Abstract
Plasma interleukin (IL)-6 concentration is increased with exercise and it has been demonstrated that contracting muscles can produce IL- The question addressed in the present study was whether the IL-6 production by contracting skeletal muscle is of such a magnitude that it can account for the IL-6 accumulating in the blood.
This was studied in six healthy males, who performed one-legged dynamic knee extensor exercise for 5 h at 25 W, which represented 40% of peak power output (Wmax). Arterial-femoral venous (a-fv) differences over the exercising and the resting leg were obtained before and every hour during the exercise. Leg blood flow was measured in parallel by the ultrasound Doppler technique. IL-6 was measured by enzyme-linked immunosorbent assay (ELISA).
Arterial plasma concentrations for IL-6 increased 19-fold compared to rest. The a-fv difference for IL-6 over the exercising leg followed the same pattern as did the net IL-6 release. Over the resting leg, there was no significant a-fv difference or net IL-6 release. The work was produced by 2.5 kg of active muscle, which means that during the last 2 h of exercise, the median IL-6 production was 6.8 ng min−1 (kg active muscle)−1 (range, 3.96-9.69 ng min−1 kg−1).
The net IL-6 release from the muscle over the last 2 h of exercise was 17-fold higher than the elevation in arterial IL-6 concentration and at 5 h of exercise the net release during 1 min was half of the IL-6 content in the plasma. This indicates a very high turnover of IL-6 during muscular exercise. We suggest that IL-6 produced by skeletal contracting muscle contributes to the maintenance of glucose homeostasis during prolonged exercise.
Physical exercise induces the release of a cascade of cytokines, including tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-1 receptor antagonist, TNF receptors and IL-10 (Sprenger et al. 1992; Drenth et al. 1995; Nehlsen-Cannarella et al. 1997; Pedersen & Hoffman-Goetz, 2000). In response to exercise, the plasma concentration of IL-6 increases more than that of any other cytokine examined (Pedersen & Hoffman-Goetz, 2000). Thus, following a marathon race, the level of plasma IL-6 is typically increased 100-fold (Ostrowski et al. 1999), the increase being equivalent to that observed in patients with severe infections (Hack et al. 1997; Giannoudis et al. 1998; Bruunsgaard et al. 1999). An earlier study on eccentric exercise suggested that the exercise-induced increase in IL-6 was associated with muscle damage (Bruunsgaard et al. 1997). However, recent studies have not supported this finding (Ostrowski et al. 1998a, 1999; Croisier et al. 1999), and it is likely that IL-6 is produced as a direct consequence of concentric and eccentric muscle contractions and is involved in the later repair mechanisms in relation to muscle damage.
The level of IL-6 increases with the duration of exercise and is also related to the intensity of the exercise (Ostrowski et al. 1998a). Interestingly, compared to pre-exercise levels, IL-6 mRNA was markedly elevated in muscle biopsies obtained from the quadriceps muscle immediately after a marathon race (Ostrowski et al. 1998b). In agreement with this finding, IL-6 mRNA was elevated in rat muscle subjected to either concentric or eccentric electrically stimulated contractions (Jonsdottir et al. 2000). These studies strongly indicate that IL-6 is produced locally in muscles in response to both muscle contractions and inflammation, but do not answer the question of whether the locally produced IL-6 can account for the large increase in the circulating concentrations of IL-6. The present study aimed to determine whether this was the case by measuring the magnitude of a possible IL-6 release from contracting skeletal muscles. Subjects performed 5 h of one-legged concentric exercise. Blood for cytokine measurement was obtained from catheters placed in both femoral veins and one artery, which provided the arterial-femoral venous (a-fv) difference. Together with leg blood flow this enabled us to study the net IL-6 release from working as well as from resting skeletal muscle.
METHODS
Subjects
Six healthy, physically active, but not regularly training male subjects, of mean age 26 years (range, 22-33 years), mean weight 77 kg (range, 75-82 kg) and mean height 1.83 m (range, 1.82-1.84 m) participated in the study. The subjects were informed about the possible risks and discomfort involved before giving their informed, written consent to participate. The study was performed according to the Declaration of Helsinki and was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark.
Protocol
Each subject underwent preliminary exercise tests to familiarise themselves with the exercise model and to obtain a measure of their work capacity. Dynamic knee extension work was performed on a modified ergometer described previously (Andersen et al. 1985). In essence, the subjects sat in a chair with the ankle of the right leg attached to a bar, which was connected to the ergometer. The exercise consisted of ‘kicking’, i.e. contractions of the knee extensors by moving the ankle over a range of ≈60 deg (from 90 to 30 deg angle). The reposition of the leg was fully passive due to gravity and the momentum of the wheel. After they became familiar with this exercise model, the subjects underwent a maximal exercise test to determine their individual knee extensor peak power output (Wmax,ke). This was done by increasing the load by 10 W every 2 min until exhaustion. After 1 h of rest they performed 3 h of one-legged knee extensor exercise at 40%Wmax,ke as a practice. On the day of the experiment, which was at least a week later, the subjects were instructed to eat two slices of bread with marmalade at 07.00 h and to report to the laboratory at 08.00 h. The subjects changed and remained supine for the next 3 h. After 10 min in the supine position, the femoral artery and vein from the leg to be exercised and the femoral vein of the resting leg were cannulated under local anaesthesia (lidocaine, 20 mg ml−1). The Seldinger technique was used to insert the catheters (20 gauge; Ohmeda, Wiltshire, UK; Berneus et al. 1954). The femoral arterial catheter was inserted ≈2-5 cm below the inguinal ligament and advanced ≈5-10 cm in the proximal direction. The femoral venous catheter was inserted ≈2 cm below the inguinal ligament and advanced ≈5 cm in the distal direction. The distal orientation of the femoral venous catheter is crucial since otherwise the blood is contaminated with blood draining from the lower abdomen and the saphenous vein as discussed previously (van Hall et al. 1999). The subjects performed one-legged knee extensor exercise for 5 h at 40%Wmax,ke. Blood samples were obtained just before the exercise started and then after each hour of exercise. Shortly before each measurement a cuff below the knee around the calf muscles was temporarily inflated to a suprasystolic (≈240 mmHg) blood pressure to eliminate blood contribution of the lower leg (van Hall et al. 1999). At each sample point the femoral arterial blood flow was measured with the ultrasound Doppler technique as previously validated (Radegran, 1997). An ultrasound Doppler (model CFM 800; Vingmed Sound, Horten, Norway) equipped with an annular phased array transducer probe (11.5 mm diameter; Vinmed Sound) operating at an imaging frequency of 7.5 MHz and variable Doppler frequencies of 4.0-6.0 MHz (high-pulsed repetition frequency mode, 4-36 kHz) was used. The site for vessel diameter determination and blood velocity measurements in the common femoral artery was distal to the inguinal ligament but above the bifurcation into the superficial and profund femoral branch. The femoral artery was isolated at a fixed perpendicular angle. The diameter was determined along the central path of the ultrasound beam where the best spatial resolution is achieved. The blood velocity and flow during exercise were specifically analysed in relation to the muscle contraction force (strain gauge) profile (Radegran, 1997).
Measurement of IL-6
Blood samples for cytokine measurement were drawn into pre-cooled glass tubes containing EDTA. The tubes were spun immediately at 2200 g for 15 min at 4°C. The plasma was stored at -80°C until analyses were performed. For IL-6 measurement, high-sensitivity ELISA kits from R&D Systems (Minneapolis, MN, USA) were used. According to R&D Systems the IL-6 ELISA kit is insensitive to the addition of the recombinant forms of the soluble IL-6 receptor and the measurements, therefore, correspond to both soluble and receptor-bound cytokine. The intra-assay coefficient of variation (c.v.) was 5.9%.
Statistics
The blood flow data were normally distributed and are shown as means ±s.e.m. To analyse changes over time a one-way repeated measures analysis of variance (one-way ANOVA) was used. If significance was indicated, Student’s paired t test was used to test for significant differences between pre-exercise and exercise values. Plasma IL-6 concentrations were not distributed normally, therefore these data are presented as medians and quartiles. Changes over time were tested using a non-parametric Friedman test (P < 0.01); if this was significant, pairwise comparisons were done using a non-parametric Wilcoxon test. The Wilcoxon test was also used to test for significant differences between exercising and resting legs at the different time points. P < 0.05 was accepted as significant.
RESULTS
The arterial plasma concentration of IL-6 increased from 0.74 ng l−1 (0.67-1.50 ng l−1) at rest to 14.13 ng l−1 (11.62- 16.75 ng l−1) after 5 h of one-legged knee exercise (Fig. 1A). There was a gradual increase in the arterial plasma IL-6 concentration with exercise, but a more substantial increase in IL-6 concentration occurred after 3 h of exercise. At rest the arterial and femoral venous plasma IL-6 concentrations were similar. Over the first 3 h of exercise the a-fv difference for IL-6 was increased slightly for both the contracting and resting leg, but it was only significant for the exercising leg at the 2 and 3 h time points. Thereafter, there was a very marked further elevation in the a-fv difference for IL-6 over the exercising leg, whereas in the resting leg the a-fv difference was not significantly greater than the pre-exercise level. The peak a-fv difference in the exercising leg reached 9.77 ng l−1 (7.82-13.74 ng l−1; Fig. 1B).
Figure 1. Plasma IL-6 data for 6 male subjects measured before and every hour during 5 h of one-legged concentric exercise.
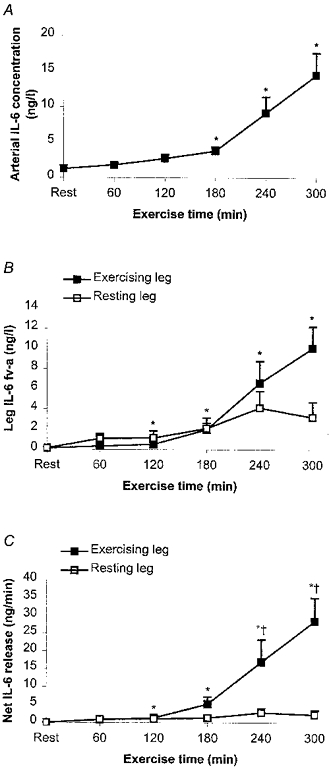
Data are presented as medians and quartiles. A, average of arterial IL-6 plasma concentration. B, average of arterial-femoral venous (a-fv) differences for exercising and resting leg, measured as venous plasma IL-6 concentration minus arterial plasma IL-6 concentration. Note that only increases in the exercising leg are significant. C, net release of IL-6 from exercising and resting leg (Ficks principle: blood flow w a-fv differences). As with the a-fv IL-6 differences, only the increase in the exercising leg is significant and there is a significant difference between the exercising and resting leg at 4 and 5 h. *Significant difference from pre-exercise value (P < 0.05); [dagger]significant difference between exercising and resting leg (P < 0.05).
The blood flow in, respectively, the exercising and resting leg before exercise was 0.40 ± 0.07 and 0.36 ± 0.08 l min−1 (Fig. 2). With one-legged knee exercise the blood flow increased to 2.40 ± 0.18 l min−1 in the exercising leg, whereafter it was in essence unaltered (mean blood flow during exercise, measured every hour, was 2.56 ± 0.08 l min−1). In the resting leg the mean blood flow during exercise was 0.63 ± 0.04 l min−1. This resulted in a continuous net IL-6 release from the exercising leg during the exercise, calculated by multiplying the a-fv difference by the blood flow. At 3 h the net IL-6 release amounted to 3.92 ng min−1 (1.61- 8.59 ng min−1) with a 2- and 2-fold further increase at time points 4 and 5 h, respectively. Thus, the maximal net IL-6 release from the exercising leg was ≈1000-fold higher than before the exercise started. In contrast, the resting leg had an unchanged net IL-6 release with neither significant uptake nor significant release during the 5 h exercise period. The net IL-6 release in the exercising leg was significantly higher than the net release in the resting leg at 4 and 5 h of exercise (Fig.1C).
Figure 2. Blood flow in the exercising and resting leg measured by ultrasound Doppler.
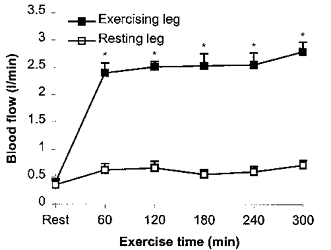
Data are means +s.e.m.*Significant difference from pre-exercise (Rest) value (P < 0.05).
DISCUSSION
The present study demonstrates that during exercise the net skeletal muscle IL-6 production increases strikingly and can account for the exercise-related high plasma IL-6 concentration. It is noteworthy that the large elevation in IL-6 was the result of exercise performed by only 2.5 kg of muscle, which during the last 2 h of exercise produced 6.8 ng IL-6 min−1 (kg muscle)−1 (3.96-9.69 ng min−1 kg−1).
When comparing the release of IL-6 from the contracting muscle with the amount accumulated in the body, it is quite apparent that the release markedly surpassed the rate of accumulation. The increase in arterial plasma IL-6 concentration during the last 2 h of exercise was 10.04 ng l−1 (8.30-12.00 ng l−1). Assuming that the IL-6 produced is diluted in the extracellular space (12 l), the total amount of IL-6 accumulated during the last 2 h of exercise was about 120.48 ng (99.60-144.08 ng) or 1.00 ng min−1 (0.83-1.20 ng min−1). This implies that the net release of IL-6 from the active leg was ≈17-fold higher than the rate of IL-6 accumulation. Moreover, the net IL-6 release from the exercising leg during 1 min at 5 h of exercise was approximately half of the IL-6 content in the blood. Indeed, the turnover rate of IL-6 in the human body must be very high when exercising and can be estimated to be 16 ng min−1 (9.07-23 ng min−1) simply by subtracting the amount accumulated in the last 2 h of exercise from the net IL-6 production, assuming that the active muscle is the only source of IL-6 production in the body during exercise.
Previous exercise studies on cytokines have examined eccentric exercise (Rohde et al. 1997; Hellsten et al. 1997; Bruunsgaard et al. 1997), combined concentric and eccentric exercise (Ostrowski et al. 1998a,b, 1999) or pure concentric exercise (Ullum et al. 1994; Bruunsgaard et al. 1997). In general, eccentric exercise has provoked higher plasma IL-6 concentrations than concentric exercise (Bruunsgaard et al. 1997; Utter et al. 1999). This difference has been attributed to eccentric exercise-induced muscle damage. In the present study, we aimed to study the effect of muscle contraction on the production of IL-6 and exclude the possible influence of the eccentric exercise-induced muscle damage by using a concentric exercise model. One-legged dynamic knee extensor exercise represents a pure concentric exercise model where high force was produced per unit muscle mass engaged in exercise, with the advantage of using the other leg as control (Andersen et al. 1985; Richardson & Saltin, 1998). Thus, in the light of the present results, there is the possibility that one stimulus for IL-6 production by skeletal muscle is the force of the contraction. The amount of IL-6 produced by the active muscle is impressive, not only because of the small muscle mass that is active, but also because the workload, in relative terms, was as low as 40% of Wmax,ke. It is not possible to transfer these high production values to models where a large fraction of muscle mass is engaged in the concentric exercise. This is because when the exercise is confined to a relatively small muscle mass, the weight-specific power output is higher than in running or bicycling (Blomstrand et al. 1997).
It is a most remarkable finding that the knee extensors of the leg are able to produce an increase in plasma IL-6 concentration to reach a level comparable to that obtained during severe infections (Bruunsgaard et al. 1999). In infectious diseases, the source of cytokine production is the monocytes (Hack et al. 1997). It remains to be determined which cells in the contracting muscles are the source of IL-6 production. The time course for monocyte infiltration into active muscle is much longer than that for the release of IL-6 by contracting skeletal muscles (Smith, 1991). Furthermore, there is no relationship between the degree of muscle damage and monocyte infiltration on the one hand and the magnitude of eccentric exercise-related IL-6 production on the other (Bruunsgaard et al. 1997). There is, however, no question that the IL-6 is produced in the contracting skeletal muscle. We have reported earlier that IL-6 mRNA is present in human skeletal muscle after strenuous exercise (Ostrowski et al. 1998b) as well as in rat muscle after electrically stimulated concentric or eccentric contractions (Jonsdottir et al. 2000). In addition, IL-6 mRNA was not demonstrated in blood mononuclear cells, either before or after exercise (Ostrowski et al. 1998b). Moreover, it has been reported that regenerating mice myofibres are able to produce IL-6 (Kurek et al. 1996). In a recent report, it was shown that myoblasts produce IL-6 in response to inflammatory stimuli, and it has also been suggested that muscle cells are able to produce IL-6 in response to muscle injury (Gallucci et al. 1998). Hence, contracting muscle cells are likely to be one source of IL-6 production.
In sepsis, the main role of IL-6 is to stimulate the liver to produce acute phase proteins, such as C reactive protein (Hack et al. 1997). The exercise-induced elevation in IL-6 results in only a minor effect on the liver production of acute phase proteins (Pedersen & Hoffman-Goetz, 2000). It is striking that the exercise-induced increase in IL-6 appears to be a close function of exercise duration. IL-6 has been shown to markedly inhibit insulin-stimulated increases in glycogen deposition in rat hepatocyte cultures (Kanemaki et al. 1998). Furthermore, IL-6 was shown to inhibit glycogen synthase activity and accelerate glycogen phosphorylase activity (Kanemaki et al. 1998). Moreover, it has been demonstrated that injection of recombinant human IL-6 (rhIL-6) into humans increases the fasting blood glucose concentration in a dose-dependent manner (Tsigos et al. 1997). Another study, also including humans, showed increased hepatic glucose output in response to injection of rhIL-6, concomitantly with a higher glucose metabolism (Stouthard et al. 1995). It has also been shown that consuming carbohydrate during exercise diminishes the exercise-induced increase in IL-6 (Nehlsen-Cannarella et al. 1997; Nieman et al. 1998). Therefore, the possibility exists that IL-6 produced by contracting skeletal muscles directly or indirectly mediates the hepatic glucose output necessary to maintain the blood glucose level as the usage of blood glucose by skeletal muscles is markedly enhanced in prolonged exercise. We therefore propose that IL-6 is produced by the active skeletal muscle and exerts its effect in a hormone-like manner.
References
- Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. Journal of Applied Physiology. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Bernéus B, Carlsten A, Holmgren A, Seldinger SI. Percutaneous catheterization of peripheral arteries as a method for blood sampling. Scandinavian Journal of Clinical Laboratory Investigation. 1954;6:217–221. [PubMed] [Google Scholar]
- Blomstrand E, Radegran G, Saltin B. Maximum rate of oxygen uptake by human skeletal muscle in relation to maximal activities of enzymes in the Krebs cycle. Journal of Physiology. 1997;501:455–460. doi: 10.1111/j.1469-7793.1997.455bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Galbo H, Halkjaer KJ, Johansen TL, Maclean DA, Pedersen BK. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. Journal of Physiology. 1997;499:833–841. doi: 10.1113/jphysiol.1997.sp021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Skinhoj P, Qvist J, Pedersen BK. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. Journal of Infectious Diseases. 1999;180:551–554. doi: 10.1086/314873. [DOI] [PubMed] [Google Scholar]
- Croisier JL, Camus G, Venneman I, Deby-Dupont G, Juchmes-Ferir A, Lamy M, Crielaard JM, Deby C, Duchateau J. Effects of training on exercise-induced muscle damage and interleukin 6 production. Muscle and Nerve. 1999;22:208–212. doi: 10.1002/(sici)1097-4598(199902)22:2<208::aid-mus8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Drenth JP, van Uum SH, van Deuren M, Pesman GJ, van der Ven-Jongekrijg J, van der Meer JWM. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo Tnf-alpha and IL-1 beta production. Journal of Applied Physiology. 1995;79:1497–1503. doi: 10.1152/jappl.1995.79.5.1497. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Provenzano C, Mazzarelli P, Scuderi F, Bartoccioni E. Myoblasts produce IL-6 in response to inflammatory stimuli. International Immunology. 1998;10:267–273. doi: 10.1093/intimm/10.3.267. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, Smith RM, Banks RE, Windsor AC, Dickson RA, Guillou PJ. Stimulation of inflammatory markers after blunt trauma. British Journal of Surgery. 1998;85:986–990. doi: 10.1046/j.1365-2168.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- Hack CE, Aarden LA, Thijs LG. Role of cytokines in sepsis. Advances in Immunology. 1997;66:101–195. doi: 10.1016/s0065-2776(08)60597-0. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Frandsen U, Orthenblad N, Sjodin B, Richter EA. Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. Journal of Physiology. 1997;498:239–248. doi: 10.1113/jphysiol.1997.sp021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. Journal of Physiology. 2000;528:157–163. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaki T, Kitade H, Kaibori M, Sakitani K, Hiramatsu Y, Kamiyama Y, Ito S, Okumura T. Interleukin 1beta and interleukin 6, but not tumor necrosis factor alpha, inhibit insulin-stimulated glycogen synthesis in rat hepatocytes. Hepatology. 1998;27:1296–1303. doi: 10.1002/hep.510270515. [DOI] [PubMed] [Google Scholar]
- Kurek JB, Nouri S, Kannourakis G, Murphy M, Austin L. Leukemia inhibitory factor and interleukin-6 are produced by diseased and regenerating skeletal muscle. Muscle and Nerve. 1996;19:1291–1301. doi: 10.1002/(SICI)1097-4598(199610)19:10<1291::AID-MUS6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, Bailey EM, Warren BJ, Utter A, Davis JM. Carbohydrate and the cytokine response to 2. 5 h of running. Journal of Applied Physiology. 1997;82:1662–1667. doi: 10.1152/jappl.1997.82.5.1662. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Utter A, Davis JM, Williams F, Butterworth DE. Influence of mode and carbohydrate on the cytokine response to heavy exertion. Medicine and Science in Sports and Exercise. 1998;30:671–678. doi: 10.1097/00005768-199805000-00005. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. Journal of Physiology. 1998a;513:889–894. doi: 10.1111/j.1469-7793.1998.889ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. Journal of Physiology. 1999;515:287–291. doi: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. Journal of Physiology. 1998b;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration and adaption. Physiological Reviews. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. Journal of Applied Physiology. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Saltin B. Human muscle blood flow and metabolism studied in the isolated quadriceps muscles. Medicine and Science in Sports and Exercise. 1998;30:28–33. doi: 10.1097/00005768-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Rohde T, MacLean DA, Richter EA, Kiens B, Pedersen BK. Prolonged submaximal eccentric exercise is associated with increased levels of plasma IL-6. American Journal of Physiology. 1997;273:E85–91. doi: 10.1152/ajpendo.1997.273.1.E85. [DOI] [PubMed] [Google Scholar]
- Smith LL. Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Medicine and Science in Sports and Exercise. 1991;23:542–551. [PubMed] [Google Scholar]
- Sprenger H, Jacobs C, Nain M, Gressner AM, Prinz H, Wesemann W, Gemsa D. Enhanced release of cytokines, interleukin-2 receptors, and neopterin after long-distance running. Clinical Immunology and Immunopathology. 1992;63:188–195. doi: 10.1016/0090-1229(92)90012-d. [DOI] [PubMed] [Google Scholar]
- Stouthard JM, Romijn JA, Van der Pol T, Endert E, Klein S, Bakker PJ, Veenhof CH, Sauerwein HP. Endocrinologic and metabolic effects of interleukin-6 in humans. American Journal of Physiology. 1995;268:E813–819. doi: 10.1152/ajpendo.1995.268.5.E813. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Papanicolaou DA, Kyrou I, Defensor R, Mitsiadis CS, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. Journal of Clinical Endocrinology and Metabolism. 1997;82:4167–4170. doi: 10.1210/jcem.82.12.4422. [DOI] [PubMed] [Google Scholar]
- Ullum H, Haahr PM, Diamant M, Palmo J, Halkjaer KJ, Pedersen BK. Bicycle exercise enhances plasma IL-6 but does not change IL-1 alpha, IL-1 beta, IL-6, or Tnf-alpha pre-mRNA in BMNC. Journal of Applied Physiology. 1994;77:93–97. doi: 10.1152/jappl.1994.77.1.93. [DOI] [PubMed] [Google Scholar]
- Utter AC, Kang J, Nieman DC, Williams F, Robertson RJ, Henson DA, Davis JM, Butterworth DE. Effect of carbohydrate ingestion and hormonal responses on ratings of perceived exertion during prolonged cycling and running. European Journal of Applied Physiology. 1999;80:92–99. doi: 10.1007/s004210050563. [DOI] [PubMed] [Google Scholar]
- van Hall G, Gonzalez-Alonso J, Sacchetti M, Saltin B. Skeletal muscle substrate metabolism during exercise: methodological considerations. Proceedings of the Nutrition Society. 1999;58:899–912. doi: 10.1017/s0029665199001202. [DOI] [PubMed] [Google Scholar]


