Abstract
Detection of the scrapie-associated protease-resistant prion protein (PrPres) in sheep brains in the early phase after intracerebral inoculation of the scrapie agent has not been documented. Fourteen 4-mo-old, genetically susceptible lambs (QQ homozygous at codon 171 of the PrP gene) were obtained for this study. Twelve lambs were inoculated intracerebrally with a brain suspension from sheep naturally affected with scrapie, and 2 served as uninoculated controls. Two inoculated animals were euthanized at each of 6 times postinoculation (1 h to 6 wk), and their brains were collected for histopathological study, for detection of PrPres by the Western blot technique and an immunohistochemical (IHC) method, and for the detection of scrapie-associated fibrils (SAF) by negatively stained electron microscopy (EM). Microscopic lesions associated with introduction of the inoculum were seen in the brains of inoculated animals at all 6 times. However, both the Western blot and IHC techniques did not detect PrPres after the initial 3 d postinoculation, nor did EM detect SAF in any of the samples. From these findings, it is presumed that until host amplification has occurred, the concentration of PrPres in inoculum is insufficient for detection by currently available techniques.
Scrapie is a naturally occurring transmissible spongiform encephalopathy (TSE) of sheep and goats. Infection by the causative agent leads to accumulation of an abnormal, protease-resistant form of prion protein (PrPres) in the central nervous system (CNS) and in other tissues. Detection of PrPres in tissues and characteristic histopathological changes in the brain are the basis of currently available methods for postmortem diagnosis of scrapie (1).
During experimental interspecies transmission of TSE (scrapie and chronic wasting disease) by the intracerebral route (2,3,4), researchers interpreted PrPres detection in inoculated animals as evidence of successful transmission. However, at that time, there was no directed effort to prove that the PrPres was amplified PrPres rather than residual inoculum. Therefore, the primary objective of this study was to determine if scrapie inoculum could be detected in brains of sheep by currently available methods (5) within the first few weeks after intracerebral inoculation.
Fourteen 4-mo-old lambs were assigned to inoculated (n = 12) or control (n = 2) groups. All lambs were QQ homozygous at codon 171 of the PrP gene (which is associated with susceptibility to scrapie). The inoculated animals were housed 2 per pen in a containment facility of biosafety level 2 at the National Animal Disease Center (NADC), Ames, Iowa. They were fed pelleted growth and maintenance rations that contained no ruminant protein, and clean water was available ad libitum. The control animals were kept with the scrapie-free sheep flock at NADC. Personnel wore protective clothing while in the isolation facility and showered when leaving the facility.
The inoculum was prepared from a pool of 13 scrapie-affected sheep brains [all positive by immunohistochemical (IHC) testing] from 7 flocks. The tissue was ground in a mechanical grinder, gentamicin was added at a concentration of 100 μg/mL, and the final concentration of 10% weight per volume was made with phosphate-buffered saline.
The lambs were inoculated intracerebrally with 1 mL of the inoculum, as described previously (2). Briefly, the animals were sedated with xylazine, a midline incision was made in the skin at the junction of the parietal and frontal bones, and a 1-mm hole was drilled through the calvarium. The inoculum was injected into the midbrain with a 22-gauge, 9-cm-long needle while the needle was being withdrawn from the brain. The skin incision was closed with a single suture. The control lambs were not inoculated.
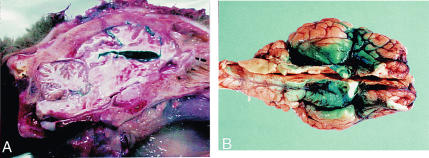
At 6 different times postinoculation (1 h, 1 d, 3 d, 1 wk, 2 wk, and 6 wk), 2 animals were euthanized and their brains removed in toto. Four samples of 4-mm-wide coronal sections of each brain (rostral cerebrum, caudal cerebrum including midbrain, cerebellum, and obex) were taken for histopathological and IHC study. Parallel coronal sections at each of the 4 sites were frozen for Western blot testing for PrPres (6), using the Prionics-check (University of Zurich-Irchel, Winterhurerstrasse, Zurich, Switzerland), and for negatively stained electron microscopy (EM) for scrapie- associated fibrils (SAF) (7). The control animals were euthanized at 6 wk and their brains similarly examined. These tissues were searched for the inoculum because of the results of a previous experiment (A.N. Hamir: unpublished observations), in which anesthetized sheep were intracerebrally injected with 1 mL of methylene blue, then euthanized. When the brains were examined, the dye was found to be widely distributed within the ventricles and leptomeninges (Figures 1A, 1B).
Figure 1. Sheep brain intracerebrally injected with methylene blue to determine the distribution of the dye within the organ. A) Longitudinal section of the head shows the dye within the brain parenchyma, in the ventricles, and in the meninges. B) Brain removed from the skull to show the distribution of the dye in the meninges.
The brain tissues were immersion-fixed in 10% buffered formalin, processed for routine histopathological study, and embedded in paraffin. Sections 5 μm thick were stained with hematoxylin and eosin (HE) and by an IHC method (8) for detection of PrPres using a cocktail of 2 monoclonal antibodies, as described previously (1). Each group of slides subjected to the IHC method included a positive-control tissue: brain stem from a sheep with clinical scrapie.
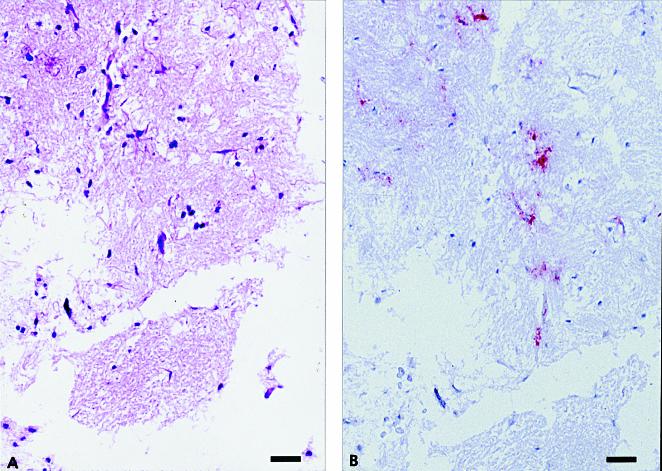
The inoculum was tested for PrPres by the Western blot technique and the IHC method and for SAF by EM. For IHC testing, a 1-mL sample was placed in a gelatin capsule, immersion-fixed in 10% buffered formalin, processed for routine histopathological study, embedded in paraffin, sectioned at 5 μm, and stained by HE and the IHC method. The results of the Western blot technique and EM were positive. Microscopic examination of the inoculum revealed variable-sized particles of partially autolyzed brain tissue (Figure 2A). Although a few aggregates of PrPres were present in sections stained by the IHC method (Figure 2B), the positively stained material was seen only in very small, scattered foci and was unlike the heavy staining usually observed in the obex of sheep with clinical scrapie.
Figure 2. Formalin-fixed inoculum of sheep scrapie agent, processed for routine histopathological study. A) Variable-sized particles of partially autolyzed brain tissue. Hematoxylin and eosin (HE); bar = 50 μm. B) An adjacent section shows staining (red) of protease-resistant prion protein (PrPres) within the brain particles. Stained for PrPres by an immunohistochemical (IHC) method; bar = 50 μm.
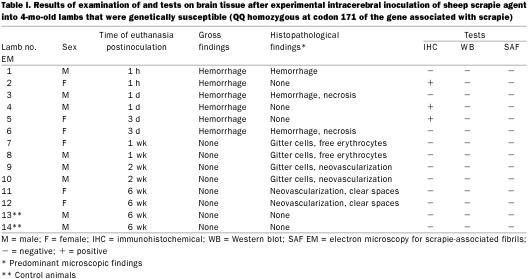
Results of examination of the brain tissue are summarized in Table I. Gross examination revealed variable-sized, locally extensive areas of acute subdural hemorrhage over the dorsal surface of the cerebrum, as well as at the base of the brain, in lambs euthanized at the first 3 times (1 h, 1d, and 3 d). The amount of hemorrhage was considerably less in the animals euthanized 3 d after inoculation. Also, a streak of acute hemorrhage, probably representing the site of intracerebral inoculation, was seen on the cut section of brains from the 1st 2 lambs that were euthanized.
Table I.
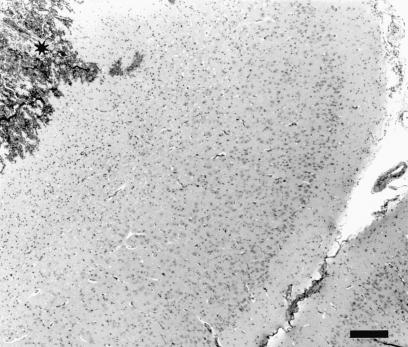
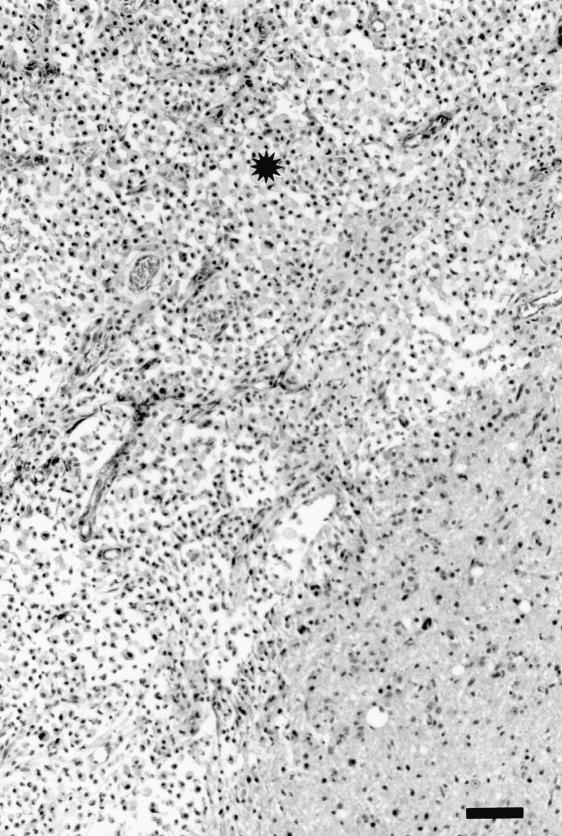
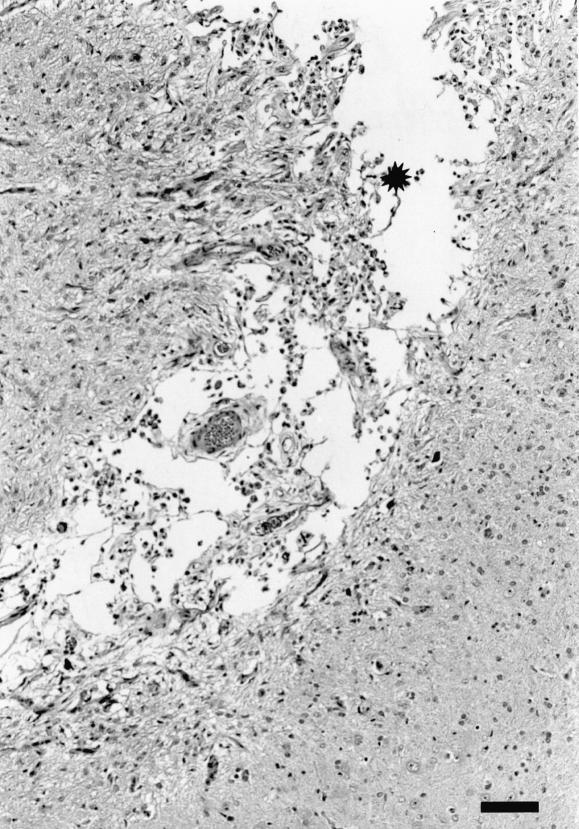
Microscopic lesions were found in either the cerebrum or the midbrain (at the inoculation site) in 9 of the 12 inoculated animals; 1 sheep euthanized at each of 1 h, 1 d, and 3 d postinoculation had no lesions in the examined brain sections. The lesions were variable (acute to chronic), and when present in both sheep euthanized at a particular time were histopathologically similar. In the sheep euthanized within 1 h postinoculation, there were locally extensive areas of acute hemorrhage in the meninges as well as the brain parenchyma (Figure 3). In the animals that were euthanized on day 1 or day 3 postinoculation, there were track-like necrotic areas, with some acute hemorrhage at the periphery of the necrotic material (Figure 4). The blood vessels around the necrotic foci were prominent, and there was mildly increased cellularity in the perivascular areas. In the sheep euthanized 1 and 2 wk postinoculation, necrotic foci were not visible. Such areas appeared to have been replaced by large numbers of macrophages with vacuolated cytoplasm (gitter cells), some free erythrocytes, and early neovascularization (Figure 5). In the sheep euthanized 6 wk postinoculation, there were small, variable-sized, clear foci (cavity formation) surrounded by some gitter cells and thin-walled blood vessels within and at the periphery of the cellular infiltrate (Figure 6).
Figure 3. Cerebrum of sheep inoculated intracerebrally with sheep scrapie agent and euthanized 1 h postinoculation. Note focus of acute hemorrhage (*). HE; bar = 200 μm.
Figure 4. Cerebrum of sheep inoculated intracerebrally with sheep scrapie agent and euthanized 3 d postinoculation. A track-like area of necrosis and hemorrhage (*) extends from the meninges (m) into the brain parenchyma. HE; bar = 200 μm.
Figure 5. Cerebrum of sheep inoculated intracerebrally with sheep scrapie agent and euthanized 2 wk postinoculation. At the inoculation site (*) is a homogeneous population of gitter cells and thin-walled blood vessels. HE; bar = 100 μm.
Figure 6. Cerebrum of sheep inoculated intracerebrally with sheep scrapie agent and euthanized 6 wk postinoculation. At the inoculation site (*) are multifocal clear areas devoid of tissue, only a few gitter cells, and some thin-walled blood vessels. The surrounding neuropil shows areas of neovascularization. HE; bar = 100 μm.
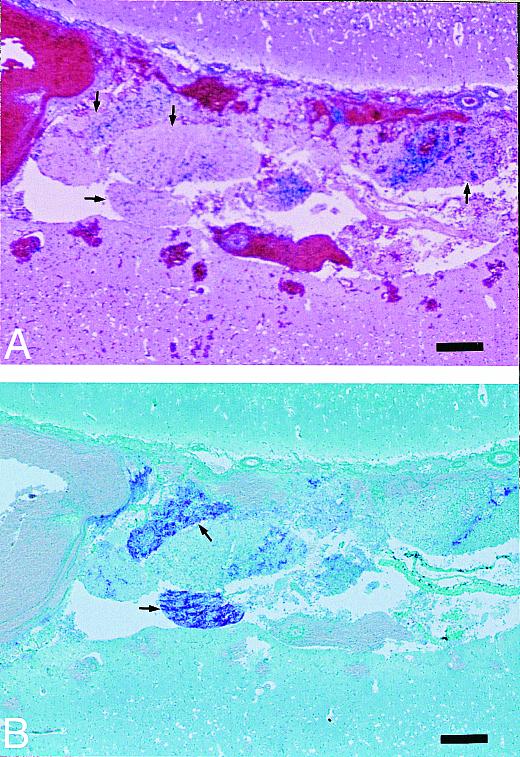
Scrapie-infected inoculum was detected in the HE-stained brain sections from the inoculated sheep for up to 3 d postinoculation. The inoculated material consisted of small, partially autolyzed particles of neural tissue surrounded by acute hemorrhage; they were easily differentiated from the host brain tissue (Figure 7A).
Figure 7. Cerebrum of sheep inoculated intracerebrally with sheep scrapie agent and euthanized 1 d postinoculation. A) Small, variable-sized particles of inoculum (neural tissue) are present at the inoculation site (arrows). HE; bar = 200 μm. B) An adjacent section shows PrPres staining within 2 neuronal particles (arrows) at the inoculation site. Stained for PrPres by an IHC method; bar = 200 μm.
PrPres was seen in sections stained by the IHC method from only 3 inoculated sheep (1 animal each euthanized 1 h, 1 d, or 3 d postinoculation) and only within the inoculated neural tissue (Figure 7B). However, none of the sections were positive for PrPres by the Western blot technique, and all were negative for SAF by negatively stained EM.
At 1 h, 1 d, and 3 d postinoculation, there was extensive hemorrhage and tissue necrosis at the site of inoculation. This was followed by intense infiltration of the damaged area by macrophages, observed between 1 and 2 wk postinoculation. Finally, there was a reparative process, characterized by removal of hemorrhage and dead tissue and the beginnings of cavity formation in the neuropil, observed at 6 wk postinoculation. The initial histopathological findings indicate that during this time it would be possible for some of the inoculated material to be transported to other organs via the blood vessels. Therefore, if such animals were allowed to live longer (2 to 3 y) and to develop clinical scrapie, it would not be surprising to detect PrPres in lymphoid as well as CNS tissues.
After 3 d postinoculation, it was not possible to detect PrPres by either Western blot or IHC testing in brain tissues of sheep that were experimentally inoculated with a scrapie-positive inoculum. Also, there was no evidence of SAF by EM in any of the samples. Similar results have been obtained in mice brains inoculated with a preparation of mouse-adapted Creutzfeldt–Jakob disease (CJD) agent (9). CJD is another TSE. In that study, PrPres was not detected in the brains until 4 wk postinoculation, by an immunoblotting technique. In naturally occurring sheep scrapie, there is also a period after infection in which infectivity cannot be detected in any of the animal's tissues, and this period may last more than 8 mo (5). The inability to detect PrPres in the mouse study was attributed to rapid spreading of the inoculated material into the brain tissue, which is compatible with the previous findings in sheep (Figures 1A, 1B). Alternatively, it could be that the techniques currently available (1) for the detection of TSE agents in mice and sheep are not sensitive enough to detect very small quantities of PrPres. Therefore, except for the initial detection of PrPres by the IHC method (up to 3 d postinoculation), it can be concluded that if a TSE agent is detected in the brain of an animal experimentally inoculated with 1 mL of a 10% suspension, it can be assumed that the agent has amplified to a level sufficient for detection with currently available methods.
Footnotes
Acknowledgments
We thank Dr. Lawayne Nusz for assistance with the animal inoculations. Expert technical assistance was provided by Judy Stasko, Martha Church, Jean Donald, and Jennifer Slovak at NADC and Linda Davis and Donna Barnicle at the Veterinary Laboratories Agency, Weybridge, England.
Address correspondence and reprint requests to Dr. Amir N. Hamir, tel: 515-663-7544, fax: 515-663-7458, e-mail: ahamir@nadc.ars.usda.gov
Received November 13, 2001. Accepted April 5, 2002.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
References
- 1.Hamir AN, Miller JM, Schmerr MJ, et al. Diagnosis of preclinical and subclinical scrapie in a naturally infected sheep flock utilizing currently available postmortem diagnostic techniques. J Vet Diagn Invest 2001;13:152–154. [DOI] [PubMed]
- 2.Cutlip RC, Miller JM, Race RE, et al. Intracerebral transmission of scrapie to cattle. J Infect Dis 1994;169:814–820. [DOI] [PubMed]
- 3.Cutlip RC, Miller JM, Lehmkuhl HD. Second passage of a US scrapie agent in cattle. J Comp Pathol 1997;117:271–275. [DOI] [PubMed]
- 4.Hamir AN, Cutlip RC, Miller JM, et al. Preliminary findings on the experimental transmission of chronic wasting disease agent of mule deer to cattle. J Vet Diagn Invest 2001;13:91–96. [DOI] [PubMed]
- 5.Detwiler LA. Scrapie. Rev Sci Tech Off Int Epizoot 1992;11: 491–537. [DOI] [PubMed]
- 6.Schaller O, Fatzer R, Stack MJ, et al. Validation of a Western immunoblotting procedure for bovine PrPSc detection and its use as a rapid surveillance method for the diagnosis of bovine spongiform encephalopathy (BSE). Acta Neuropathol 1999; 98:437–443. [DOI] [PubMed]
- 7.Stack MJ, Keyes P, Scott AC. The diagnosis of bovine spongiform encephalopathy and scrapie by the detection of fibrils and the abnormal protein isoform. In: Baker HF, Ridley RM, eds. Methods in Molecular Medicine — Prion Diseases. Totowa, New Jersey: Humana Press, 1996:85–104.
- 8.Miller JM, Jenny AL, Taylor WD, et al. Detection of prion protein in formalin-fixed brain by hydrated autoclaving immunohistochemistry for diagnosis of scrapie in sheep. J Vet Diagn Invest 1994;6:366–368. [DOI] [PubMed]
- 9.Nakaoke R, Sakaguchi S, Atarashi R, et al. Early appearance but lagged accumulation of detergent-insoluble prion protein in the brains of mice inoculated with a mouse-adapted Creutzfeldt–Jakob disease agent. Cell Mol Neurobiol 2000; 20:717–330. [DOI] [PMC free article] [PubMed]