Abstract
While a pathway for Ca2+ accumulation into mitochondria has long been established, its functional significance is only now becoming clear in relation to cell physiology and pathophysiology. The observation that mitochondria take up Ca2+ during physiological Ca2+ signalling in a variety of cell types leads to four questions: (i) ‘What is the impact of mitochondrial Ca2+ uptake on mitochondrial function?’ (ii) ‘What is the impact of mitochondrial Ca2+ uptake on Ca2+ signalling?’ (iii) ‘What are the consequences of impaired mitochondrial Ca2+ uptake for cell function?’ and finally (iv) ‘What are the consequences of pathological [Ca2+]c signalling for mitochondrial function?’ These will be addressed in turn. Thus: (i) accumulation of Ca2+ into mitochondria regulates mitochondrial metabolism and causes a transient depolarisation of mitochondrial membrane potential. (ii) Mitochondria may act as a spatial Ca2+ buffer in many cells, regulating the local Ca2+ concentration in cellular microdomains. This process regulates processes dependent on local cytoplasmic Ca2+ concentration ([Ca2+]c), particularly the flux of Ca2+ through IP3-gated channels of the endoplasmic reticulum (ER) and the channels mediating capacitative Ca2+ influx through the plasma membrane. Consequently, mitochondrial Ca2+ uptake plays a substantial role in shaping [Ca2+]c signals in many cell types. (iii) Impaired mitochondrial Ca2+ uptake alters the spatiotemporal characteristics of cellular [Ca2+]c signalling and downregulates mitochondrial metabolism. (iv) Under pathological conditions of cellular [Ca2+]c overload, particularly in association with oxidative stress, mitochondrial Ca2+ uptake may trigger pathological states that lead to cell death. In the model of glutamate excitotoxicity, microdomains of [Ca2+]c are apparently central, as the pathway to cell death seems to require the local activation of neuronal nitric oxide synthase (nNOS), itself held by scaffolding proteins in close association with the NMDA receptor. Mitochondrial Ca2+ uptake in combination with NO production triggers the collapse of mitochondrial membrane potential, culminating in delayed cell death.
The existence of a pathway that allows mitochondria to accumulate Ca2+ has been firmly established for about 40 years. Nevertheless, only recently has a consensus begun to emerge concerning the physiological significance of the pathway. The central question that has dogged us has been, apparently so simply: ‘Do mitochondria take up Ca2+ during the normal processes involved in the routine business of [Ca2+]c signalling?’ Alternatively, as long proposed: ‘Is the pathway only active under particular conditions or in pathological states?’ It is striking that in any contemporary meeting about mitochondrial physiology, a number of presentations will focus significantly on the mechanism, process and impact of mitochondrial Ca2+ uptake. At The Journal of Physiology Symposium which originated this series of reviews, we have seen the experiments of Rizzuto et al. (2000, this issue of The Journal of Physiology) using recombinant site-directed aequorins that have gone so far in resolving these questions. The functional importance of this pathway is reflected by the presentation from Maechler & Wollheim (2000, this issue) which focuses on the biochemical and functional consequences of mitochondrial Ca2+ uptake in pancreatic β-cells, while that of Hajnóczky et al. (2000, this issue) focuses again on the transfer of Ca2+ from the ER to mitochondria and on the consequence for cell death when combined with proapoptotic factors, such as ceramide. Crompton (2000, this issue) discusses the mitochondrial permeability transition pore, a pathological consequence of mitochondrial Ca2+ accumulation. Inevitably there is a significant overlap in our various contributions, for which I make no apology, as one of the pleasing aspects of the recent development in this area is the way in which different approaches and different technologies have provided data which (generally) converge towards a consensus view. In this brief review, I propose to discuss the functional consequences of the mitochondrial Ca2+ uptake in a variety of models.
Perhaps it is inevitable that many questions remain about the comparative cell physiology of mitochondrial Ca2+ handling. Nevertheless, once mitochondrial Ca2+ uptake is demonstrable under physiological conditions, this leads directly to a number of interrelated questions. ‘What is the impact of Ca2+ signalling on mitochondrial function?’ Then, conversely: ‘What is the impact of mitochondrial Ca2+ uptake on [Ca2+]c signalling?’ Both of these questions then have pathophysiological ramifications: ‘What is the impact of impaired mitochondrial Ca2+ uptake on [Ca2+]c signalling?’ and ‘What is the impact of abnormal [Ca2+]c signalling on mitochondrial function?’
Pathways of mitochondrial Ca2+ accumulation
Given the sophistication of our understanding of many ion channels and membrane transporters, our ignorance about the molecular properties of the Ca2+ uptake pathway seems remarkable. Mitochondria take up Ca2+ primarily through a uniporter, whose molecular nature still eludes us. Some data suggest that this might act like a channel, opening with increased probability once the local [Ca2+]c rises (for review, see Rizzuto et al. 2000, this issue, and Gunter et al. 1998). The influx of Ca2+ into the matrix by this route is dependent on the electrochemical potential gradient for Ca2+. This is developed and maintained firstly by the process of mitochondrial respiration, which establishes a large potential gradient, the mitochondrial membrane potential (ΔΨm), generally estimated to be in the order of 150-200 mV or so negative to the cytosol, together with a low resting intramitochondrial Ca2+ concentration [Ca2+]m, maintained primarily by the mitochondrial Na+-Ca2+ exchanger. The experimental collapse of ΔΨm using an uncoupler is a simple and much used experimental tool to explore the consequence of preventing mitochondrial Ca2+ accumulation. Similarly, the collapse of ΔΨm as a response to pathological states – during anoxia or as a response to disordered mitochondrial respiration, for example – will also limit mitochondrial Ca2+ uptake and may contribute to the emergent cellular pathophysiology. This pathway has often been referred to as the Ruthenium Red (RuR)-sensitive uptake pathway, despite the very poor specificity of RuR, which inhibits Ca2+ flux through a variety of different channels. In addition to this uniporter, another pathway has been described recently as the rapid uptake pathway (Sparagna et al. 1995), proposed by the authors as a mechanism for the rapid uptake of Ca2+ at physiological concentrations. The comparative cell physiology and expression of this mechanism remain to be evaluated. The re-equilibration of mitochondrial Ca2+ is largely achieved through the activity of the mitochondrial Na+-Ca2+ exchanger, an exchanger distinct from that found in the plasmalemma, and Na+ is then exchanged for protons through a rapid Na+-H+ exchange. It has been suggested that during hypoxia, mitochondria may become Ca2+ loaded by the reversal of the Na+-Ca2+ exchanger (Griffiths et al. 1998). This would require that the intracellular Ca2+ concentration must be very high, that mitochondria must also be Na+ loaded and that the rapid Na+-H+ exchange be suppressed.
Impact of mitochondrial Ca2+ uptake on mitochondrial function
The major targets of the mitochondrial Ca2+ import pathway are the dehydrogenases of the TCA cycle, as the rate-limiting enzymes are all upregulated by Ca2+-dependent processes, as discussed by Maechler & Wollheim (2000) and Rizzuto et al. (2000) (this issue, and see McCormack et al. 1990 for review). Remarkably, it is possible to observe this biochemical activation in single cells by measuring an increase in the autofluorescence of NADH (signalling an increased NADH/NAD+ ratio) following a rise in [Ca2+]c in adrenal cortical cells (Pralong et al. 1992), in sensory neurons (Duchen, 1992), and in hepatocytes (Hajnóczky et al. 1995). A similar process was associated with a measurable increase in oxygen consumption in Limulus photoreceptors (Fein & Tsacopoulos, 1988). Therefore it was reasonable, even some 10 years ago, to infer that modest and physiological Ca2+ signals must be associated with significant movement of Ca2+ into mitochondria, even before that rise in [Ca2+]m was itself directly demonstrable. The trace shown in Fig. 1A illustrates the change in NADH autofluorescence recorded from a single mouse sensory neuron following a very brief (100 ms) depolarisation to initiate voltage-gated Ca2+ influx. The change in signal was entirely dependent on external Ca2+ and was blocked by microinjection of the cell with Ruthenium Red (Duchen, 1992), demonstrating the dependence of the response on mitochondrial Ca2+ uptake. It is perhaps worth noting that the literature concerning mitochondrial Ca2+ uptake is currently dominated by studies of [Ca2+]c signalling in non-excitable cells, and therefore concerns mitochondrial Ca2+ uptake following release of Ca2+ from ER, while the experiment illustrated in Fig. 1A relates to the impact on mitochondrial function of Ca2+ influx through voltage-gated Ca2+ channels in an excitable cell. It is also noteworthy that the [Ca2+]c transient associated with this stimulus lasted ∼10 s (measured independently, and not shown) while the increased autofluorescence typically lasted minutes, often as long as 5-10 min. The activation of dehydrogenases also stimulates mitochondrial respiration leading to an increase in ΔΨm (see Robb-Gaspers et al. 1998) driving an increase in ATP production (see Rizzuto et al. 2000, this issue).
Figure 1. Consequences of mitochondrial Ca2+ uptake for mitochondrial function.
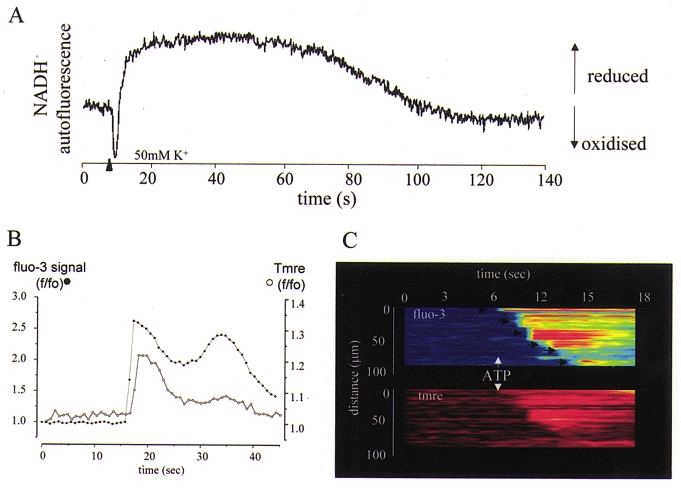
A, changes in NADH autofluorescence, excited at 350 nm and recorded at 450 nm, are shown following a 100 ms pulse of 50 mm KCl, which was used to depolarise a mouse sensory neuron and thence to raise [Ca2+]c. The autofluorescence initially showed a transient decrease attributable to a transient depolarisation of ΔΨm that accompanies the Ca2+ flux into mitochondria (see below and Fig. 4). This was then superseded by a prolonged increase in signal (increased NADH/NAD+ ratio) which is attributed to activation of the dehydrogenases of the TCA cycle by a high intramitochondrial [Ca2+]. The entire response was blocked by microinjection of the cell with Ruthenium Red (not shown). B, a rise in [Ca2+]c causes a transient mitochondrial depolarisation. Rat cortical astrocytes were loaded with tetramethyl-rhodamine ethyl ester (TMRE) and the [Ca2+]c indicator fluo-3, and imaged simultaneously on a confocal microscope (Zeiss 510CLSM). Application of ATP to a single cell raised [Ca2+]c (•) by IP3-dependent mobilisation from ER stores, and caused a small transient mitochondrial depolarisation (○), signalling mitochondrial Ca2+ uptake (an increase in TMRE fluorescence signals mitochondrial depolarisation – see Boitier et al. 1999). The same phenomenon is seen on a larger scale as [Ca2+]c waves are propagated through a network of interconnected astrocytes, as shown in C. Here, ATP application initiated a wave that propagated from cell to cell, imaged as described above. The steps from cell to cell are indicated by arrows. The simultaneous measurement of ΔΨm reveals a wave of mitochondrial depolarisation propagating through the network.
Mitochondrial Ca2+ import is an electrogenic process, as the movement of Ca2+ is not countered by any other ion exchange and therefore acts like an inward current tending to depolarise the mitochondrial membrane. This is observed as a small and transient depolarisation of the mitochondrial membrane in response to the rising phase of the [Ca2+]c transient – the period of maximal Ca2+ flux (Figs 1B and C and 4A and see Duchen, 1992; Peuchen et al. 1996b), which would then be superseded by an increase in potential as the slower enzyme activation takes over. The traces shown in Fig. 1B and C show the small mitochondrial depolarisation seen in association with a rise in [Ca2+]c in cortical astrocytes in culture. Here, application of an agonist that mobilised Ca2+ from IP3-sensitive stores (in this case ATP, acting at P2u receptors – see Peuchen et al. 1996a) raises [Ca2+]c and, simultaneously, a small transient depolarisation of the mitochondrial membrane is measurable. ATP application can also initiate an intercellular [Ca2+]c wave that propagates from cell to cell through a network, probably by a combination of IP3 movement through gap junctions and via the propagated release of ATP from cell to cell (see Guthrie et al. 1999; Wang et al. 2000). The traces in Fig. 1C show the propagation of the signal through a monolayer of cortical astrocytes in which [Ca2+]c and ΔΨm were measured simultaneously. Note that the mitochondrial depolarisation closely follows the spread of the [Ca2+]c signal, again demonstrating that the [Ca2+]c signal must, of necessity, be accompanied by mitochondrial Ca2+ uptake.
Figure 4. Effects of toxic glutamate exposure on mitochondrial membrane potential in hippocampal neurons.
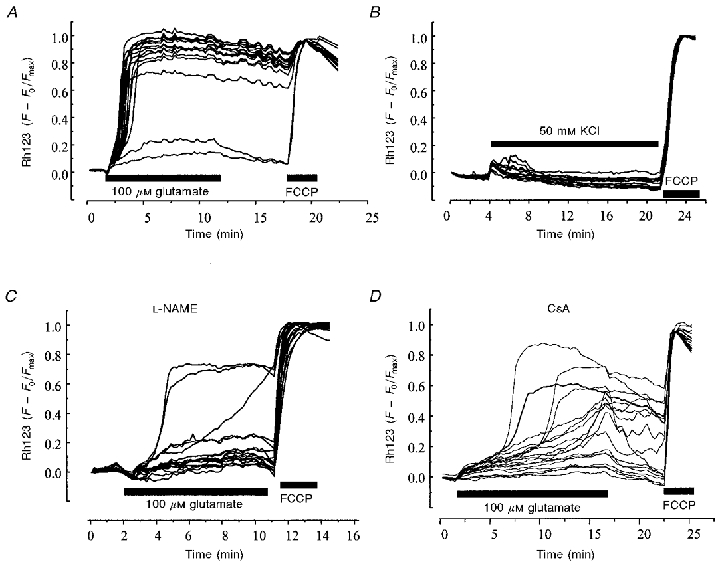
Changes in ΔΨm in rat hippocampal neurons during changes in [Ca2+]c caused by the prolonged application of 100 μm glutamate (A), 50 mm KCl (B), 100 μm glutamate in the presence of the NOS inhibitor L-NAME (100 μm; C) and in the presence of cyclosporin A (CsA, 200 nM; D). In each case, records were obtained using rat hippocampal neurons in culture. Fluorescence signals were recorded from cells loaded with both rhodamine 123 and fura-2 (or its low affinity equivalent fura-2FF) to measure mitochondrial membrane potential and [Ca2+]c simultaneously from a field of 20-30 cells. An increase in the rhodamine 123 signal represents a depolarisation of the mitochondrial membrane (see Keelan et al. 1999), as indicated by the response to FCCP shown at the end of each experiment. The rhodamine signals were normalised between baseline as 0, and the full depolarisation with FCCP as 1.0. In each case, the early changes in [Ca2+]c were not significantly different for any of these manipulations.
In addition to the use of mitochondrially targeted aequorin (described by Rizzuto et al. 2000, this issue, and see Brini et al. 1995), it has recently become possible to measure Ca2+ uptake into mitochondria directly using the fluorescent [Ca2+] indicator rhod-2. The AM ester of this dye is positively charged and so partitions between cytosol and mitochondria in response to the mitochondrial potential. Once de-esterified, it is trapped in the compartment, and the localisation is clearly shown when cells are co-loaded with other mitochondrial staining dyes such as mitotracker green or when they are transfected with mitochondrially targeted green fluorescent protein (GFP). In our hands, rhod-2 loading almost always leaves a significant cytosolic signal. It is only really appropriate to assume that the rhod-2 signal from a cell is mitochondrial if positive measures are taken to ensure the loss of the cytosolic component (e.g. by whole-cell patch clamp which dialyses out the cytosolic free dye; Babcock et al. 1997). Most measurements of rhod-2 fluorescence in most cell types have shown that [Ca2+]c signals within a reasonably physiological range are associated with a rise in [Ca2+]m (Fig. 2B). Perhaps this has been most directly demonstrated in the work of David et al. (1998) who have shown changes in [Ca2+]m with trains of action potentials in the nerve terminals of the lizard neuromuscular junction. In most published work, the rise in [Ca2+]m is significantly slow compared with the rise in [Ca2+]c and tends also to outlast the cytosolic signal, the exact timing varying apparently between cell types (see Jou et al. 1996; Babcock et al. 1997; Ricken et al. 1998; Boitier et al. 1999; Drummond et al. 2000). In our experiments, a transient rise in [Ca2+]c lasting just 20 s following IP3-mediated release from ER stores in rat astrocytes was followed by a rise in [Ca2+]m that could last ∼10 min (Boitier et al. 1999). Similarly, perhaps, the rise in NADH autofluorescence that signals increased dehydrogenase activity could last 5-10 min after a [Ca2+]c transient lasting only 10 s following voltage-gated Ca2+ influx in mouse sensory neurons (Fig. 1, and see Duchen, 1992). Interestingly, it seems likely that a significant proportion of the Ca2+ entering mitochondria may not appear as free ionised Ca2+ in the matrix, but might rather be present either bound to phosphate or to phospholipids (e.g. see David, 1999), and so measurements of ionised free [Ca2+]m may usually give an underestimate of total matrix [Ca2+].
Figure 2. Impact of mitochondrial Ca2+ uptake on the propagation of [Ca2+]c waves in rat cortical astrocytes.
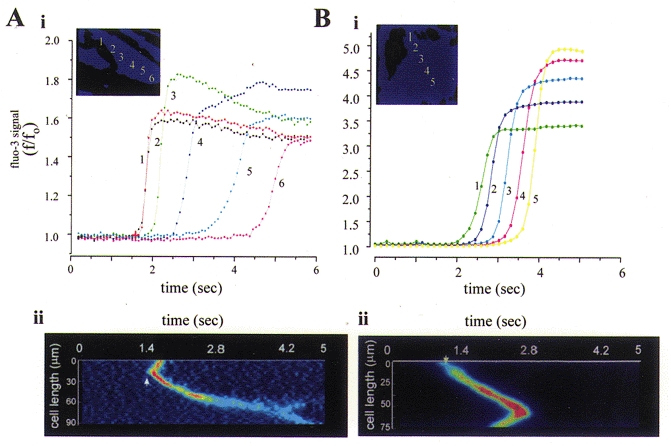
A, brief application of a threshold concentration of ATP to an adult astrocyte in culture caused a wave of [Ca2+]c which propagated across the cell. The cell was loaded with the [Ca2+]c indicator fluo-3 and imaged using a fast readout cooled CCD camera (Hamamatsu 4880). Plots of intensity with time are shown in Ai for successive points in space at ≈10 μm intervals across the cell as indicated by the inset. It should be clear that both the rate of propagation and the rate of rise of the [Ca2+]c signal attenuate progressively as the wave progresses along the cell. The mean rate of propagation of the signal was 25 μm s−1. The progress of the wavefront is also illustrated in Aii which shows the intensity profile along a line selected along the long axis of the cell with successive image frames from a sequence of just 6 s. The image sequence was first differentiated so that signal is seen only in those pixels in which the signal has changed, and therefore shows the wavefront only. The same principles were applied after depolarisation of ΔΨm in order to limit mitochondrial Ca2+ uptake and data are shown in B i and ii. Now the [Ca2+]c signal clearly propagated faster, with no loss of momentum as it progressed across the cell, and the rate of rise of the signal was sustained throughout the progression of the wave. The mean rate of propagation was now 40 μm s−1.
It might seem that mitochondrial Ca2+ uptake will only occur during a substantial global rise in [Ca2+]c, when the Ca2+ concentration throughout the cytosol reaches levels that exceed a high apparent Kd for Ca2+ (see, for example, Nicholls & Crompton, 1980, for review). In fact, the work discussed by Rizzuto et al. (2000) and by Hajnóczky et al. (2000) in this issue demonstrates that mitochondrial Ca2+ uptake during global [Ca2+]c signalling is probably facilitated by structural organisation of the organelles within the cell (see also Rizzuto et al. 1998). The proximity of mitochondria to ER Ca2+ release sites underlies the generation of microdomains of high [Ca2+]c close to the IP3-gated Ca2+ release channels of the ER. The same principles might apply to the localisation of mitochondria and Ca2+ influx sites in excitable cells (Lawrie et al. 1996), although the data here are far less developed. Our own data also suggest similar close contacts between mitochondria and ER Ca2+ release sites, revealed in a completely different way, and apparently operating spontaneously even in the absence of global [Ca2+]c signals. In several cell types, we have observed apparently spontaneous transient depolarisations of ΔΨm with time constants of the order of 100 ms or so. These events are clearly dependent on the release of Ca2+ from internal stores. In cardiomyocytes, for example, they were completely abolished by blockade of the SR Ca2+ release channel with ryanodine, or by chelation of Ca2+ by loading cells with BAPTA-AM. They were also blocked by inhibition of mitochondrial Ca2+ uptake by diaminopentane pentammine cobalt (DAPPAC), a novel inhibitor of the uniporter (Duchen et al. 1998 and see Crompton & Andreeva, 1994). Data such as these suggest that mitochondrial Ca2+ uptake is far more tightly integrated into the routine business of cell signalling than previously thought, operating at a subtle and continuous level below the threshold of global Ca2+ signals.
We need then to ask what is the functional significance of mitochondrial Ca2+ uptake. The continuous charging of mitochondria with Ca2+ will sustain the activation state of the mitochondrial dehydrogenases, and may be important in sustaining mitochondrial energy production. The failure of this mechanism has been demonstrated in the cardiomyopathic hamster, where a reduction in mitochondrial Ca2+ loading is associated with a gradual deterioration of energetic state (Di Lisa et al. 1993). While we understand remarkably little about the pathophysiology of the chronic mitochondrial diseases such as the inherited mutations of the respiratory chain, an expected consequence might be a loss of ΔΨm, which will depress mitochondrial Ca2+ accumulation (as demonstrated by Brini et al. 1999). The consequent downregulation of the TCA cycle might in turn depress mitochondrial ATP generation, ultimately leading to cellular pathophysiology.
Modulation of [Ca2+]c signals by mitochondrial Ca2+ uptake
Mitochondrial Ca2+ uptake also plays an intimate role in the fundamental events involved in cellular [Ca2+]c signalling. The precise features of the interplay between mitochondria and cellular [Ca2+]c signalling seem to vary between cells. In excitable cells, a fairly consistent pattern has emerged over the past 10 years or so. Thus, in sensory neurons (Werth & Thayer, 1994), central neurons (Wang & Thayer, 1996), sympathetic neurons (Friel & Tsien, 1994), chromaffin cells (Babcock et al. 1997) and at the neuromuscular junction (David et al. 1998), Ca2+ influx through voltage-gated Ca2+ channels initiates a rapid rise in [Ca2+]c which then shows a biphasic pattern of recovery, consisting of a rapid component with a subsecond time constant followed by a slower component that may take minutes. Inhibition of mitochondrial Ca2+ uptake slows the initial rate of recovery of the [Ca2+]c transient but also removes a slow secondary phase of recovery. This latter component of the [Ca2+]c response is also blocked by inhibition of the mitochondrial Na+-Ca2+ exchanger (White & Reynolds, 1997). This sequence of responses has been taken to show that mitochondria take up Ca2+ during the rapid increase in [Ca2+]c, and, by actively removing Ca2+ from the cytosol, accelerate the recovery of the transient. However, the slower re-equilibration of cytosolic and mitochondrial Ca2+ pools through the Na+-Ca2+ exchange maintains a slower plateau phase of relatively high [Ca2+]c. This plateau has in turn been implicated in processes such as the post-tetanic potentiation of synaptic transmission, as synaptic vesicle fusion is enhanced in response to renewed Ca2+ influx that arrives during the plateau phase (Tang & Zucker, 1997; David et al. 1998). Thus what seems to be a simple consequence of the thermodynamic properties of the mitochondrial transport pathways has substantial functional implications for the synaptic circuitry of the organism.
Mitochondria also have a significant impact on the spatiotemporal features of the evolving [Ca2+]c signal in inexcitable cells, largely reflecting the Ca2+ sensitivity of the Ca2+-permeable channels involved. In many cell types, [Ca2+]c signals following the mobilisation of Ca2+ from internal stores evolve as waves that propagate across the cell from an initiation site, often with oscillatory repeats if the stimulus is maintained. We found that in rat cortical astrocytes the [Ca2+]c wave generated within single cells in response to a low dose of ATP, which acts at P2U receptors (Peuchen et al. 1996a) to mobilise IP3-releasable Ca2+, propagates at an average rate of about 25 μm s−1 (Boitier et al. 1999). Associated with this was a wave of mitochondrial depolarisation that was dependent on the integrity of the Ca2+ store (abolished, for example, by pretreatment with thapsigargin to empty the store), suggesting that there is a flux of Ca2+ into mitochondria during the progression of the wave, even though the global rise in [Ca2+]m detectable with rhod-2 was rather slower and more difficult to identify (see above). When mitochondrial Ca2+ uptake is inhibited, by collapsing ΔΨm while protecting the cell from ATP depletion with oligomycin (which limits mitochondrial ATP consumption by the F1F0-ATPase in reverse mode), then the wave propagated almost twice as fast, reaching velocities of 35-40 μm s−1. Also it is very striking that, in control cells, both the rate of rise and the rate of propagation of the wave tended to slow progressively as it travelled across the cell. This gradual decline in propagation disappeared if mitochondrial Ca2+ uptake was blocked, and the waves then maintained a more consistent velocity and rate of rise (see Fig. 2B), suggesting disclosure of a Ca2+-induced Ca2+ release (CICR) process. All these data suggest a mechanism outlined in the cartoon shown in Fig. 3. This scheme proposes that Ca2+ uptake by mitochondria close to the ER Ca2+ release sites lowers the local [Ca2+]c into a range below the concentration at which Ca2+ sensitises IP3-mediated Ca2+ release. A similar model could explain the observations of Tinel et al. (1999), who showed that Ca2+ uptake by the mitochondria of pancreatic acinar cells, in which the mitochondria are arranged in a band between the two poles of these polarised cells, tends to restrict the spread of a [Ca2+]c signal from the apical pole through to the luminal pole. Mitochondria are acting essentially as a spatial buffer in these systems, restricting the propagation of [Ca2+]c signals by mopping up Ca2+ and limiting the concentrations required for (IP3-dependent) CICR.
Figure 3. Scheme to illustrate the interplay between mitochondria and ER [Ca2+]c signalling.
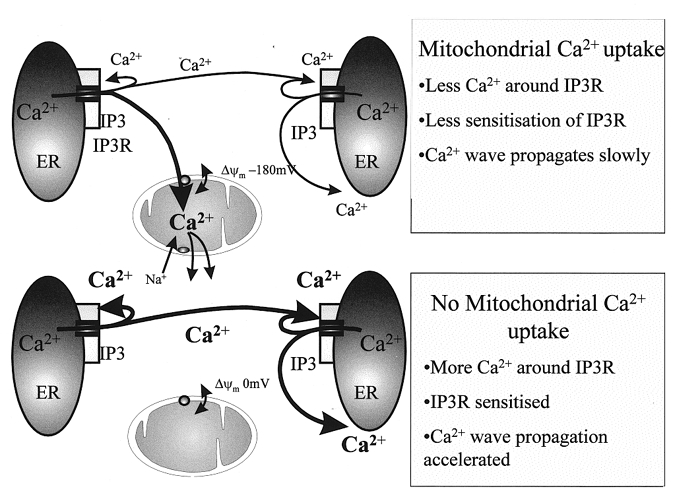
These cartoons show a scheme to account for the modulation of [Ca2+]c signalling by mitochondrial Ca2+ uptake in inexcitable cells. The local removal of Ca2+ seems able to buffer [Ca2+]c in local microdomains close to the ER Ca2+ release channels. As IP3-regulated channels are themselves sensitive to [Ca2+]c, this provides a local regulation of their open probability, which serves to regulate the rate and extent of propagation of the signal. When mitochondrial Ca2+ uptake is disabled, this modulation is removed, local [Ca2+]c will be allowed to rise, and will exert feedback regulation on the IP3 receptor (IP3R). The precise consequence may vary depending on the class of IP3 receptor expressed by the cell.
Curiously, in Xenopus oocytes, mitochondrial Ca2+ uptake seemed to have the opposite consequence for the characteristics of the [Ca2+]c wave (Jouaville et al. 1995). In that system, energisation of mitochondria (i.e. increasing mitochondrial membrane potential) by provision of substrate increased the coordination of waves through the oocyte cytosol and slowed the rate of propagation of the wave. It was argued that this could be explained as a consequence of the bell-shaped Ca2+ sensitivity of IP3-dependent Ca2+ release. Thus, in the absence of mitochondrial uptake, the local [Ca2+]c close to the mouth of the IP3-sensitive Ca2+ release channel might rise above the peak of the bell-shaped curve, and so the channel open probability begins to fall. This will tend to reduce the sensitivity of the propagating mechanism and slow the rate of wave propagation. Energisation allows mitochondria to accumulate Ca2+, lowering the local [Ca2+]c towards the peak of the bell-shaped curve, increasing the sensitivity of the channels to IP3, and so enhancing the rate of wave propagation. Thus, in one model, mitochondrial uptake slows wave propagation, while in the other, it speeds the process. Why should they be different? One explanation may lie in the different properties of the different classes of IP3 receptors. Currently, it seems likely that only the IP3 type I receptor shows the bell-shaped sensitivity to Ca2+ described by Iino (1990) and by Bezprozvanny et al. (1991), while the type II and III classes of receptor may show a more linear sensitivity to Ca2+ (Hagar et al. 1998). The astrocytes appear to express predominantly type II receptors (see, for example, Sheppard et al. 1997; E. Boitier, S. Brind & M. R. Duchen, unpublished observations) whilst the oocytes express predominantly type I. If this proves to be correct and reproducible in other systems, it implies that the influence of mitochondrial Ca2+ uptake on the spatiotemporal features of the [Ca2+]c may depend on the predominant class of IP3 receptors expressed by the cells. A similar mechanism can be invoked to account for the mitochondrial regulation of ‘capacitative’ Ca2+ influx in lymphocytes (Hoth et al. 1997), where again, local mitochondrial Ca2+ buffering seems to keep the local microdomains of [Ca2+]c close to the Ca2+-sensitive influx channels low enough to keep the channels open and allow sustained influx. Simpson & Russell (1998; a review) have also described amplification sites in the propagation of [Ca2+]c waves along fine processes in oligodendrocytes, which are enriched in mitochondria and in ER, which co-localise. In that system, though, the impact of removing mitochondrial Ca2+ uptake was highly variable, and so the models are rather more difficult to interpret.
Perhaps one of the more surprising recent observations in this field comes from a recent study by Zimmermann (2000). Many cells respond to continuous agonist exposure by generating repetitive oscillatory [Ca2+]c signals whose oscillatory frequency tends to increase with agonist concentration. Zimmermann (2000) found that in permeabilised cells in which the ER Ca2+ content was measured, addition of exogenous IP3 only generated an oscillatory efflux of Ca2+ from the ER when mitochondria were energised. This response could be blocked by collapse of ΔΨm or by inhibition of the uptake pathway with RuR. That mitochondrial Ca2+ uptake should have such a profound impact on the patterning of [Ca2+]c signals seems remarkable and again demonstrates the tight integration of mitochondria into the process of cellular [Ca2+]c signalling.
Mitochondrial Ca2+ uptake and cell pathology
It seems that, in some circumstances, mitochondrial Ca2+ uptake can switch from a useful physiological regulatory mechanism to a potentially harmful process that can initiate the progression towards cell death (e.g. see Ichas & Mazat, 1998). These processes have been mostly attributed to situations arising when mitochondrial Ca2+ accumulation is accompanied by some form of oxidative stress. Analogous processes will be dealt with in other models by Hajnóczky et al. (2000, this issue). One such model which we have been exploring is that of glutamate excitotoxicity. It is well established that the excitatory neurotransmitter glutamate may accumulate massively in the CNS during a period of anoxia or ischaemia, largely through reversal of the glutamate transporter in response to the changes in ionic homeostasis that follow ischaemia (Szatkowski & Attwell, 1994). It is also well established that application of glutamate to many classes of neurons in culture may cause cell death; this has long been attributed to ‘cellular Ca2+ overload’ (for review, see Choi, 1994). While the cell death is clearly Ca2+ dependent, the downstream processes that couple the rise in [Ca2+]c to cell death have been less clear. The involvement of mitochondrial Ca2+ uptake in this process has been fairly clearly demonstrated by some surprisingly simple experiments that show that preventing mitochondrial Ca2+ uptake by depolarising mitochondria with a mitochondrial uncoupler is neuroprotective (Stout et al. 1998).
A number of laboratories found that an early feature of the response to toxic glutamate exposure is the depolarisation of mitochondrial membrane potential (see, amongst others, Schinder et al. 1996; Khodorov et al. 1996; Nieminen et al. 1996) and see Fig. 4A. Our own contribution focused on the relationship between the change in [Ca2+]c and the change in ΔΨm (see Vergun et al. 1999 and Keelan et al. 1999). One obvious question was whether the change in ΔΨm is simply an inevitable consequence of an unusually large Ca2+ load, or whether there was something else contributing specifically to the glutamate response. It turns out that apparently very similar changes in [Ca2+]c may be associated with mitochondrial responses that vary enormously in time course and amplitude. For example, 100 μm glutamate exposure for 10 min raises [Ca2+]c to a similar degree in cells that have been in culture only a short time (7-10 days) and in those that have been in culture for 12-14 days, but in the latter, mitochondria undergo an almost complete collapse of potential and 24 h later ∼70 % of the cells will be dead, while in the ‘younger’ cells, the mitochondria undergo only a small reversible loss of potential and the cells do not die. Similarly, although glutamate often causes considerable calcium loading, prolonged depolarisation with high potassium (50 mm) can in some cells raise [Ca2+]c into the same range as seen with glutamate (when measured with fura-2, but also with 45Ca2+– see Sattler et al. 1998), and yet we have never seen any substantial mitochondrial depolarisation in response to high potassium alone (Fig. 4B), and high potassium does not cause cell death. The correlation between the loss of ΔΨm and the progression to cell death seems very strong, and so the questions have focused on why mitochondria depolarise in response to glutamate but not to some other similar [Ca2+]c load. The Ca2+ dependence of the loss of ΔΨm is clear, but in addition to the anomalies set out above, the time course of the [Ca2+]c response is generally very consistent, but the timing of the profound collapse of ΔΨm varies enormously between cells. Key to this study is the ability to correlate changes in [Ca2+]c and ΔΨm simultaneously on a cell-by-cell basis. These data together suggested that some other ‘factor’ in addition to Ca2+ must play a role in the fate of the mitochondria and thence, perhaps of the cell.
We considered a number of possible agents, including superoxide and nitric oxide, as there is extensive published data suggesting that both antioxidants and nitric oxide synthase (NOS) inhibitors are neuroprotective (e.g. see Kashii et al. 1996). In our hands, a number of potent antioxidants failed to make any impression at all on the changes in [Ca2+]c and the mitochondrial depolarisation, except those that also cause an inhibition of the NMDA-gated conductance (the conductance has redox modulatory sites that may account for these effects and may also account for at least part of the cytoprotection observed with antioxidants – see Gozlan & Ben-Ari, 1995 and Vergun et al. 2001). However, inhibition of neuronal NOS with L-NAME was significantly protective and also suppressed the mitochondrial depolarisation in a large proportion of cells without reducing the [Ca2+]c signal (Fig. 4C). Conversely, it proved possible to convert an otherwise innocuous [Ca2+]c signal, in response to K+-induced depolarisation or in response to glutamate but in ‘younger’ cells, into a toxic signal associated with a loss of mitochondrial potential if the [Ca2+]c signal was generated in the presence of exogenous NO at concentrations that had no effect on these variables when applied alone. These data are all consistent with the data published recently by Sattler et al. (1999) who demonstrated a possible mechanism for the ‘source specificity’ of glutamate toxicity. Thus, why should a Ca2+-dependent process of cell death fail to show a direct dependence on [Ca2+]c and be dependent rather on the route of Ca2+ influx? Indeed, if the mechanism involves the additional requirement of NO production, this itself is Ca2+ dependent, and so might be expected simply to reflect the Ca2+ concentration. This apparent anomaly would seem to have been resolved by the elegant experiments of Sattler et al. (1999), who showed that suppression of the expression of the scaffolding protein psd95 by introduction of anti-sense oligonucleotides did not alter the rise in [Ca2+]c in response to glutamate, but suppressed the production of NO with the result that glutamate toxicity was much reduced. Putting all these data together suggests that Ca2+ entering the cell through NMDA-gated channels causes microdomains of high [Ca2+]c which activate nNOS which is held close to the receptor by the scaffolding protein. The combination of NO and high intramitochondrial [Ca2+] then together cause the collapse of the mitochondrial potential, leading ultimately to cell death. It would be misleading to imply that this problem is solved, elegant as it seems. In some hands, it seems that there may be a far more direct and simple relationship between absolute calcium load and cell death (Hyrc et al. 1997; Stout & Reynolds, 1999), and it seems clear that there remain elements in the pathway between glutamate exposure and cell death that remain to be clarified.
The other crucial question that remains is the mechanism underlying the collapse of ΔΨm. Two major processes are likely candidates as mechanisms for a loss of ΔΨm– the opening of the mitochondrial permeability transition pore (mPTP – for review see Crompton, 2000, this issue), or damage to the respiratory chain causing a failure of the very processes that develop and sustain the potential and the gradual loss of potential due to proton leak. With the involvement of NO established, either mechanism seems plausible. NO competes with O2 at cytochrome oxidase and so may suppress respiration, and may also cause irreversible nitrosylation at complex I (see Clementi et al. 1998). A combination of NO and high intramitochondrial Ca2+ may also serve to open the mPTP. It has proven surprisingly difficult to resolve this issue, partly because the pharmacological tools available are insufficiently selective to draw unambiguous conclusions. The complexity and ambiguities inherent in this process are illustrated in the cartoon shown in Fig. 5. The mPTP is closed by cyclosporin A (Crompton et al. 1988), and the glutamate-induced mitochondrial depolarisation also is suppressed by CsA (Fig. 4D; see Nieminen et al. 1996; Vergun et al. 1999). However, CsA acts by binding to cyclophilins. At the mPTP, the cyclophilin involved is the mitochondrially localised cyclophilin D (CypD). However, CsA will also bind to the cytosolic cyclophilin, CypA, which binds to calcineurin. Calcineurin phosphorylates – and activates – NOS, a phosphorylation inhibited by CsA, and given the involvement of NO in glutamate toxicity, it seems perfectly plausible that the action of CsA is attributable to an interaction with calcineurin. To add spice to this argument, calcineurin is also modulated by the so-called FK binding protein, itself modulated by the drug FK-506, and this has also been shown to be protective in glutamate toxicity (Ankarcrona et al. 1996; Keelan et al. 1998). Improved specificity may be achieved using the cyclosporin analogue methyl valine Cs (mvCs). This agent binds to all cyclophilins, but the mvCs-CypA complex apparently does not bind to calcineurin. mvCs has been very effective in our experience at suppressing glutamate-induced mitochondrial depolarisation (J. Keelan, O. Vergun & M. R. Duchen, unpublished observations), but had no effect in cerebellar granule cells (Castilho et al. 1998). So, while it is tempting to attribute the mitochondrial depolarisation to the mPTP, it is perhaps more appropriate at present to withhold judgement until more unequivocal evidence is available. This is not a purely academic matter, as the hope is obviously that once the mechanism is fully understood, pharamacological tools may be developed which will be significantly neuroprotective during episodes of ischaemia and reperfusion in patients.
Figure 5. Cartoon to highlight the complex pharmacology of cyclosporin A.
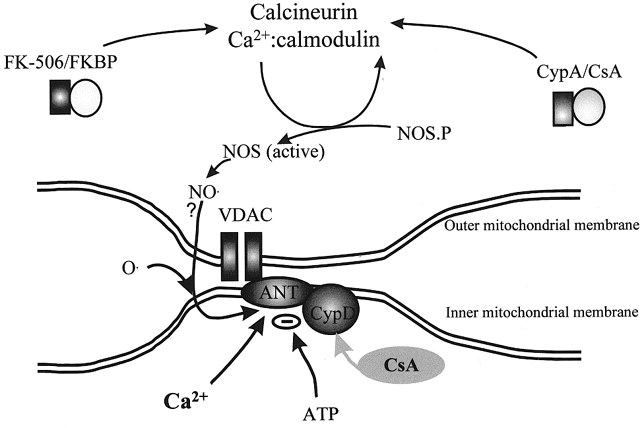
Cyclosporin A (CsA) has many actions and cannot be considered specific for the mitochondrial permeability pore. This cartoon illustrates at least two ways in which CsA may interfere with the pathways involved in glutamate excitotoxicity. A local rise in [Ca2+]c through NMDA receptors activates nNOS whose activity is itself regulated through phosphorylation by calcineurin. CsA (and FKBP, the FK-506 binding protein) inhibit the activation of NOS through calcineurin, and, as NO is required for the glutamate-induced mitochondrial depolarisation, CsA may prevent the collapse of ΔΨm through mechanisms quite unrelated to the mPTP. The mPTP itself probably consists of the adenine nucleotide translocase (ANT) and possibly also the voltage-dependent anion channel (VDAC), which form a pore under conditions in which SH groups are oxidised and when intramitochondrial [Ca2+] is high. The pore is regulated by cyclophilin D (CypD), which binds CsA. Therefore CsA may also prevent mitochondrial depolarisation by acting to prevent mPTP opening.
Acknowledgments
I would like to thank The Journal of Physiology for organising and funding the symposium which prompted this collection of reviews. I thank the Wellcome Trust, the Royal Society, the BBSRC and the Medical Research Council for financial support. I also thank Eric Boitier, Jake Jacobson, Julie Keelan, Mart Mojet and Olga Vergun who carried out much of the work described, Boris I. Khodorov for lively and provocative discussion, and D. Lilian Patterson for constant technical support in the laboratory for many years.
References
- Ankarcrona M, Dypbukt JM, Orrenius S, Nicotera P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Letters. 1996;394:321–324. doi: 10.1016/0014-5793(96)00959-3. [DOI] [PubMed] [Google Scholar]
- Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. Journal of Cell Biology. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. Journal of Cell Biology. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Marsault R, Bastianutto C, Alvarez J, Pozzan T, Rizzuto R. Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c). A critical evaluation. Journal of Biological Chemistry. 1995;270:9896–9903. doi: 10.1074/jbc.270.17.9896. [DOI] [PubMed] [Google Scholar]
- Brini M, Pinton P, King MP, Davidson M, Schon EA, Rizzuto R. A calcium signaling defect in the pathogenesis of a mitochondrial DNA inherited oxidative phosphorylation deficiency. Nature Medicine. 1999;5:951–954. doi: 10.1038/11396. [DOI] [PubMed] [Google Scholar]
- Castilho RF, Hansson O, Ward MW, Budd SL, Nicholls DG. Mitochondrial control of acute glutamate excitotoxicity in cultured cerebellar granule cells. Journal of Neuroscience. 1998;18:10277–10288. doi: 10.1523/JNEUROSCI.18-24-10277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Calcium and excitotoxic neuronal injury. Annals of the New York Academy of Sciences. 1994;747:162–171. doi: 10.1111/j.1749-6632.1994.tb44407.x. [DOI] [PubMed] [Google Scholar]
- Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proceedings of the National Academy of Sciences of the USA. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. Mitochondrial intermembrane junctional complexes and their role in cell death. The Journal of Physiology. 2000;529:11–21. doi: 10.1111/j.1469-7793.2000.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Andreeva L. On the interactions of Ca2+ and cyclosporin A with a mitochondrial inner membrane pore: a study using cobaltammine complex inhibitors of the Ca2+ uniporter. Biochemical Journal. 1994;302:181–185. doi: 10.1042/bj3020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochemical Journal. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- David G. Mitochondrial clearance of cytosolic Ca2+ in stimulated lizard motor nerve terminals proceeds without progressive elevation of mitochondrial matrix [Ca2+] Journal of Neuroscience. 1999;19:7495–7506. doi: 10.1523/JNEUROSCI.19-17-07495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Barrett JN, Barrett EF. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. The Journal of Physiology. 1998;509:59–65. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Fan CZ, Gambassi G, Hogue BA, Kudryashova I, Hansford RG. Altered pyruvate dehydrogenase control and mitochondrial free Ca2+ in hearts of cardiomyopathic hamsters. American Journal of Physiology. 1993;264:H2188–2197. doi: 10.1152/ajpheart.1993.264.6.H2188. [DOI] [PubMed] [Google Scholar]
- Drummond RM, Mix TC, Tuft RA, Walsh JV, Jr, Fay FS. Mitochondrial Ca2+ homeostasis during Ca2+ influx and Ca2+ release in gastric myocytes from Bufo marinus. The Journal of Physiology. 2000;522:375–390. doi: 10.1111/j.1469-7793.2000.t01-2-00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Ca2+-dependent changes in the mitochondrial energetics of single mouse sensory neurones. Biochemical Journal. 1992;283:41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Leyssens A, Crompton M. Transient mitochondrial depolarisations in response to focal SR calcium release in single rat cardiomyocytes. Journal of Cell Biology. 1998;142:975–988. doi: 10.1083/jcb.142.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A, Tsacopoulos M. Activation of mitochondrial oxidative metabolism by calcium ions in Limulus ventral photoreceptor. Nature. 1988;331:437–440. doi: 10.1038/331437a0. [DOI] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. Journal of Neuroscience. 1994;14:4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozlan H, Ben-Ari Y. NMDA receptor redox sites: are they targets for selective neuronal protection? Trends in Pharmacological Sciences. 1995;16:368–374. doi: 10.1016/s0165-6147(00)89077-x. [DOI] [PubMed] [Google Scholar]
- Griffiths EJ, Ocampo CJ, Savage JS, Rutter GA, Hansford RG, Stern MD, Silverman HS. Mitochondrial calcium transporting pathways during hypoxia and reoxygenation in single rat cardiomyocytes. Cardiovascular Research. 1998;39:423–433. doi: 10.1016/s0008-6363(98)00104-7. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Buntinas L, Sparagna GC, Gunter KK. The Ca2+ transport mechanisms of mitochondria and Ca2+ uptake from physiological-type Ca2+ transients. Biochimica et Biophysica Acta. 1998;1366:5–15. doi: 10.1016/s0005-2728(98)00117-0. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. Journal of Neuroscience. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III IP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnóczky G, Csordas G, Madesh M, Pacher P. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. The Journal of Physiology. 2000;529:69–81. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. Journal of Cell Biology. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrc K, Handran SD, Rothman SM, Goldberg MP. Ionized intracellular calcium concentration predicts excitotoxic neuronal death: observations with low-affinity fluorescent calcium indicators. Journal of Neuroscience. 1997;17:6669–6677. doi: 10.1523/JNEUROSCI.17-17-06669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochimica et Biophysica Acta. 1998;1366:33–50. doi: 10.1016/s0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in smooth muscle cells of the guinea pig taenia caeci. Journal of General Physiology. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou MJ, Peng TI, Sheu SS. Histamine induces oscillations of mitochondrial free Ca2+ concentration in single cultured rat brain astrocytes. The Journal of Physiology. 1996;497:299–308. doi: 10.1113/jphysiol.1996.sp021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- Kashii S, Mandai M, Kikuchi M, Honda Y, Tamura Y, Kaneda K, Akaike A. Dual actions of nitric oxide in N-methyl-D-aspartate receptor-mediated neurotoxicity in cultured retinal neurons. Brain Research. 1996;711:93–101. doi: 10.1016/0006-8993(95)01330-x. [DOI] [PubMed] [Google Scholar]
- Keelan J, Vergun O, Duchen MR. Is there a role for the mitochondrial permeability transition pore in glutamate excitotoxicity? Society for Neuroscience Abstracts. 1998;24:570.12. [Google Scholar]
- Keelan J, Vergun O, Duchen MR. Excitotoxic mitochondrial depolarisation requires both calcium and nitric oxide in rat hippocampal neurons. The Journal of Physiology. 1999;520:797–813. doi: 10.1111/j.1469-7793.1999.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorov B, Pinelis V, Vergun O, Storozhevykh T, Vinskaya N. Mitochondrial deenergization underlies neuronal calcium overload following a prolonged glutamate challenge. FEBS Letters. 1996;397:230–234. doi: 10.1016/s0014-5793(96)01139-8. [DOI] [PubMed] [Google Scholar]
- Lawrie AM, Rizzuto R, Pozzan T, Simpson AW. A role for calcium influx in the regulation of mitochondrial calcium in endothelial cells. Journal of Biological Chemistry. 1996;271:10753–10759. doi: 10.1074/jbc.271.18.10753. [DOI] [PubMed] [Google Scholar]
- McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiological Reviews. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Maechler P, Wollheim CB. Mitochondrial signals in glucose-stimulated insulin secretion in the beta cell. The Journal of Physiology. 2000;529:49–56. doi: 10.1111/j.1469-7793.2000.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Crompton M. Mitochondrial calcium transport. FEBS Letters. 1980;111:261–268. doi: 10.1016/0014-5793(80)80806-4. [DOI] [PubMed] [Google Scholar]
- Nieminen AL, Petrie TG, Lemasters JJ, Selman WR. Cyclosporin A delays mitochondrial depolarization induced by N-methyl-D-aspartate in cortical neurons: evidence of the mitochondrial permeability transition. Neuroscience. 1996;75:993–997. doi: 10.1016/0306-4522(96)00378-8. [DOI] [PubMed] [Google Scholar]
- Peuchen S, Clark JB, Duchen MR. Mechanisms of intracellular calcium regulation in adult astrocytes. Neuroscience. 1996a;71:871–883. doi: 10.1016/0306-4522(95)00515-3. [DOI] [PubMed] [Google Scholar]
- Peuchen S, Duchen MR, Clark JB. Energy metabolism of adult astrocytes in vitro. Neuroscience. 1996b;71:855–870. doi: 10.1016/0306-4522(95)00480-7. [DOI] [PubMed] [Google Scholar]
- Pralong WF, Hunyady L, Varnai P, Wollheim CB, Spat A. Pyridine nucleotide redox state parallels production of aldosterone in potassium-stimulated adrenal glomerulosa cells. Proceedings of the National Academy of Sciences of the USA. 1992;89:132–136. doi: 10.1073/pnas.89.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricken S, Leipziger J, Greger R, Nitschke R. Simultaneous measurements of cytosolic and mitochondrial Ca2+ transients in HT29 cells. Journal of Biological Chemistry. 1998;273:34961–34969. doi: 10.1074/jbc.273.52.34961. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. The Journal of Physiology. 2000;529:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO Journal. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Charlton MP, Hafner M, Tymianski M. Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. Journal of Neurochemistry. 1998;71:349–364. doi: 10.1046/j.1471-4159.1998.71062349.x. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. Journal of Neuroscience. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard CA, Simpson PB, Sharp AH, Nucifora FC, Ross CA, Lange GD, Russell JT. Comparison of Type 2 inositol 1,4,5,-trisphosphate receptor distribution and subcellular Ca2+ release sites that support Ca2+ waves in cultured astrocytes. Journal of Neurochemistry. 1997;68:2317–2327. doi: 10.1046/j.1471-4159.1997.68062317.x. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Russell JT. Role of mitochondrial Ca2+ regulation in neuronal and glial cell signalling. Brain Research Reviews. 1998;26:72–81. doi: 10.1016/s0165-0173(97)00056-8. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. Journal of Biological Chemistry. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- Stout AK, Raphael HM, Kanterewicz BI, Klann E, Reynolds IJ. Glutamate-induced neuron death requires mitochondrial calcium uptake. Nature Neuroscience. 1998;1:366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- Stout AK, Reynolds IJ. High-affinity calcium indicators underestimate increases in intracellular calcium concentrations associated with excitotoxic glutamate stimulations. Neuroscience. 1999;89:91–100. doi: 10.1016/s0306-4522(98)00441-2. [DOI] [PubMed] [Google Scholar]
- Szatkowski M, Attwell D. Triggering and execution of neuronal death in brain ischaemia: two phases of glutamate release by different mechanisms. Trends in Neurosciences. 1994;17:59–65. doi: 10.1016/0166-2236(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Tang YG, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Tinel H, Cancela JM, Mogami H, Gerasimenko JV, Gerasimenko OV, Tepikin AV, Petersen OH. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO Journal. 1999;18:4999–5008. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergun O, Keelan J, Khodorov BI, Duchen MR. Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. The Journal of Physiology. 1999;519:451–466. doi: 10.1111/j.1469-7793.1999.0451m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergun O, Sobolevsky AI, Yelshansky MV, Keelan J, Khodorov BI, Duchen MR. Exploration of the role of reactive oxygen species in glutamate neurotoxicity in rat hippocampal neurons in culture. The Journal of Physiology. 2001. in the Press. [DOI] [PMC free article] [PubMed]
- Wang GJ, Thayer SA. Sequestration of glutamate-induced Ca2+ loads by mitochondria in cultured rat hippocampal neurons. Journal of Neurophysiology. 1996;76:1611–1621. doi: 10.1152/jn.1996.76.3.1611. [DOI] [PubMed] [Google Scholar]
- Wang Z, Haydon PG, Yeung ES. Direct observation of calcium-independent intercellular ATP signaling in astrocytes. Analytical Chemistry. 2000;72:2001–2007. doi: 10.1021/ac9912146. [DOI] [PubMed] [Google Scholar]
- Werth JL, Thayer SA. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. Journal of Neuroscience. 1994;14:348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ, Reynolds IJ. Mitochondria accumulate Ca2+ following intense glutamate stimulation of cultured rat forebrain neurones. The Journal of Physiology. 1997;498:31–47. doi: 10.1113/jphysiol.1997.sp021839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann B. Control of InsP3-induced Ca2+ oscillations in permeabilized blowfly salivary gland cells: contribution of mitochondria. The Journal of Physiology. 2000;525:707–719. doi: 10.1111/j.1469-7793.2000.t01-1-00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


