathoexcitatory responses generated by the RVLM.
The rostral ventrolateral medulla (RVLM) is critical for maintaining basal sympathetic vasomotor tone and is an essential component of many sympathetic reflexes (Reis et al. 1989; Stornetta et al. 1989; Guyenet, 1990; Verberne & Guyenet, 1992; Koshiya et al. 1993; Dampney, 1994a; Sun, 1995; McCulloch et al. 1999). The C1 adrenergic neurones coincide with a region of the RVLM that regulates sympathetic nerve activity (SNA) and arterial pressure (AP) (Ross et al. 1981, 1984b; Reis et al. 1989), and these cells have been proposed to be important for the generation of sympathetic vasomotor tone and AP. The spinal axons of many C1 cells target sympathetic preganglionic neurones or their immediate vicinity (Ross et al. 1984a; Hökfelt et al. 1984; Milner et al. 1988; Jansen et al. 1995a, b). In addition, many bulbospinal C1 neurones are inhibited by increased AP (Lipski et al. 1995; Schreihofer & Guyenet, 1997; Verberne et al. 1999), a hallmark of the sympathetic vasomotor efferents that regulate blood pressure and cardiac output.
However, the C1 cells are not the only efferent projection of the RVLM towards the thoracic spinal cord. This structure also contains other highly active, bulbospinal neurones that are inhibited by stimulation of arterial baroreceptors, but do not contain tyrosine hydroxylase (TH) or phenylethanolamine-N-methyltransferase (PNMT) (Lipski et al. 1995; Schreihofer & Guyenet, 1997). The properties of these lightly myelinated, non-catecholaminergic cells (Lipski et al. 1996) suggest that they could be a source of supraspinal glutamatergic drive to sympathetic preganglionic neurones (Deuchars et al. 1995), which is essential for the generation of resting sympathetic tone and AP (Huangfu et al. 1994).
The relative contributions of the C1 cells and the non-catecholaminergic RVLM neurones to the generation of sympathetic vasomotor tone are not known. One way to address this question would be to identify deficits in the regulation of sympathetic tone that result from the selective destruction of one or the other cell type. Until recently, neither cell type could be targeted because C1 neurones are insensitive to the classic catecholaminergic neurotoxin 6-hydroxydopamine (6-OHDA; Jonsson et al. 1976), and a specific marker to target the non-catecholaminergic neurones has not been identified. However, a newly introduced immunotoxin produced by conjugating the ribosomal toxin saporin to an anti-dopamine-β-hydroxylase antibody (anti-DβH-SAP) now provides the means to lesion C1 cells while sparing non-catecholaminergic neurones in the RVLM (Wrenn et al. 1996). Membrane-bound DβH is exteriorized during exocytosis and acts as a specific receptor for the internalization of anti-DβH-SAP into noradrenergic and adrenergic neurones. Once inside, saporin blocks protein synthesis to cause the death and eventual elimination of the cell (Stripe & Barbieri, 1986). Anti-DβH-SAP has proved capable of selectively lesioning C1 neurones within the RVLM after administration into terminal fields or next to cell bodies (Wrenn et al. 1996; Madden et al. 1999; Schreihofer & Guyenet, 2000).
We have previously shown that the depletion of bulbospinal C1 cells by intraspinal microinjection of anti-DβH-SAP does not chronically alter AP or abolish sympathetic vasomotor tone in anaesthetized rats (Schreihofer & Guyenet, 2000). However, the sympathoexcitatory response to cyanide is virtually eliminated (Schreihofer & Guyenet, 2000). In the present study we examined whether the RVLM continues to maintain basal sympathetic tone after depletion of bulbospinal C1 cells with intraspinal microinjection of anti-DβH-SAP. Because this treatment also depletes bulbospinal pontine noradrenergic neurones (Schreihofer & Guyenet, 2000), we also examined the effects of selective depletion of these cells by pontine microinjection of 6-OHDA. Furthermore, we characterized the remaining barosensitive bulbospinal RVLM neurones to determine whether the lightly myelinated non-catecholaminergic cells could be maintaining SNA. Finally, we determined whether the reduced sympathoexcitatory responses in rats treated with anti-DβH-SAP could be the result of a deficit in the ability of the RVLM to activate SNA.
METHODS
Animals
Male Sprague-Dawley rats (Hilltop Laboratories, Scotsdale, PA, USA) weighed 250-275g at the time of the first surgery (injections of saporin conjugates into spinal cord) and weighed 375-425 g when used for neurophysiological experiments. All procedures were performed in accordance with National Institutes of Health and University of Virginia Animal Care and Use Guidelines.
Microinjection of saporin conjugates into thoracic spinal cord or 6-OHDA into the ventrolateral pons
Anaesthesia was induced with 5% halothane (in 100% O2) and maintained during surgery with 1.9% halothane delivered through a nose cone. The depth of anaesthesia was verified by noting the absence of responses to firm paw pinch and corneal probing. Bilateral microinjections of anti-DβH-SAP into a total of four to six spinal cord sites were made at two levels of the thoracic spinal cord (T2 and T3; total of 48 ng in a total volume of 400 nl; Chemicon, Temecula, CA, USA). Control rats were either unoperated or received four to six microinjections of saporin conjugated to goat anti-mouse IgG (IgG-SAP, total of 48 ng in a total volume of 400 nl; Chemicon). The IgG-SAP causes similar non-selective injury at the injection sites but does not alter the number of bulbospinal catecholaminergic neurones (Schreihofer & Guyenet, 2000). All microinjections were directed towards the intermediolateral cell column (0.8 mm lateral to the midline and 1.0 mm ventral to the dorsal surface). The rats were treated postoperatively with an analgesic (buprenorphine, 4 μg kg−1i.m.; Buprenex, Reckitt and Colman, Richmond, VA, USA) and an antibiotic (penicillin G Procaine, 7500 units kg−1; G. C. Hanford, Syracuse, NY, USA) and returned to standard housing conditions for 3-5 weeks.
To selectively lesion the A5 noradrenergic neurones, rats anaesthetized with halothane received two bilateral microinjections (4 injections of 500 nl each) of 6-OHDA (6 μg μl−1 in a solution of 1 mg ascorbic acid in 1 ml of 0.9% saline) into the ventrolateral pons. Injections were targeted towards A5 cell bodies using stereotaxic coordinates: 2.0 and 2.8 mm rostral to the lamboid suture, 2.2 mm lateral to the midline and 9.2 mm ventral to the dorsal surface of the brain. Each microinjection was performed over 3 min, and after waiting 5 min the pipette was withdrawn. Buprenorphine and penicillin were given as described above, and the rats were returned to standard housing conditions for 2-3 weeks.
Extracellular recording and juxtacellular labelling of RVLM units
Groups of rats previously treated with IgG-SAP (n = 6) or anti-DβH-SAP (n = 8) were instrumented for extracellular recording of RVLM units as previously described (Schreihofer & Guyenet, 1997; Verberne et al. 1999). Anaesthesia was induced with halothane (5% in 100% O2), and all surgical procedures were performed under 1.5-1.8% halothane delivered via a tracheal cannula and a ventilator. A brachial artery and vein were cannulated to measure AP and administer drugs, respectively. An inflatable snare was implanted around the abdominal aorta just below the diaphragm to manipulate upper body AP. The mandibular branch of the facial nerve was exposed on each side for implantation of a stimulating electrode to elicit facial motor nucleus field potentials. After placing the rat in a stereotaxic apparatus, a laminectomy was performed at spinal segment T1 for implantation of a bipolar stimulation electrode into the dorsolateral funiculus for antidromic activation of bulbospinal RVLM cells (Lipski, 1981; Brown & Guyenet, 1985; Schreihofer & Guyenet, 1997; Verberne et al. 1999). The interparietal plate was removed for insertion of a recording electrode into the RVLM. Upon completion of surgical procedures, the halothane was replaced by α-chloralose (70 mg kg−1, i.v. of a 30 mg ml−1 solution in 3% sodium borate, with hourly supplements of one-third of the initial dose), and the rat was allowed to stabilize for 30 min. After ensuring that this dose of α-chloralose produced an appropriate level of anaesthesia, rats were paralysed prior to the onset of recording (pancuronium bromide, i.v., 1 mg kg−1; Elkins-Sinn, Inc., Cherry Hill, NJ, USA). After the induction of neuromuscular blockade, the adequacy of anaesthesia was judged by the stability of AP and the absence of a pressor response to firm paw pinch. Rectal temperature (maintained at 37°C), end-tidal CO2 (maintained at 4.5-5.5%), and AP were monitored throughout the experiment.
RVLM units were recorded extracellularly using glass pipettes filled with 1.5% biotinamide (Molecular Probes) in 0.5 m saline (Schreihofer & Guyenet, 1997; Verberne et al. 1999). Optimal electrode resistance for recording and labelling cells was 25-38 MΩ measured in vivo. Recordings were made with an intracellular amplifier in bridge mode (Axoclamp 2A, Axon Instruments) to allow monitoring of action potentials during injection of current through the electrode. The extracellular signal was filtered (0.2-3 kHz) and a window discriminator was used to determine neuronal discharge rates.
Using a transcerebellar approach the rostral medulla was explored within 0-500 μm posterior to the caudal pole of the facial motor nucleus, which was identified with antidromic field potentials (Brown & Guyenet, 1985). Recordings were limited to spontaneously active, barosensitive units that could be antidromically activated from the thoracic spinal cord (Schreihofer & Guyenet, 1997; Verberne et al. 1999). All units were silenced by increased AP, and firing rate during baroreceptor unloading was determined after injection of nitroprusside (5 μg kg−1i.v.). The conduction velocity (m s−1) was determined by dividing the estimated distance between the unit and the stimulation electrode (35 mm) by the recorded latency to antidromically activate the unit (in ms). Both sides of the medulla were explored in each rat. The individually characterized RVLM, units were filled with biotinamide using a previously described juxtacellular labelling method (Pinault, 1996; Schreihofer & Guyenet, 1997; Verberne et al. 1999). Positive current pulses were delivered through the recording pipette (1.0-3.5 nA with a 50% duty cycle of 200 ms current pulses for 1-5 min) while the activity of the isolated cell was monitored. We have previously found that successful entrainment of the cell's activity to the current pulses produces labelling of a single cell in the vast majority of cases (Schreihofer & Guyenet, 1997). The relative positions of the labelled neurones were recorded for later phenotypic identification, and a maximum of three cells on each side was labelled in each rat. At the end of the experiment the rat was deeply anaesthetized with halothane and perfused transcardially with phosphate-buffered saline (pH 7.4) followed by formaldehyde (4% in 0.1 mm phosphate buffer, pH 7.4). The brain was removed and stored in fixative for 24 h at 4°C. Using a vibratome, coronal sections were cut (30 μm) through the medulla and stored in a cryoprotectant solution (Schreihofer & Guyenet, 1997) at -20°C.
Stimulation and inhibition of the RVLM
Groups of control rats (6 unoperated rats and 5 rats treated with IgG-SAP) and rats treated with anti-DβH-SAP (n = 8) or 6-OHDA (n = 6) underwent the same surgical procedures described above for RVLM unit recording. In addition, these rats were prepared for recording splanchnic sympathetic nerve activity (sSNA), as previously described (Koshiya et al. 1993). The left splanchnic nerve was isolated via a retroperitoneal approach, and the segment distal to the suprarenal ganglion was placed on two Teflon-coated silver wires that had been bared at the tip (250 μm bare diameter; A-M Systems, Everett, WA, USA). The nerve and wires were embedded in a dental impression material (polyvinylsiloxane; Darby Dental Supply, Westbury, NY, USA), and the wound was closed around the exiting recording wires. The sSNA signal was amplified, filtered (0.20-3 kHz; 60 Hz notch filter), full-wave rectified, and integrated in 1 s bins as described previously (Koshiya et al. 1993). The overall amplification factor (from input to output of the PCM amplifier) was x147 000 when determined with a 0.8 kHz sine wave input. The recording noise for sSNA was determined following administration of clonidine (10 μg kg−1i.v.) at the end of the experiment and subtracted from the normalized baseline value (Koshiya et al. 1993).
While the rat was still anaesthetized with 1% halothane, the RVLM was located as described above and then the recording electrode was withdrawn and was replaced by a tungsten microelectrode (monopolar, 4 MΩ; Frederick Haer & Company, Brunswick, ME, USA). Using a randomized order of preselected intensities (10-200 μA), a series of single-pulse stimulations (0.5 ms, 0.5Hz) was delivered to the left RVLM to evoke peak sSNA responses as previously described (Guyenet & Brown, 1986; Huangfu et al. 1994). Then, the anaesthetic was switched to α-chloralose, and the single-pulse stimulation protocol was repeated 1 h later. The single-pulse stimulation protocol was followed by a train stimulation protocol (45 Hz, 10-60 μA, 5 s) used to evoke a sympathetically mediated rise in AP as previously described (Ross et al. 1984b; Brown & Guyenet, 1985).
In a subset of these rats (5 rats treated with IgG-SAP and 8 rats treated with anti-DβH-SAP) the stimulation electrode was withdrawn and replaced by a glass microinjection pipette (pulled and broken to a 25 μm tip) filled with the GABAA antagonist muscimol (1 mm in 0.9% saline; Sigma Chemical Company). The solution was pressure ejected (100 nl over 20 s) into the RVLM (Koshiya et al. 1993). The muscimol injection was repeated immediately in the contralateral RVLM by withdrawing the pipette and moving 3.8 mm medio-laterally (same depth and rostro-caudal coordinates from previous side). At the end of the experiment the rat was deeply anaesthetized with 5% halothane and perfused as described above. Medullary sections (30 μm) were saved for later histological analyses.
Histology
All histological procedures have been previously described in detail (Schreihofer & Guyenet, 1997; Schreihofer et al. 1999; Stornetta et al. 1999; Verberne et al. 1999). Solutions were prepared in Tris-buffered saline (0.1 m Tris, pH 7.4) and used at room temperature unless indicated otherwise.
The extent of depletion of bulbospinal C1 cells produced by anti-DβH-SAP was evaluated following two immunohistochemical protocols performed in separate sets of sections. First, every 1 in 6 sections within the appropriate medullary levels was processed to reveal PNMT-immunoreactive (PNMT-ir) neurones. The PNMT was detected with a rabbit polyclonal antibody (1:2000; DiaSorin, Stillwater, MN, USA) followed by biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, VT, USA) and then streptavidin-indocarbocyanine (Cy3; 1:1000; Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA). Second, another series of 1 in 6 sections was processed to reveal neuropeptide Y (NPY) mRNA by in situ hybridization histochemistry followed by immunohistochemical detection of TH. We have previously determined that PNMT immunoreactivity does not survive the in situ hybridization histochemistry protocol, and that TH and PNMT are of equal diagnostic value for identifying C1 neurones at medullary levels between -12.8 and -11.2 mm caudal to bregma (Stornetta et al. 1999). For detection of NPY mRNA, an antisense riboprobe was transcribed from a 511 bp DNA template inserted into the EcoR1 site of Bluescribe M13(-) (Stratagene, La Jolla, CA, USA) supplied and previously characterized by S. Sabol (Higuchi et al. 1988). The riboprobe was transcribed using digoxigenin-11-UTP. Following hybridization the riboprobe was revealed with a sheep polyclonal digoxigenin antibody conjugated with alkaline phosphatase (Boehringer Mannheim) as described previously (Stornetta et al. 1999). Following the hybridization histochemistry protocol, TH-ir neurones were detected by incubation with a mouse monoclonal antibody (1:2000, Chemicon) and goat anti-mouse IgG-Alexa 488 (Molecular Probes) as previously described (Stornetta et al. 1999).
To examine the A5 neurones, TH immunoreactivity was detected with the mouse monoclonal primary antibody followed by a biotinylated goat anti-mouse IgG (1:200; Vector Laboratories) and then streptavidin-Cy3. Sections processed using immunohistochemistry were mounted onto gelatin-coated slides and coverslips were applied with Krystalon mounting medium (EM Diagnostic Systems, Inc., Gibbstown, NJ, USA). For sections processed to reveal NPY mRNA and TH-ir cells, coverslips were applied with Vectashield (Vector laboratories) and affixed with nail polish.
For detection of juxtacellularly labelled RVLM units, medullary sections were incubated in streptavidin-Cy3 (1:1000 with 0.1% Triton X-100 for 2 h). Sections were mounted onto uncoated slides and coverslips were applied with Vectashield (Vector Laboratories) and affixed with nail polish. The biotinamide-labelled neurones were located, photographed (details below), and drawn along with the outline of the section using Neurolucida software (Microbrightfield, Colchester, VT, USA) and a Ludl motor driven stage (Schreihofer & Guyenet, 1997). The sections containing the labelled neurones were removed from the slides and processed to reveal TH-ir neurones by a peroxidase-antiperoxidase method using diaminobenzidene as previously described (Schreihofer & Guyenet, 1997). The sections were remounted onto gelatin-coated slides, dehydrated through a series of alcohols and xylenes, and coverslips were applied with DPX (Aldrich, Milwaukee, WI, USA). To determine whether the biotinamide-labelled neurone was a C1 cell, the Neurolucida drawing of the section was optically superimposed through the binocular of the microscope using the Lucivid camera system (Schreihofer & Guyenet, 1997). To evaluate the extent of the lesion produced by anti-DβH-SAP in these rats, a series of 1 in 6 sections which did not contain biotinamide-labelled neurones was removed from the uncoated slides after incubation with streptavidin-Cy3 and processed to reveal PNMT-ir cells as described above.
Quantification of lesions produced by anti-DβH-SAP or 6-OHDA
Sections were examined under an epifluorescence microscope. Cell counts included all neuronal perikarial profiles, regardless of whether a nucleus was detectable. Neuroanatomical nomenclature and planes of sections are in accord with Paxinos & Watson (1998), with anterior-posterior (A-P) levels in relation to bregma. The ventral quadrant of both sides of the section was plotted, and C1 and A5 profile counts reflect an average of the two sides of the medulla in each animal.
To evaluate the depletion of C1 neurones in the RVLM in rats treated with anti-DβH-SAP, the number of PNMT-ir profiles was counted from three A-P levels (-11.96, -11.8 and -11.6 mm) corresponding to the rostral RVLM where C1 cells with a spinal projection predominate. Counts of PNMT-ir cell profiles in these most rostral A-P levels produces an estimate that is highly correlated to the percentage depletion of the bulbospinal C1 cells (Schreihofer & Guyenet, 2000). Counts from two more caudal A-P levels (-13.0 and -12.8 mm) corresponding to a region of the VLM with C1 cells that project to the diencephalon but not to the cord (Stornetta et al. 1999) served to show consistency of staining between groups.
Counts of PNMT-ir neurones in the RVLM produce an approximate 12% underestimation of the bulbospinal C1 cell loss (Schreihofer & Guyenet, 2000) due to interspersed C1 cells that project to the hypothalamus but not to the spinal cord (Stornetta et al. 1999; Verberne et al. 1999). Therefore, C1 cells in the RVLM were also counted in relation to their expression of NPY mRNA. The vast majority of C1 neurones with axonal projections to the hypothalamus express NPY mRNA, but most of the C1 cells with spinal axonal projections do not appear to express NPY mRNA (Stornetta et al. 1999). Therefore, counts of TH-ir neurones without NPY mRNA served to estimate the depletion of bulbospinal C1 neurones, whereas counts of TH-ir neurones with NPY mRNA served to demonstrate consistency of staining between groups.
To determine the extent of depletion of A5 neurones, TH-ir neurones were counted from eight A-P levels (-10.4 to -9.0 mm) in control rats (n = 5), rats treated with anti-DβH-SAP (n = 6) and rats treated with 6-OHDA (n = 6).
Examples of PNMT-ir cells, TH-ir cells, and cells with NPY mRNA reaction product were photographed with 35 mm film (1600 ASA push process colour slide film) at x250 or x400 magnification. The slides were scanned on a flatbed scanner (1000 dots inch−1; Ultra Saphir, Young Phillips, Richmond, VA, USA) and acquired as Adobe Photoshop documents (Adobe Systems, Mountain View, CA, USA). Multiple photomicrograph figures were assembled in Photoshop as previously described (Schreihofer & Guyenet, 2000).
Data analyses and statistics
All physiological variables were monitored on a chart recorder and simultaneously stored on a video cassette recorder via a digitizer interface (Vetter 3000A; frequency range, DC to 22 kHz; Vetter Digital, Rebersburg, VA, USA). Mean AP (MAP), integrated sSNA, and integrated rate histograms of RVLM single unit activity were analysed using a Metrabyte Dash-16 A/D interface and custom-designed software (Koshiya et al. 1993).
The effects of anti-DβH-SAP, IgG-SAP or 6-OHDA on the number of cell counts were analysed by two-way ANOVA. The effects of these treatments on physiological variables were analysed by one-way ANOVA, ANOVA by rank, or two-way ANOVA for repeated measures as appropriate. When significant ANOVA F values were obtained, pair-wise comparisons were made with the Student-Newman-Keuls test. Results are expressed as means ±s.e.m. Significance was set at P < 0.05.
RESULTS
Depletion of C1 and A5 neurones by anti-DβH-SAP or 6-OHDA
Treatment with anti-DβH-SAP dramatically reduced the number of rostral PNMT-ir neurones in the RVLM (73 ± 3%; A-P levels, -11.8 and -11.6 mm) compared to control rats (Figs 1A and B, and 2A), but did not alter the number of PNMT-ir cells at more caudal levels of the ventral medulla (A-P levels, -13.0 and -12.8 mm; Fig. 2A). Treatment with anti-DβH-SAP selectively depleted the rostral C1 neurones that do not express NPY mRNA (84 ± 2%; A-P, levels -11.8 and -11.6 mm; Fig. 2B). In contrast, the number of C1 cells with NPY mRNA was not significantly altered by treatment with anti-DβH-SAP (example of staining in Fig. 1E and F; see also Fig. 2B).
Figure 1. Immunohistochemical identification of PNMT-ir C1 neurones, TH-ir A5 neurones and a TH-ir C1 neurone with NPY mRNA in control rats and rats treated with anti-DβH-SAP.

A, PNMT-ir C1 neurones within the rostral ventrolateral medulla (RVLM) revealed with streptavidin-indocarbocyanine (Cy3) in a control rat (A-P level, -11.8 mm). Arrow indicates one of many PNMT-ir neurones. B, section from the same medullary level shown in A from a rat treated with anti-DβH-SAP. Arrow indicates a PNMT-ir neurone. C, TH-ir A5 neurones revealed with Cy3 in a control rat (A-P level, -9.8 mm). Arrow indicates one of many TH-ir neurones. D, section from the same medullary level shown in C from a rat treated with anti-DβH-SAP. Arrow indicates a TH-ir A5 neurone. E, TH-ir C1 neurones (arrow, asterisk) revealed with Alexa 488 in the RVLM from a control rat. F, same area of the section as in E but shown in brightfield to reveal hybridization reaction product for NPY mRNA. Arrow indicates a TH-ir neurone with NPY mRNA. Asterisk indicates a TH-ir neurone with no NPY mRNA. 7n, facial nerve; LSO, lateral superior olive. Scale bar, 200 μm for A–D and 50 μm for E and F. Group data are shown in Fig. 2.
Figure 2. Catecholaminergic neurones are depleted by treatment with anti-DbH-SAP or 6-OHDA.

A, number of PNMT-ir C1 neurones per side of the RVLM in unoperated control rats (▪ n = 6) and rats injected with anti-DbH-SAP (□; n = 8). The number of PNMT-ir neurones at the 3 rostral levels (A-P, -11.96, -11.8 and -11.6 mm caudal to bregma) was substantially reduced in rats treated with anti-DbH-SAP. In contrast, the number of PNMT-ir neurones at the 2 caudal levels (A-P, -13.0 and -12.8 mm) was unaffected by treatment with anti-DbH-SAP. B, number of TH-ir C1 neurones with NPY mRNA (NPY+) or without NPY mRNA (no NPY) in control rats (n = 4; filled symbols) and rats treated with anti-DbH-SAP (n = 5; open symbols). Treatment with anti-DbH-SAP reduced the number of TH-ir neurones with no NPY mRNA without altering the number of TH-ir neurones with NPY mRNA. *P < 0.05 compared to control rats by ANOVA and Student-Newman-Keuls test. C, number of TH-ir A5 neurones per side of the ventrolateral pons in unoperated control rats (n = 5, 6), rats injected with anti-DbetaH-SAP (n = 6;), and rats treated with 6-OHDA (n = 6;). The number of TH-ir cells was reduced substantially at all medullary levels examined in rats treated with anti-DbH-SAP or 6-OHDA.
Spinal microinjection of anti-DβH-SAP dramatically reduced the number of TH-ir neurones in the A5 cell group (95 ± 1%; Figs 1C and D, and 2C), as shown previously (Schreihofer & Guyenet, 2000). Bilateral pontine microinjection of 6-OHDA produced a comparable depletion of TH-ir neurones in the A5 cell group (89 ± 2%; Fig. 2C).
Properties of presympathetic RVLM neurones after treatment with anti-DβH-SAP
In rats treated with IgG-SAP the latency for antidromic activation of barosensitive RVLM units ranged from 4.5 to 82 ms with 42% of the cells conducting at < 1 m s−1, 33% of the cells at 1-3 m s−1, and 25% at > 3 m s−1 (Fig. 3A and C), as seen in unoperated rats (Schreihofer & Guyenet, 1997). Also, as noted previously (Schreihofer & Guyenet, 1997), under chloralose anaesthesia there appeared to be an inverse relationship between the basal firing rate and the latency of antidromic activation (Fig. 3A).
Figure 3. Treatment with anti-DβH-SAP effectively depletes slowly conducting, slow firing barosensitive bulbospinal RVLM neurones.
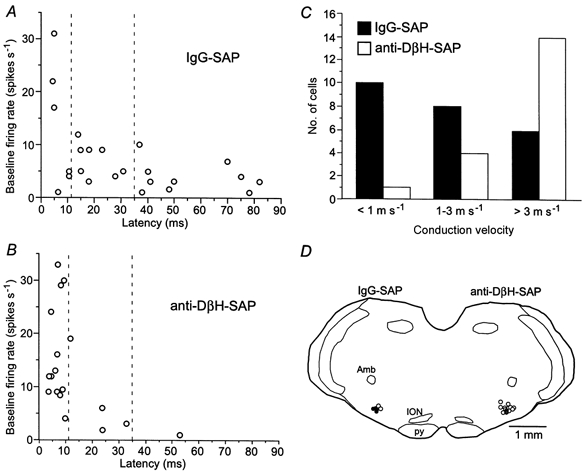
A, relationship between baseline firing rates and antidromic latencies of neurones recorded in rats treated with IgG-SAP (24 cells in 6 rats). Dashed lines correspond to categories of conduction velocities depicted in C. B, relationship between baseline firing rates and antidromic latencies of neurones recorded in rats treated with anti-DβH-SAP (19 cells in 8 rats). Dashed lines correspond to categories of conduction velocities depicted in C. C, number of recorded RVLM units categorized into 3 ranges of conduction velocity based on previous findings regarding TH immunoreactivity (Schreihofer & Guyenet, 1997). The distribution of conduction velocities was significantly different between the two groups (chi-squared test, P < 0.01). D, location of recorded RVLM neurones that were evaluated for TH immunoreactivity from rats treated with IgG-SAP (left) and rats treated with anti-DβH-SAP (right). Units were placed onto a representative coronal section at A-P level -11.8 mm. ○, cells with no TH immunoreactivity; •, TH-ir cells with intense immunoreactivity (left side) and very light immunoreactivity (right side). Amb, nucleus ambiguus; ION, inferior olivary nuclei; py, pyramidal tract. Group data are shown in Table 1.
In rats treated with anti-DβH-SAP, which showed a 72 ± 4% depletion of rostral PNMT-ir cells, barosensitive bulbospinal units were encountered in the RVLM (Fig. 3B–D and 4). More electrode tracts were required to find a comparable number of appropriate units, presumably due to the elimination of a large proportion of barosensitive bulbospinal RVLM cells. The average conduction velocity of the recorded cells was higher in rats treated with anti-DβH-SAP than in rats treated with IgG-SAP (Table 1, Fig. 3A and B), due to the paucity of cells found in the lower conduction velocity ranges (Fig. 3B and C). Although basal MAP was unchanged (104 ± 2 mmHg in IgG-SAP-treated rats vs. 99 ± 3 mmHg in anti-DβH-SAP-treated rats), the average basal firing rate of presympathetic RVLM neurones was higher in rats treated with anti-DβH-SAP compared to rats treated with IgG-SAP (Table 1, Fig. 3A and B). However, examination of cells with comparable conduction velocities (> 3 m s−1) revealed that the firing rates of this class of neurones were not altered by treatment with anti-DβH-SAP (Table 1, Fig. 3A and B).
Figure 4. Representative recordings of a presympathetic RVLM unit, splanchnic sympathetic nerve activity and mean arterial pressure from a rat treated with anti-DβH-SAP.

A, simultaneous recording of mean arterial pressure (MAP, top trace), RVLM unit activity (integrated rate histogram in 1 s bins, middle trace) and splanchnic sympathetic nerve activity (sSNA; full-wave rectified and integrated, 1 s bins, bottom trace). MAP was raised by aortic occlusion (AOc) and decreased with sodium nitroprusside (SNP). B, antidromic activation at 4.5 ms of the unit shown in A (5 consecutive stimuli delivered to the spinal cord at arrow with stimulation artifact truncated). The asterisk indicates a spontaneous collision. C, entrainment of the same RVLM unit using depolarizing pulses (bars above unit trace) to introduce biotinamide into the cell. This cell was not TH-ir (not shown).
Table 1.
Characteristics of barosensitive bulbospinal RVLM neurones in rats treated with anti-DβH-SAP or IgG-SAP
| IgG-SAP | Anti-DβH-SAP | |
|---|---|---|
| All recorded cells | ||
| Conduction velocity (ms−1) | 2.3 ± 0.5 (24) | 4.7 ± 0.6 (19)* |
| Baseline FR (spike s−1) | 7.3 ± 1.5 (24) | 13.3 ± 2.2 (19)* |
| Maximum FR (spike s−1) | 10.7 ± 2.0 (18) | 20.1 ± 2.9 (19)* |
| Fast-conducting cells | ||
| Conduction velocity (m s−1) | 5.6 ± 0.6 (6) | 5.8 ± 0.6 (14) |
| Baseline FR (spike s−1) | 13.3 ± 4.9 (6) | 15.9 ± 2.5 (14) |
| Maximum FR (spikes s−1) | 25.7 ± 3.5 (3) | 24.4 ± 2.9 (14) |
Mean ± S.E.M. (number of cells) values are presented for RVLM units recorded in rats treated with IgG-SAP or anti-DβH-SAP. Maximum firing rate (FR) was determined during nitroprusside-induced hypotension. The FR and conduction velocity between the two groups were compared by Student's t test.
P < 0.05.
In rats treated with IgG-SAP, six of the 24 recorded cells were filled with biotinamide for later determination of TH immunoreactivity. In agreement with our previous findings (Schreihofer & Guyenet, 1997), four out of six of the cells were TH-ir (example in Fig. 5A and B; location in the RVLM in Fig. 3D), with three of the TH-ir cells having conduction velocities of < 1 m s−1, and the other three cells conducting in the 1-3 m s−1 range. In rats treated with anti-DβH-SAP, 13 of the 19 recorded cells were filled with biotinamide (example of juxtacellular entrainment of activity in Fig. 4C). In contrast to the unlesioned group, the majority of labelled cells (11 of 13) were not TH-ir (example in Fig. 5C and D; location in the RVLM in Fig. 3D). Eight of the non-TH-ir cells had conduction velocities > 3 m s−1, and the other thre cells had conduction velocities in the 1-3 m s−1 range. The two other cells (conduction velocities > 3 m s−1) displayed a very light TH immunoreactivity (example in Fig. 5E and F), similar to the light TH immunoreactivity observed previously in a few faster conducting presympathetic neurones (Schreihofer & Guyenet, 1997).
Figure 5. Phenotypic identification of barosensitive bulbospinal RVLM units filled juxtacellularly with biotinamide and processed to reveal TH immunoreactivity.

A, photomicrograph of a single neurone filled with biotinamide and revealed with Cy3 (arrow) from a rat treated with IgG-SAP. The antidromic latency for this neurone was 48 ms. B, photomicrograph of the same area of the section as in A but shown in brightfield. The biotinamide-labelled cell is clearly TH-ir. C, photomicrograph of a single neurone filled with biotinamide and revealed with Cy3 (arrow) from a rat treated with anti-DβH-SAP. The antidromic latency of this neurone was 3.5 ms. D, photomicrograph of the same area of the section as in C but shown in brightfield. The biotinamide-labelled cell was not TH-ir (arrow), but was adjacent to a TH-ir neurone (asterisk). E, photomicrograph of a single neurone filled with biotinamide and revealed with Cy3 (arrow) from a rat treated with anti-DβH-SAP. The antidromic latency of this neurone was 6 ms. F, photomicrograph of the same area of the section as in E but shown in brightfield. The biotinamide-labelled neurone was very lightly TH-ir (arrow) compared to other TH-ir cells in the section (asterisk). Scale bar, 50 μm.
sSNA and pressor responses evoked by electrical stimulation within the RVLM
Single-pulse electrical stimulation of the RVLM evokes two excitatory peak responses in sympathetic nerves, but the role of the C1 neurones in generating one or both of these peaks has remained a matter of speculation (Guyenet & Brown, 1986; Morrison et al. 1988; Morrison, 1993; Huangfu et al. 1994). We examined the sSNA responses to electrical stimulation of the RVLM under two anaesthetic conditions in control rats (6 unoperated rats and 5 rats treated with IgG-SAP) and rats treated with anti-DβH-SAP or 6-OHDA. Because the responses in rats treated with IgG-SAP were comparable to those seen in the unoperated rats the two groups were combined to form the control group for statistical analyses.
Under 1% halothane, an anaesthetic condition that causes unmyelinated C1 cells to discharge at a high resting rate (15-20 spikes s−1; Sun & Guyenet, 1986), the single-pulse stimulation (typical RVLM site in Fig. 6A) evoked two intensity-dependent peaks in sSNA (apex of peak I at 60-65 ms and peak II at 142-154 ms; Fig. 6B, and 7A and B). Under α-chloralose, which is associated with a lower resting discharge rate of presympathetic RVLM neurones (Schreihofer & Guyenet, 1997), the single-pulse stimulation also elicited an intensity-dependent sSNA peak in the range 60-65 ms (peak I; Fig. 6C and 7C). However, under α-chloralose the threshold to elicit this peak was much lower, and the peak amplitude measured in relation to the resting (prestimulation) baseline was larger than the responses seen under halothane (Fig. 6C and 7C). Under α-chloralose, peak II was very small and highly variable in the range of intensities examined (example in Fig. 6C). Therefore, this peak was not systematically analysed under this anaesthetic condition.
Figure 6. Representative sSNA responses evoked by single-pulse stimulation of the RVLM in a rat treated with IgG-SAP or anti-DβH-SAP.
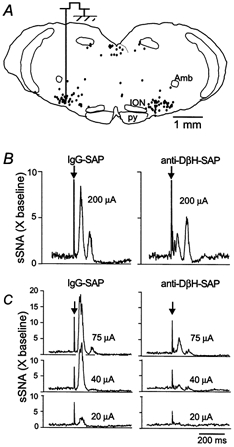
A, illustration of a representative RVLM stimulation site at A-P level 1.8 mm. •, PNMT-ir C1 cells plotted from a representative section. B, 2 evoked sSNA responses (peak I and peak II; baseline = 1; X baseline = multiple of baseline) under halothane (left, rat treated with IgG-SAP; right, rat treated with anti-DβH-SAP). C, evoked sSNA responses under α-chloralose at 3 stimulus intensities (left, rat treated with IgG-SAP; right, anti-DβH-SAP). Arrows in B and C indicate RVLM stimulation artifacts.
Figure 7. Effects of treatment with anti-DβH-SAP or 6-OHDA on RVLM-evoked increases in sSNA and MAP.

A, under halothane the amplitude of sSNA peak I produced by single-pulse stimulation of the RVLM (0.5 Hz, 0.5 ms) is reduced in rats treated with anti-DβH-SAP (n = 8), but not in rats treated with 6-OHDA (n = 6). B, under halothane the amplitude of sSNA peak II produced by single-pulse stimulation of the RVLM is not affected by treatment with anti-DβH-SAP or 6-OHDA. C, under α-chloralose the amplitude of sSNA peak I produced by single-pulse stimulation of the RVLM is reduced in rats treated with anti-DβH-SAP, but not in rats treated with 6-OHDA. D, under α-chloralose the rise in MAP caused by repetitive stimulation of the RVLM (44 Hz, 0.5 ms, 5 s trains) was significantly attenuated by treatment with anti-DβH-SAP but not 6-OHDA. Asterisks indicate significant differences from control rats, P < 0.05.
In rats treated with the anti-DβH-SAP, the amplitude of peak I was greatly reduced compared to control rats under both anaesthetic conditions (Figs 6B and C, and 7A and C). Under halothane the amplitude of peak II was unaffected by treatment with anti-DβH-SAP (Figs 6B and 7B). Treatment with 6-OHDA produced no deficits in either peak sSNA response under either anaesthetic condition compared to control rats (Fig. 7A–C). Low intensity, repetitive stimulation of the RVLM produces a rise in MAP due to an increase in SNA (Ross et al. 1984b; Brown & Guyenet, 1985). Under α-chloralose anaesthesia, repetitive stimulation of the RVLM (10-60 μA) produced an intensity-dependent rise in MAP in control rats that was significantly attenuated in rats treated with anti-DβH-SAP (Fig. 7D). In contrast, the stimulation-evoked increases in MAP were not altered by treatment with 6-OHDA (Fig. 7D).
Inhibition of sSNA and depressor responses produced by bilateral microinjection of muscimol into the RVLM
Rats treated with anti-DβH-SAP clearly have a sympathetic vasomotor tone that is modulated by changes in AP (Fig. 8A and B, and 9; Schreihofer & Guyenet, 2000), but whether the RVLM continues to drive SNA in the absence of the majority of bulbospinal C1 cells is not known. Estimation of the absolute level of baseline sSNA revealed no difference between groups (0.35 ± 0.14 μV in control rats and 0.30 ± 0.05 μV in rats treated with anti-DβH-SAP). Bilateral injection of muscimol into the RVLM produced equivalent decreases in sSNA in control rats (n = 6) and rats treated with anti-DβH-SAP (n = 8), which were comparable to the inhibition produced by saturation of the baroreflex by a phenylephrine-induced increase in MAP (Fig. 8A and B, and 9).
Figure 8. Bilateral inhibition of the RVLM decreases sSNA and MAP in a control rat (A) and a rat treated with anti-DβH-SAP (B).

Baroreflex-evoked changes in sSNA were elicited by injection of phenylephrine (PE; 2 μg kg−1, i.v.) and sodium nitroprusside (SNP; 5 μg kg−1, i.v.). After recovery, muscimol (100 pmol in 100 nl) was microinjected into the RVLM (representative site depicted in Fig. 6A) on the left side (Musc-L) and then on the right side (Musc-R). In both animals inhibition of the RVLM with muscimol produced a decrease in MAP and virtual elimination of sSNA.
Figure 9. Treatment with anti-DβH-SAP does not alter the decreases in sSNA and MAP produced by bilateral inhibition of the RVLM.

A, baseline sSNA (Base) was normalized to 100% prior to injections of muscimol. Bilateral microinjection of muscimol (100 pmol in 100 nl on each side) decreased sSNA to levels comparable to those seen after increasing MAP with PE (2 μg kg−1, i.v.) with no differences between control rats (treated with IgG-SAP; n = 5) and rats treated with anti-DβH-SAP (n = 8). B, bilateral microinjection of muscimol produced a decrease in MAP that was not different between the two groups.
A, baseline sSNA (Base) was normalized to 100% prior to injections of muscimol. Bilateral microinjection of muscimol (100 pmol in 100 nl on each side) decreased sSNA to levels comparable to those seen after increasing MAP with PE (2 μg kg−1, i.v.) with no differences between control rats (treated with IgG-SAP; n = 5) and rats treated with anti-DβH-SAP (n = 8). B, bilateral microinjection of muscimol produced a decrease in MAP that was not different between the two groups.
Baseline MAP was not affected by treatment with anti-DβH-SAP (Fig. 9B). Bilateral inhibition of the RVLM with muscimol produced the same large decrease in MAP that was observed in control rats (Figs 8A and B, and 9B).
DISCUSSION
The present study provides several new findings regarding the generation of sympathetic vasomotor tone by the RVLM. Firstly, treatment with anti-DβH-SAP effectively depleted bulbospinal C1 cells but spared a class of barosensitive bulbospinal RVLM neurones that express little or no TH. Secondly, the RVLM remained critical for the generation of resting sympathetic vasomotor tone after depletion of the vast majority of bulbospinal C1 cells. Finally, lesion of bulbospinal C1 adrenergic neurones reduced the ability of electrical stimulation within the RVLM to increase sSNA and MAP above basal levels. These findings suggest that the non-C1 barosensitive bulbospinal RVLM neurones have a sympathoexcitatory function and are able to maintain SNA in the absence of most C1 cells. However, the bulbospinal C1 neurones appear to be critical for the full expresssion of sympathoexcitatory responses produced by the RVLM.
Selectivity and extent of depletion of bulbospinal catecholaminergic neurones by anti-DβH-SAP
We confirm that injections of anti-DβH-SAP into the thoracic spinal cord produce massive lesions of bulbospinal catecholaminergic neurones, including C1 and A5 neurones (Schreihofer & Guyenet, 2000). A principal advantage of this lesion protocol is that it causes a very effective depletion of bulbospinal C1 cells but spares the C1 cells that do not project to the spinal cord (Tucker et al. 1987). This protocol also avoids potential non-specific damage to the RVLM or to the sympathetic preganglionic neurones giving rise to the sympathetic nerve recorded in the present study. Intraparenchymal microinjections of anti-DβH-SAP in doses that effectively deplete catecholaminergic neurones inevitably cause some local tissue damage and a persistent gliosis (present study; Madden et al. 1999; Schreihofer & Guyenet, 2000). Identical local effects are seen with injection of saporin conjugated to a mouse IgG (present study; Schreihofer & Guyenet, 2000), suggesting that the damage is due to the non-selective uptake of saporin. However, the non-selective lesions are circumscribed and have no detectable effect on the behaviour or rate of growth of the animals (present study; Schreihofer & Guyenet, 2000). In addition, animals treated with IgG-SAP do not display the reduced sympathoexcitatory responses observed in rats treated with anti-DβH-SAP, suggesting that these effects are due to the depletion of catecholaminergic neurones.
The extensive depletion of C1 cells after microinjection of anti-DβH-SAP restricted to the upper thoracic spinal cord is in agreement with the notion that these cells project to multiple levels of the spinal cord (McAllen, 1990). Extensive collateralization is also indicated by electrophysiological data (Barman & Gebber, 1985), and by the finding that a large proportion of bulbospinal C1 cells are infected by peripheral injections of retrogradely transported virus into a single sympathetic ganglion regardless of the targeted ganglion (Janssen et al. 1995a,b). In addition, anti-DβH-SAP may be taken up by axons of passage. In this case, however, the uptake is selective for catecholaminergic fibres, because the number of non-catecholaminergic bulbospinal neurones in the RVLM and mid-line raphe nuclei is not affected by treatment with anti-DβH-SAP (see also Schreihofer & Guyenet, 2000). Nevertheless, a conceivable limitation of our protocol is that upper thoracic injections of anti-DβH-SAP could have spared C1 cells that exclusively control splanchnic vasoconstrictor preganglionic neurones. However, this possibility is not likely for several reasons. First, there is no compelling evidence that a distinct class of C1 cells controls splanchnic vasoconstrictor efferents. Although microinjections of glutamate or GABA into the RVLM can produce some degree of differential activation or inhibition of sympathetic efferents (e.g. Campos & McAllen, 1997; Ootsuka & Terui, 1997), it is far from certain that the phenomenon is due to the differential recruitment of C1 cells. Second, we did observe very clear deficits in splanchnic nerve function during stimulation of the RVLM of rats injected with anti-DβH-SAP, indicating that the innervation of splanchnic preganglionic cells had not been spared. Third, the massive depletion of C1 cells following injection of anti-DβH-SAP into a restricted number of spinal segments is consistent with the notion of extensive collateralization of bulbospinal barosensitive cells or significant uptake by catecholaminergic fibres of passage. Therefore, our depletion of bulbospinal cells is very likely to include those that innervate splanchnic preganglionic neurones.
Anti-DβH-SAP also lesions the C3 cells that project to the spinal cord and produces an even greater depletion of bulbospinal noradrenergic neurones (present data; Schreihofer & Guyenet, 2000). The noradrenergic cells affected include virtually all A5 cells, the caudal and ventral portions of the locus coeruleus that project to the spinal cord and many A7 cells. Experiments designed to control for the loss of A5 cells were performed in the present study. These experiments indicated that the selective lesion of A5 cells does not mimic the effects of the combined lesion of adrenergic and noradrenergic neurones. However, we did not investigate the consequences of lesions restricted to the locus coeruleus or A7 neurones. Therefore, it remains possible that some of the deficits we attribute to the lesion of C1 cells could be due in part to the loss of these other bulbospinal noradrenergic neurones or bulbospinal C3 adrenergic neurones.
Counts of C1 cells, even when made at the extreme rostral end of the RVLM, underestimate the depletion of bulbospinal C1 neurones by anti-DβH-SAP (Schreihofer & Guyenet, 2000), because this region also contains some C1 cells that do not project to the spinal cord (Stornetta et al. 1999). In order to obtain a more precise measurement of the loss of the bulbospinal C1 neurones, in the present study we also examined the extent to which C1 cells devoid of NPY mRNA were depleted. We have recently shown that all C1 cells with a hypothalamic axonal projection express high levels of NPY mRNA, whereas most bulbospinal C1 cells do not contain detectable levels of NPY mRNA (Stornetta et al. 1999). The results in the present study confirm that C1 cells with no NPY mRNA project specifically to the cord, because they are massively and selectively depleted by intraspinal injections of anti-DβH-SAP. The depletion of the C1 cells devoid of NPY mRNA averaged 84%, whereas the depletion of all PNMT-ir profiles in the same region was only 73%. This 11% difference is consistent with our previous finding of a 12% underestimation of the depletion of bulbospinal PNMT-ir cells (retrogradely labelled from the spinal cord) by using counts of rostral PNMT-ir neurones (Schreihofer & Guyenet, 2000). Therefore, counts of C1 cells devoid of NPY mRNA appear to provide a reliable index of the loss of bulbospinal C1 cells. In the present study, using these criteria we estimated an 84% average loss of bulbospinal C1 cells in rats treated with anti-DβH-SAP.
The RVLM generates sympathetic vasomotor tone after lesion of C1 neurones
After elimination of most bulbospinal catecholaminergic neurones, rats have a normal resting MAP with sympathetic tone that is modulated by cardiopulmonary reflexes (Schreihofer & Guyenet, 2000). The present study confirms the lack of effect of these lesions on resting MAP and shows that the average integrated voltage signal recorded in the splanchnic nerve at rest is not altered by treatment with anti-DβH-SAP. The administration of the GABAA receptor agonist muscimol into the RVLM of rats treated with anti-DβH-SAP produced the same degree of hypotension and reduction of sSNA as in control rats. These data indicate that SNA contributes equally to AP in the two groups, and that sympathetic vasomotor tone continues to be generated by the RLVM after treatment with anti-DβH-SAP.
One interpretation of these data is that C1 cells are essential for the generation of sympathetic tone, but an 84% depletion of bulbospinal C1 cells is insufficient to produce a physiological change. In this case, the preganglionic neurones could be re-innervated by the surviving cells or compensate for a reduced C1 cell input by some postsynaptic adaptive response. This possibility is compatible with observations made in other systems, most prominently the nigrostriatal dopaminergic pathway. In this system, dopaminergic cells must be reduced by more than 80% for the appearance of symptomatic changes in extrapyramidal motor function (Zigmond & Stricker, 1984; Chiueh et al. 1985). If similar adaptive mechanisms were to account for the lack of effect of C1 cell lesions on resting sSNA, this would suggest that C1 cells could indifferently reinnervate a variety of target neurones. The possibility cannot be dismissed at present, but seems unlikely given that several rats with more than a 90% lesion of the C1 cells still had sSNA and a normal resting MAP (present study; Schreihofer & Guyenet, 2000).
The second interpretation is that C1 cells do not make an essential contribution to resting sympathetic vasomotor tone, so their destruction produces little effect on SNA and resting MAP. This hypothesis requires the existence of other types of presympathetic RVLM neurones that survive treatment with anti-DβH-SAP. In intact animals, a large fraction of the faster conducting barosensitive bulbospinal RVLM neurones do not contain TH or PNMT (Lipski et al. 1995; Schreihofer & Guyenet, 1997), and these neurones appear to be spared by treatment with anti-DβH-SAP. In addition, the basal firing rates of the surviving bulbospinal barosensitive cells were not different when compared to those of presympathetic neurones with the same conduction velocity in unoperated rats (Schreihofer & Guyenet, 1997) or rats treated with IgG-SAP (present study). Thus, the present results are compatible with the possibility that non-catecholaminergic presympathetic neurones generate the bulk of the excitatory drive to sympathetic nerves under resting conditions.
A sympathoexcitatory role for bulbospinal C1 cells
Although rats treated with anti-DβH-SAP displayed no differences from control rats under resting conditions, the present study uncovered significant deficits in the lesioned animals in response to stimulation of the RVLM region. Because these deficits were absent in animals with selective lesion of A5 neurones, they are more likely to reflect the destruction of bulbospinal C1 neurones.
As previously described (Guyenet & Brown, 1986; Morrison et al. 1988; Huangfu et al. 1994), single-pulse electrical stimulation of the RVLM produced two excitatory peaks in sSNA. The shorter latency peak (60-65 ms; peak I) appears to be mediated in large part by the release of glutamate (Morrison et al. 1989; Huangfu et al. 1994; Deuchars et al. 1995). The latency of this response is consistent with the activation of bulbospinal RVLM neurones with lightly myelinated axons, which includes a portion of the C1 cells and all the non-catecholaminergic barosensitive neurones (Schreihofer & Guyenet, 1997). The attenuation of peak I in rats treated with anti-DβH-SAP may be interpreted in at least two ways. One possibility is that the lesion of C1 cells eliminates a source of catecholamines that facilitate the excitatory drive of sympathetic preganglionic neurones by non-catecholaminergic presympathetic RVLM neurones. In agreement with this interpretation, activation of postsynaptic α1-adrenergic receptors potentiates synaptic responses onto preganglionic neurones (Yoshimura et al. 1987a,b; Polosa et al. 1988). Alternatively, the elimination of C1 neurones may remove a source of glutamate that is released by the C1 cells with lightly myelinated spinal axons (Morrison et al. 1989; Dampney, 1994b). In either case, the C1 cells appear to be essential for the full expression of this sympathoexcitatory response, and this deficit may contribute to the reduced sympathoexcitatory response to cyanide that we observed in rats treated with anti-DβH-SAP (Schreihofer & Guyenet, 2000).
In contrast, the amplitude of the longer latency excitatory peak of sSNA (peak II) was not altered by treatment with anti-DβH-SAP. Because the slowly conducting C1 cells were eliminated in these rats, peak II is not likely to be generated by the stimulation of this class of neurone, contrary to previous speculation (Guyenet & Brown, 1986; Morrison et al. 1988; Huangfu et al. 1994). Although peak II is attenuated by intraspinal administration of the α-adrenergic antagonists phentolamine and prazosin, these drugs also reduce the sympathoexcitatory peak produced by stimulation of the adjacent raphe pallidus (Huangfu et al. 1994). Therefore, these data indicate that peak II is not produced by the stimulation of slowly conducting C1 neurones in the RVLM, but instead may be produced by fibres of passage from cells originating in the raphe pallidus (Morrison, 1993).
As previously shown (Ross et al. 1984b; Brown & Guyenet, 1985), repetitive electrical stimulation of the RVLM raised MAP in an intensity-dependent manner. The attenuation of this pressor response in rats treated with anti-DβH-SAP is consistent with the reduction in evoked sSNA observed in these animals. This result agrees with the finding that stimulation of cell bodies within the RVLM by microinjection of glutamate produces a smaller increase in MAP in rats that have received injections of anti-DβH-SAP directly into the RVLM (Madden et al. 1999). Importantly, both studies indicate that the pressor response to RVLM stimulation is only attenuated, not eliminated, by massive lesions of C1 cells. Because the selective destruction of A5 neurones produced no such deficit, the attenuation of the pressor effect of RVLM stimulation in rats treated with anti-DβH-SAP is most probably due to the loss of the adrenergic neurones.
Summary and conclusions
The present data suggest that adrenergic C1 neurones may not play a dominant role in generating the sympathetic vasomotor tone of anaesthetized animals under resting conditions. Instead, this role may be fulfilled by lightly myelinated, non-catecholaminergic presympathetic RVLM neurones, which are spared by treatment with anti-DβH-SAP. However, the deficits observed during stimulation of the RVLM in rats treated with anti-DβH-SAP support the prevalent theory that C1 cells have a sympathoexcitatory role (Dampney, 1994b). The attenuation of the short latency evoked sSNA response in rats treated with anti-DβH-SAP suggests that the lightly myelinated C1 cells contribute to the activation of sympathetic preganglionic neurones, either by the release of glutamate or via a catecholaminergic facilitation of glutamatergic excitation by non-C1 neurones. However, the precise function of the unmyelinated C1 cells in the stimulation of sympathetic preganglionic neurones is less clear. The persistence of the long latency evoked sSNA response to stimulation of the RVLM in rats treated with anti-DβH-SAP suggests that slowly conducting C1 cells are not responsible for this sympathoexcitatory response. However, these cells may provide a level of catecholaminergic tone that influences the general excitability of preganglionic neurones. Nevertheless, most bulbospinal adrenergic cells are activated during stressful conditions such as sustained hypotension, haemorrhage, or activation of peripheral chemoreceptors (Li & Dampney, 1994; Chan & Sawchenko, 1994; Janssen et al. 1995a;Schreihofer & Guyenet, 2000), and they are likely to make a significant contribution to the stimulation of sympathetic vasomotor tone under these conditions.
Acknowledgments
This work was supported by a grant from the National Institutes of Health to P.G.G. (HL 28785).
References
- Barman SM, Gebber GL. Axonal projection patterns of ventrolateral medullospinal neurons. Journal of Neurophysiology. 1985;53:1551–1566. doi: 10.1152/jn.1985.53.6.1551. [DOI] [PubMed] [Google Scholar]
- Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circulation Research. 1985;56:359–369. doi: 10.1161/01.res.56.3.359. [DOI] [PubMed] [Google Scholar]
- Campos RR, McAllen RM. Cardiac sympathetic premotor neurons. American Journal of Physiology. 1997;272:R615–620. doi: 10.1152/ajpregu.1997.272.2.R615. [DOI] [PubMed] [Google Scholar]
- Chan RKW, Sawchenko PE. Spatially and temporally differentiated patterns of c-fos expression in brain-stem catecholaminergic cell groups induced by cardiovascular challenges in the rat. Journal of Comparative Neurology. 1994;348:433–460. doi: 10.1002/cne.903480309. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Burns RS, Markey SP, Jacobowitz DM, Kopin IJ. Primate model of parkinsonism: selective lesion of nigrostriatal neurons by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine produces an extrapyramidal syndrome in rhesus monkeys. Life Sciences. 1985;36:213–218. doi: 10.1016/0024-3205(85)90061-x. [DOI] [PubMed] [Google Scholar]
- Dampney RAL. The subretrofacial vasomotor nucleus: Anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Progress in Neurobiology. 1994a;42:197–227. doi: 10.1016/0301-0082(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiological Reviews. 1994b;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Morrison SF, Gilbey MP. Medullary-evoked EPSPs in neonatal rat sympathetic preganglionic neurones in vitro. Journal of Physiology. 1995;487:453–463. doi: 10.1113/jphysiol.1995.sp020892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. Role of the ventral medulla oblongata in blood pressure regulation. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 145–167. [Google Scholar]
- Guyenet PG, Brown DL. Nucleus paragigantocellularis lateralis and lumbar sympathetic discharge in the rat. American Journal of Physiology. 1986;250:R1081–1094. doi: 10.1152/ajpregu.1986.250.6.R1081. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Yang H-YT, Sabol SL. Rat neuropeptide Y precursor gene expression: mRNA structure, tissue distribution, and regulation by glucocorticoids, cyclic AMP, and phorbol ester. Journal of Biological Chemistry. 1988;263:6288–6295. [PubMed] [Google Scholar]
- Hökfelt T, Johansson O, Goldstein M. Central catecholamine neurons as revealed by immunohistochemistry with special reference to adrenaline neurons. In: Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy, Classical Transmitters in the CNS. Vol. 2. Amsterdam: Elsevier; 1984. pp. 157–276. part I. [Google Scholar]
- Huangfu D, Hwang L-J, Riley TA, Guyenet PG. Role of serotonin and catecholamines in sympathetic responses evoked by stimulation of rostral medulla. American Journal of Physiology. 1994;266:R338–352. doi: 10.1152/ajpregu.1994.266.2.R338. [DOI] [PubMed] [Google Scholar]
- Jansen ASP, VanNguyen X, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: Basis of the fight-or-flight response. Science. 1995a;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- Jansen ASP, Wessendorf MW, Loewy AD. Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Research. 1995b;683:1–24. doi: 10.1016/0006-8993(95)00276-v. [DOI] [PubMed] [Google Scholar]
- Jonsson G, Fuxe K, Hökfelt T, Goldstein M. Resistance of central phenylethanolamine-N-methyltransferase containing neurons to 6-hydroxydopamine. Medical Biology. 1976;54:421–426. [PubMed] [Google Scholar]
- Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Research. 1993;609:174–184. doi: 10.1016/0006-8993(93)90871-j. [DOI] [PubMed] [Google Scholar]
- Li Y-W, Dampney RAL. Expression of Fos-like protein in the brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience. 1994;61:613–634. doi: 10.1016/0306-4522(94)90439-1. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytical tool in the study of the central nervous system. Journal of Neuroscience Methods. 1981;4:1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Lipski J, Kanjhan R, Kruszewska B, Rong W. Properties of presympathetic neurones in the rostral ventrolateral medulla in the rat: an intracellular study in vivo. Journal of Physiology. 1996;490:729–744. doi: 10.1113/jphysiol.1996.sp021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Kanjhan R, Kruszewska B, Smith M. Barosensitive neurons in the rostral ventrolateral medulla of the rat in vivo: Morphological properties and relationship to C1 adrenergic neurons. Neuroscience. 1995;69:601–618. doi: 10.1016/0306-4522(95)92652-z. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Dampney RA. Vasomotor neurons in the rostral ventrolateral medulla are organized topographically with respect to type of vascular bed but not body region. Neuroscience Letters. 1990;110:91–96. doi: 10.1016/0304-3940(90)90793-9. [DOI] [PubMed] [Google Scholar]
- McCulloch PF, Panneton WM, Guyenet PG. The rostral ventrolateral medulla mediates the sympathoactivation produced by chemical stimulation of the rat nasal mucosa. Journal of Physiology. 1999;516:471–484. doi: 10.1111/j.1469-7793.1999.0471v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Ito S, Rinaman L, Wiley RG, Sved AF. Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DβH-saporin. American Journal of Physiology. 1999;277:R1063–1075. doi: 10.1152/ajpregu.1999.277.4.R1063. [DOI] [PubMed] [Google Scholar]
- Milner TA, Morrison SF, Abate C, Reis DJ. Phenylethanolamine N-methyltransferase containing terminals synapse directly on sympathetic preganglionic neurons in the rat. Brain Research. 1988;448:205–222. doi: 10.1016/0006-8993(88)91258-9. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Raphe pallidus excites a unique class of sympathetic preganglionic neurons. American Journal of Physiology. 1993;265:R82–89. doi: 10.1152/ajpregu.1993.265.1.R82. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Ernsberger P, Milner TA, Callaway J, Gong A, Reis DJ. A glutamate mechanism in the intermediolateral nucleus mediates sympathoexcitatory responses to stimulation of the rostral ventrolateral medulla. In: Ciriello J, Caverson MM, Polosa C, editors. Progress in Brain Research, The Central Neural Organization of Cardiovascular Control. Vol. 8. Amsterdam: Elsevier; 1989. pp. 159–169. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Milner TA, Reis DJ. Reticulospinal vasomotor neurons of the rat rostral ventrolateral medulla: Relationship to sympathetic nerve activity and the C1 adrenergic cell group. Journal of Neuroscience. 1988;8:1286–1301. doi: 10.1523/JNEUROSCI.08-04-01286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, Terui N. Functionally different neurons are organized topographically in the rostral ventrolateral medulla of rabbits. Journal of the Autonomic Nervous System. 1997;67:67–78. doi: 10.1016/s0165-1838(97)00094-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: Morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or neurobiotin. Journal of Neuroscience Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Polosa C, Yoshimura M, Nishi S. Electrophysiological properties of sympathetic preganglionic neurons. Annual Review of Physiology. 1988;50:541–551. doi: 10.1146/annurev.ph.50.030188.002545. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Ruggiero DA, Morrison SF. The C1 area of the rostral ventrolateral medulla oblongata. A critical brainstem region for control of resting and reflex integration of arterial pressure. American Journal of Hypertension. 1989;2:363–374S. [PubMed] [Google Scholar]
- Ross CA, Armstrong DM, Ruggiero DA, Pickel VM, Joh TH, Reis DJ. Adrenaline neurons in the rostral ventrolateral medulla innervate thoracic spinal cord: a combined immunocytochemical and retrograde transport demonstration. Neuroscience Letters. 1981;25:257–262. doi: 10.1016/0304-3940(81)90401-8. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: Selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. Journal of Comparative Neurology. 1984a;228:168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. Journal of Neuroscience. 1984b;4:474–494. doi: 10.1523/JNEUROSCI.04-02-00474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. Journal of Comparative Neurology. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Sympathetic reflexes in rat after depletion of bulbospinal catecholaminergic neurons. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2000;279:R729–742. doi: 10.1152/ajpregu.2000.279.2.R729. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Bs¯tzinger neurons express mRNA for glycinergic transporter 2. Journal of Comparative Neurology. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Akey PJ, Guyenet PG. Location and electrophysiological characterization of rostral medullary adrenergic neurons that contain neuropeptide Y mRNA in rat medulla. Journal of Comparative Neurology. 1999;415:482–500. doi: 10.1002/(sici)1096-9861(19991227)415:4<482::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Morrison SF, Ruggiero DA, Reis DJ. Neurons of rostral ventrolateral medulla mediate somatic pressor reflex. American Journal of Physiology. 1989;256:R448–462. doi: 10.1152/ajpregu.1989.256.2.R448. [DOI] [PubMed] [Google Scholar]
- Stripe F, Barbieri L. Ribosome-inactivating proteins up to date. FEBS Letters. 1986;195:1–8. doi: 10.1016/0014-5793(86)80118-1. [DOI] [PubMed] [Google Scholar]
- Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Progress in Neurobiology. 1995;47:157–233. doi: 10.1016/0301-0082(95)00026-8. [DOI] [PubMed] [Google Scholar]
- Sun MK, Guyenet PG. Effect of clonidine and gamma-aminobutyric acid on the discharges of medullo-spinal sympathoexcitatory neurons in the rat. Brain Research. 1986;368:1–17. doi: 10.1016/0006-8993(86)91036-x. [DOI] [PubMed] [Google Scholar]
- Tucker DC, Saper CB, Ruggiero DA, Reis DJ. Organization of central adrenergic pathways: I. Relationships of ventrolateral medullary projections to the hypothalamus and spinal cord. Journal of Comparative Neurology. 1987;259:591–603. doi: 10.1002/cne.902590408. [DOI] [PubMed] [Google Scholar]
- Verberne AJM, Guyenet PG. Medullary pathway of the Bezold-Jarisch reflex in the rat. American Journal of Physiology. 1992;263:R1195–1202. doi: 10.1152/ajpregu.1992.263.6.R1195. [DOI] [PubMed] [Google Scholar]
- Verberne AJM, Stornetta RL, Guyenet PG. Properties of C1 and other ventrolateral medullary neurones with hypothalamic projections in the rat. Journal of Physiology. 1999;517:477–494. doi: 10.1111/j.1469-7793.1999.0477t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn CC, Picklo MJ, Lappi DA, Robertson D, Wiley RG. Central noradrenergic lesioning using anti-DBH-saporin: anatomical findings. Brain Research. 1996;740:175–184. doi: 10.1016/s0006-8993(96)00855-4. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Polosa C, Nishi S. Slow EPSP and the depolarizing action of noradrenaline on sympathetic preganglionic neurons. Brain Research. 1987a;414:138–142. doi: 10.1016/0006-8993(87)91334-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Polosa C, Nishi S. Noradrenaline-induced afterdepolarization in cat sympathetic preganglionic neurons in vitro. Journal of Neurophysiology. 1987b;57:1314–1324. doi: 10.1152/jn.1987.57.5.1314. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Stricker EM. Parkinson's disease: studies with an animal model. Life Sciences. 1984;35:5–18. doi: 10.1016/0024-3205(84)90147-4. [DOI] [PubMed] [Google Scholar]


