The interleukins are part of a larger class of polypeptides known as cytokines. These are messenger molecules that transmit signals between various cells of the immune system. They are mostly secreted from macrophages and lymphocytes and their production is induced in response to injury or infection. Their actions influence other cells of the immune system as well as other tissues and organs including the liver and brain.
The paper by Steensberg et al. (2000) in this issue of The Journal of Physiology is (to my knowledge) the first study to describe an increase in the net release of interleukin-6 (IL-6) from exercising skeletal muscle in humans. Using the arterio-venous concentration difference technique, it was shown that the active (but not the resting) upper leg of humans released significant amounts of IL-6 into the circulation during prolonged single-limb exercise. The magnitude of the IL-6 release could more than account for the observed increase in the plasma IL-6 content. This suggests that active muscles (rather than blood leukocytes) are the major source of IL-6 production during exercise and that turnover (production and clearance) of this cytokine is quite high during exercise. Until recently it was thought that increases in the plasma levels of certain cytokines, including IL-1b, IL-6, IL-8 and tumour necrosis factor, during exercise were exclusively derived from blood leukocytes and lymphoid tissue. The study by Steensberg et al. (2000) helps to explain why the concentration of plasma cytokines such as IL-6 rises during exercise, whereas in vitro cytokine production by blood leukocytes is inhibited during and after exercise. This is in keeping with the view that prolonged strenuous exercise results in a temporary depression of immune function and an activation of the acute phase response with some similarities to trauma and sepsis.
Of course, we cannot be absolutely certain from this study that the IL-6 comes from the muscle fibres themselves. One limitation of the arterio-venous difference method is that it cannot distinguish which specific tissues are releasing IL-6 (e.g. it could be from the muscle fibres, satellite cells, tissue macrophages, nerve endings, the endothelium of the muscle capillary bed, adipose tissue, or bone, etc.). However, it does seem most likely that the release is actually from the contracting myofibres. This notion is supported by evidence presented in another paper recently published in The Journal of Physiology (Jonsdottir et al. 2000) which shows that mRNA for IL-6 is produced in muscle following electrically stimulated muscle contractions in the rat. mRNA for IL-6 was identified in isolated myofibres following a period of intermittent contractions. The mRNA was only found in muscles that had been activated; no mRNA for IL-6 was found in resting muscles of the contralateral leg. Furthermore, eccentric and concentric muscle actions were associated with similar increases in IL-6 mRNA levels, which suggests that the production of IL-6 is not solely in response to muscle tissue damage, which is seen primarily after eccentric exercise.
So now it seems that IL-6 can actually be produced in active muscle fibres and that the release of IL-6 from active muscle does not require the presence of muscle fibre injury. Furthermore, IL-6 may have other roles in the body apart from its influence on immune cells. In the study by Steensberg et al. (2000), net IL-6 release was only substantially increased after more than 2 h of single-limb exercise and the authors suggest a hormone-like glucoregulatory role for this cytokine, which seems reasonable given the known effects of IL-6 on liver glucose output (Tsigos et al. 1997). This idea is supported by other studies which have shown that the plasma IL-6 response to exercise is attenuated when carbohydrate is ingested during exercise (Nehlsen-Cannarella et al. 1997). We have also carried out a study in Birmingham which showed that the plasma IL-6 response to exercise is elevated when subjects begin exercise in a glycogen-depleted state (Gleeson & Bishop, 2000). It is an intriguing possibility that the IL-6 response may be a signal indicating that muscle glycogen stores are reaching critically low levels and that the active muscles’ reliance on blood glucose as a source of energy is on the increase. This may signal the liver to increase its glucose output in order to prevent a drastic fall in the blood glucose concentration (Fig. 1).
Figure 1.
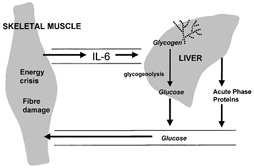
An energy crisis (e.g. glycogen depletion) or tissue damage in exercising skeletal muscle may provide the signal for increased IL-6 production and release. The elevated plasma IL-6 concentration then stimulates hepatic glycogenolysis and glucose release, helping to maintain blood glucose homeostasis. IL-6 also promotes acute phase protein and insulin-like growth factor synthesis, which may play a role in muscle adaptation to exercise.
Several cytokines have effects on the brain and are known to affect mood and sensations of fatigue. Could it be that muscle IL-6 output also acts as a mechanism contributing to the development of central fatigue in prolonged exercise? Other known effects of IL-6 include the stimulation of hepatic synthesis and release of acute phase proteins, production of insulin-like growth factor and release of anti-inflammatory cytokines such as IL-1-receptor antagonist. These effects may have implications for the recovery, repair and adaptation of muscle in the post-exercise period.
References
- Gleeson M, Bishop NC. Immunology and Cell Biology. 2000;78:554–561. doi: 10.1111/j.1440-1711.2000.t01-6-.x. [DOI] [PubMed] [Google Scholar]
- Jonsdottir IH, Scherling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Journal of Physiology. 2000;528:157–163. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, Bailey EM, Warren BJ, Utter A, Davis JM. Journal of Applied Physiology. 1997;82:1662–1667. doi: 10.1152/jappl.1997.82.5.1662. [DOI] [PubMed] [Google Scholar]
- Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Journal of Physiology. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


