Abstract
A role of the immune system in muscular adaptation to physical exercise has been suggested but data from controlled human studies are scarce. The present study investigated immunological events in human blood and skeletal muscle by immunohistochemistry and flow cytometry after eccentric cycling exercise and multiple biopsies.
Immunohistochemical detection of neutrophil- (CD11b, CD15), macrophage- (CD163), satellite cell- (CD56) and IL-1β-specific antigens increased similarly in human skeletal muscle after eccentric cycling exercise together with multiple muscle biopsies, or multiple biopsies only.
Changes in immunological variables in blood and muscle were related, and monocytes and natural killer (NK) cells appeared to have governing functions over immunological events in human skeletal muscle.
Delayed onset muscle soreness, serum creatine kinase activity and C-reactive protein concentration were not related to leukocyte infiltration in human skeletal muscle.
Eccentric cycling and/or muscle biopsies did not result in T cell infiltration in human skeletal muscle. Modes of stress other than eccentric cycling should therefore be evaluated as a myositis model in human.
Based on results from the present study, and in the light of previously published data, it appears plausible that muscular adaptation to physical exercise occurs without preceding muscle inflammation. Nevertheless, leukocytes seem important for repair, regeneration and adaptation of human skeletal muscle.
Muscular adaptation to physical stress is of significant importance for normal muscular development and function. Without stimulation from physical activity, muscle tissue will undergo atrophy and decreased functional capacity. In addition, muscular adaptation to physical exercise is indispensable for increased physical performance with training. The mechanisms responsible for maintaining normal muscle function in healthy individuals are largely unknown, as the processes involved in adapting muscle tissue to changes in functional demand have not been clarified. The involvement of several different systems, including the nervous, neuroendocrine, vascular and immune systems, is most probably inevitable (Grounds, 1991; Felten et al. 1993; Ottaway & Husband, 1994; Chambers & McDermott, 1996). Exercise, especially if strenuous and including eccentric muscle contractions, is believed to induce local muscle damage resulting in the release of various substances such as intracellular proteins, cytokines and chemokines, ultimately resulting in an inflammatory response (Shek & Shephard, 1998). This local inflammation may include complement activation, upregulation of adhesion molecule expression on leukocytes and endothelium with subsequent migration and infiltration of positively selected blood leukocytes into the affected tissue (Tidball, 1995). Suggestions have been made that the immune system may play an important role in adaptation to physical stress (Fielding & Evans, 1997), but few studies have yet been conducted to directly investigate this hypothesis. One possible explanation for the lack of investigations may be the lack of applicable analytical methods. Some of the methodological limitations have now been overcome, and numerous studies have investigated the effect of exercise on circulating blood leukocytes regarding absolute number, percentage distribution and in vitro function (Pedersen, 1997). These studies are important for understanding the systemic function of the immune system but give only limited information about local immunological events in peripheral tissue. It may be argued that leukocytes in circulation in fact represent a population of cells on their way towards participation in ongoing tissue surveillance, repair and adaptation.
Knowledge regarding the interaction between circulating and tissue leukocytes may be used as a means to understand muscular adaptation to physical exercise, the mechanisms behind the overtraining syndrome in athletes and overuse injuries in conjunction with monotonous work tasks. In a clinical setting, an inflammatory-inducing exercise model can be used in the study of inflammatory muscle diseases as well as in designing exercise protocols for the elimination of some of the disabling symptoms associated with these diseases.
The intention of this study was to perform quantitative and qualitative analysis of leukocytes in blood and quantitative analysis of leukocytes in human muscle tissue in response to exercise-induced stress. Based on current knowledge regarding the inflammatory response in tissue, a selection of common leukocyte membrane antigens believed to be involved in the exercise-induced activation of the immune system was investigated (Table 2). Cytokines can be mediators of the inflammatory response in muscle tissue, and some cytokines have previously been detected in muscle tissue from patients with chronic muscle inflammation (Lundberg et al. 1997). Thus, five different cytokines were investigated in this study (Table 2). Because the intention in this study was to investigate inflammatory events, there were concerns raised regarding the effects of multiple biopsies in the same muscle. A control group, which did not exercise, was therefore included to investigate the effects of multiple muscle biopsies on local and systemic immunological variables.
Table 2.
Staining panel for immunohistochemistry
| Antigen* | Clone | Supplier | Dilution | Expression, function |
|---|---|---|---|---|
| CD3 | T3–4B5 | DP | 1:400 | T cells, T cell receptor accessory molecule |
| CD4 | MT310 | DP | 1:100 | T helper, monocytes, macrophages, all APC, MHC II binding |
| CD8 | DK25 | DP | 1:100 | T cytotoxic, MHC I binding |
| CD11b | 2LPM19c | DP | 1:800 | Granulocytes, C3b1 receptor (CR3) |
| CD15 | C3D-1 | DP | 1:20 | Granulocytes |
| CD56 | MOC-1 | DP | 1:100 | NK cells, satellite cells, dendritic cells, (N-CAM) |
| CD79α | JCB117 | DP | 1:20 | B cells |
| CD163 | Ber-MAC3 | DP | 1:200 | Human macrophages |
| IL-1α | Mix | CG | 1:500 | Macrophages, fibroblasts, endothelial cell, smooth muscles. Pro-inflmmatory effects |
| IL-1β | Mix | CG | 1:500 | Macrophages, fibroblasts, endotelial cell, smooth muscles |
| IL-1ra | Mix | BMA | 1:100 | Blocks IL-1α and IL-1β function |
| IL-6 | AF206 | RD | 2μg ml−1 | Macrophages, T2 helper cells. Pro-inflammatory. (Goat polyclonal) |
| IL-6 | MQ2–6A3 | PH | 2 μg ml−1 | Macrophages, T2 helper cells. Pro-inflammatory. (Rat monoclonal) |
| TNFα | Mab-1 | PH | 1:83 | Macropahges. Induces cytokine production, weight-loss in chronic inflammation |
| Endothelium | EN4 | SB | 1:100 | Human endothelium |
| Neg. control | IgG1 | DP | 1:20 | Aspergillus niger glucose oxidase antibody |
APC. antigen presenting cells; DP, DAKOPTTS AB, Älsvjö, Sweden; SB, SANBIO, Netherlands; PH, Pharmingen, USA; CG, Immunocontact, Ciba-Geigen, Switzerland; BMA, Biomedicals AG, Switzerland; RD, R&D Systems, UK.
Cluster of differentiation (CD). Neg. control, negative control.
METHODS
Subjects
Thirteen healthy male subjects with a mean age of 23.9 years (range 19-32) and a mean body mass of 74.9 kg (range 63-95) participated in the study. All subjects were physically active on a regular basis (mean maximal oxygen uptake during concentric cycling (V̇O2,max) = 3.66 l min−1; range 2.80-4.57). After receiving oral and written information about the study, subjects signed an informed consent and were randomly assigned to either exercise or control groups. The study conformed with the Declaration of Helsinki, and was approved by the Ethics Committee at the Karolinska Institute (Dnr: 97-044).
Exercise protocol
Not later than 2 days before the eccentric cycling exercise each subject's maximal oxygen uptake during concentric cycling was determined (Medical Graphics Corp. CPX system, St Paul, MN, USA). A standard incremental cycling test was performed, starting at a work rate of 100 W at 60 revolutions min−1 with a 50 W increase in work rate every 2 min until exhaustion. Subjects were asked not to perform any strenuous or unaccustomed exercise from 7 days before the eccentric cycling until the last muscle and blood sample was taken.
The electrically powered bicycle used in this study has previously been used for eccentric cycling exercise (Friden et al. 1983). It consists of an electrical motor, an electrical induction clutch and a modified cycle ergometer. Subjects were instructed to maintain 60 r.p.m. for 30 min at a work rate equal to the highest concentric cycling work rate maintained for 2 min during the concentric cycling V̇O2,max test. This protocol is similar to the one used by Friden et al. (1983). The work rate was chosen after a series of pilot trials on separate subjects, where different eccentric work rates relative to the subject's concentric V̇O2,max were tested in order to determine the highest eccentric work rate possible to maintain for 30 min. All subjects performed eccentric cycling at 250 or 300 W. In a few cases the work rate had to be decreased at the end of exercise due to extreme fatigue. The eccentric exercise can thus be considered maximal or close to maximal for most subjects, with respect to eccentric muscular exercise capacity. The O2 uptake (V̇O2) was measured during the last 5 min of exercise, and blood lactate samples were taken 3 min post exercise.
Blood samples
Blood from a fingertip capillary was collected 3 min post testing for determination of blood lactate concentration using the YSI 2300 STAT lactate analyser (YSI Corp., Yellow Springs, OH, USA).
Immediately prior to each muscle biopsy, venous blood samples were drawn from a forearm vein before, immediately after and 6, 24 and 48 h, and 4 and 7 days post exercise. Blood samples for analysis of catecholamines were only drawn before and immediately after exercise. Five millilitre samples of blood were collected (1) into Vacutainer tubes containing ethylendiaminetetraacetic acid (EDTA; Becton Dickinson, France) for leukocyte analysis by flow cytometry, (2) into untreated Vacutainer tubes for analysis of C-reactive protein (CRP), cortisol, albumin, sex hormone-binding globulin (SHBG) and testosterone and (3) into heparinized tubes (Becton Dickinson) for catecholamine and creatine kinase (CK) analysis.
Flow cytometry
Determination of different subsets of leukocytes (Table 1 and Table 2) was accomplished by flow cytometry with three-colour analyses. The method for three-colour flow cytometry analysis has been described in detail previously (Lenkei & Andersson, 1995) and is based on a high degree of standardization.
Table 1.
Antigens investigated on lymphocytes and monocytes in blood
| Antigen * | Function | Expression |
|---|---|---|
| Lymphocytes | ||
| CD3 | Associated with the T cell receptor. Involved in signal transduction | T cells |
| CD4 | Accessory molecule for TCR antigen recognition, MHC II interaction | T helper cells, monocytes |
| CD5 | Signal transduction | T cell, B and NK cell subsets |
| CD8 | Accessory molecule for TCR antigen recognition, MHC I interaction | T cytotoxic/supressor cells |
| CD11b | Integrin, adhesion to vascular endothelium, receptor for C3b1 | Granulocytes, monocytes, NK cells, T cells |
| CD16 | Low affinity Fc receptor for IgG | NK, monocytes, neutrophils |
| CD20 | Regulates B cell activation and proliferation | B cells |
| CD23 | Triggers monokine release, pro-inflammatory, IgE synthesis | B cells, monoytes |
| CD45 | Cytoplasmic phiosphatase activity, signal transduction, apoptosis | All leukocytes |
| CD56 | Homotypic adheions | NK cells, T cell subset, some B cells |
| CD57 | Neuroadhesion molecule | NK cells, T cells |
| CD62L | Selectin, homing receptor on leukocytes | Blood B, T and NK cells, monocytes, granulocytes |
| DR | MHC II subunit | Antigen-presenting cells |
| Monocytes | ||
| CD14 | LPS binding protein. Activation marker. Induces oxidative burst | Monocytes |
Cluster of differentiation (CD). TCR, T cell receptor; MHC, major histocompatibility complex; LPS, lipopolysaccharide.
White blood cell count and differentials were estimated with a Coulter STKS haemocytometer (Beckman Coulter Inc., Fullerton, CA, USA). Because cell numbers were determined in whole blood, corrections for changes in plasma volume were not made.
C-reactive protein and albumin
C-reactive protein (CRP) and albumin were analysed by means of particle-enhanced immunonephelometry (Dade Behring Marburg, Germany).
Cortisol
Total serum cortisol was determined by a standard immunofluorescent method (Department of Clinical Chemistry, Karolinska Hospital, Solna, Sweden).
Testosterone, sex hormone binding globulin (SHGB) and albumin
Serum testosterone and SHGB concentration were determined by a standard time-resolved fluoroimmunoassay (Kit B050-101 and B070-101 respectively; AutoDelphia, Wallac Oy, Finland). The biologically available (free) testosterone concentration (not bound to SHGB or albumin) was calculated as T(1 + 0.601C) where T is unbound testosterone and C is plasma albumin concentration (personal communication from Regina Solborg, Department of Clinical Chemistry, Karolinska Hospital, Solna, Sweden).
Creatine kinase
Creatine kinase (CK) activity was measured using a standard laboratory kit (CK MPR2, Boehringer-Mannheim, Germany) and a DU-70 spectrophotometer (Beckman Instruments AB, Bromma, Sweden).
Catecholamines
Adrenaline, noradrenaline and dopamine were analysed in heparinized plasma, using high-pressure liquid chromatography (HPLC) with electrochemical detection (CMA Microdialysis AB, Solna, Sweden) and a Nucleosil-100 SA 5 μm column (NC100-5SA-250D, Hichrome, Berks, UK). The coefficient of variance (n = 8) was 4.6 % for noradrenaline, 6.0 % for adrenaline and 7.4 % for dopamine.
Muscle biopsies
Muscle biopsies were taken from the vastus lateralis using the forceps biopsy technique. The first, second, fourth and sixth biopsies were taken in the left leg and the third, fifth and seventh biopsies were taken in the right leg. The first biopsy in each leg was taken in the distal part of the muscle and each subsequent biopsy was taken approximately 2 cm proximal to the previous one. This procedure was used in order to minimize the influence of one biopsy on the following one. After local epidermal anaesthesia (Carbocain 20 mg ml−1, ASTRA, Södertälje, Sweden) a 1.5 cm incision was made through the dermis, epimysium and perimysium and a 50-100 mg muscle tissue sample removed. The muscle sample was placed in Tissue-Tek medium (Miles Laboratories, Elkhart, IN, USA), frozen in isopropanol in liquid nitrogen within 1 min and stored at -70°C before sectioning (6 μm). Sections were placed on Superfrost glass slides (Novakemi AB, Enskede, Sweden), air dried overnight (leukocyte antigens) or fixed in 2 % formaldehyde and air dried for 30 min (cytokines) and stored at -70°C until staining.
Muscle samples from four subjects (two exercised and two controls) were also stained using Gomoris trichrome method for altered muscle fibres and for acid phosphatase (Department of Neurophathology, Huddinge Hospital, Sweden).
Immunhistochemistry
The Vectastain ABC Elite chemicals and rapid staining protocol were used (Vector Laboratories, Burlingame, CA, USA) when investigating expression of leukocyte phenotypic antigens with monoclonal antibodies in muscle tissue sections (Table 2). For cytokine staining, a modified Vectastain protocol was used.
Image analysis
For quantification of positively stained tissue sections, a semi-automated image analysis system was used (Leica Microsystems, Kista, Sweden). The system consists of a high resolution 3CCD camera (DXC-950P, Sony Corporation, Tokyo, Japan) mounted on a Leica Fluovert FS microscope and connected to the QWin 500IW v.2.2 image analysis system (Leica Microsystems, Kista, Sweden) via a stabilized power supply (Inmac Power Line Conditioner 8831-7, Inmac, Santa Clara, CA, USA).
Analyses were performed on three separate areas of each muscle section for a total area of approximately 1.5 mm2 per antigen and biopsy. The analyses included detection of positively stained tissue, manual counting of the total number of muscle fibres, measurement of detected area and counting of positively stained leukocyes by the QWin system. All CD antigens were detected on leukocytes only, except CD56 which was also present on satellite and muscle cells. The proportions of CD56 positive satellite and muscle cells of all muscle cells were calculated by manual counting. Because identical statistical results were obtained when using detected leukocyte number or areas, only ‘area’ results are presented. The detection threshold for positively stained tissue was set for each antigen using the pre-exercise biopsy and kept constant for all seven biopsies from the same subject. The optimal threshold for each individual and each antigen was determined. The coefficient of variance for repeated measurement of the same tissue section was 0.08 % (n = 39).
Muscle soreness
At the time points of blood and muscle samples, delayed onset of muscle soreness (DOMS) at rest was estimated by the subject's rating on a 0-10 subject rating scale (0 = no soreness and 10 = very, very sore).
Statistical analysis
The StatView software (Abacus Concepts, Inc., Berkeley, CA, USA) was used for all statistical analyses. Due to the abnormal distribution in resting numbers of most blood leukocyte phenotypes in a larger population (n = 57) (Lenkei & Andersson, 1995), as well as in blood and muscle samples in the present study (skewness > 2.0), non-parametric methods were used. The Mann-Whitney procedure tested for within-group changes and the Wilcoxon signed rank test was employed for between group comparisons. Non-parametric methods were also applied for investigating CK and DOMS changes. Multiple regression was used to investigate relationships between variables. A strict statistical approach was used with r2 > 0.80 accepted as significant in the model, but variables where one single outlier determined the correlation were excluded. Outliers were judged by a dependent versus a fitted plot. Two predictor variables were used only if they were not correlated to each other (P > 0.2), for example, the prediction of CD11b in muscle in the exercise group where the testosterone/cortisol quota (an indicator of metabolic stress) and eccentric V̇O2 are not correlated (Table 8 and Fig. 1).
Table 8.
Multiple regression analysis of changes in antigen expression in muscle
| Antigen | Time | Predictor variables | r2 | P | |
|---|---|---|---|---|---|
| CD11b (leukocytes) | Exercise | 24 h | Test/Cort post exercise (−) | 0.0065 | |
| 0.97 | |||||
| Eccentric VO2 (l min−1) (+) | 0.0027 | ||||
| Control | 24 h | NK (CD56 + CD16 + CD57-CD3-) cell number at rest (+) | 0.99 | 0.0005 | |
| CD56 (satellite cells) | Exercise | 24 h | Change in CD45 expression on CD14 + DR- monocytes from rest to post) (−) | 0.0039 | |
| 0.92 | |||||
| Exercise | 48 h | CD14 expression on DR+ monocytes at 6 h (−) | 0.0030 | ||
| 0.87 | |||||
| Change in CD11b expression on CD62L+ monocytes from rest to 6 h (−) | 0.0005 | ||||
| Control | 24 h | ? | |||
| CD163 (macrophages) | Exercise | 48 h | CD11b in muscle post exercise (+) | < 0.0001 | |
| 0.98 | |||||
| CD45 expression on CD14 + DR+ monocytes at rest(+) | 0.0003 | ||||
| Control | 48 h | CD11b in muscle at 6 h (−) | 0.0085 | ||
| 0.98 | |||||
| 4 days | Change in CD11b expression on CD62L+ monocytes from rest to 4 days (+) | 0.0063 | |||
| 0.92 | |||||
| IL-1α | Exercise | No change | |||
| Control | 6 h | NK (CD56 + CD16 + CD57-CD3-) cell number at rest (+) | 0.91 | 0.0081 | |
| IL-1β | Exercise | Post | Excentric VO2 (ml min−1 kg−1) (−) | 0.0095 | |
| 0.95 | |||||
| CD14 expression on DR+ monocytes (rest)(−) | 0.0421 | ||||
| Control | ? |
Exercise, n = 7; control, n = 5. Positive (+) or negative (−) correlation in parenthesis; r2, multiple correlation coefficient adjusted for multiple predictor variables; P, individual probability for each predictor variable.
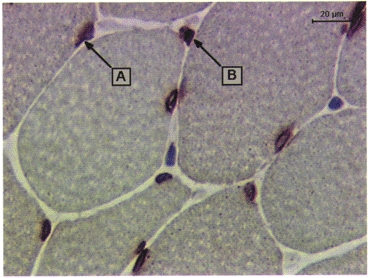
Figure 1.
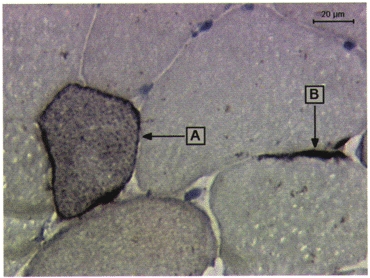
Demonstration of regenerating muscle cells (A) and activated satellite cells (B) by the expression of CD56 in human muscle tissue.
RESULTS
Eccentric cycling exercise for 30 min resulted in significant changes in several measured variables in blood and muscle tissues. However, the procedure with multiple muscle biopsies and blood samples resulted in similar changes in control subjects (Table 3, Table 4 and Table 5). Multiple regression analysis revealed that the mechanisms causing similar changes in the exercise and control groups may or may not be the same (Table 8 and Fig 5, Fig 6, Fig 7, Fig 8, Fig 9 and Fig 10).
Table 3.
Significantly changed leukocyte phenotypes in bllod before and after ecentric cycling exericse
| Phenotype | Rest | Post | 6 h | 24 h | 48 h | 4 days | 7 days | |
|---|---|---|---|---|---|---|---|---|
| LPK (cells ml-1) | Exercise | 5200 | 5400 | 8600 | 5700 | 5400 | 5500 | 5700 |
| (4300–6300) | (4600–7300)*‡ | (6900–10000)* | (5000–7800)* | (3300–10900) | (5500–7000)* | (4700–7100) | ||
| Control | 5500 | 5600 | 6900 | 6200 | 5700 | 5200 | 5500 | |
| (3500–8700) | (3700–7800)‡ | (4000–9900) | (4600–9500) | (3400–8300) | (3900–8100) | (4700–6100) | ||
| Lymphocytes (cells ml−1) | Exercise | 1955 | 1998 | 2066 | 2185 | 1999 | 1978 | 2033 |
| (1765–2311) | (1756–2585) | (1813–2669) | (1937–2737)*‡ | (1526–2462) | (1727–2070) | (1653–2403) | ||
| Control | 2470 | 2106 | 2284 | 2297 | 1772 | 2357 | 2095 | |
| (1573–4013) | (1420–3350)* | (1526–3399) | (1374–3498)*‡ | (1526–2462) | (1395–3225)* | (1636–2282) | ||
| Lymphocytes (%) | Exercise | 38 | 34 | 25*† | 35 | 39 | 34 | 40 |
| (33–45) | (31–43) | (22–32)*‡ | (30–46) | (22–47) | (26–40) | (26–47) | ||
| Control | 45 | 42 | 34 | 39 | 33 | 42 | 41 | |
| (42–49) | (38–45)* | (28–45)* | (24–45)* | (24–42)‡ | (27–51)* | (33–51) | ||
| Neutrophils (cells μl-1) | Exercise | 2500 | 2900 | 5300 | 3000 | 2700 | 3000 | 2600 |
| (1800–3600) | (2100–4200)* | (4000–6500)* | (2200–4400) | (400–8600) | (2300–4300)* | (1800–4400) | ||
| Control | 2200 | 2700 | 3900 | 3500 | 3500 | 2500 | 2400 | |
| (1500–3700) | (1600–3600) | (1800–5800)* | (2100–5500) | (1700–4900) | (1450–4800) | (1800–3100) | ||
| Neutrophils (%) | Exercise | 49‡ | 49 | 62 | 52 | 53 | 53 | 46 |
| (42–55)‡ | (45–56) | (55–67)* | (43–58) | (39–71) | (46–60) | (38–62) | ||
| Control | 41 | 45 | 57 | 47 | 56 | 49 | 45 | |
| (38–45) | (39–51)* | (45–64)* | (41–67)* | (45–67) | (36–64) | (35–56) | ||
| Monocytes (cells μl-1) | Exercise | 474 | 564*† | 687* | 551* | 440 | 612* | 612 |
| (288–514) | (338–669)*‡ | (444–1060)* | (356–656)* | (347–485) | (442–683)* | (442–683) | ||
| Control | 610 | 541‡ | 662 | 800 | 429 | 598 | 527 | |
| (213–827) | (206–748)‡ | (292–806) | (282–1016) | (169–769) | (397–681) | (283–779) | ||
| Monocytes (%) | Exercise | 9 | 9 | 10 | 8 | 7 | 11* | 11 |
| (6–10) | (5–11) | (6–13) | (6–10) | (5–9) | (8–12)* | (8–12) | ||
| Control | 9 | 8 | 8 | 9 | 9 | 8 | 10 | |
| (7–11) | (7–12) | (6–9) | (5–13) | (4–12) | (7–11) | (6–14) | ||
| Lymphocytes CD3+(cells μl-1) | Exercise | 1402 | 1252 | 1490 | 1503 | 1350 | 1272* | 1433 |
| (1326–1572) | (1134–1811) | (1134–2017) | (1240–1910) | (992–1916) | (1088–1417)* | (1153–1668) | ||
| Control | 1538 | 1437 | 1276* | 1342* | 1171 | 1608 | 1348 | |
| (593–3372) | (670–2786) | (558–2594) | (439–2661)* | (395–2371) | (651–2645) | (873–2069) | ||
| CD3+(%) | Exercise | 73 | 69 | 68 | 67 | 75 | 67* | 71 |
| (66–77) | (59–76) | (61–78) | (56–76) | (63–81) | (59–73)* | (60–82) | ||
| Control | 64 | 72 | 57 | 66 | 66 | 68 | 66 | |
| (51–85) | (54–87) | (44–81) | (41–82) | (54–83) | (53–84) | (56–74) | ||
| CD8+CD11b+(cells μl-1) | Exercise | 160 | 248* | 176 | 339 | 133 | 163 | 215 |
| (77–351) | (142–435)* | (111–333) | (206–404) | (76–294) | (121–329) | (111–317) | ||
| Control | 215 | 174 | 254 | 305 | 185 | 182 | 157 | |
| (82–317) | (83–249) | (86–336) | (102–456) | (4–402) | (67–384) | (82–246) | ||
| CD8+CD11b+(%) | Exercise | 9 | 12* | 10 | 14 | 8 | 9 | 11 |
| (3–19) | (7–21)* | (8–15) | (9–16) | (4–15) | (7–17) | (6–16) | ||
| Control | 8 | 7 | 8 | 11 | 8 | 7 | 7 | |
| (3–13) | (3–11) | (2–16) | (4–18) | (−1 to 22) | (4–16) | (4–10) | ||
| CD8+CD62L+(cells μl-1) | Exercise | 386 | 365 | 367 | 481* | 373 | 349 | 357 |
| (312–440) | (275–544) | (269–540) | (349–631)* | (238–487) | (295–478) | (307–492) | ||
| Control | 408 | 384 | 324 | 445 | 340 | 473 | 363 | |
| (161–785) | (185–677) | (202–467) | (157–733) | (95–622) | (218–620) | (125–608) | ||
| CD8+CD62L+(%) | Exercise | 19 | 19 | 17 | 22 | 18 | 20 | 20 |
| (16–22) | (15–22) | (15–21) | (17–24) | (14–22) | (16–20) | (16–24) | ||
| Control | 17 | 18 | 13 | 18 | 18 | 18 | 17 | |
| (11–22) | (13–22) | (7–22) | (12–23) | (11–25) | (14–21) | (8–24) | ||
| Proportion of CD4+ of all CD62L+(%) | Exercise | 83 | 83 | 76 | 86*† | 76 | 85 | 83 |
| (69–88) | (73–87) | (66–83) | (78–90) | (71–85) | (78–89) | (77–88) | ||
| Control | 71 | 68 | 73 | 80 | 80 | 77 | 73 | |
| (59–85) | (53–81) | (56–85) | (64–86) | (70–89) | (68–90) | (67–83) | ||
| Proportion of CD8+ of all CD62L+(%) | Exercise | 46 | 47 | 53 | 55* | 49 | 64* | 60* |
| (40–56) | (43–60) | (43–64) | (51–64) | (42–58) | (49–70) | (48–69) | ||
| Control | 49 | 44 | 40 | 52 | 53 | 46 | 41 | |
| (27–64) | (26–65) | (18–66) | (33–63) | (20–79) | (35–65) | (27–66) | ||
| CD20+(cells μl-1) | Exercise | 161† | 174† | 236* | 208 | 171 | 166 | 190 |
| (125–223) | (144–196) | (181–270) | (152–261) | (156–203) | (133–199) | (98–283) | ||
| Control | 289 | 211 | 258 | 226 | 177 | 267 | 269 | |
| (184–354) | (164–303) | (141–395) | (176–305) | (98–265) | (147–332) | (212–398) | ||
| CD20+(%) | Exercise | 8 | 8 | 11* | 9 | 9 | 9 | 8 |
| (7–10) | (6–10) | (8–13) | (7–11) | (7–13) | (7–11) | (6–13) | ||
| Control | 12 | 12 | 12 | 12 | 10 | 12 | 14 | |
| (5–16) | (6–15) | (6–17) | (6–16) | (2–19) | (6–17) | (11–16) | ||
| CD20+CD23+(cells μl-1) | Exercise | 97 | 98† | 142 | 138 | 104 | 95 | 95 |
| (69–127) | (72–123) | (105–167) | (90–165) | (70–125) | (51–125) | (48–159) | ||
| Control | 161 | 145 | 148 | 139 | 95 | 154 | 178 | |
| (89–193) | (105–157) | (98–203) | (121–161) | (58–157) | (93–187) | (129–220) | ||
| CD20+CD23+(%) | Exercise | 5 | 5 | 7* | 5 | 5 | 5 | 5 |
| (4–6) | (4–6) | (5–7) | (4–7) | (3–6) | (3–7) | (3–7) | ||
| Control | 6 | 6 | 6 | 6 | 6 | 8 | 8 | |
| (3–9) | (3–9) | (3–10) | (3–10) | (1–11) | (3–10) | (6–10) | ||
| CD56+CD16+CD57+CD3-(cells μl-1) | Exercise | 190 | 210*†‡ | 155 | 221 | 109† | 211 | 192 |
| (111–230) | (167–277) | (110–192) | (117–317) | (80–161) | (140–243) | (75–278) | ||
| Control | 173 | 102*‡ | 133 | 182 | 206 | 151 | 154 | |
| (113–215) | (50–198) | (78–260) | (52–376) | (79–345) | (100–276) | (18–320) | ||
| CD56+CD16+CD57+CD3-(%) | Exercise | 10 | 11‡ | 8 | 9 | 7‡ | 10 | 9 |
| (6–11) | (8–14) | (5–11) | (6–13) | (4–9) | (8–13) | (4–13) | ||
| Control | 8 | 6‡ | 6 | 9 | 13‡ | 7 | 8 | |
| (3–10) | (2–9) | (2–13) | (2–17) | (3–22) | (2–16) | (−16) | ||
| CD56+CD16+CD57-CD3-(cells μl-1) | Exercise | 136 | 155*‡ | 128 | 155* | 115 | 150 | 159 |
| (61–256) | (86–304) | (87–214) | (56–372) | (84–136) | (105–227) | (62–283) | ||
| Control | 104 | 82*‡ | 92 | 105 | 134 | 130 | 131 | |
| (55–188) | (36–162) | (54–183) | (45–263) | (73–209) | (102–151) | (42–205) | ||
| CD56+Cd16+CD57-CD3-(%) | Exercise | 7 | 8*†‡ | 7 | 7 | 5* | 7 | 8 |
| (4–11) | (5–15) | (4–11) | (4–15) | (3–9) | (5–13) | (3–13) | ||
| Control | 5 | 4‡ | 5 | 6 | 8 | 6 | 7 | |
| (2–8) | (2–7) | (2–8) | (2–12) | (3–13) | (3–19) | (1–10) | ||
| Monocytes CD14+(cells μl-1) | Exercise | 468 | 534* | 660* | 538* | 413 | 599* | 605 |
| (279–528) | (321–630) | (424–1136) | (344–643) | (334–465) | (482–786) | (429–665) | ||
| Control | 603 | 529 | 641 | 780 | 418 | 592 | 518 | |
| (203–790) | (202–725) | (286–770) | (258–998) | (161–753) | (390–666) | (276–770) | ||
| CD14+(%) | Exercise | 97 | 94 | 98 | 98 | 97 | 98* | 98 |
| (95–98) | (91–97) | (95–99) | (95–99) | (94–99) | (97–99) | (96–99) | ||
| Control | 95 | 98 | 97 | 98 | 98 | 99* | 99 | |
| (92–99) | (96–99) | (95–98) | (95–99) | (96–98) | (96–99) | (98–99) | ||
| CD14+DR+ (cells μl-1) | Exercise | 421 | 504* | 553* | 338 | 360 | 494* | 451 |
| (257–487) | (300–586) | (338–1026) | (236–488) | (294–430) | (376–696) | (356–583) | ||
| Control | 567 | 465 | 571 | 627 | 350 | 422 | 433 | |
| (203–717) | (192–659) | (279–688) | (242–817) | (108–652) | (328–556) | (270–647) | ||
| CD14+DR+(%) | Exercise | 88 | 89 | 84* | 73* | 88* | 84 | 89 |
| (86–93) | (82–94) | (72–91) | (56–84) | (82–92) | (73–91) | (74–94) | ||
| Control | 90 | 91 | 90 | 82 | 78 | 86 | 89 | |
| (84–95) | (85–96) | (84–95) | (77–90) | (65–94) | (72–93) | (76–99) | ||
| CD62L+CD4+ (cells μl-1) | Exercise | 424 | 501* | 631* | 534* | 355 | 569* | 544 |
| (242–500) | (284–621) | (366–1092) | (304–637) | (193–434) | (338–761) | (306–623) | ||
| Control | 323 | 320 | 393 | 731 | 407 | 571 | 475 | |
| (112–626) | (155–527) | (255–612) | (250–900) | (100–752) | (363–655) | (276–680) | ||
| CD62L+CD4+ (%) | Exercise | 90 | 89 | 93 | 95† | 82* | 96 | 91 |
| (64–102) | (62–103) | (61–104) | (66–107) | (48–98) | (60–104) | (61–105) | ||
| Control | 82 | 86 | 93 | 91 | 94 | 96 | 91 | |
| (44–105) | (49–106) | (62–102) | (64–94) | (67–110) | (90–98) | (86–96) | ||
| CD62L+CD4-(cels μl-1) | Exercise | 4 | 5 | 16*† | 8 | 10* | 8 | 5 |
| (1–14) | (−3 to 32) | (1–47) | (3–16) | (−11 to 69) | (−3 to 32) | (2–10) | ||
| Control | 9 | 5 | 2 | 13 | 10 | 5 | 20 | |
| (−3 to 33) | (−16) | (−1 to 10) | (−10 to 52) | (−19) | (−1 to 20) | (−9 to 53) | ||
| CD62L+CD4-(%) | Exercise | 2 | 1 | 2* | 1 | 2 | 1 | 1 |
| (0–4) | (0–6) | (0–9) | (−2 to 9) | (1–5) | (−1 to 7) | (0–2) | ||
| Control | 3 | 1 | 1 | 2 | 2 | 1 | 1 | |
| (0–5) | (0–3) | (0–2) | (0–8) | (0–4) | (−1 to 5) | (−1 to 5) | ||
| PropCD14+ of all DR+ (%) | Exercise | 91 | 93 | 86* | 74*† | 89* | 85* | 92 |
| (89–95) | (89–97) | (75–93) | (59–86) | (85–95) | (74–92) | (76–97) | ||
| Control | 95 | 93 | 93 | 84 | 81 | 88 | 90 | |
| (89–99) | (88–98) | (88–97) | (78–94) | (68–97) | (73–96) | (77–101) |
Exercise, n = 7; control, n = 5. LPK, leukocyte plasma concentration. Data are median values with 95% confidence intervals in parentheses
P < 0.05 compared to rest (Wilcoxon signed rank test)
P < 0.05 compared to control (Mann-Whitney test)
P < 0.01 between change from rest in exercise compared to control group (Mann-Whitney test); P± n = 4 due to missing value.
Table 4.
Significantly changed mean fluoresence intensity (MFI) on circulating lymphocytes and monocytes before and after eccentric exercise
| Antigen | Rest | Post | 6 h | 24 h | 48 h | 4 days | 7 days | |
|---|---|---|---|---|---|---|---|---|
| Lym CD20 sub CD5- | Exercise | 602 | 599 | 584* | 540* | 555* | 510* | 547* |
| (573–630) | (568–629) | (543–613) | (502–579) | (536–583) | (498–545) | (505–582) | ||
| Control | 571 | 583 | 563 | 541 | 517 | 530 | 511 | |
| (553–601) | (549–599) | (550–584) | (521–557) | (461–591) | (215–706) | (487–540) | ||
| Lym CD4 sub CD3+ | Exercise | 810† | 811† | 806† | 812† | 796 | 801 | 795 |
| (764–834) | (807–819) | (796–816) | (803–820) | (791–805) | (793–806) | (748–795) | ||
| Control | 799 | 796 | 791 | 790 | 796 | 802 | 791 | |
| (790–803) | (787–804) | (788–798) | (778–798) | (770–816) | (791–808) | (782–803) | ||
| Lym CD45 sub DR+ | Exercise | 708 | 694 | 699 | 703 | 703* | 701 | 698 |
| (692–717) | (679–715) | (692–712) | (689–717) | (769–714) | (691–710) | (682–708) | ||
| Control | 708 | 713 | 709 | 700* | 704 | 701 | 691 | |
| (693–726) | (693–723) | (693–725) | (686–713) | (688–718) | (692–712) | (673–711) | ||
| Lym CD8 sub CD3+ | Exercise | 625 | 619* | 609 | 606 | 599* | 597 | 593* |
| (573–699) | (580–635) | (591–629) | (578–631) | (572–609) | (559–615) | (535–613) | ||
| Control | 597 | 585 | 583 | 569 | 571 | 582 | 556 | |
| (567–627) | 585 | 583 | 569 | 571 | 582 | 556 | ||
| (567–627) | (555–622) | (546–620) | (507–658) | (486–651) | (519–630) | (517–581) | ||
| Mon CD11b sub 62L+ | Exercise | 653 | 663 | 700 | 662 | 584 | 701 | 683 |
| (567–719) | (572–723) | (595–747) | (566–728) | (511–659) | (611–745) | (544–770) | ||
| Control | 598 | 578 | 615 | 658 | 701 | 672* | 574 | |
| (472–715) | (455–729) | (483–748) | (530–754) | (572–794) | (656–724) | (501–643) | ||
| Mon CD14 sub DR+ | Exercise | 804 | 801 | 795* | 757* | 772* | 756* | 754 |
| (785–821) | (778–818) | (770–801) | (733–782) | (751–796) | (717–788) | (716–786) | ||
| Control | 787 | 781 | 782 | 760* | 743 | 744* | 751 | |
| (763–805) | (704–816) | (773–800) | (734–776) | (681–829) | (727–764) | (692–807) | ||
| Mon CD14 sub DR- | Exercise | 779 | 755 | 772 | 743 | 747* | 740* | 733 |
| (752–789) | (707–783) | (743–789) | (726–767) | (693–766) | (685–767) | (697–768) | ||
| Control | 755 | 769 | 750 | 733* | 711 | 737* | 735 | |
| (758–791) | (701–818) | (712–796) | (655–784) | (645–805) | (714–760) | (651–815) | ||
| Mon CD4 sub CD62L+ | Exercise | 690 | 697 | 631*‡ | 615* | 705†‡ | 598* | 610* |
| (669–707) | (669–704) | (595–661) | (589–669) | (657–725) | (581–646) | (577–676) | ||
| Control | 699 | 703 | 683*‡ | 654* | 583‡ | 600* | 688 | |
| (648–743) | (642–743) | (622–727) | (594–712) | (511–701) | (585–623) | (627–734) | ||
| Mon CD4 sub CD62L- | Exercise | 685 | 693 | 617* | 641 | 693† | 632* | 626* |
| (671–699) | (663–717) | (588–655) | (608–685) | (631–727) | (571–670) | (602–680) | ||
| Control | 701 | 697 | 683 | 669 | 616 | 634 | 711 | |
| (647–735) | (639–733) | (611–726) | (646–699) | (576–641) | (604–650) | (659–801) | ||
| Mon CD45 sub CD14+DR+ | Exercise | 686 | 679 | 678 | 659*‡ | 678†‡ | 657* | 659 |
| (668–698) | (661–696) | (670–686) | (628–675) | (664–699) | (632–686) | (663–700) | ||
| Control | 684 | 682 | 685 | 670‡ | 656 | 668* | 674 | |
| (670–703) | (663–701) | (657–712) | (651–684) | (642–675) | (626–685) | (630–705) | ||
| Mon CD45 sub CD14+DR- | Exercise | 650‡ | 641 | 660 | 627* | 642 | 614* | 631 |
| (631–685) | (606–662) | (642–665) | (598–644) | (611–673) | (577–653) | (595–667) | ||
| Control | 668 | 659 | 646 | 631* | 616 | 631 | 640 | |
| (657–681) | (647–678) | (614–685) | (580–670) | (599–638) | (599–648) | (585–689) |
Exercise, n = 7; control, n = 5. Data are median values with 95% confidence intervals in parentheses
P < 0.05 compared to control (Mann-Whitney test)
P < 0.05 compared to rest (Wilcoxon signed rank test); § P < 0.01 between change from rest in exercise compared to control group (Mann-Whitney test). MFI for CD antigen on phenotype subset; for example, Lym CD45 sub DR+ indicates CD45 expression on DR+ lymphocyte.
Table 5.
Expression of leukocyte and cytokine antigens in human skeletal muscle after eccentric (percentage of stained area of total muscle section area analysed)
| Antigen | Rest | Post | 6 h | 24 h | 48 h | 4 days | 7 days | |
|---|---|---|---|---|---|---|---|---|
| CD3 | Exercise | 0.01 | 0.02 | 0.03* | 0.03 | 0.02 | 0.02 | 0.01 |
| (0.0.1) | (−0.02 to 0.09) | (−0.01 to 0.11) | (−0.01 to 0.12) | (0.01–0.04) | (0.0.10) | (0–0.04) | ||
| Control | 0.03 | 0.01 | 0,02 | 0.03 | 0.02 | 0.03 | 0.02 | |
| (0.01–0.04) | (−0.03) | (−0.02 to 0.06) | (−0.01 to 0.08) | (−0.01 to 0.07) | (−0.05 to 0.19) | (−0.02 to 0.07) | ||
| CD4 | Exercise | 0.01 | 0.01 | 0.02† | 0.03 | 0.05 | 0.04 | 0.03 |
| (−0.01 to 0.08) | (−0.04 to 0.15) | (0–0.09) | (0–0.8) | (0–0.18) | (0.02–0.06) | (−0.01 to 0.13) | ||
| Control | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.04* | 0.29 | |
| (0–0.01) | (−0.01 to 0.04) | (0–0.01) | (−0.05 to 0.16) | (−0.09 to 0.24) | (−0.08 to 0.39) | (−0.21 to 0.80) | ||
| CD8 | Exercise | 0.01 | 0.01 | 0.02† | 0.03 | 0.01 | 0 | 0.02 |
| (−0.01 to 0.06) | (−0.01 to 0.06) | (−0.02 to 0.12) | (0.0–0.05) | (−0.02 to 0.08) | (−0.01 to 0.07) | (0.0.04) | ||
| Control | 0.01 | 0.01 | 0 | 0.01 | 0.01 | 0.01 | ||
| (0–0.02) | (0–0.02) | (0–0.01) | (−0.01 to 0.05) | (−0.01 to 0.03) | (0–0.02) | (0–0.02) | ||
| CD11b | Exercise | 0.02 | 0.04 | 0.06 | 0.24* | 0.32* | 0.06* | 0.03 |
| (0–0.10) | (−0.02 to 0.20) | (−0.01 to 0.29) | (0.02–0.67) | (0.07–0.70) | (0.02–0.22) | (−0.01 to 0.22) | ||
| Control | 0.02 | 0.06 | 0.05 | 0.16* | 0.11 | 0.05* | 0.04 | |
| (0.0.07) | (−0.27 to 0.72) | (0.01 to 0.08) | (−0.02 to 0.49) | (−0.03 to 0.25) | (−0.24 to 0.75) | (−0.28 to 0.64) | ||
| CD15 | Exercise | 0.03 | 0.03 | 0.04† | 0.08 | 0.13 | 0.01 | 0.02† |
| (−0.02 to 0.12) | (0–0.12) | (−0.05 to 0.024) | (−0.12 to 0.61) | (−0.03 to 0.60) | (−0.02 to 0.08) | (0.01–0.02) | ||
| Control | 0 | 0.01 | 0.01 | 0.02 | 0.02* | 0.02 | 0.15 | |
| (0–0.01) | (−0.39 to 0.86) | (0–0.02) | (−0.09 to 0.30) | (−0.01 to 0.10) | (−0.06 to 0.27) | (−0.03 to 0.15) | ||
| CD56 | Exercise | 0.07 | 0.03† | 0.01 | 0.03 | 0.08 | 0.01 | 0.03 |
| (0.03–0.13) | (−0.04 to 0.17) | (−0.03 to 0.17) | (0–0.08) | (−0.24 to 0.86) | (0–0.10) | (0.01–0.13) | ||
| Control | 0.01 | 0.05 | 0.05 | 0.05 | 0.03 | 0.07 | 0.02 | |
| (0–0.13) | (0.03–0.11) | (0.03 to 0.15) | (−0.08 to 0.29) | (0.01 to 0.07) | (−0.015 to 0.55) | (−0.08 to 0.21) | ||
| CD79a | Exercise | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 |
| (−0.03 to 0.08) | (0–0.01) | (0–0.01) | (0–0.01) | (−0.03 to 0.09) | (−0.03 to 0.08) | (−0.01 to 0.02) | ||
| Control | 0.01 | 0.01 | 0 | 0 | 0* | 0.01 | 0 | |
| (0.01) | (0.01) | (0–0.01) | (−0.01 to 0.04) | (0–0) | (0–0.01) | (0.002) | ||
| CD163 | Exercise | 0.01 | 0.02 | 0.03 | 0.09 | 0.15* | 0.04 | 0.03 |
| (0.0.09) | (0–0.06) | (0.01–0.09) | (0.02–0.19) | (0.03–0.29) | (0.01–0.17) | (0.01–0.09) | ||
| Control | 0.01 | 0.01 | 0.03 | 0.03 | 0.01 | 0.17* | 0.68 | |
| (0–0.03) | (0–0.03) | (0–0.06) | (−0.02 to 0.18) | (−0.08 to 0.28) | (−0.07 to 0.058) | (−0.84 to 2.66) | ||
| CD56 on muscle cells (% stained fibres) | Exercise | 0 | 0.27 | 1.63* | 1.39* | 2.71* | 0.82 | 1.81 |
| (−0.15 TO 0.69) | (−0.06 to 1.24) | (0.36–2.65) | (0.33–3.07) | (0.12–10.28) | (0.07–1.73) | (−0.077 to 6.53) | ||
| Control | 0 | 0 | 0 | 2.02* | 0.72 | 1.36 | 0.48 | |
| (−0.33 to 0.70) | (−0.44 to 0.94) | (−1.11 to 3.35) | (0.08–3.90) | (−3.37 to 9.44) | (−0.25–2.50) | (−0.08 to 0.86) | ||
| IL-1α | Exercise | 0.86 | 1.03 | 1.31 | 0.86 | 1.66 | 1.32 | 0.22 |
| (−10.5 to 5.90) | (−0.62 to 7.19) | (0.21 to 6.96) | (0.23 to 1.92) | (−3.89 to 13.8) | (−3.54 to 14.2) | (−0.05 to 1.29) | ||
| Control | 1.49 | 1.90* | 2.31* | 1.59 | 3.09 | 1.73 | 0.68 | |
| (0.07–2.53) | (−3.23 to 11.2) | (0.45–4.03) | (0.29–3.64) | (0.79–4.37) | (0.79–2.26) | (−2.08 to 6.33) | ||
| IL1β | Exercise | 1.20 | 1.95* | 1.65* | 2.00 | 0.96 | 1.42 | 1.42 |
| (0.55–1.96) | (0.82–3.57) | (1.07–3.29) | (−0.18 to 6.41) | (0.07–2.56) | (−0.47 to 6.13) | (0.89–3.12) | ||
| Control | 1.10 | 1.89* | 3.72 | 2.46 | 3.12 | 1.90 | 1.42 | |
| (−1.36 to 6.26) | (−0.48 to 7.53) | (−0.10 to 6.28) | (−0.07 to 6.57) | (0.36–4.64) | (0.85–3.80) | (0.27–3.81) |
Exercise, n = 7; control, n = 5. Data are median values with 95% confidence intervals parentheses
P < 0.05 compared to control (Mann-Whitney test)
P < 0.05 compared to rest (Wilcoxon signed rank test).
Figure 5.
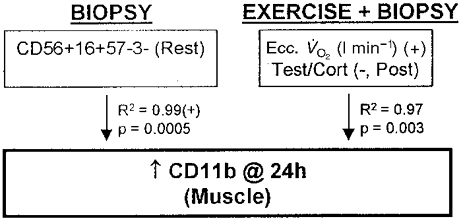
Regression model for an increased neutrophil number (CD11b) in human skeletal muscle due to three biopsies or biopsies + eccentric cycling exercise. Ecc. V̇O2, V̇O2 during eccentric cycling; Post, post exercise.
Figure 6.
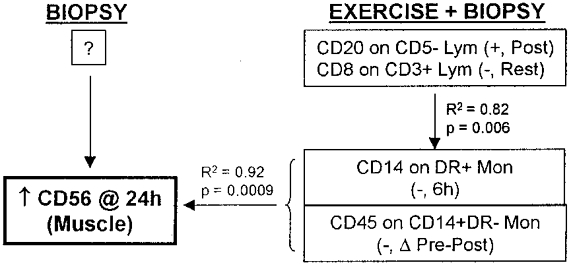
Regression model for an increased percentage of satellite cells (CD56) in human skeletal muscle due to three biopsies + eccentric cycling exercise. No model could be determined for the effect of biopsies only. Lym, lymphocytes; Mon, monocytes; Post, post exercise; ΔPre-Post, change in pre- vs. post-exercise values.
Figure 7.
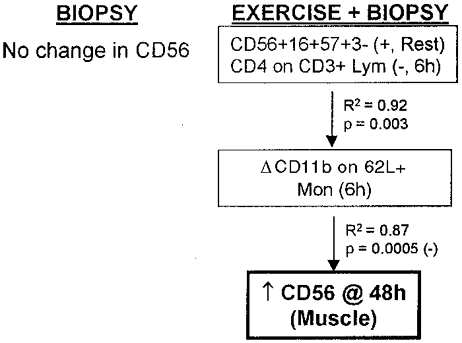
Regression model for an increased percentage of satellite cells (CD56) in human skeletal muscle due to four biopsies + eccentric cycling exercise. No change in the percentage of satellite cells occurred at 48 h due to the effect of biopsies only.
Figure 8.
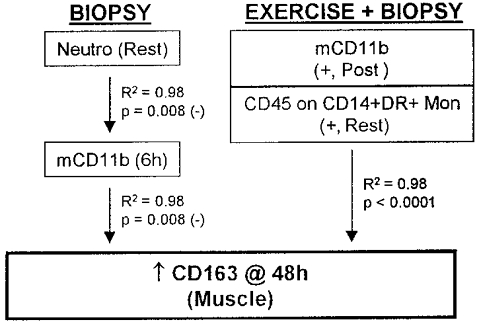
Regression model for an increased number of macrophages (CD163) in human skeletal muscle due to four biopsies or biopsies + eccentric cycling exercise.
Figure 9.
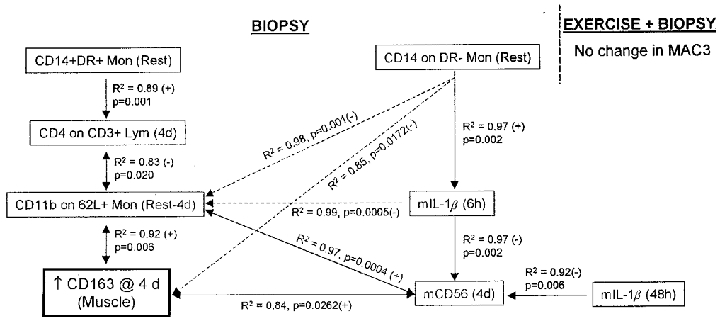
Regression model for an increased number of macrophages (CD163) in human skeletal muscle due to five biopsies. There was no change in macrophage number due to biopsies + eccentric exercise. 4d, 4 days; m, muscle.
Figure 10.
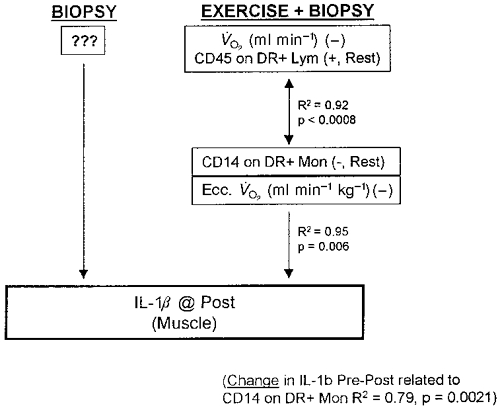
Regression model for an increased IL-1β expression in human skeletal muscle due to one biopsy + eccentric cycling exercise. No model was found for one biopsies alone. 4d, 4 days.
Eccentric exercise intensity
During the last 5 min of eccentric exercise, the subjects’V̇O2 was 1.20 l min−1 (1.08-1.31 l min−1) (mean (95 % confidence interval)) equivalent to 37 % (31-41 %) of concentric cycling V̇O2,max. Blood lactate 3 min post exercise was 2.6 mm (1.5-3.7 mm).
Muscle soreness, muscle damage and leukocyte phenotype
Subjects in the exercise group experienced severe delayed onset of muscle soreness (DOMS), peaking 24 or 48 h after exercise, while the control group reported no muscle soreness (Table 6). Because DOMS is a non-linear variable, the use of correlation statistics between DOMS and other measured linear variables may not be done. Grouping subjects in ‘muscle soreness’ and ‘no muscle soreness’ groups and using regression analysis resulted in a significant positive correlation between DOMS and CD4 expression on CD3+ lymphocytes post exercise (r2= 0.61, P = 0.001). CK activity in blood, a reported marker of muscle damage (Friden et al. 1989) increased by 123 % at 24 h and CRP, a marker of systemic inflammation and tissue damage increased by 392 % 24 h after exercise (Table 6). The CK activity at 24 h post exercise was not correlated to exercise intensity (percentage V̇O2,max; eccentric V̇O2 (l min−1 or l min−1 kg−1)), leukocyte infiltration or other indicators of muscle damage, but was correlated positively to cortisol (at 6 h) and free testosterone (at 24 h) concentrations in both the exercised (r2= 0.74, P = 0.029) and control (r2= 0.97, P = 0.013) groups (both groups together r2= 0.80, P = 0.0003). CRP was always < 10 μg min−1, but the significantly increased CRP concentration at 24 h in the exercise group correlated with CD8 expression on CD3+ lymphocytes post exercise (r2= 0.90, P = 0.0006).
Table 6.
DOMS, CK and CRP after eccentric exercise
| Variable | Time after work | |||||||
|---|---|---|---|---|---|---|---|---|
| Rest | Post | 6 h | 24 h | 48 h | 4 days | 7 days | ||
| DOMS (0–10) | Exercise | 0 | 0 | 2.5 | 6.0 | 5.5 | 3.0 | 0 |
| (0.8–4.2) | (4.1–7.2) | (1.7–5.7) | (1.8–4.8) | (−0.7 to 2.7) | ||||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CK (U l−1) | Exercise | 53.5 | 60.5* | 119.0* | 119.5* | 84.2* | 61.5† | 68.0 |
| (42.0–70.3) | (47.2–81.2) | (22.8–310.0) | (51.1–258.9) | (62.0–129.9) | (49.8–79.6) | (36.0–132.6) | ||
| Control | 247.0 | 241.0 | 241.0 | 179.0 | 134.9 | 121.7 | 86.2 | |
| (30.8–347.6) | (29.1–347.2) | (56.5–369.8) | (77.3–344.9) | (45.9–382.1) | (44.9–269.1) | (39.0–142.3) | ||
| CRP (μg ml−1) | Exercise | 0.25 | 0.28 | 0.25 | 1.23 | 0.82 | 0.63 | 0.38 |
| (0.11–0.58) | (0.11–0.61) | (−8.55 to 20.6) | (0.58–1.78) | (0.44–1.30) | (−7.11 to 18.9) | (−1.70 to 5.68) | ||
| Control | 0.37 | 0.37 | 0.38 | 0.64 | 0.55 | 0.57 | 0.35 | |
| (0.25–0.75) | (0.24–0.75) | (0.24–0.74) | (0.40–0.74) | (−0.15 to 2.14) | (−0.28 to 2.04) | (−0.27 to 1.40) | ||
Exercise, n = 7; control, n = 5. Median (95% confidence interval)
P < 0.05compared to control (Mann-Whitney test)
P < 0.05 compared to rest (Wilcoxon signed rank test).
Muscle biopsies from four subjects (two exercised and two controls) stained using Gomori's trichrome procedure, which detects abnormal mitochondria, and for acid phosphatases did not show any signs of altered muscle fibres or enzyme expression. Further, in seven biopsies from one subject with severe muscle soreness, no change in desmin immunostaining was detected at any time point.
Leukocytes in blood
The total number of circulating white blood cells increased significantly in the exercise group compared to rest, peaking at 6 h and lasting for 24 h post exercise, but never differed significantly from the control group (Table 3). The increase at 6 h was mostly due to the increase in neutrophils and T (CD3+) cell numbers (r2= 0.98, P < 0.0001).
Neutrophils
Compared to rest, neutrophil number increased similarly in both groups (Table 3), peaking in the third blood sample, collected 6 h after exercise. In the exercise group, the neutrophil number at 6 h correlated to the expression of CD4 on CD62L- monocytes at rest (r2= 0.78, P = 0.002) but not to catecholamines or cortisol in the same sample. The post-exercise adrenaline concentration correlated to neutrophil numbers at 6 h (r2= 0.84, P = 0.022). In the control group, the neutrophil number at 6 h correlated to both resting plasma concentration of noradrenaline (r2= 0.96, P = 0.003) and the changes in noradrenaline from the first to second sampling times (r2= 0.97, P = 0.001).
Lymphocytes
Lymphocyte numbers increased, and peaked at 24 h in the exercise group, but decreased to a minimum at the same time in the control group (Table 3). In both groups, however, the lymphocyte number in sample 4 (at 24 h) was correlated to the T (CD3+) cell number at rest (exercise group: r2= 0.97, P = 0.0003; control group: r2= 0.90, P = 0.0085). The change in lymphocyte number from rest to 24 h post exercise related to the pre- to post-exercise changes in adrenaline (r2= 0.96, P = 0.0005). As seen in Table 3 and Table 4, the T and B cell populations seemed to be more affected in the exercise group than in the control group, with changes in absolute numbers, percentage and cell surface receptor density (MFI). Although several values changed compared to rest, few were significantly different from the control group (Table 3 and Table 4).
Monocytes
Blood monocyte number (CD14+ cells) increased in the exercise group only (Table 3), with a first increase from immediately after exercise lasting for 24 h with a peak at 6 h. This increase was related to the oxygen consumption during the eccentric exercise (r2= 0.92, P = 0.002). A second increase in monocyte number occurred in the sixth blood sample 4 days after exercise. This increase correlated to neutrophil and free testosterone levels at 24 h (r2= 0.98, P = 0.0002). The number of several monocyte subpopulations increased in samples taken from immediately after, to up to 4 days after exercise, while no changes were seen in the control group (Table 3). Again, few values are significantly different from controls. At the same time, the receptor density of CD4, CD14 and CD45 was down-regulated in both the exercise and control groups (Table 4).
NK cells
Circulating NK (CD56+CD16+CD57-CD3-) cells increased immediately and 24 h after exercise, while the number decreased in the second sample in the control group (Table 3). The median NK cell number was, however, never significantly different between the two groups, even though the change in number differed. The number of the subset of NK cells expressing CD57 (Leu 7), (CD56+CD16+CD57+CD3-) increased after exercise and was significantly higher than in the control group, in which the number actually decreased in the second sample. At 48 h post exercise, the number of CD56+CD16+CD57+CD3- cells was significantly lower in the exercise group than in the control group.
No regression model could be found for the increased NK (CD56+CD16+CD57-CD3-) cell number in the post-exercise blood sample. Increases in adrenaline and noradrenaline did not correlate to the changes. A decrease in CD57- NK cell number between the first and the second blood sample in the control group was positively correlated to CD20 expression on CD5- lymphocytes in the second blood sample (r2= 0.81, P = 0.024). If the effect of the adrenaline concentration in the second sample was added, the correlation increased (r2= 0.99, P = 0.007). When exercise and control groups were combined, a weak correlation was found between CD57- NK cells and adrenaline in the second blood sample (r2= 0.56, P = 0.008).
Changes in CD56+CD16+CD57+CD3- cell numbers after exercise correlated positively to the resting noradrenaline concentration (r2= 0.71, P = 0.021). The correlation with the increase in adrenaline was weak (r2= 0.56, P = 0.056), but the combined effect of noradrenaline and adrenaline was positively correlated (r2 = 0.91, P = 0.013). In the control group, the decrease in CD57+ NK cells was not correlated to catecholamines but was negatively correlated to the change in proportion of CD14+ monocytes of all DR+ monocytes between the first and the second sample (r2= 0.97, P = 0.001).
Also of some interest is the finding that the change in CD57+ NK cells due to exercise was positively correlated to the subjects’V̇O2,max (r2= 0.63, P = 0.021), while the change in CD57- NK cells was not (r2= 0.08, P = 0.53).
Thus, distinct subpopulations of NK cells respond somewhat differently in response to eccentric exercise and/or multiple muscle biopsies. CD57+ NK cells appear to be more affected by catecholamines and more related to physical exercise than CD57- NK cells.
Antigen expression in muscle
All antigens investigated, except CD79α (B cell marker) were detected to some extent in all muscle sections analysed (Table 5). The main antigens detected were, however, markers for leukocytes/neutrophils (CD11b and CD15), macrophages (CD163), activated satellite cells (CD56) and IL-1α and IL-1β.
T (CD3, CD4 and CD8) and B (CD79α) cells were scarce in healthy human skeletal muscle tissue, both at rest and after exercise and/or multiple biopsies.
Leukocytes/neutrophils (CD11b/CD15)
CD11b, the complement 3bi receptor, was detected on between 0.02 and 1.2 % (mininimum and maximum) of the total section area. The increase in CD11b detection was significant, compared to rest, at 24 h, 48 h and 4 days post exercise. A similar increase occurred in the control group (biopsies only; Table 5) and the change in the exercise group was therefore never significantly different from control. In the exercise group, detection of CD11b peaked at 24 h post exercise and correlated positively to eccentric V̇O2 (l min−1) and negatively to the testosterone/cortisol ratio post exercise (r2= 0.97, P = 0.003). Increased CD11b detection in the control group also peaked at 24 h, but correlated to resting NK (CD56+CD16+CD57-CD3-) cell number (r2= 0.99, P = 0.0005). CD15 displayed a similar pattern as CD11b, with a detected staining between 0 and 1.3 % of total section area (Table 5).
Activated satellite cells (CD56)
Antibodies to CD56 stained between 0 and 1.9 % (minimum and maximum) of the total muscle section area. Most of the staining was located on the membrane of small muscle cells, or cells of polygonal shapes (Fig. 1). These cells are believed to be activated satellite cells or myoblasts (Illa et al. 1992) and are therefore a sign of regenerating muscle fibres. Very few non-muscle cells (e.g. potential NK or neuroectodermal cells) expressing CD56 were observed. As with CD11b, the proportion of CD56+ muscle cells (of all muscle cells) increased in both the exercise and control group, with a peak at 24 h in control, and at 48 h in exercise subjects (Table 5).
Macrophages (CD163)
Detection of the macrophage-specific antigen CD163 revealed that between 0 and 2.3 % (minimum and maximum) of the total muscle section area consisted of macrophages. The percentage of stained area increased similarly in both the exercise and control group, with peaks at 48 h and 4 days, respectively. The largest area of macrophages staining positive was seen in the 6th biopsy (taken at 4 days) from two control subjects. Macrophages were located at the edge in both biopsies, suggesting that the previous biopsy from the same leg (the 4th biopsy taken at 24 h) had a direct effect on the tissue obtained in the 6th biopsy (Fig. 2).
Figure 2.
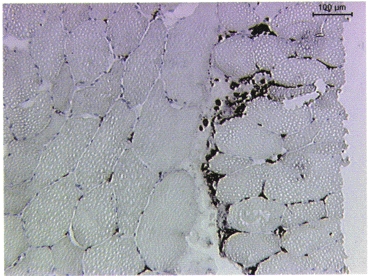
Infiltration of macrophages in human muscle tissue, located at the periphery of the biopsy.
IL-1α
Intracellular interleukin-1α (IL-1α) was detected in most muscle sections (0-8.1 % of section area), but changed compared to rest in the control group only, with the median peak increase 6 h after the first biopsy (Table 5). IL-1α detection was located in both muscle and non-muscle cells, endothelial cells being a likely non-muscle cell source when comparing IL-1α and EN-4 (endothelium) staining patterns.
IL-1β
Almost all muscle sections contained intracellular IL-1β (Table 5 and Fig. 3). IL-1β was located in both muscle and non-muscle cells.
Figure 3.
Staining of IL-1β in human skeletal muscle. IL-1β appears located in muscle cells (A) and non-muscle cells (B).
IL-1ra and TNFα
These antigens were not detectable in any of the 90 biopsies analysed.
IL-6
Biopsies from two exercised subjects and one control subject were stained with two different IL-6 antibodies, one rat monoclonal and one goat polyclonal (Table 2). The goat polyclonal IL-6 antibody gave an even background stain in all sections, while the rat monoclonal IL-6 antibody resulted in a few distinctly stained areas (Fig. 4). IL-6 was found in low levels (0-0.02 % of section area; data not shown) within both muscle and non-muscle cells and was only changed in the 7th biopsy from the control subject (0.045 %).
Figure 4.
IL-6 expression in human skeletal muscle cells (A).
DISCUSSION
The present study investigated immunological variables in blood and muscle tissue before and after strenuous eccentric cycling exercise together with multiple biopsies or multiple biopsies only. The results clearly demonstrate that with respect to infiltrating neutrophils and macrophages, satellite cell activation and IL-1β detection, eccentric cycling exercise and multiple biopsies cause similar changes in adult human skeletal muscle (Table 4, Table 5 and Table 6). T and B cell antigens were only detected in minute quantities in muscle tissue samples (Table 5). In the blood, eccentric cycling exercise resulted in significantly larger changes than multiple biopsies, although multiple biopsies also induced changes in some blood leukocyte phenotypes and hormones (Table 3, Table 4, Table 5 and Table 7). In both groups, immunological changes in muscle (i.e. a 12-fold increase in CD11b) were vastly larger than leukocyte changes in blood (usually less than 2-fold). Individual changes varied significantly (large confidence intervals; Table 3, Table 4 and Table 5). This could be explained by the fact that the cells of the immune system have memory and undergo somatic mutation. The response to identical stress events can therefore differ from one time point to another, even in the same individual. In some cases, similar immunological changes in exercised and non-exercised muscle related to different causal mechanisms (Fig 5, Fig 6, Fig 7, Fig 8, Fig 9 and Fig 10). Thus, even if eccentric cycling exercise induces immunological changes in human skeletal muscle, the exercise-induced changes are inferior to the changes inflicted by the biopsy procedure itself.
Table 7.
Hormone response to eccentric cycling exercise
| Time after work | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hormone (nmol l−1) | Rest | Post | 6 h | 24 h | 48 h | 4 days | 7 days | |
| Adrenaline | Exercise | 0.28 | 0.63*† | — | — | — | — | — |
| (0.13–0.38) | (0.33–1.00) | |||||||
| Control | 0.018 | 0.17 | — | — | — | — | — | |
| (0.09–0.29) | (0.02–0.43) | |||||||
| Noradrenaline | Exercise | 2.11 | 5.24*† | — | — | — | — | — |
| (1.34–3.16) | (3.46–6.53) | |||||||
| Control | 2.09 | 2.13 | — | — | — | — | — | |
| (1.26–3.06) | (1.24–3.24) | |||||||
| Dopamine | Exercise | 0.10 | 0.23 | — | — | — | — | — |
| (0–0.33) | (0.12–0.34) | |||||||
| Control | 0.17 | 0.16 | — | — | — | — | — | |
| (0.07–0.30) | (0.03–0.37) | |||||||
| Cortisol | Exercise | 379 | 369 | 221 | 412 | 372 | 357 | 421 |
| (195–501) | (224–461) | (86.6–380) | (388–445) | (302–427) | (332.431) | (370–482) | ||
| Control | 404 | 321* | 236*361 | 293 | 348 | 327 | ||
| (225–482) | (903–435) | (39.7–379) | (238–497) | (178–444) | (234–455) | (184–533) | ||
| Free testosterone | Exercise | 10.9 | 12.0 | 8.40 | 11.7 | 11.9 | 11.9 | 13.0 |
| (5.88–13.99) | (11.0–13.8) | (5.44–10.1) | (8.35–17.7) | (10.2–12.6) | (10.2–13.0) | (11.5–15.3) | ||
| Control | 12.6 | 13.3 | 10.8 | 14.4 | 12.3 | 12.3 | 12.7 | |
| (8.78–16.6) | (6.47–16.4) | (6.05–14.1) | (11.2–17.7) | (9.59–15.3) | (9.69–14.3) | (8.52–17.3) | ||
| Total testosterone | Exercise | 19.4 | 20.2 | 14.6 | 22.26 | 19.5 | 19.6 | 24.1* |
| (10.3–23.6) | (18.5–23.6) | (16.1–27.2) | (8.56–23.6) | (16.7–23.2) | (17.1–22.9) | (19.0–26.0) | ||
| Control | 22.8 | 22.4 | 18.7* | 23.4 | 21.5 | 20.2 | 22.3 | |
| (15.8–28.3) | (12.4–28.2) | (11.7–24.0) | (20.6–29.8) | (17.5–27.3) | (17.5–25.8) | (14.0–31.3) | ||
Exercise, n = 7; control, n = 5, Median (95% confidence interval)
P < 0.05 compared to control (Mann-Whitney test)
P < 0.05 compared to rest (Wilcoxon signed rank test).
Previous publications have indicated that biopsies affect adjacent muscle fibres and serum enzymes (Aronson et al. 1998). Few investigators of exercise-induced changes in human muscle have considered these results in their study design. Conclusions regarding exercise-induced damage, leukocyte infiltration and inflammation in human muscle tissue based on results from studies with multiple muscle biopsies should therefore be reconsidered (Friden et al. 1983; Cannon et al. 1989; Smith, 1991; Friden & Lieber, 1992; Fielding et al. 1993; Hellsten et al. 1997).
It has been suggested that DOMS could be caused by acute inflammation (Smith, 1991; MacIntyre et al. 1995). However, human studies where exercise-induced muscle damage has been directly measured are few and some of the often cited original investigations were performed either on electrically stimulated (Lieber et al. 1996) or on exercised (Armstrong et al. 1983) animal muscle and lack statistical comparison with non-exercised control muscle (Friden et al. 1983; Fielding et al. 1993) or both (Kuipers, 1994).
A temporal association between infiltration of neutrophils, macrophages, satellite cell activation and DOMS was observed in the present study (Table 5 and Table 6). Because immunological changes in muscle were similar in the exercise and control groups, and no statistical correlation was found between DOMS and immunohistochemical analyses or inflammation markers in blood (CRP, T and B cells, neutrophils, monocytes), it can be concluded that DOMS in men is not caused by cellular or humoral inflammation. The present results obtained with different staining procedures (including Gomori trichrome, acid phosphatase and desmin) challenge the notion that eccentric cycling exercise causes significant cytoskeletal disruption and inflammation in human skeletal muscle. In contrast to the conclusion reached by others (Shek & Shephard, 1998), the lack of significant exercise-induced inflammation in the present study excludes eccentric cycling as a good model for studying muscular inflammatory diseases in human.
No correlations between CK activity in blood and leukocyte phenotypes in blood or muscle were found at any time point. The peak CK activity at 24 h post exercise was, however, correlated to the combined effect of cortisol and testosterone. This observation supports the opinion that CK is not a reliable marker of exercise-induced muscle damage (Kuipers, 1994; Warren et al. 1999). Instead, it supports observations that CK activity in blood may be linked to sex hormones because CK usually increases more in males than females in response to similar physical activity (Evans & Cannon, 1991; Kuipers, 1994). In one of the few studies where actual muscle damage and CK were measured, Fielding et al. (1993) found no correlation between z-band damage and CK activity in serum (for review see Warren et al. (1999)). If we accept the argument that CK activity in venous or capillary blood is not a relevant marker of muscle damage, conclusions made in studies (including our own observations: Malm et al. 1999) of ‘exercise-induced muscle damage’, where CK is the only indicator of muscle damage, should be re-evaluated. Because the expression of CK in differentiating satellite cells increases (Grounds, 1991), and the peak increase in serum CK occurs at the same time as peak CD56 expression in muscle (Table 5 and Table 6), it is suggested, but not proven, that serum CK is more related to exercise-induced muscle adaptation than damage.
Due to the complexity of the human immune system, investigations of immunological events in humans demand elaborate models. At present, use of the intact organism is inevitable because it is the only model that contains all variables, known and unknown. Because of the synergism and antagonism within the immune system, exclusion of one or more factors renders the functional outcome unpredictable. Consequently, results from in vitro and animal models are important for understanding single events, but have limited validity when describing the effects of voluntary physical exercise on the human immune system and muscle tissue (Grounds, 1991). In an attempt to describe the interactions between local and systemic events, multiple regression analysis was used to find likely causes of changed immunoreactivity in the analysed muscle samples (Fig 5, Fig 6, Fig 7, Fig 8, Fig 9 and Fig 10).
Identical changes in CD11b detection in the exercised and control groups (Fig. 5) correlated to resting numbers of circulating NK cells (CD56+CD16+CD57-CD3-) in the control group. A combination of eccentric V̇O2 and the testosterone/cortisol quota (an indicator of metabolic stress) was responsible for changes in the exercise group. A direct link between circulating NK cells and neutrophil infiltration in damage muscle has not been reported. Not all functions of the NK cell are known and, as stated by Tidball (1995), ‘non-muscle cells may play a complex and essential role in regulating the muscle repair process’. Mechanically damaged muscle fibres require circulating leukocytes for repair (Robertson et al. 1992) and NK cells may thus have a regulatory function in muscle repair. Physical exercise and a decreased testosterone/cortisol quota (increased physical stress), resulting in increased accumulation of neutrophils in muscle tissue in the exercised group, are in agreement with previous investigations of neutrophil accumulation in muscle tissue after downhill running (Fielding et al. 1993). Neutrophils are known to induce tissue destruction (Dallegri & Ottonello, 1997), but also to release factors necessary for repair and immunomodulation (Pyne, 1994). In the study by Fielding et al. (1993), neutrophil accumulation was related to z-band damage. Contrary to the findings by Fielding et al., we did not find a correlation between neutrophil and IL-1β in muscle (r < 0.4, P > 0.25 at all times, Spearman rank test). The correlation in the study by Fielding et al. (1993) was, however weak (r = 0.36, P < 0.06, Spearman rank test). The detected increase in CD11b can have different effects depending on the cells bearing the molecule. As identification of the different leukocyte subsets expressing CD11b in muscle tissue was not done, our study cannot address this issue.
The complex events involved in satellite cells activation are described in detail in several excellent reviews (Grounds & Yablonka-Reuveni, 1993; Schultz & McCormick, 1994; Chambers & McDermott, 1996). Expression of CD56 (neural cell adhesion molecule, N-CAM) is a reliable marker for activated satellite cells and regenerating and degenerating muscle fibres in humans (Illa et al. 1992). CD56+ muscle cells are suggested to represent activated embryonal myoblasts (Illa et al. 1992). In this study, we have not distinguished between satellite cells and regenerating or degenerating muscle fibres, but we refer to them as ‘CD56+ muscle cells’ because small cells without distinct morphological appearance of either satellite or muscle cells were not counted. The proportion of CD56+ muscle cells of all muscle fibres increased in both groups but in the control group CD56 was only significantly increased from rest in the 4th biopsy at 24 h (Table 5). Because satellite cells are activated upon increased functional demand, and by a number of factors including crush, contusion, cut, local anaesthetics, exercise, stretch, cold and compression (Chambers & McDermott, 1996), the different activation patterns seen in Fig 6 and Fig 7 are not surprising. Development of functional muscle cells also needs an appropriate and changing cellular environment depending on the stage of maturation (Grounds, 1991), different growth factors (Husmann I. et al. 1996) and the presence of macrophages (Lescaudron et al. 1999). The presence of blood-borne macrophages as an early event in regeneration muscle after grafting (Lescaudron et al. 1999) suggests an important relationship between monocytes and CD56+ muscle cells (Fig 5, Fig 6 and Fig 7). Monocyte activation includes shedding of the CD14 membrane receptor (Bazil & Strominger, 1991). Because of the governing roll of lymphocytes in the immune system and the multiple functions of CD14 (Hirohata & Oka, 1993), the relationships between CD14 on monocytes, the number of T and B lymphocytes and CD56+ muscle cells (Fig 6 and Fig 7) are in line with previously published data. The appearance of CD56+ muscle cells before macrophage infiltration in both groups (Table 5) indicates a process of regeneration rather than necrosis (St Pierre & Tidball, 1994). The complex interactions between CD56+ muscle cells, tissue macrophages, circulating monocytes and local cytokine production in the present study are therefore not surprising (Fig 5, Fig 6, Fig 7, Fig 8, Fig 9 and Fig 10). Illa et al. (1992) speculated that there may be a link between different cells and tissues expressing CD56, and that CD56 may facilitate cytotoxic lymphocyte-induced muscle fibre injury. Hohlfeld & Engel (1994) have shown that myoblasts can be lysed by NK cells. The present study demonstrates an indirect relationship in vivo between circulating NK cells and CD56+ muscle cells via monocytes (Fig. 7). Previous studies have revealed the importance of CD11b in the adhesion and migration of monocytes (Ley, 1996), as well as the necessity of macrophages for muscle development (Illa et al. 1992; Schultz & McCormick, 1994; Chambers & McDermott, 1996; Husmann et al. 1996; Massimino et al. 1997; Lescaudron et al. 1999).
CD11b is a determining factor for cell migration and activation (Dallegri & Ottonello, 1997), while CD62L induces leukocyte rolling, intracellular signalling and cytokine production (Berton et al. 1996). A plausible explanation for the negative correlation between CD11b on monocytes at 6 h and CD56+ muscle cells at 48 h is that by downregulating the surface expression of CD11b, monocytes may avoid firm adhesion while still inducing signalling and local cytokine production. This explanation is supported by the fact that infiltrating macrophages do not appear in muscle before the 5th biopsy at 48 h and the correlation between IL-1β expression in muscle and monocytes (Fig 9 and Fig 10).
It can be concluded that satellite cells are activated in response to muscle biopsies, as they express CD56, but that exposure to eccentric cycling exercise alters the activation pattern.
The role of macrophages in muscle cell development, regeneration and repair has been discussed above and elsewhere (Tidball & St Pierre, 1996; Lescaudron et al. 1999). It has been concluded that, at least in the rat, there are at least two different types of macrophages, ED1+ associated with muscle necrosis and ED2+ associated with muscle regeneration (St Pierre & Tidball, 1994). In our human subjects, the distinction between different macrophage subsets were not made and there is a possiblity that the different causes of increased CD163 expression at 48 h are due to different macrophage subpopulations (Fig. 8). An interesting observation is that whereas the increased number of macrophages correlated negatively to muscle CD11b expression in the control group, there was a positive correlation between macrophages and muscle CD11b in the exercised group. In the control group, macrophages also correlated negatively to blood neutrophil number at rest, and it is possible that a low neutrophil availability resulted in increased macrophage infiltration in response to multiple biopsies. This could be in line with the suggestion made by Aronson et al. (1998) that activation of MAP kinases in response to local injury (needle or open biopsy) can facilitate repair of damage cells and also with the findings of Robertson et al. (1992) that the sealing of injured myofibres is not dependent on infiltrating leukocytes. Thus, extensive neutrophil presence in damaged muscle tissue may cause excessive lesions (Weiss, 1989) and inhibit repair. On the other hand, neutrophils are influenced by their local environment and, in synergy with monocytes and after strenuous exercise, may function as immunoregulatory cells by releasing cytokines (Dallegri & Ottonello, 1997). This may explain the positive correlation between macrophages and neutrophils after eccentric exercise (Fig. 8 and Table 8).
Most of the complex interactions between tissue macrophages, CD56+ muscle cells, tissue IL-1β and circulating monocytes shown in Fig. 5 is supported by data from in vitro and in vivo studies. Multiple regression analysis with the increase in macrophages as the dependent variable revealed two separate lines of causes. One involves T helper cell activity (CD4 on CD3+ lymphocytes) and HLA-DR+ blood monocytes (HLA, human leukocyte antigen). The other line of explanation involves muscle IL-1β expression and HLA-DR- blood monocytes. This indicates that there may be one antigen-dependent and one antigen-independent cause of macrophage accumulation in human muscle tissue after multiple biopsy tissue injury. The two different causes for the same muscular event are, however not necessarily exclusive. In addition to the multiple roles of monocytes and macrophages in muscle tissue repair and adaptation discussed above, these cells can also function as antigen-presenting cells for CD4+ cells when expressing HLA class II. Because monocyte function can be influenced by factors such as exercise and age (Fielding & Evans, 1997; Malm et al. 1999) resting monocyte status may result in different responses to similar immunological challenges in different individuals. This could be the case for both pathways shown in Fig. 9. CD11b on monocytes is vital for migration and upregulated upon activation (Lundahl et al. 1996) thereby explaining the correlation between increased CD11b expression on blood monocytes and increased percentages of tissue macrophages.
Muscle IL-1β expression at both 6 and 48 h was negatively correlated to the percentage of CD56+ muscle cells (Fig. 9). IL-1β is a pro-inflammatory cytokine, and this negative correlation is in line with the concept that satellite cell activation is a process of repair and regeneration, and not inflammation. IL-1β is reported to be associated with N-CAM (CD56) expression in regenerating muscle cells in inflammatory myopathies (Authier et al. 1997). IL-1β has also been reported to increase in response to downhill running (Fielding et al. 1993), but these findings may be due to the biopsy procedure used and so be in agreement with the present data.
IL-1β in the second biopsy increased similarly in both groups, but our results indicate different causes. In agreement with other studies, IL-1β increased after exercise (Cannon et al. 1989; Fielding et al. 1993) and the post exercise expression in muscle correlated negatively (r2= 0.96, P = 0.006) to eccentric V̇O2 (ml min−1 kg−1) and monocyte activation at rest (CD14 on DR+ monocytes alone r2= 0.79, P = 0.002). This observation is surprising because it indicates that IL-1β increases more with less oxidative stress. Resting monocyte status was, however, a larger determinant (79 %) for IL-1β accumulation in muscle than the oxidative stress due to exercise (17 %). Previous factors such as physical training may therefore be of importance. Although IL-1β apparently increases with muscle injury and is associated with CD56+ regenerating muscle fibres (Authier et al. 1997), our study reveals that the relationship is negative, as a decrease in IL-1β in muscle relates to an increase in CD56+ muscle cells. Thus, a decrease in IL-1β appears to be beneficial for muscle adaptation and regeneration. This agrees with several other studies where IL-1β has been demonstrated to have a role in protein degradation and muscle atrophy and not in inflammation (Authier et al. 1997). It has therefore been suggested that IL-1β produced by muscle cells is not pro-inflammatory but involved in muscle adaptation (Authier et al. 1997). Increased IL-1β in the control group was not explained by any variable measured in this study, but may be due to local factors such as the protein kinase cascade pathway, which is known to increase after a muscle biopsy (Aronson et al. 1998). These findings demonstrate that IL-1β expression in muscle is increased after exercise, but also that a needle biopsy results in a similar increase within 60 min.
The observed increase in IL-1α in the third biopsy in the control group was strictly related to the number of NK cells (CD56+CD16+CD57-CD3-) at rest (Table 8). Because the third biopsy was taken in the left leg, which had not previously been biopsied, the increase in IL-1α must be related to systemic and not local events.
As discussed above, NK cells are suggested to have a controlling role of immunological events in muscle, and CD57+ NK cells may be more related to exercise than CD57- NK cells. This argument is supported by the correlation between CD57+ NK cells and satellite cells in the exercise group (Fig. 7) and between the change in CD57+ NK cells after eccentric exercise and the subjects V̇O2,max (see Results). The finding that CD57- NK cells are correlated to increased neutrophil infiltration (Fig. 5) and increased IL-1α expression in response to multiple biopsies, suggests that CD57- NK cells have a role in tissue regeneration after direct physical trauma.
The present study did not find a correlation between resting NK cell numbers (CD57+ or CD57-) and aerobic physical capacity (V̇O2,max, l min−1 or ml min−1 kg−1). Others have suggested that NK cell function is elevated in well-trained subjects and that NK cells are the leukocyte population most influenced by physical exercise (Nieman et al. 1989; Pedersen, 1997). Consequently, it appears that NK cells are influenced by previous physical exercise and training as well as acute exercise. Based on current results, NK cells are also involved in governing skeletal muscle response, but distinguishing between different NK cell subpopulations when performing analyses is necessary.
Exercise can increase plasma IL-6 concentration (Bruunsgaard et al. 1997; Suzuki et al. 1999), and a relationship with muscle damage (plasma CK activity) has been proposed (Bruunsgaard et al. 1997; Suzuki et al. 1999) but also debated (Croisier et al. 1999). In one published study IL-6 was detected in muscle homogenate after, but not before marathon running (Ostrowski et al. 1998). The divergent findings may be due to the several factors, including; (1) the small amounts of IL-6 detected within muscle fibres in 21 muscle biopsies in the present study (< 0.01 % of the total area in all samples) as well as in the study by Ostrowski et al. (1998) (picogram range), and (2) unspecific staining with one antibody clone. These results may indicate that IL-6 is produced by human muscle cells in response to prolonged exercise, but not specifically to eccentric exercise. Because of the increased detection of IL-6 in one control subject in response to the muscle biopsies, caution should be exercised when planning future studies.
In summary, the main conclusions from the present study are:
Eccentric cycling exercise affects immunological variables in both blood and skeletal muscle.
Multiple biopsies can have effects similar to eccentric cycling exercise in human skeletal muscle. Results from several previous studies may therefore need reconsideration.
Muscle adaptation to physical exercise may occur without the classical signs of inflammation (i.e. neutrophil and macrophage accumulation).
Circulating NK cells and monocytes may have a governing function over muscular events.
Due to the lack of T cell infiltration, eccentric cycling exercise is not a suitable model for studying myositis.
Serum CK activity is not related to muscle inflammation.
DOMS is not related to muscle inflammation but is suggested to be related more to muscle adaptation than to damage.
Hormonal changes in blood are related to leukocyte changes in blood but not in muscle tissue.
Throughout human evolution, our immune system has evolved to protect the body from infection and damage, and adapt it to new functional demands. It is therefore no surprise that the monocytes and NK cells seem to be more involved in adaptation to physical exercise than T and B cells. Because physical activity is a primary event in human evolution, it is anomalous that a tissue is damaged by the very task for which it has evolved, namely, in the case of skeletal muscle, physical activity. If the presence of leukocytes in human skeletal muscle after exercise is established, it could reflect a functional role of leukocytes in muscle adaptation rather than degeneration. From an evolutionary standpoint, prompt adaptation without further damage would be beneficial.
Acknowledgments
We thank Berit Sjöberg from the Department of Physiology and Pharmacology, Karolinska Institute, Stockholm for analyses of catecholamines, Regina Solborg from the Department of Clinical Chemistry, Karolinska Hospital, Solna for hormone analyses, Inger Nennesmo and Kristina Lindqvist from the Department of Neuropathology, Huddinge Hospital for histochemical staining, and participating subjects for enduring a painful week of DOMS. This study was in part supported by a grant from Centrum för idrottsforskning, Stockholm, Sweden.
References
- Armstrong R B, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. Journal of Applied Physiology. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Aronson D, Wojtaszewski JF, Thorell A, Nygren J, Zangen D, Richter EA, Ljungqvist O, Fielding RA, Goodyear LJ. Extracellular-regulated protein kinase cascades are activated in response to injury in human skeletal muscle. American Journal of Physiology. 1998;275:C555–561. doi: 10.1152/ajpcell.1998.275.2.C555. [DOI] [PubMed] [Google Scholar]
- Authier FJ, Chazaud B, Mhiri C, Eliezer-Vanerot MC, Poron F, Barlovatz-Meimon G, Gherardi RK. Interleukin-1 expression in normal motor endplates and muscle fibers showing neurogenic changes. Acta Neuropathologica. 1997;94:272–279. doi: 10.1007/s004010050703. [DOI] [PubMed] [Google Scholar]
- Authier FJ, Mhiri C, Chazaud B, Christov C, Cherin P, Barlovatz-Meimon G, Gherardi RK. Interleukin-1 expression in inflammatory myopathies: evidence of marked immunoreactivity in sarcoid granulomas and muscle fibres showing ischaemic and regenerative changes. Neuropathology and Applied Neurobiology. 1997;23:132–140. [PubMed] [Google Scholar]
- Bazil V, Strominger JL. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. Journal of Immunology. 1991;147:1567–1574. [PubMed] [Google Scholar]
- Berton G, Yan SR, Fumagalli L, Lowell CA. Neutrophil activation by adhesion: mechanisms and pathophysiological implications. International Journal of Clinical and Laboratory Research. 1996;26:160–177. doi: 10.1007/BF02592978. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Galbo H, Halkjaer-Kristensen J, Johansen TL, MacLean DA, Pedersen BK. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. The Journal of Physiology. 1997;499:833–841. doi: 10.1113/jphysiol.1997.sp021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JG, Fielding RA, Fiatarone MA, Orencole SF, Dinarello CA, Evans WJ. Increased interleukin 1 beta in human skeletal muscle after exercise. American Journal of Physiology. 1989;257:R451–455. doi: 10.1152/ajpregu.1989.257.2.R451. [DOI] [PubMed] [Google Scholar]
- Chambers RL, McDermott JC. Molecular basis of skeletal muscle regeneration. Canadian Journal of Applied Physiology. 1996;21:155–184. doi: 10.1139/h96-014. [DOI] [PubMed] [Google Scholar]
- Croisier JL, Camus G, Venneman I, Deby-Dupont G, Juchmes-Ferir A, Lamy M, Crielaard JM, Deby C, Duchateau J. Effects of training on exercise-induced muscle damage and interleukin 6 production. Muscle and Nerve. 1999;22:208–212. doi: 10.1002/(sici)1097-4598(199902)22:2<208::aid-mus8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Dallegri F, Ottonello L. Tissue injury in neutrophilic inflammation [see comments] Inflammation Research. 1997;46:382–391. doi: 10.1007/s000110050208. [DOI] [PubMed] [Google Scholar]
- Evans WJ, Cannon JG. The metabolic effects of exercise-induced muscle damage. Exercise and Sports Science Reviews. 1991;19:99–125. [PubMed] [Google Scholar]
- Felten DL, Felten SY, Bellinger DL, Madden KS. Fundamental aspects of neural-immune signaling. Psychotherapy and Psychosomatics. 1993;60:46–56. doi: 10.1159/000288679. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Evans WJ. Aging and the acute phase response to exercise: implications for the role of systemic factors on skeletal muscle protein turnover. International Journal of Sports Medicine. 1997;18(suppl. 1):S22–27. doi: 10.1055/s-2007-972697. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG. Acute phase response in exercise. III. Neutrophil and IL-1β accumulation in skeletal muscle. American Journal of Phsiology. 1993;265:R166–172. doi: 10.1152/ajpregu.1993.265.1.R166. [DOI] [PubMed] [Google Scholar]
- Friden J, Lieber RL. Structural and mechanical basis of exercise-induced muscle injury. Medicine and Science in Sports and Exercise. 1992;24:521–530. [PubMed] [Google Scholar]
- Friden J, Sfakianos PN, Hargens AR. Blood indices of muscle injury associated with eccentric muscle contractions. Journal of Orthopedic Research. 1989;7:142–145. doi: 10.1002/jor.1100070120. [DOI] [PubMed] [Google Scholar]
- Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. International Journal of Sports Medicine. 1983;4:170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- Grounds MD. Towards understanding skeletal muscle regeneration. Pathology Research and Practice. 1991;187:1–22. doi: 10.1016/S0344-0338(11)81039-3. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Yablonka-Reuveni Z. Molecular and cell biology of skeletal muscle regeneration. Molecular and Cellular Biology of Human Disorders Series. 1993;3:210–256. doi: 10.1007/978-94-011-1528-5_9. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Frandsen U, Orthenblad N, Sjodin B, Richter EA. Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. Journal of Physiology. 1997;498:239–248. doi: 10.1113/jphysiol.1997.sp021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohata S, Oka H. Modulation of T cell production of interferon-gamma by human monocytes: effect of engagement of CD14 on monocytes. Cell Immunology. 1993;151:270–282. doi: 10.1006/cimm.1993.1237. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Engel AG. The immunobiology of muscle. Immunology Today. 1994;15:269–274. doi: 10.1016/0167-5699(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Husmann I, Soulet L, Gautron J, Martelly I, Barritault D. Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Review. 1996;7:249–258. doi: 10.1016/s1359-6101(96)00029-9. [DOI] [PubMed] [Google Scholar]
- Illa I, Leon-Monzon M, Dalakas MC. Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Annals of Neurology. 1992;31:46–52. doi: 10.1002/ana.410310109. [DOI] [PubMed] [Google Scholar]
- Kuipers H. Exercise-induced muscle damage. International Journal of Sports Medicine. 1994;15:132–135. doi: 10.1055/s-2007-1021034. [DOI] [PubMed] [Google Scholar]
- Lenkei R, Andersson B. Determination of the antibody binding capacity of lymphocyte membrane antigens by flow cytometry in 58 blood donors. Journal of Immunological Methods. 1995;183:267–277. doi: 10.1016/0022-1759(95)00064-h. [DOI] [PubMed] [Google Scholar]
- Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscular Disorders. 1999;9:72–80. doi: 10.1016/s0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovascular Research. 1996;32:733–742. [PubMed] [Google Scholar]
- Lieber RL, Thornell LE, Friden J. Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. Journal of Applied Physiology. 1996;80:278–284. doi: 10.1152/jappl.1996.80.1.278. [DOI] [PubMed] [Google Scholar]
- Lundahl J, Hallden G, Skold CM. Human blood monocytes, but not alveolar macrophages, reveal increased CD11b/CD18 expression and adhesion properties upon receptor-dependent activation. European Respiration Journal. 1996;9:1188–1194. doi: 10.1183/09031936.96.09061188. [DOI] [PubMed] [Google Scholar]
- Lundberg I, Ulfgren AK, Nyberg P, Andersson U, Klareskog L. Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis and Rheumatism. 1997;40:865–874. doi: 10.1002/art.1780400514. [DOI] [PubMed] [Google Scholar]
- MacIntyre DL, Reid WD, McKenzie DC. Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Sports Medicine. 1995;20:24–40. doi: 10.2165/00007256-199520010-00003. [DOI] [PubMed] [Google Scholar]
- Malm C, Lenkei R, Sjodin B. Effects of eccentric exercise on the immune system in men. Journal of Applied Physiology. 1999;86:461–468. doi: 10.1152/jappl.1999.86.2.461. [DOI] [PubMed] [Google Scholar]
- Massimino ML, Rapizzi E, Cantini M, Libera LD, Mazzoleni F, Arslan P, Carraro U. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochemical and Biophysical Research Communications. 1997;235:754–759. doi: 10.1006/bbrc.1997.6823. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Johanssen LM, Lee JW. Infectious episodes in runners before and after a roadrace. Journal of Sports Medicine and Physical Fitness. 1989;29:289–296. [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. The Journal of Physiology. 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway CA, Husband AJ. The influence of neuroendocrine pathways on lymphocyte migration. Immunology Today. 1994;15:511–517. doi: 10.1016/0167-5699(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Exercise Immunology. Georgetown, MD, USA: R. G. Landes Company; 1997. [Google Scholar]
- Pyne DB. Regulation of neutrophil function during exercise. Sports Medicine. 1994;17:245–258. doi: 10.2165/00007256-199417040-00005. [DOI] [PubMed] [Google Scholar]
- Robertson TA, Grounds MD, Papadimitriou JM. Elucidation of aspects of murine skeletal muscle regeneration using local and whole body irradiation. Journal of Anatomy. 1992;181:265–276. [PMC free article] [PubMed] [Google Scholar]
- St Pierre BA, Tidball JG. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. Journal of Applied Physiology. 1994;77:290–297. doi: 10.1152/jappl.1994.77.1.290. [DOI] [PubMed] [Google Scholar]
- Schultz E, McCormick KM. Skeletal muscle satellite cells. Reviews in Physiology Biochemistry and Pharmacology. 1994;123:214–246. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- Shek PN, Shephard RJ. Physical exercise as a human model of limited inflammatory response. Canadian Journal of Physiology and Pharmacology. 1998;76:589–597. doi: 10.1139/cjpp-76-5-589. [DOI] [PubMed] [Google Scholar]
- Smith LL. Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Medicine and Science in Sports and Exercise. 1991;23:542–551. [PubMed] [Google Scholar]
- Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q, Sugawara K, Yamaya K, Sato K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. Journal of Applied Physiology. 1999;87:1360–1367. doi: 10.1152/jappl.1999.87.4.1360. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory cell response to acute muscle injury. Medicine and Science in Sports and Exercise. 1995;27:1022–1032. doi: 10.1249/00005768-199507000-00011. [DOI] [PubMed] [Google Scholar]
- Tidball JG, St Pierre BA. Apoptosis of macrophages during the resulution of muscle inflammation. Journal of Leukocyte Biology. 1996;59:380–388. doi: 10.1002/jlb.59.3.380. [DOI] [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Medicine. 1999;27:43–59. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. New England Journal of Medicine. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]


