Abstract
Cardiovagal baroreflex sensitivity (BRS), the arterial baroreflex-mediated change in the R-R interval per unit change in systolic blood pressure, decreases with advancing age in sedentary adult humans. We determined the effects of regular aerobic exercise on the age-related decline in cardiovagal BRS.
In the cross-sectional study, 133 healthy men 18-79 years of age who were either sedentary, performing moderate aerobic exercise, or endurance exercise trained were studied. Among the sedentary men, cardiovagal BRS (phase IV of Valsalva’s manoeuvre) was progressively lower (P < 0·05) in the middle-aged (≈33 %) and older (≈60 %) groups compared with the young group. In contrast, cardiovagal BRS was similar in the young and middle-aged men in the moderate exercise and endurance-trained groups. Cardiovagal BRS was lower (P < 0·05) in the older exercising men, but the magnitude of decline across age (≈30 %) was only half as great as that in sedentary men. Cardiovagal BRS was 40-75 % greater (P < 0·05) in middle-aged and older men who exercised regularly compared with their sedentary peers.
In the intervention study, a 3 month aerobic exercise intervention (primarily walking) increased cardiovagal BRS by an average of 25 % (P < 0·05) in 13 previously sedentary middle-aged and older (56 ± 1 years) healthy men.
Our results demonstrate for the first time that regular aerobic exercise: (1) attenuates the age-associated decline in cardiovagal BRS; and (2) partially restores the loss of cardiovagal BRS in previously sedentary middle-aged and older healthy men.
Cardiovagal baroreflex sensitivity (BRS) can be defined as the slope of the relation between the R-R interval (interval between successive R waves of the ECG) and systolic blood pressure during an acute change in arterial blood pressure (Smyth et al. 1969). Cardiovagal BRS appears to be an important physiological determinant of electrical stability in the heart, especially during ischaemia (Billman et al. 1982; Schwartz et al. 1988; Cerati & Schwartz, 1991), and also is believed to contribute to the beat-to-beat control of arterial blood pressure (Sagawa, 1983).
Cardiovagal BRS decreases with age in sedentary humans (Gribbin et al. 1971; Ebert et al. 1992; Laitinen et al. 1998). We have been interested in the potential beneficial influence of habitual exercise on these age-related changes. In our initial set of cross-sectional studies (Davy et al. 1996, 1998), we used an indirect measure of cardiovagal BRS (i.e. spontaneous BRS; Bertinieri et al. 1985; Parati et al. 1988; Blaber et al. 1995). We found that cardiovagal BRS was: (1) higher in endurance exercise-trained compared with sedentary healthy postmenopausal women (Davy et al. 1996, 1998); and (2) lower in both endurance-trained and sedentary postmenopausal women compared with their respective premenopausal controls (Davy et al. 1998). Using the same measure, we subsequently found that cardiovagal BRS was not changed by 12 weeks of relatively low-intensity aerobic exercise training in previously sedentary postmenopausal women with mildly elevated systolic blood pressure (Davy et al. 1997). Thus, using an intervention study design we were unable to confirm the favourable influence of regular exercise on cardiovagal BRS that we had observed in our earlier cross-sectional studies (Davy et al. 1996, 1998).
One possibility is that our measure of cardiovagal BRS was not sensitive enough to detect either differences across age in sedentary compared with exercising adults or increases in response to exercise training. Indeed, the validity of this technique has recently been questioned (Watkins et al. 1996; Pitzalis et al. 1998). Another possibility is that the exercise ‘stimulus’ used in our intervention study (Davy et al. 1997) was not sufficiently strong to augment cardiovagal BRS to the levels observed in endurance-trained middle-aged and older subjects (Davy et al. 1996, 1998).
In the present investigation we re-tested two working hypotheses: (1) habitual aerobic exercise is associated with a delayed and/or attenuated reduction in cardiovagal BRS with age in healthy adult humans; and (2) regular moderate-intensity aerobic exercise can increase cardiovagal BRS in previously sedentary middle-aged and older adult humans. To do so, we used two different experimental approaches. To test the first hypothesis, using a cross-sectional design we determined cardiovagal BRS in groups of young, middle-aged and older healthy adult men who were either sedentary, performing moderate aerobic exercise, or engaged in strenuous endurance exercise training. To test the second hypothesis, cardiovagal BRS was determined in a group of the sedentary middle-aged and older men before and after a 3 month aerobic exercise intervention.
For both studies, we measured cardiovagal BRS using Valsalva’s manoeuvre (Eckberg & Sleight, 1992; Smith et al. 1996) because it: (1) is non-invasive and, thus, appropriate for use in older adults; (2) provides results that correlate strongly with invasive (‘gold standard’) techniques (Palmero et al. 1981); and (3) depicts population differences in cardiovagal BRS as well as invasive techniques (James et al. 1996). Finally, in the present investigation we studied men because they tend to demonstrate greater age-related declines in cardiovagal BRS than women (Laitinen et al. 1998). As such, we reasoned that any modulatory influence of habitual exercise on age-related changes should be more apparent in this gender.
METHODS
Subjects
A total of 133 healthy men voluntarily participated in the cross-sectional study. Subjects were classified into one of 9 groups based on their age and habitual physical activity habits. The three age groups comprised consecutive ∼20 year age ranges: young (18-37 years), middle-age (38-56 years) and older (57-79 years). Based on their exercise habits over the prior 2 years, the three physical activity groups were defined as ‘sedentary’ (no regular physical activity), ‘moderate exercise’ (moderately vigorous aerobic exercise 3-5 days week−1), or ‘endurance-trained’ (strenuous endurance exercise > 5 days week−1 and participating in local road race competitions). For the exercise intervention study, 13 previously sedentary middle-aged to older men were studied before and after a 3 month period of aerobic exercise training.
All subjects were normotensive (< 140/90 mmHg), non-smokers, non-obese, not taking any drugs known to affect cardiovascular and autonomic function, and had no overt disease as assessed by medical questionnaire, complete blood chemistries and haematological evaluation. Subjects 40 years and older were further evaluated by physical examination and by resting and maximal treadmill exercise electrocardiograms (ECG).
This study was performed according to the Declaration of Helsinki, and approved by the Human Research Committee of the University of Colorado at Boulder. Written informed consent was obtained from all participants after the nature, purpose and risks of the study were explained.
Measurements
Prior to testing subjects abstained from caffeine and fasted for a minimum of 4 h. Physically active men were studied 20-24 h after their last exercise bout to avoid the acute effects of exercise.
Cardiovagal baroreflex sensitivity
Cardiovagal BRS was determined using Valsalva’s manoeuvre as described previously (Eckberg & Sleight, 1992; Smith et al. 1996). After 15 min in the seated upright position subjects performed a Valsalva manoeuvre after a deep inspiration. Subjects were asked to maintain an expiratory mouth pressure of 40 mmHg for 10 s in duration by blowing through a 1 inch diameter tube against an open glottis so as to direct the pressure development into the thorax. Mouth pressure was measured with a pressure transducer, and was displayed visually on an oscilloscope calibrated and set to the target pressure to provide visual feedback to the subject. Subjects breathed regularly after release of strain. R-R interval (ECG) and arterial blood pressure (BP; Finapres, Ohmeda, Englewood, CO, USA) were measured continuously. The right hand used for BP monitoring was positioned at heart level to avoid hydrostatic effects. Finapres-derived BP was used because it is obtained non-invasively, and values agree closely with simultaneously recorded intra-arterial BP during the Valsalva manoeuvre (Imholz et al. 1988). Typical ECG, BP and mouth pressure responses are shown in Fig. 1. Subjects performed two Valsalva manoeuvres at 5 min intervals during which time heart rate and BP returned to baseline levels.
Figure 1. Typical ECG, blood pressure and mouth pressure responses to Valsalva’s manoeuvre.
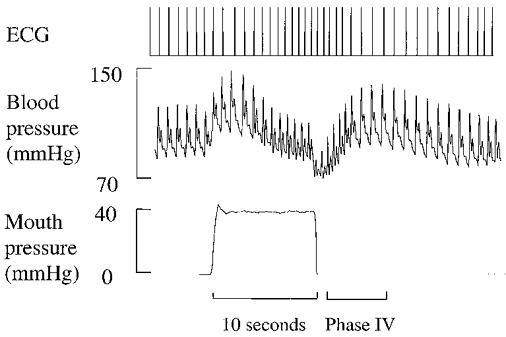
Phase IV (overshoot phase) is apparent shortly after release of mouth pressure when blood pressure increases from the systolic nadir to the peak, resulting in arterial baroreflex-mediated R-R interval prolongation.
Data for assessment of cardiovagal BRS were recorded and stored after analog-to-digital conversion at 500 Hz (CODAS, Dataq Instruments, Akron, OH, USA) onto a PC computer for later off-line analysis. Cardiovagal BRS was assessed during phase IV (overshoot phase) of the Valsalva manoeuvre, which occurs shortly after release of strain (Fig. 1). Cardiovagal BRS was determined as previously described (Palmero et al. 1981) and recommended by Eckberg & Sleight (1992). Briefly, consecutive systolic BP values during phase IV of the Valsalva manoeuvre were linearly regressed against corresponding (lag 1) R-R intervals from the time point where R-R intervals begin to lengthen and continued to the point of maximal systolic BP elevation. Typical results from data analysis are depicted in Fig. 2. The slope of this relation was used as a measure of cardiovagal BRS if the linear regression coefficient exceeded r = 0.80 and if systolic BP values overshot baseline values by > 15 mmHg. Using these criteria, the agreement between this technique and the ‘Oxford technique’ is maximized (r = 0.91) (Palmero et al. 1981). The average of slopes from two repeated trials was used to reduce variability associated with baroreflex testing. The reproducibility of the measurements was established in 15 subjects varying in age who were studied on 2 days ∼3 months apart. The coefficient of variation for the repeated assessment was 10 ± 2 %. This value corresponds closely with reported values of variation for both the Valsalva manoeuvre and for the ‘Oxford technique’ (Toyry et al. 1995; Laitinen et al. 1998).
Figure 2. Results of linear regression of systolic blood pressure and corresponding (lag 1) R-R intervals during phase IV of Valsalva’s manoeuvre.
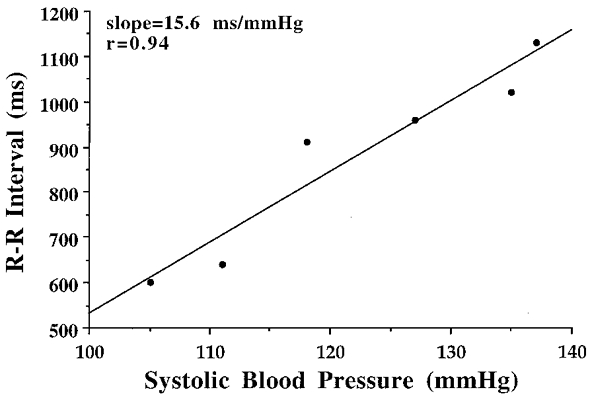
Regression begins during phase IV at the time of R-R interval prolongation (lag 1) in response to systolic blood pressure increases. Cardiovagal baroreflex sensitivity is expressed as the slope of this relation.
Heart rate variability
Heart rate variability (HRV) was determined using both time and frequency domain measures. Data were obtained via ECG from 300 s time series collected from subjects in the supine position during paced breathing (0.25 Hz) after 15 min of rest. The mean R-R interval and R-R interval standard deviation (RRSD) were calculated as the mathematical mean and standard deviation of these time series. Power spectral analysis of R-R intervals based on the Welch algorithm (Welch, 1967) was performed as previously described in detail by our laboratory (Davy et al. 1996). Briefly, 300 s time series of R-R intervals were interpolated by a cubic spline function at 4 Hz to assure equidistant time points, divided into three equal overlapping segments, detrended, Hanning filtered, and fast-Fourier transformed into their respective frequency components. The spectral estimates were based on the averaged periodograms. The spectral power in the high frequency (HF) band was assessed via integration of power in the frequency range between 0.15 and 0.35 Hz. Records were visually inspected to assure that the HF band was centred on the paced breathing frequency of 0.25 Hz. As excepted, the HF power of all subjects was centred very close to the paced breathing frequency (± 0.01 Hz), and thus analysis ranges and reported values were based on the 0.15-0.35 Hz range for the entire study population.
Resting arterial blood pressure and heart rate
Chronic resting BP was measured over the brachial artery using a semi-automated device (Dinamap XL, Johnson&Johnson, Arlington, TX, USA) with subjects in the sitting position according to American Heart Association guidelines. The average of three consecutive measures is reported. Resting heart rate was determined from the ECG.
Treadmill exercise test
To assess aerobic fitness, subjects performed incremental treadmill exercise using a modified Balke protocol as previously described (Stevenson et al. 1994). Maximal oxygen consumption (V̇O2,max) was measured using on-line computer-assisted open circuit spirometry. Additionally, heart rate and rating of perceived exertion were measured throughout the exercise and total exercise time to exhaustion was recorded.
Body composition
Fat mass and fat-free mass were measured using dual energy X-ray absorptiometry (Lunar Radiation, Madison, WI, USA).
Exercise intervention study
After completion of baseline measurements, 13 sedentary middle-aged to older men participated in a 3 month exercise intervention consisting of daily walking. Subjects were asked to walk 5-7 days week−1, 40-50 min day−1, at 65-80 % of their individual maximal heart rate determined during maximal exercise testing. As their fitness improved, some subjects integrated jogging into their exercise sessions in order to provide the necessary stimulus to maintain their heart rate within the prescribed range. Adherence to the exercise program was documented every 2 weeks based on data downloaded directly from heart rate monitors (Polar Electro Inc., Woodbury, NY, USA) and from subject diaries.
Statistical analysis
Data for the cross-sectional study were assessed via two-way (age × training status) analysis of variance. In the case of a significant F value, the Newman-Keuls post hoc test for multiple comparisons was used to assess differences between specific group means. Paired t tests were used to assess differences before compared with after the exercise intervention. Significance was set a priori at P < 0.05. All data are presented as means ±s.e.m. Univariate correlation and regression analyses were used to assess the strength of relations among variables of interest. Frequency estimates of HF HRV were natural logarithm transformed (lnHF) to adjust for skewness in their distribution and to allow application of parametric statistical analyses.
RESULTS
Cross-sectional study
Subject characteristics
The physical characteristics of the subjects in the cross-sectional study are presented in Table 1. There were no group differences in height or systolic BP. Diastolic BP increased with age within the normotensive range (P < 0.01), but was unrelated to exercise status. Body mass was not related to age, but was progressively lower with increasing habitual exercise status (P < 0.01). Body fat percentage increased with age and was inversely related to habitual exercise status (P < 0.05). V̇O2,max declined across age within each exercise status; the lowest values were observed in the sedentary men and the highest values in the endurance-trained men (P < 0.01). Heart rate at rest was unrelated to age, but generally was lower with increasing exercise status (P < 0.01). There was no difference in the number of years of prior exercise between the moderate exercise (17 ± 3) and endurance-trained (15 ± 2) groups (P > 0.05). However, consistent with our study design, in addition to their greater intensity of training the endurance-trained men exercised more frequently (5.5 ± 0.2 vs. 3.9 ± 0.2 days week−1, P < 0.0001) and for a longer duration (65 ± 3 vs. 47 ± 4 min session−1, both P < 0.0001) compared with the moderate exercise group.
Table 1.
Characteristics of subjects in the cross-sectional study
| Sedentary | Moderate exercise | Endurance trained | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Young | Middle | Older | Young | Middle | Older | Young | Middle | Older | |
| No. of subjects (n) | 16 | 18 | 15 | 9 | 10 | 16 | 18 | 16 | 15 |
| Age (years) | 27 ± 1 | 49 ± 1* | 63 ± 2*† | 26 ± 1 | 49 ± 2* | 62 ± 1*† | 28 ± 1 | 48 ± 2* | 64 ± 1*† |
| Height (cm) | 181 ± 2 | 176 ± 1 | 178 ± 2 | 177 ± 3 | 177 ± 2 | 173 ± 2 | 179 ± 2 | 174 ± 2 | 176 ± 2 |
| Body mass (kg) | 86.1 ± 4.2 | 84.6 ± 3.3 | 81.1 ± 1.8 | 78.6 ± 2.5 | 82.2 ± 3.1 | 79.6 ± 2.5 | 73.1 ± 1.6‡ | 72.0 ± 2.0 | 73.2 ± 1.6 |
| Body fat (%) | 21 ± 1 | 25 ± 2 | 26 ± 1* | 15 ± 2‡ | 21 ± 2* | 24 ± 1* | 11 ± 1‡ | 15 ± 1‡ | 18 ± 1*‡ |
| V̇O2.max (ml kg−1 min−1) | 42 ± 1 | 36 ± 1* | 31 ± 1*† | 50 ± 3‡ | 44 ± 2*‡ | 35 ± 1*†‡ | 61 ± 1‡§ | 53 ± 1*‡§ | 40 ± 2*†‡§ |
| Heart rate (beats min−1) | 61 ± 3 | 61 ± 2 | 64 ± 3 | 65 ± 2 | 57 ± 2 | 60 ± 2 | 53 ± 2§ | 49 ± 1‡ | 51 ± 3‡§ |
| Systolic BP (mmHg) | 112 ± 3 | 114 ± 4 | 113 ± 4 | 113 ± 4 | 114 ± 4 | 118 ± 4 | 111 ± 2 | 108 ± 3 | 115 ± 4 |
| Diastolic BP (mmHg) | 60 ± 2 | 70 ± 3* | 70 ± 2* | 60 ± 3 | 69 ± 3 | 70 ± 2 | 63 ± 1 | 65 ± 1 | 67 ± 2 |
P < 0.05 vs. young subjects within same activity group
P < 0.05 vs. middle-aged subjects within same activity group
P < 0.05 vs. sedentary subjects of same age group
P < 0.05 vs. moderately exercising subjects of same age group; V̇O2.max, maximal oxygen consumption; BP, blood pressure.
Cardiovagal BRS
Values for cardiovagal BRS are presented in Fig. 3. In the sedentary men, cardiovagal BRS was progressively lower with increasing age (young group, 17.8 ± 1.8 ms mmHg−1; middle-aged group, 11.9 ± 1.3 ms mmHg−1; older group, 7.1 ± 0.6 ms mmHg−1; all P < 0.05). In contrast, cardiovagal BRS was not different between the young and middle-aged men in the moderate exercise and endurance-trained groups (16.6 ± 2.1 vs. 16.5 ± 1.6 ms mmHg−1 and 17.7 ± 1.2 vs. 17.6 ± 1.1 ms mmHg−1, respectively, P > 0.05), although it was lower in the older men in these groups (11.1 ± 1.4 and 12.4 ± 1.3 ms mmHg−1; P < 0.05 vs. the other two age groups).
Figure 3. Cardiovagal baroreflex sensitivity of subjects in the cross-sectional study.
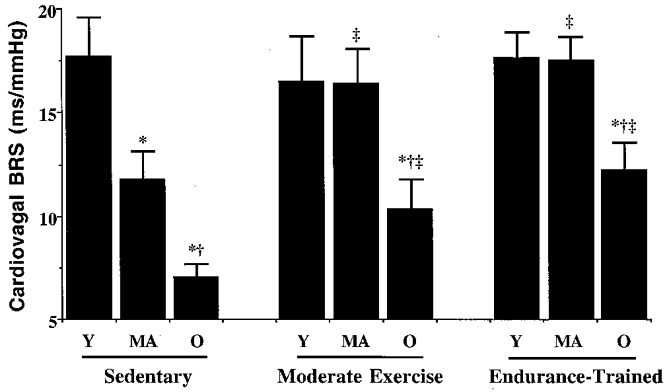
*P < 0.05 vs. young subjects (Y) within same activity group; †P < 0.05 vs. middle-aged subjects (MA) within same activity group; ‡P < 0.05 vs. sedentary subjects of same age group; Y, young; MA, middle age; O, older.
Cardiovagal BRS was not associated with physical activity status among the young men. However, the middle-aged and older men in the moderate exercise and endurance-trained groups demonstrated greater cardiovagal BRS than their age-matched sedentary peers (P < 0.05). Cardiovagal BRS was not different in the moderately exercising and endurance-trained men within either the middle-aged or the older age groups.
Heart rate variability
Values for HRV are presented in Table 2. RRSD declined 64 % with age in the sedentary men (P < 0.05). The decline in RRSD with age was 38 % in the men who performed moderate exercise and 18 % in the endurance-trained men (P > 0.05). RRSD was higher in middle-aged and older endurance-trained men compared with sedentary men of the same age (P < 0.05). Qualitatively similar results were obtained using the frequency domain measure of HRV. Natural logarithm transformed values of HF R-R interval variability (lnHF) declined in sedentary men, showing a greater relative decline (22 %; P < 0.05) across age than occurred with the exercising men (10-11 %; P > 0.05). lnHF was higher in endurance-trained men than in sedentary older men (P < 0.05), and this also tended to be the case in the middle-aged men (P = 0.10). lnHF tended to be higher in the moderately exercising men than in the sedentary older men (P = 0.10).
Table 2.
Heart rate variability measures in the cross-sectional study
| Sedentary | Moderate exercise | Endurance trained | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Young | Middle | Older | Young | Middle | Older | Young | Middle | Older | |
| No. of subjects (n) | 16 | 18 | 15 | 9 | 10 | 16 | 18 | 16 | 15 |
| RRi (ms) | 1014 ± 43 | 1023 ± 42 | 958 ± 40 | 929 ± 35 | 1075 ± 44 | 1010 ± 25 | 1143 ± 31§ | 1236 ± 27‡§ | 1216 ± 56‡§ |
| RRSD (ms) | 73 ± 10 | 40 ± 4* | 26 ± 3* | 56 ± 8 | 49 ± 5 | 35 ± 3 | 65 ± 7 | 60 ± 4‡ | 53 ± 7‡ |
| InHF (ln(ms2)) | 11.1 ± 0.3 | 9.6 ± 0.2* | 8.6 ± 0.3* | 10.5 ± 0.5 | 9.8 ± 0.4 | 9.3 ± 0.2 | 11.1 ± 0.3 | 10.7 ± 0.2 | 10.0 ± 0.4‡ |
P < 0.05 vs. young subjects within same activity group
P < 0.05 vs. sedentary subjects of same age group
P < 0.05 vs. moderately exercising subjects of same age group; RRi, R-R interval; RRSD, R-R interval standard deviation; lnHF, high frequency R-R interval variability.
Correlations
Several subject characteristics were related (P < 0.05) to cardiovagal BRS in the pooled study population, including percentage body fat (r = -0.40), V̇O2,max (r = 0.50), resting systolic BP (r = -0.27), resting diastolic BP (r = -0.33) and resting heart rate (r = -0.38). Physiological characteristics related to RRSD and lnHF included percentage body fat (r = -0.45 and -0.49, respectively), V̇O2,max (r = 0.47 and 0.55), resting systolic BP (r = -0.20 and -0.25), resting diastolic BP (r = -0.35 and -0.38), resting heart rate (r = -0.48 and -0.46) and cardiovagal BRS (r = 0.58 and 0.54).
Exercise intervention study
All 13 middle-aged and older men completed the aerobic exercise intervention. Subjects exercised for an average of 13.5 ± 1.0 weeks, 5.3 ± 0.3 sessions week−1, 45 ± 2 min session−1, at 72 ± 1 % of maximal heart rate. The aerobic exercise intervention did not alter body mass, percentage body fat, or BP (Table 3). Aerobic exercise training increased treadmill exercise time by ∼20 % (P < 0.001), and reduced heart rate and ratings of perceived exertion during standardized submaximal exercise (workload of ∼70 % of baseline V̇O2,max) (P < 0.01); V̇O2,max and resting heart rate were not significantly changed.
Table 3.
Characteristics of subjects in the exercise intervention study
| Before | After | |
|---|---|---|
| Age (years) | 56 ± 1 | — |
| Body mass (kg) | 85.6 ± 4.0 | 84.9 ± 4.0 |
| Body fat (%) | 29 ± 2 | 27 ± 2 |
| Systolic BP (mmHg) | 117 ± 4 | 119 ± 3 |
| Diastolic BP (mmHg) | 76 ± 2 | 78 ± 2 |
| Heart rate (beats min−1) | 65 ± 3 | 66 ± 4 |
| V̇O2.max (ml kg−1 min−1) | 30.0 ± 1.2 | 30.9 ± 1.3 |
| Treadmill time to exhaustion (min) | 9.6 ± 0.3 | 11.5 ± 0.4* |
| Submaximal exercise heart rate (beats min−1)† | 149 ± 2 | 140 ± 3* |
| Submaximal exercise RPE (units)† | 13 ± 1 | 11 ± 1* |
P < 0.05 vs. values before exercise intervention. V̇O2.max, maximal oxygen consumption; BP, blood pressure; RPE, rating of perceived exertion.
Treadmill walking at ∼70% of baseline V̇O2.max.
Cardiovagal BRS responses to aerobic exercise training are presented in Fig. 4. Regular aerobic exercise increased cardiovagal BRS ∼25 % (from 7.9 ± 0.8 to 9.8 ± 0.9 ms mmHg−1; P < 0.05) in the overall group. Values for cardiovagal BRS were higher after exercise training in nine of the 13 men, with an average increase in the nine men of ∼50 %. There were no significant changes following the intervention in mean R-R interval (938 ± 41 vs. 962 ± 47 ms), RRSD (30 ± 3 vs. 36 ± 6 ms), or lnHF (8.6 ± 0.3 vs. 8.8 ± 0.8 ln(ms2)) (all P > 0.05). There were no significant physiological correlates of the change in cardiovagal BRS in response to the exercise intervention in either the nine ‘responders’ or in the overall group of subjects.
Figure 4. Cardiovagal baroreflex sensitivity before and after the aerobic exercise intervention.
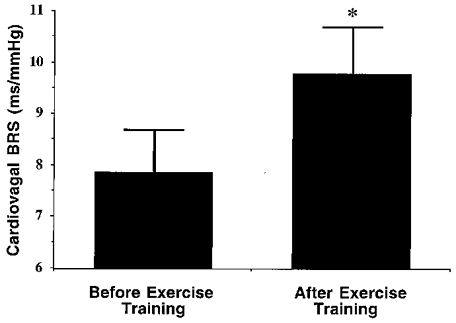
*P < 0.05 vs. before exercise.
DISCUSSION
The major findings from the present study were as follows. First, in contrast to sedentary healthy men, cardiovagal BRS appears to be maintained well into middle age in men who regularly perform aerobic exercise. Second, the magnitude of the decline in cardiovagal BRS with age appears to be markedly attenuated in men who exercise regularly. Third, moderate aerobic exercise appears to be a sufficient stimulus to attenuate the age-associated decline in cardiovagal BRS in healthy men. Fourth, regular aerobic exercise can increase cardiovagal BRS in previously sedentary middle-aged and older men. To our knowledge, these are the first experimental data to show that regular exercise can both lessen the age-related decline in cardiovagal BRS and augment the low baseline levels in sedentary middle-aged and older men.
Several studies in sedentary humans, some of which included older subjects with systolic hypertension, have reported that cardiovagal BRS declines 55-70 % from young to older adulthood (Gribbin et al. 1971; Ebert et al. 1992; Laitinen et al. 1998). Consistent with these previous observations, in the present study cardiovagal BRS was 60 % lower in older men than in young healthy sedentary men. The reduction in cardiovagal BRS in sedentary adults was observed by middle age, with that group demonstrating a 33 % lower BRS than the young controls. In contrast to the sedentary men, there was no difference in cardiovagal BRS between the young and middle-aged men in either the moderate exercise or the endurance-trained groups. Cardiovagal BRS was lower in the older men in both of these groups, but the magnitude of the difference between young and older men (∼30 %) was only half as great as that observed in the sedentary men.
There were no differences in cardiovagal BRS among the young men in any of the groups. This presumably was because sedentary young healthy men have normal (high) cardiovagal BRS and, therefore, a further beneficial influence of exercise would not be expected (ceiling effect). This is consistent with the earlier findings of Kingwell et al. (1992) who found in young adults that 4 weeks of endurance training did not change baroreflex sensitivity, although the sympathetic component of the baroreflex response was reduced. Somers et al. (1991) have shown that regular endurance exercise is associated with greater cardiovagal BRS in patients with elevated blood pressure who normally demonstrate reduced BRS. Consistent with this, in the present study cardiovagal BRS was 40-75 % higher in the middle-aged and older exercising men than in sedentary men of the same age. Thus, a favourable association between regular exercise and cardiovagal BRS was clearly observed at older ages in which baseline BRS was reduced. Importantly, based on the results of our cross-sectional study, moderate exercise appeared to be enough to delay and attenuate the age-associated decline in cardiovagal BRS – no additional benefit was demonstrated in middle-aged and older men who performed more strenuous and prolonged endurance training.
A related, but separate, question addressed in the present investigation was whether the age-related reduction in cardiovagal BRS could be at least partially restored by performing regular aerobic exercise. The results of our exercise intervention study provide experimental support for this concept. The increases in cardiovagal BRS observed with regular exercise were not related to increases in aerobic fitness, changes in body mass/composition, or reductions in resting heart rate (increase in R-R interval) or BP. It is important to emphasize that the increases in cardiovagal BRS were achieved within a 3 month period using a type (primarily walking), frequency, and intensity of exercise that most, if not all, healthy middle-aged and older men are able to perform.
Three previous intervention studies in middle-aged and older adults found no change in cardiovagal BRS in response to regular aerobic exercise (Sheldahl et al. 1994; Bowman et al. 1997; Davy et al. 1997), whereas Spina et al. (1994) actually reported a decrease in cardiovagal BRS after endurance exercise training. One explanation for the differences in results between the present study and the three earlier investigations showing no change was the use of a less intense exercise stimulus in the earlier studies. Specifically, the total volume of the exercise performed in the present study (5-6 days week−1 for ∼45 min session−1 for 13.5 weeks) was greater than that performed in these prior investigations (< 3 days week−1 for ∼20-43 min session−1 for 6-12 weeks). The wide variety of methods used to measure cardiovagal BRS is another likely contributor. For example, measurement of spontaneous cardiovagal BRS using the sequence method as in our earlier study (Davy et al. 1996) showed no difference before compared with after endurance exercise training in 10 subjects in whom measurements were performed in the present intervention study (13.5 ± 3.5 vs. 12.9 ± 1.8 ms mmHg−1; P > 0.05). The differences between the present results and those of Spina and colleagues (Spina et al. 1994) may be linked to at least two other methodological factors. Their use of heart rate as an indicator, when combined with the reduction in resting heart rate which they observed with exercise training, would effectively reduce cardiovagal BRS, whereas the use of R-R interval responsiveness would probably have shown, at the very least, no change. In addition, they employed a non-bolus drug infusion technique that probably produced a different baroreflex stimulus than that used in the present study. Finally, the increase in cardiovagal BRS observed with endurance training in the present study but not in our prior intervention studies (Davy et al. 1997) could be explained by the different genders of the subjects.
In our previous cross-sectional and intervention studies using the sequence technique (Davy et al. 1996, 1997, 1998), we found that spontaneous cardiovagal BRS was associated with HRV. This suggested that spontaneous cardiovagal BRS might be physiologically linked to tonic vagal modulation of heart rate, at least in the context of ageing and habitual exercise. In the present cross-sectional study, we also observed an association between HRV and Valsalva-derived cardiovagal BRS across age and habitual exercise groups. However, our exercise intervention resulted in a substantial increase in cardiovagal BRS without a significant increase in our measures of tonic vagal modulation of heart rate. This suggests that these events may not be coupled in a mechanistic ‘cause and effect’ manner, and that moderate-intensity endurance exercise training can augment cardiovagal BRS without obvious corresponding increases in tonic vagal modulation of heart rate. It also suggests that the prior associations noted for these two variables may have been due to the fact that measurement of spontaneous cardiovagal BRS via the sequence technique is influenced by HRV. Alternatively, fluctuations in R-R interval represent respiratory gating of vagal motoneurones (Gilbey et al. 1984). It is possible that respiration influences on HRV were maximal in our subjects before training (Eckberg et al. 1988), and that an increase in cardiovagal BRS of the magnitude demonstrated would not be expected to increase this respiratory influence, and therefore HRV, further.
We can only speculate on the mechanisms by which regular aerobic exercise is associated with augmented cardiovagal BRS in middle-aged and older men. In our intervention study we found no physiological correlates of increases in cardiovagal BRS among the individual subjects. One possibility is that habitual exercise results in increased compliance in the large cardiothoracic arteries where the baroreceptors are located (ascending aorta and carotid sinus; Tanaka et al. 2000). The stiffening of these segments is believed to contribute to the age-related decline in cardiovagal BRS by impairing the transduction of BP-related stimuli which would reduce baroreceptor afferent responsiveness (Rowe, 1987). Enhanced arterial compliance associated with habitual exercise would presumably act to augment stimulus transduction and afferent responsiveness. In addition, reductions in muscarinic receptor density in the sino-atrial node with advancing age may contribute to the decline in cardiovagal BRS (Poller et al. 1997; Brodde et al. 1998). If so, regular exercise may increase cardiovagal BRS in middle-aged and older humans by augmenting muscarinic receptor density. Another possibility is that an increase in blood volume contributed to the increase in cardiovagal BRS with our exercise intervention. We did not measure blood volume in the present study, but the results of previous studies indicate that blood volume does not appear to increase in middle-aged and older adults in response to moderate exercise training programmes (Hagberg et al. 1989; Stachenfeld et al. 1998). Moreover, blood volume has been reported to influence the absolute blood pressure responses during the Valsalva manoeuvre, but not the values for cardiovagal BRS per se (Luster et al. 1996; Fritsch-Yelle et al. 1999). Finally, it is possible that habitual exercise somehow modulates CNS integration of afferent feedback to enhance cardiovagal BRS.
There are experimental limitations of our study that should be mentioned. First, we studied only healthy men; the effects of regular exercise on cardiovagal BRS in healthy women or in adults with chronic disease may differ from those reported here. Second, the Valsalva manoeuvre-derived method employed to measure cardiovagal BRS is a complex stimulus for cardiovascular reflexes (Eckberg & Sleight, 1992; Smith et al. 1996). For example, this procedure may produce acute baroreflex resetting (Smith et al. 1996). If the resetting differed among groups (cross-sectional study) and/or changed with exercise training (intervention study), it could have influenced our results. We are not aware of any experimental evidence, however, that would support such a potential effect. Moreover, muscle sympathetic nervous system activity increases during the early portion of the Valsalva manoeuvre (Smith et al. 1996). If sympathetic activity to the sino-atrial node in the heart also increases during this period, and if the increases were different among groups or changed with training, it could have influenced our results. However, we have demonstrated that reflex control of muscle sympathetic activity does not change with age (Ng et al. 1994), and is not significantly influenced by the endurance-trained state (Seals, 1991). Thus, we do not believe that changes in sympathetic nervous activity had an important influence on our results. Finally, it is recognized that controversy exists regarding which variable, heart rate or R-R interval, is most appropriate for determining the sensitivity of cardiovagal baroreflex. Our rationale for using the latter measure in the present study is as follows. Because alterations in BP induced by the Valsalva manoeuvre are both rapid and transient, the reflex chronotropic response is produced primarily by changes in cardiac-vagal nerve activity (Elisberg et al. 1953). Increases in cardiac-vagal tone appear to be protective against the development of ventricular fibrillation and cardiac sudden death during acute exposure to cardiac ischaemia (Cerati & Schwartz, 1991). Thus, methods that allow assessment of changes in cardiac-vagal nerve firing rate can provide physiologically insightful and clinically relevant information for human function and health. In this context, changes in the stimulus to the arterial baroreceptors cause proportional, linear changes in cardiac-vagal nerve firing which, in turn, result in corresponding changes in R-R interval, which is not the case with heart rate (Carlsten et al. 1957; Angell-James & Lumley, 1975; Borst et al. 1983).
In addition to possible influences on cardiac electrical stability (Billman et al. 1982, 1984; Schwartz et al. 1988; Cerati & Schwartz, 1991), the modulatory effects of age and habitual endurance exercise on cardiovagal BRS may have important implications for arterial blood pressure control. For example, cardiovagal BRS declines with age in sedentary subjects, whereas blood pressure variability increases (Zito et al. 1991; Imai et al. 1997; Davy et al. 1998). The opposite is the case with subjects undertaking endurance exercise, which is associated with augmented cardiovagal BRS and reduced blood pressure variability in middle-aged and older adults (Davy et al. 1996, 1998; Stevenson et al. 1997; Seals et al. 1999).
In conclusion, the results of the present study provide experimental evidence supporting the hypothesis that regular aerobic exercise both attenuates the age-associated decline in cardiovagal BRS, and partially restores the loss of cardiovagal BRS in previously sedentary middle-aged and older healthy men. This could have important physiological implications for the maintenance of myocardial electrical stability and/or control of arterial blood pressure in older adults.
Acknowledgments
We thank Yoli Casas, Linda Shapiro, Jayne Semmler and Teresa Wilson for their technical assistance. This study was supported by National Institutes of Health awards AG06537, AG13038 and AG16071 (D.R.S.), AG00847 (H.T.) and HL03840 (C.A.D.).
References
- Angell-James JE, Lumley JSP. Changes in the mechanical properties of the carotid sinus region and carotid sinus nerve activity in patients undergoing carotid endarterectomy. The Journal of Physiology. 1975;244:80–81P. [PubMed] [Google Scholar]
- Bertinieri G, DiRienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. Journal of Hypertension. 1985;3(suppl. 3):S79–81. [PubMed] [Google Scholar]
- Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–879. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- Billman GE, Schwartz PJ, Stone HL. The effects of daily exercise on susceptibility to sudden cardiac death. Circulation. 1984;69:1182–1189. doi: 10.1161/01.cir.69.6.1182. [DOI] [PubMed] [Google Scholar]
- Blaber A, Yamamoto Y, Hughson R. Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. American Journal of Physiology. 1995;37:H1682–1687. doi: 10.1152/ajpheart.1995.268.4.H1682. [DOI] [PubMed] [Google Scholar]
- Borst C, Karemaker JM, Dunning AJ, Bouman LN, Wagner J. Frequency limitation in the human baroreceptor reflex. Journal of the Autonomic Nervous System. 1983;9:381–397. doi: 10.1016/0165-1838(83)90003-6. [DOI] [PubMed] [Google Scholar]
- Bowman AJ, Clayton RH, Murray A, Reed JW, Subhan MMF, Ford GA. Effects of aerobic exercise training and yoga on the baroreflex in healthy elderly persons. European Journal of Clinical Investigation. 1997;27:443–449. doi: 10.1046/j.1365-2362.1997.1340681.x. [DOI] [PubMed] [Google Scholar]
- Brodde OE, Konschak U, Becker K, Ruter F, Poller U, Jakubetz J, Radke J, Zerkowski HR. Cardiac muscarinic receptors decrease with age. Journal of Clinical Investigation. 1998;101:471–478. doi: 10.1172/JCI1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten A, Folkow B, Hamberger CA. Cardiovascular effects of direct vagal stimulation in man. Acta Physiologica Scandinavica. 1957;41:68–76. doi: 10.1111/j.1748-1716.1957.tb01510.x. [DOI] [PubMed] [Google Scholar]
- Cerati D, Schwartz PJ. Single cardiac vagal fiber activity, acute myocardial ischemia, and risk for sudden death. Circulation Research. 1991;69:1389–1401. doi: 10.1161/01.res.69.5.1389. [DOI] [PubMed] [Google Scholar]
- Davy KP, DeSouza CA, Jones PP, Seals DR. Elevated heart rate variability in physically active young and older adult women. Clinical Science. 1998;94:579–584. doi: 10.1042/cs0940579. [DOI] [PubMed] [Google Scholar]
- Davy KP, Miniclier NL, Taylor JA, Stevenson ET, Seals DR. Elevated heart rate variability in physically active postmenopausal women: a cardioprotective effect? American Journal of Physiology. 1996;271:H455–460. doi: 10.1152/ajpheart.1996.271.2.H455. [DOI] [PubMed] [Google Scholar]
- Davy KP, Willis WL, Seals DR. Influence of exercise training on heart rate variability in post-menopausal women with elevated arterial blood pressure. Clinical Physiology. 1997;17:31–40. doi: 10.1046/j.1365-2281.1997.01010.x. [DOI] [PubMed] [Google Scholar]
- Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. American Journal of Physiology. 1992;263:H798–803. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Rea RF, Andersson OK, Hedner T, Pernow J, Lundberg JM, Wallin BG. Baroreflex modulation of sympathetic activity and sympathetic neurotransmitters in humans. Acta Physiologica Scandinavica. 1988;133:221–231. doi: 10.1111/j.1748-1716.1988.tb08401.x. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease. Oxford, UK: Clarendon Press; 1992. [Google Scholar]
- Elisberg EI, Miller G, Weinberg SL, Katz LN. The effect of the Valsalva Maneuver on the circulation. II. The role of the autonomic nervous system in the production of the overshoot. American Heart Journal. 1953;45:227–236. doi: 10.1016/0002-8703(53)90183-5. [DOI] [PubMed] [Google Scholar]
- Fritsch-Yelle JM, Convertino VA, Schlegel TT. Acute manipulations of plasma volume alter arterial pressure responses during Valsalva maneuvers. Journal of Applied Physiology. 1999;86:1852–1857. doi: 10.1152/jappl.1999.86.6.1852. [DOI] [PubMed] [Google Scholar]
- Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. The Journal of Physiology. 1984;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circulation Research. 1971;29:424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Montain SJ, Martin WH, Ehsani AA. Effect of exercise training in 60- to 69-year-old persons with essential hypertension. American Journal of Cardiology. 1989;64:348–353. doi: 10.1016/0002-9149(89)90533-x. [DOI] [PubMed] [Google Scholar]
- Imai Y, Aihara A, Ohkubo T, Nagai K, Tsuji I, Minami N, Satoh H, Hisamichi S. Factors that affect blood pressure variability: a community-based study in Ohasama, Japan. American Journal of Hypertension. 1997;10:1281–1289. doi: 10.1016/s0895-7061(97)00277-x. [DOI] [PubMed] [Google Scholar]
- Imholz BPM, van Montfrans GA, Settels JJ, van der Hoeven GMA, Karemaker JM, Weiling W. Continuous non-invasive blood pressure monitoring: reliability of the Finapres device during the Valsalva manoeuvre. Cardiovascular Research. 1988;22:390–397. doi: 10.1093/cvr/22.6.390. [DOI] [PubMed] [Google Scholar]
- James MA, Robinson TG, Panerai RB, Potter JF. Arterial baroreceptor-cardiac reflex sensitivity in the elderly. Hypertension. 1996;28:953–960. doi: 10.1161/01.hyp.28.6.953. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Dart AM, Jennings GL, Korner PI. Exercise training reduces the sympathetic component of the blood pressure-heart rate baroreflex in man. Clinical Science. 1992;82:357–362. doi: 10.1042/cs0820357. [DOI] [PubMed] [Google Scholar]
- Laitinen T, Hartikainen J, Vanninen E, Niskanen L, Geelen G, Lansimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. Journal of Applied Physiology. 1998;84:576–583. doi: 10.1152/jappl.1998.84.2.576. [DOI] [PubMed] [Google Scholar]
- Luster EA, Baumgartner N, Adams WC, Convertino VA. Effects of hypovolemia and posture on responses to the Valsalva maneuver. Aviation, Space, and Environmental Medicine. 1996;67:308–313. [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. American Journal of Physiology. 1994;267:H344–353. doi: 10.1152/ajpheart.1994.267.1.H344. [DOI] [PubMed] [Google Scholar]
- Palmero HA, Caeiro TF, Iosa DJ, Bas J. Baroreceptor reflex sensitivity index derived from phase 4 of the Valsalva maneuver. Hypertension. 1981;3:II-134–II-137. doi: 10.1161/01.hyp.3.6_pt_2.ii-134. [DOI] [PubMed] [Google Scholar]
- Parati G, DiRienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension. 1988;12:214–219. doi: 10.1161/01.hyp.12.2.214. [DOI] [PubMed] [Google Scholar]
- Pitzalis MV, Mastropasqua F, Passantino A, Massari F, Ligurgo L, Forleo C, Balducci C, Lombardi F, Rizzon P. Comparison between noninvasive indices of baroreceptor sensitivity and the phenylephrine method in post-myocardial infarction patients. Circulation. 1998;97:1362–1367. doi: 10.1161/01.cir.97.14.1362. [DOI] [PubMed] [Google Scholar]
- Poller U, Nedelka G, Radke J, Ponicke K, Brodde OE. Age-dependent changes in cardiac muscarinic receptor function in healthy volunteers. Journal of the American College of Cardiology. 1997;29:187–193. doi: 10.1016/s0735-1097(96)00437-8. [DOI] [PubMed] [Google Scholar]
- Rowe JW. Clinical consequences of age-related impairments in vascular compliance. American Journal of Cardiology. 1987;60:68–71G. doi: 10.1016/0002-9149(87)90594-7. [DOI] [PubMed] [Google Scholar]
- Sagawa K. Baroreflex control of systemic arterial pressure and vascular bed. In: Abboud FM, Shepherd JT, Geiger SR, editors. Handbook of Physiology, section 2, The Cardiovascular System, Peripheral Circulation and Organ Blood Flow. III. Bethesda, MD, USA: American Physiological Society; 1983. pp. 453–496. part 2. [Google Scholar]
- Schwartz PJ, Vanoli E, Badiale MS, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden cardiac death: new insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78:969–979. doi: 10.1161/01.cir.78.4.969. [DOI] [PubMed] [Google Scholar]
- Seals DR. Hypertension. Vol. 17. Dallas: 1991. Sympathetic neural adjustments to stress in physically trained and untrained humans; pp. 36–43. [DOI] [PubMed] [Google Scholar]
- Seals DR, Stevenson ET, Jones PP, DeSouza CA, Tanaka H. Lack of age-associated elevations in 24-h systolic and pulse pressures in women who exercise regularly. American Journal of Physiology. 1999;277:H947–955. doi: 10.1152/ajpheart.1999.277.3.H947. [DOI] [PubMed] [Google Scholar]
- Sheldahl LM, Ebert TJ, Cox B, Tristani FE. Effect of aerobic training on baroreflex regulation of cardiac and sympathetic function. Journal of Applied Physiology. 1994;76:158–165. doi: 10.1152/jappl.1994.76.1.158. [DOI] [PubMed] [Google Scholar]
- Smith ML, Beightol LA, Fritsch-Yelle JM, Ellenbogen KA, Porter TR, Eckberg DL. Valsalva’s maneuver revisited: a quantitative method yielding insight into human autonomic control. American Journal of Physiology. 1996;271:H1240–1249. doi: 10.1152/ajpheart.1996.271.3.H1240. [DOI] [PubMed] [Google Scholar]
- Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man: a quantitative method of assessing baroreflex sensitivity. Circulation Research. 1969;24:109–121. doi: 10.1161/01.res.24.1.109. [DOI] [PubMed] [Google Scholar]
- Somers VK, Conway J, Johnston J, Sleight P. Effects of endurance training on baroreflex sensitivity and blood pressure in borderline hypertensives. Lancet. 1991;333:1363–1368. doi: 10.1016/0140-6736(91)93056-f. [DOI] [PubMed] [Google Scholar]
- Spina RJ, Bourey RE, Ogawa T, Ehsani AA. Effects of exercise training on alpha-adrenergic mediated pressor responses and baroreflex function in older subjects. Journal of Gerontology. 1994;49:B277–281. doi: 10.1093/geronj/49.6.b277. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Mack GW, DiPietro L, Morocco TS, Jozsi AC, Nadel ER. Regulation of blood volume during training in post-menopausal women. Medicine and Science in Sports and Exercise. 1998;30:92–98. doi: 10.1097/00005768-199801000-00013. [DOI] [PubMed] [Google Scholar]
- Stevenson ET, Davy KP, Jones PP, DeSouza CA, Seals DR. Blood pressure risk factors in healthy postmenopausal women: physical activity and hormone replacement. Journal of Applied Physiology. 1997;82:652–660. doi: 10.1152/jappl.1997.82.2.652. [DOI] [PubMed] [Google Scholar]
- Stevenson ET, Davy KP, Seals DR. Maximal aerobic capacity and total blood volume in highly trained middle-aged and older female endurance athletes. Journal of Applied Physiology. 1994;77:1691–1696. doi: 10.1152/jappl.1994.77.4.1691. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Toyry J, Mantysaari M, Hartkainen J, Lansimies E. Day-to-day variability of cardiac autonomic regulation parameters in normal subjects. Clinical Physiology. 1995;15:39–46. doi: 10.1111/j.1475-097x.1995.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Grossman P, Sherwood A. Noninvasive assessment of baroreflex control in borderline hypertension comparison with the phenylephrine method. Hypertension. 1996;28:238–243. doi: 10.1161/01.hyp.28.2.238. [DOI] [PubMed] [Google Scholar]
- Welch P. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short modified periodograms. IEEE Transactions in Audio Electroaccoustics. 1967;15:70–73. [Google Scholar]
- Zito M, Parati G, Omboni S, Cervone C, Ulian L, D’Aviero M, Abate G, Mancia G. Effect of ageing on blood pressure variability. Journal of Hypertension. 1991;9:S328–329. [PubMed] [Google Scholar]


