Abstract
We examined, in the anaesthetised cat, the influence of the neuronal ensembles producing spontaneous negative cord dorsum potentials (nCDPs) on segmental pathways mediating primary afferent depolarisation (PAD) of cutaneous and group I muscle afferents and on Ia monosynaptic activation of spinal motoneurones.
The intraspinal distribution of the field potentials associated with the spontaneous nCDPs indicated that the neuronal ensembles involved in the generation of these potentials were located in the dorsal horn of lumbar segments, in the same region of termination of low-threshold cutaneous afferents.
During the occurrence of spontaneous nCDPs, transmission from low-threshold cutaneous afferents to second order neurones in laminae III-VI, as well as transmission along pathways mediating PAD of cutaneous and Ib afferents, was facilitated. PAD of Ia afferents was instead inhibited.
Monosynaptic reflexes of flexors and extensors were facilitated during the spontaneous nCDPs. The magnitude of the facilitation was proportional to the amplitude of the ‘conditioning’ spontaneous nCDPs. This led to a high positive correlation between amplitude fluctuations of spontaneous nCDPs and fluctuations of monosynaptic reflexes.
Stimulation of low-threshold cutaneous afferents transiently reduced the probability of occurrence of spontaneous nCDPs as well as the fluctuations of monosynaptic reflexes.
It is concluded that the spontaneous nCDPs were produced by the activation of a population of dorsal horn neurones that shared the same functional pathways and involved the same set of neurones as those responding monosynaptically to stimulation of large cutaneous afferents. The spontaneous activity of these neurones was probably the main cause of the fluctuations of the monosynaptic reflexes observed under anaesthesia and could provide a dynamic linkage between segmental sensory and motor pathways.
Rudomin et al. (1987, 1990) used spike triggered averaging of dorsal root and ventral root potentials to disclose possible connections of intermediate nucleus interneurones with afferent fibres and/or spinal motoneurones. They found one set of interneurones (class I) whose spontaneous activity was associated with short-latency glycinergic inhibitory potentials in motoneurones as well as in the ventral roots (iVRPs), which were produced without concurrent dorsal root potentials (DRPs). These inhibitory neurones had collaterals to Clarke's column and appeared to be the same as those mediating non-reciprocal postsynaptic inhibition of Ib origin (Harrison & Jankowska, 1985). The activity of the second set of interneurones (class II) was instead associated with the generation of short-latency DRPs and iVRPs. It was suggested that these were GABAergic interneurones directly connected with the intraspinal terminals of group I muscle afferents and spinal motoneurones, where they produced PAD and postsynaptic inhibition, respectively (Rudomin et al. 1987, 1990).
The spontaneous activity of class I and class II interneurones usually appeared in synchrony with a negative cord dorsum potential lasting 40–60 ms that was initiated 20–30 ms before the interneuronal action potentials. It was assumed that this cord dorsum potential was generated by the spontaneous activity of a population of neurones located in the most superficial regions of the spinal cord. It was not clear, however, if these were ‘command’ interneurones modulating transmission along the pathways activated by class I and class II interneurones, or if they were driven together with the intermediate nucleus interneurones by some common input, but had no synaptic interactions with them.
Although spontaneous cord dorsum potentials were recorded in the cat spinal cord more than 50 years ago (see Bremer, 1941; ten Cate, 1950), there have been few studies addressing their origin and functional significance. Mark & Gasteiger (1953) found that in the anaesthetised cat, spontaneous cord dorsum potentials were still recorded after acute spinal transection and deafferentation, and suggested that these potentials were generated by intrinsic spinal mechanisms. The spontaneous potentials appeared to be largest in the dorsal grey matter (Gasteiger & Ichikawa, 1963; see also Gasteiger, 1969) and their amplitude was increased by spinalisation.
Spontaneous spinal cord potentials have been also recorded in the behaving cat (Kasprzak & Gasteiger, 1970). They appear to be essentially the same but of lower amplitude and frequency as those recorded in anaesthetised preparations. They changed during alert and relaxed wakefulness states and by tactile stimulation of the skin.
Polysynaptic reflexes tested during the appearance of the sharp negative cord dorsum potentials in the anaesthetised cat showed a significantly greater amplitude and variability than control reflexes. However, monosynaptic reflexes (MSRs) showed no consistent amplitude changes that could be related to the variations of the spontaneous nCDPs (Molt & Gasteiger, 1976).
The purpose of the present study was to disclose the actions of the neurones involved in the generation of the spontaneous nCDPs on the reflex pathways mediating PAD of cutaneous and muscle afferents, as well as their influence on the amplitude and variability of Ia monosynaptic reflexes. Disclosure of these actions may provide valuable clues to assess changes in spinal function in humans after cord lesions, in addition to standard tests based on the analysis of segmentally and supraspinally evoked cord dorsum potentials (Stuhmeier et al. 1993; Khan et al. 1994; Polo et al. 1994; Sindou et al. 1994).
The results obtained indicate that in the anaesthetised cat spontaneous, large-amplitude nCDPs are generated by the synchronous activation of a population of dorsal horn neurones that respond monosynaptically to stimulation of low-threshold cutaneous afferents. Activity of this set of neurones affects impulse transmission in segmental pathways mediating PAD of muscle and cutaneous afferents, and also appears to be the main source of the variability of Ia MSRs, both by presynaptic (Rudomin & Dutton, 1969a; Rudomin et al. 1969, 1975; Rudomin & Madrid, 1972) and postsynaptic mechanisms (Gossard et al. 1994). It is suggested that the ensemble of spontaneously active dorsal horn neurones sets a background level of transmission regulation in sensory and motor spinal pathways. Some preliminary reports have been already published (Manjarrez et al. 1996, 1997, 1998; Rojas-Piloni et al. 1999).
METHODS
General procedures
Guidelines contained in NIH publication 85–23 revised in 1985, on the principles of laboratory animal care were followed throughout. All experiments were approved by the Institutional Animal Ethics Committee. Briefly, experiments were carried out in adult cats initially anaesthetised with pentobarbitone (35 mg kg−1, i.p.), supplemented during the dissection and recording periods with additional doses of 10 mg kg−1, i.v. as necessary to maintain deep anaesthesia. Adequacy of anaesthesia was assessed by verifying that the pupils were constricted, and that blood pressure was stable (between 100 and 120 mmHg) and was not affected by noxious stimulation to the skin.
After the surgical procedures, the animals were paralysed and artificially respired. Tidal air volume was adjusted to have a 4 % CO2 concentration in the expired air. The lumbo-sacral and low thoracic spinal segments were exposed and the left L5 to S1 ventral roots sectioned. The left posterior biceps and semitendinosus (PBSt), gastrocnemius-soleus (GS), sural (SU) and superficial peroneus (SP) nerves were dissected and sectioned, and their central ends prepared for stimulation. Monosynaptic reflexes (MSRs) produced by GS or PBSt nerve stimulation were recorded from the central ends of the sectioned L6 or L7 ventral rootlets.
Exposed tissues were covered with mineral oil to prevent desiccation and kept at constant temperature (37°C) by means of radiant heat.
Stimulation and recording
Evoked and spontaneous nCDPs were monopolarly recorded from the surface of dorsal horn with a silver ball electrode against an indifferent electrode placed on the paravertebral muscles. Evoked and spontaneous dorsal root potentials were recorded with two electrodes, one placed distally on the central end of a sectioned L6 or L7 dorsal rootlet, and the other close to, but not touching, the spinal cord. In addition, one glass micropipette filled with 1.2 M NaCl (1.2–1.7 MΩ) was used to record intraspinal extracellular field potentials (EFPs) from the L6-L7 segments.
Sampling and averaging were triggered either by spontaneously occurring nCDPs of selected amplitudes, or by pulses preceding the stimuli applied to cutaneous or muscle afferents. Spinal potentials were recorded with the pre-amplifier filters set to 0.3 Hz in the low range and 10 kHz in the high range. During the experiment all potentials and triggering pulses were stored on digital tape for further processing.
Stimulation procedures
The interaction between the neuronal sets producing the spontaneous nCDPs and neurones responding to stimulation of cutaneous or muscle afferents (evoked responses) was examined using the following protocol. Every time a spontaneous nCDP crossed a fixed voltage level, set with a window discriminator (World Precision Instruments), a TTL pulse was sent to the input channel of a control device that triggered a sequence of no stimulus, a stimulus at a delay after the nCDP, or a stimulus >500 ms from a nCDP (control). Potentials from each condition were collected separately. On the completion of one cycle of stimulation, the control device prevented initiation of the next cycle until after 2 s. Sample size was 64 in all cases. Cutaneous nerves (SU or SP) were stimulated with single pulses of 1.1 to 1.2 times the strength of the most excitable fibres (T). Muscle nerves were stimulated with single pulses or trains of pulses (2 or 3 pulses, 300 Hz), 1.1 to 3T.
Isopotential contours
Intraspinal recordings were made in several experiments at various depths (300 μm intervals), in three to four parallel tracks separated by 400 μm. At each depth the amplitude of the averaged evoked monosynaptic intraspinal field potential was measured 2 ms after the onset of the afferent volley, while the amplitude of the EFP was measured 2 ms after the maximum of the averaged spontaneous nCDP. These values were used to construct a matrix to calculate with SigmaPlot v. 4.1 the isopotential contour lines. This program uses an inverse distance method to generate interpolated Z values for an evenly spaced XY grid from XYZ triplet data. The isopotential contours for the spontaneous and the evoked potentials were superposed on the metric plane of the dorsal horn derived from histological reconstruction (see Willis et al. 1973).
Spectral analysis
Spectral analysis of 1.5 min recordings of cord dorsum potentials obtained in the absence of purposeful stimulation of sensory nerves was made in eight experiments, using the fast Fourier transform of the Chaos Data Analyser program of the American Insitute of Physics Academic Software. Data were acquired with a sampling rate of 250 Hz. The same program was used for autocorrelation analysis of cord dorsum potentials.
Histology
At the end of the experiment each animal was killed with a pentobarbitone overdose and perfused with 10 % formalin, and the spinal cord removed while leaving the recording micropipette in place. After complete fixation and dehydration, the lumbosacral segments were placed in a solution of methyl salicylate for clearing and subsequently cut transversally to obtain a section containing the electrode, and photographed.
RESULTS
General features of the spontaneous nCDPs
As already described by Gasteiger & Ichikawa (1963), the spontaneous activity that is recorded from the cord dorsum of the anaesthetised cat comprises negative potentials of variable amplitude and duration (from 50 to 150 μV and from 25 to 50 ms, respectively), and this has been confirmed in the present series of observations (Fig. 1A).
Figure 1. Power spectra of the spontaneous cord dorsum potentials.
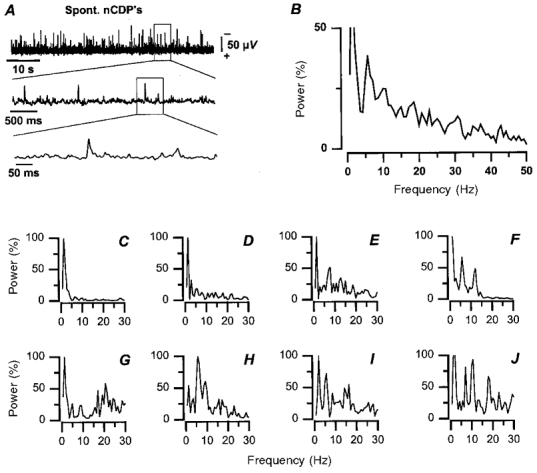
A, recordings of spinal cord dorsum potential at the L6 segmental level with different time bases, as indicated. Negativity upward. B, power spectrum obtained by averaging the power spectra of the 8 experiments illustrated in C-J. Abscissa, frequency. Ordinates, power as percentage of the maximum attained in each experiment (100 %). Power spectra computed from continuous recordings lasting 1.5 min.
Spectral analysis of the cord dorsum potentials has revealed that some frequencies occur more often than others. In most (7/8) experiments the dominant frequencies were between 1 and 2 Hz (Fig. 1C-J). In most experiments there were also higher-frequency components, some in the range 5–7 Hz (Fig. 1E, F and H-J) and others in the range 10–12 Hz (Fig. 1F and J).
Averaging the spectra obtained from eight experiments indicates that the dominant frequencies varied between 1 and 10 Hz (Fig. 1B), in agreement with data published by Gasteiger & Ichikawa (1963). The present analysis on the location and functional organisation of the spinal neurones involved in the generation of spontaneous nCDPs was restricted to the analysis of potentials occurring in the low-frequency range (<2 Hz), because of the conditions imposed by the stimulation protocol (see Methods). Yet, it is clear from traces such as those of Fig. 1A that there were no cyclic changes that could be attributed to episodes of fictive locomotion. In fact, autocorrelation analysis provided no evidence of such rhythmic activity in seven experiments.
Intraspinal location of neurones producing the spontaneous nCDPs
To investigate the location of the neurones involved in the generation of the spontaneous nCDPs, simultaneous recordings were made from the cord dorsum and intraspinally at different depths. A window discriminator was used to generate a pulse each time the spontaneous nCDP exceeded a fixed value and was not preceded by another event for at least 2 s (Fig. 2A). This pulse was used to trigger the averaging of the CDPs and of the simultaneously recorded EFPs.
Figure 2. Intraspinal distribution of spontaneous and cutaneous-evoked potentials.
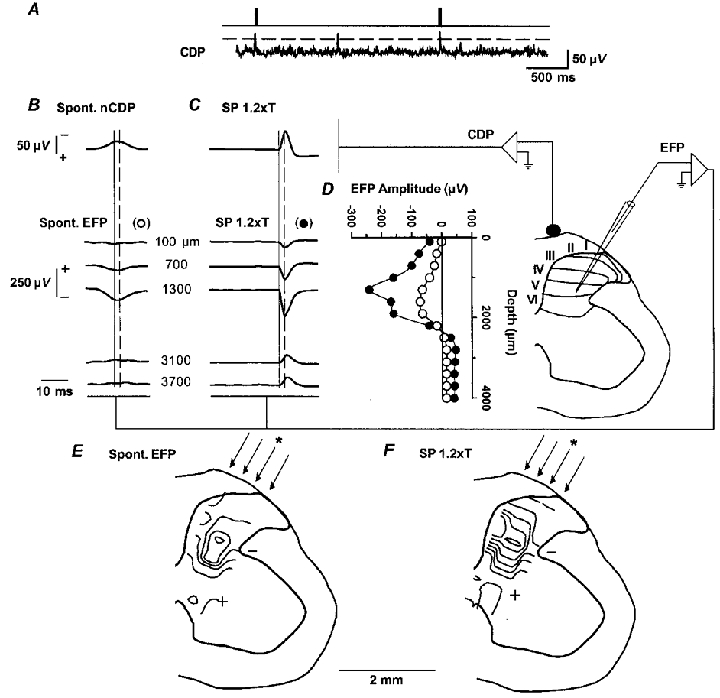
A, sample of spontaneous cord dorsum potentials recorded from the surface at the L6 lumbar segment. The upper trace shows the pulses generated by the window discriminator when the spontaneous nCDPs exceeded a prefixed level (shown by the interrupted horizontal line). These pulses were used to generate trigger pulses (upper trace) for the averaging procedure. B, averages of spontaneous nCDPs and corresponding extracellular field potentials (EFPs) recorded at various intraspinal depths, as indicated. C, same, but CDPs and intraspinal field potentials were produced by SP nerve stimulation (single pulses of 1.2T at 0.5 Hz). Interrupted vertical lines in B and C indicate time of amplitude measurements, that were made 2 ms after the peak of the spontaneous nCDP or after the arrival of the afferent volley, respectively. D, plot of amplitude of averaged spontaneous (^) and SP-evoked EFPs (•) recorded at different intraspinal depths derived from the records partly illustrated in B and C. E and F, isopotential contours of spontaneous and SP-evoked EFPs obtained in the same experiment from a series of 4 parallel tracks, as indicated by the arrows. Micropipette position while recording EFPs in B and C is indicated by asterisks. Isopotential fields have been superposed on a transverse section of the L6 spinal segment. In this and other figures, cord dorsum potentials, negativity upward. EFPs, positivity upward.
The upper trace in Fig. 2B shows the average of the spontaneous nCDPs obtained with this procedure, and the traces below show the averages of the spontaneous EFPs, recorded at different intraspinal depths. The open circles in Fig. 2D show the amplitude changes of these potentials measured 2 ms after the peak of the spontaneous nCDP, as indicated by the vertical lines in Fig. 2B. These potentials were negative in the dorsal-most regions, acquired their maximal amplitude between 1200 and 1700 μm depth and reverted to a slight positivity in more ventral regions of the spinal cord. A similar distribution within the dorsal horn of the spontaneous field potentials was obtained in nine experiments (Fig. 3A and B). It thus seems that these potentials were generated within the dorsal horn, in the same region where the largest cutaneous afferents terminate (see Willis et al. 1973; Willis & Coggeshall, 1991).
Figure 3. Intraspinal distribution of field potentials produced during the spontaneous nCDPs and by stimulation of cutaneous nerves.
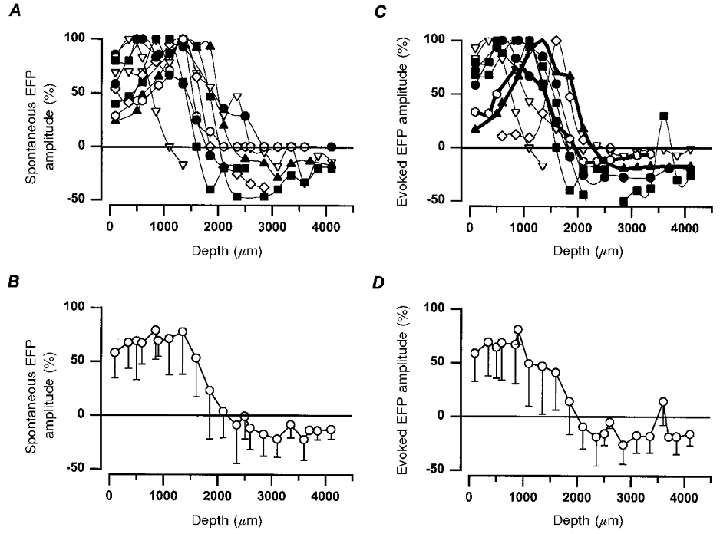
A, field potentials associated with spontaneous nCDPs. Ordinate, percentage amplitude (relative to maximum) of field potentials measured 2 ms after the peak of the spontaneous nCDPs. Abscissa, depth from cord surface at the L6 segmental level. Data from 9 different experiments. B, mean and s.d. of the data depicted in A. C and D, the same, but intraspinal distribution of the field potentials produced by stimulation of the SU nerve (7 experiments) and SP (2 experiments, thick lines) with pulses of 1.2T. Amplitude measurements were made 2 ms after the initiation of the N-wave in the cord dorsum. In A and C data from the same experiment are indicated with the same symbol.
To further test this assumption, we recorded, in the same experiment, the intraspinal field potentials produced by stimulation of the SP nerve with pulses of 1.2T (Fig. 2C). The postsynaptic component of the SP-evoked EFP, measured 2 ms after the onset of the afferent volley (vertical lines in Fig. 2C), had a similar intraspinal distribution to the spontaneous EFP. It was also negative in the dorsal-most regions, became maximal at a depth between 1200 and 1700 μm, and reverted to positivity in more ventral regions (see filled symbols in Figs 2D and 3C and D).
In three experiments we recorded the spontaneous and evoked potentials in three to four parallel microelectrode tracks, separated by 400 μm. Figure 2E displays the isopotential maps derived from the EFPs associated with the spontaneous nCDPs, and Fig. 2F, the contour maps obtained from the SP-evoked EFPs. These data were obtained from the same experiment as that of Fig. 2B-D. It may be seen that in both cases the largest negativity, indicating the most active regions (see Willis et al. 1973), was confined to layers III-VI. However, the data depicted in Fig. 3 suggest that neurones in more dorsal layers may also contribute to the generation of the spontaneous nCDPs (see Discussion).
Synaptic interaction between neurones producing spontaneous nCDPs and neurones activated by stimulation of cutaneous afferents
The finding that the neurones involved in the generation of spontaneous nCDPs had a similar intraspinal location to neurones monosynaptically activated by cutaneous afferents raised the question of whether the two sets belong to the same or to different populations. This question was approached by examining the interaction between activity in both sets of neurones by testing the effect of the spontaneous activation of dorsal horn neurones on the cord dorsum and intraspinal postsynaptic field potentials evoked by stimulation of low-threshold cutaneous afferents. The spontaneous nCDPs were used to trigger, with a variable delay, a single pulse that was used to stimulate a cutaneous nerve (see Methods).
The upper traces in Fig. 4A-C show the averages of the cord dorsum potentials, and the lower traces the averages of the extracellular field potentials recorded at a depth of 1300 μm within the dorsal horn. The negative potential recorded from the cord dorsum (N-wave), as well as the EFP produced by stimulation of low-threshold SU afferents (1.15T) are due to synaptic activation of dorsal horn neurones (Eccles et al. 1963b; Willis et al. 1973; Willis & Coggeshall, 1991). The positive potential (P-wave) that follows the N-wave after stimulation of cutaneous nerves is generated, at least in part, by the intraspinal current flows involved in the generation of PAD of cutaneous and Ib afferents (Eccles et al. 1963a,b).
Figure 4. Facilitation of intraspinal postsynaptic field potentials produced by low-threshold cutaneous afferents during the occurrence of spontaneous nCDPs.
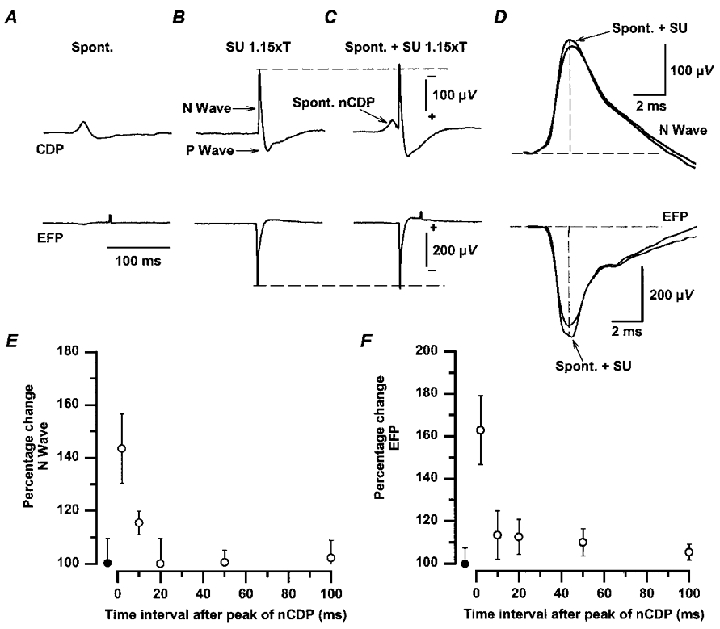
A-C, upper traces, averages of CDPs recorded from the L6 lumbar segment. Lower traces, averages of EFPs recorded at 1300 μm depth in the same segment. A, spontaneous potentials (Spont.). B, responses produced by stimulation of the SU nerve with single pulses of 1.15T. C, SU-evoked responses preceded by spontaneous nCDPs (arrow). D, superimposed control and conditioned SU-evoked CDPs and EFPs (spont. + SU) with expanded time base. Conditioned responses in D were obtained after subtraction of the spontaneous potentials shown in A. Amplitude of evoked CDPs and of EFPs was measured at a fixed time after the initiation of the N-wave (2 ms) as indicated by the vertical lines in D. E and F, mean time course of the effects produced during the occurrence of spontaneous nCDPs on the amplitude of the N-waves and of the EFPs evoked by SU stimulation (single pulses of 1.1–1.2T) in 3 experiments. Ordinates, percentage changes relative to control. Abscissa, time interval between the peak of the ‘conditioning’ spontaneous nCDP and the application of the test stimulus. •, control responses. Vertical bars, s.d. Further explanations in text.
The SU-evoked N-waves and the corresponding EFPs were facilitated when preceded by a spontaneous nCDP (Fig. 4C and D). Facilitation was largest at the peak of the spontaneous nCDP, and was practically over at 100 ms, as illustrated in Fig. 4E and F, which shows the means and standard deviations of the effects obtained in three experiments with SU nerve stimulation in the range 1.1–1.2T.
Effects of spontaneously activated dorsal horn neurones on pathways producing PAD of cutaneous afferents
The P-waves produced by stimulation of the SU nerve were also increased during the occurrence of the spontaneous nCDPs (upper traces in Figs 4B and C and 5B and C), probably because of facilitation of impulse transmission along the pathways mediating PAD of cutaneous afferents. To test this possibility further, we examined the changes in the cutaneously evoked DRPs recorded from a fine dorsal rootlet in the same (L6) segment during the occurrence of the spontaneous nCDPs.
Figure 5. DRPs and P-waves evoked by stimulation of low-threshold cutaneous afferents are facilitated during the occurrence of spontaneous nCDPs.
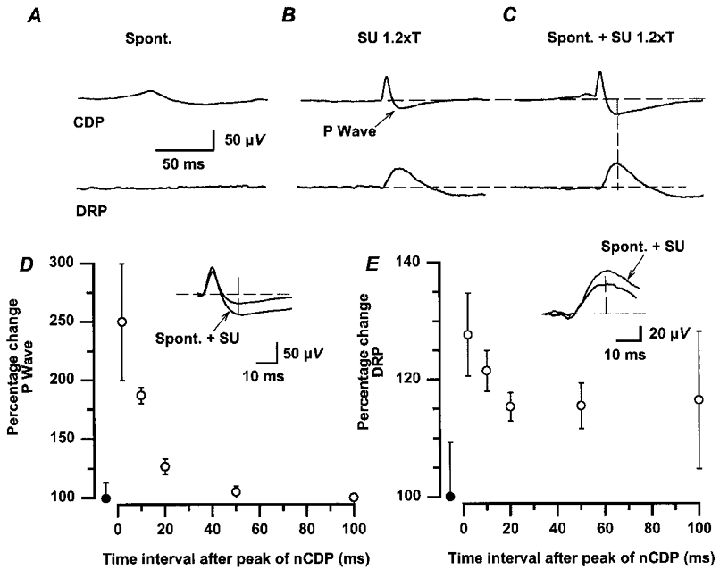
Same experimental protocol as that of Fig. 4. A, averaged spontaneous nCDPs and DRPs. B, CDPs and DRPs evoked by stimulation of the SU nerve (single pulses, 1.2T). C, SU-evoked potentials preceded at a constant interval by spontaneous nCDPs. Insets in D and E show, with faster time base, superimposed traces of the control and facilitated P-waves and DRPs (spont. + SU) illustrated in B and C. Amplitude of responses was measured at a fixed time after the initiation of the N-wave (20 ms) as indicated by the vertical lines in C. D and E, mean time course of change in P-waves and DRPs obtained in 6 experiments. Ordinates, percentage changes relative to control. Abscissa, time interval between the peak of the ‘conditioning’ spontaneous nCDP and the application of the test stimulus. •, control responses. Vertical bars, s.d.
In the example of Fig. 5, the averaged spontaneous nCDP was followed by a small positive component that was not associated with a spontaneous DRP (Fig. 5A). Stimulation of the SU nerve, with pulses of 1.2T, produced in the cord dorsum an N-wave followed by a P-wave, and a small DRP (of about 50 μV; Fig. 5B). Both the SU-evoked P-waves and the DRPs were facilitated when preceded by spontaneous nCDPs (Fig. 5C; see insets in Fig. 5D and E). The facilitation of the P-waves and of the DRPs was also largest at the peak of the ‘conditioning’ spontaneous nCDPs and decayed slowly thereafter. Unlike the facilitation of the P-wave, some facilitation of the DRPs was still observed 50–100 ms after the maximum of the spontaneous nCDPs (Fig. 5D and E). It thus seems that the neurones producing the spontaneous nCDPs have facilitatory actions on the interneurones mediating the PAD of cutaneous afferents, and possibly also of Ib afferents (see below), thus resembling the actions produced by electrical stimulation of low-threshold cutaneous afferents (see Eccles et al. 1963a,b; Rudomin et al. 1986).
Effects of spontaneous activity of dorsal horn neurones on DRPs produced by stimulation of group I muscle afferents
In five experiments we examined the effects of the spontaneously active sets of dorsal horn neurones on the pathways mediating PAD of Ia and Ib afferents. Figure 6 illustrates the results obtained in one of them. In this experiment occurrence of spontaneous nCDPs appeared associated with a rather small DRP (Fig. 6A). Stimulation of the PBSt nerve with a train of 3 pulses of 1.15T strength, activating Ia afferents only (see inset in Fig. 6B), produced a small DRP as well as a P-wave in the cord dorsum (Fig. 6B). Both of them were depressed when preceded by spontaneous nCDPs (Fig. 6C and D).
Figure 6. Changes in dorsal root potentials evoked by stimulation of group I muscle afferents during the occurrence of spontaneous nCDPs.
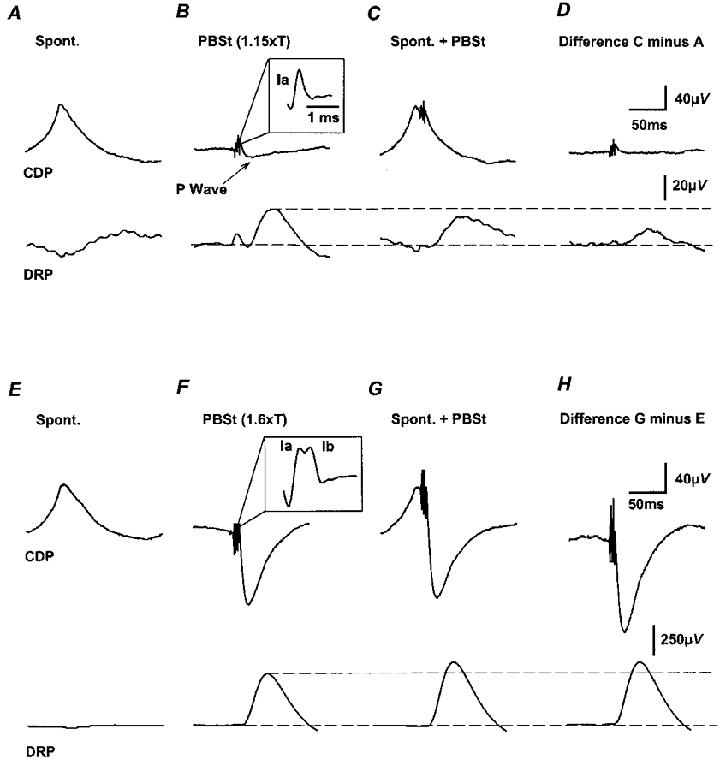
A-D, inhibition of DRPs evoked by stimulation of Ia PBSt afferents. Upper traces, cord dorsum potentials. Lower traces, DRPs. A, DRPs generated in synchrony with the spontaneous nCDPs. B, DRPs produced by stimulation of the PBSt nerve with a train of 3 pulses of 300 Hz and 1.15T. Inset shows the last Ia volley in the train with an expanded time base. C, PBSt-induced DRPs preceded by the spontaneous nCDPs. D, difference between traces C and A, showing depression of the Ia-evoked P-waves and DRPs. E-H, the same as A-D but PBSt stimulus intensity was increased to 1.6T to activate Ia and Ib afferents, as shown by the inset in F. Note facilitation of the P-wave and of the DRPs.
In contrast, the DRPs and P-waves produced with stronger stimuli applied to the PBSt nerve (1.6T), which activated both Ia and Ib afferents, were facilitated when preceded by the spontaneous nCDPs (Fig. 6E-H). Pooling data from five different experiments we observed that the P-waves and DRPs evoked by Ia volleys (1.1–1.4T) delivered at the peak of the spontaneous nCDPs were depressed to mean values of 69 ± 17 % (s.d.) and 38 ± 12 % of control (100 %), respectively. In contrast, the P-waves and DRPs produced by concurrent stimulation of Ia and Ib afferents with pulses of 1.4–1.7T, were increased to 160 ± 20 and 120 ± 5 % of control, respectively.
The differential effects of the spontaneous nCDPs on the P-waves and DRPs produced either by Ia or Ib muscle afferents resembled the actions produced by stimulation of low-threshold cutaneous afferents on these same pathways, as showed by Lund et al. (1965) (see also Rudomin et al. 1983, 1986; Enríquez et al. 1996).
Depression of MSRs produced by presynaptic inhibition is reversed during the spontaneous nCDPs
The observations depicted in Fig. 6A-D indicate that the PAD of group Ia afferents is reduced during the occurrence of the spontaneous nCDPs. It was therefore anticipated that during the appearance of the spontaneous nCDPs there would be a reduction of presynaptic inhibition of Ia fibres.
This possibility was examined in six experiments, and Fig. 7 illustrates the results obtained in one of them. Figure 7A, shows the averaged CDPs and monosynaptic reflexes, produced by three pulses of 1.5T strength applied to the GS nerve. Conditioning stimulation with a train of two pulses at 300 Hz, applied to the PBSt nerve before the test Ia-GS volley, depressed the monosynaptic reflex to 40 % of control (taken as 100 %; Fig. 7B). This depression, which has been attributed to presynaptic inhibition (Lund et al. 1965; Rudomin et al. 1974, 1983), was partially reversed by conditioning stimulation of the SU nerve (Fig. 7C). A similar reduction of the PBSt-induced inhibition of the GS monosynaptic reflexes was observed during the occurrence of the spontaneous nCDPs (Fig. 7D).
Figure 7. Presynaptic inhibition of monosynaptic reflexes is reduced during the occurrence of spontaneous nCDPs.
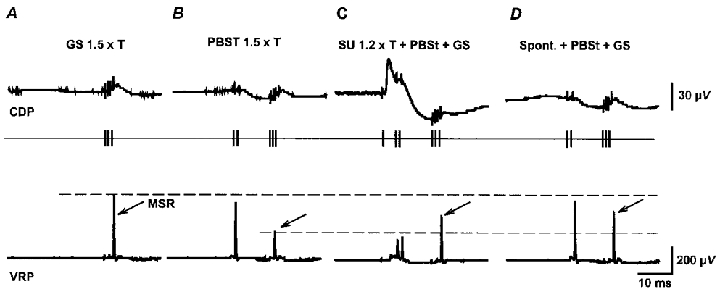
A, averaged CDPs (upper traces) and MSRs (lower traces) recorded from the L7 ventral root (VRP) after stimulation of the GS nerve (3 pulses, 1.5T). B, inhibition of MSRs by conditioning stimulation of the PBSt nerve (2 pulses, 1.5T) applied before the test GS volley. C, reduction of inhibition by additional stimulation of the SU nerve (single pulses of 1.2T). D, reduction of inhibition of MSRs during the occurrence of spontaneous nCDPs. Arrows indicate test GS monosynaptic reflexes.
These findings were confirmed in five other experiments. Conditioning stimulation of the PBSt nerve with strengths varying between 1.2 and 1.5T (as in Fig. 7B) depressed the MSRs to 45 ± 12 % of the amplitude of the unconditioned reflexes (control). The additional stimulation of the SU nerve with strengths of 1.2–2T (as in Fig. 7C) reduced the PBSt-induced inhibition of the MSRs, now of 77 ± 18 % of control. When the PBSt stimulus was preceded by spontaneous nCDPs (as in Fig. 7D) the inhibition was also reduced and the MSRs were 65 ± 18 % of control.
Interaction between the spontaneously activated dorsal horn neurones and monosynaptic reflexes
Gasteiger & Brust-Carmona (1964) and Molt & Gasteiger (1976) examined the possible relation between the amplitude variations of the spontaneous cord dorsum potentials and the fluctuations of monosynaptic reflexes, but found no evidence in this regard. On the other hand, our finding that during the spontaneous activity of the dorsal horn neurones there is a reduction of the PAD of group Ia afferents (Fig. 6A-D), as well as a partial reversion of the presynaptic inhibition of monosynaptic reflexes (Fig. 7D), suggests instead that changes in the spontaneous activity of the laminae III-VI neurones will induce correlated variations in the amplitude of the monosynaptic reflexes.
Figure 8A illustrates the facilitation of the GS monosynaptic reflexes tested during the occurrence of the spontaneous nCDPs. In this experiment the stimulating pulse was generated at a constant time interval after the peak of the spontaneous nCDPs (5 ms), as shown by the upper traces. The window discriminator was used in the ‘within mode’ to select series of spontaneous nCDPs with different mean amplitudes (six in this case). Separate averages were calculated for each series. It may be seen that the larger the amplitude of the ‘conditioning’ spontaneous nCDPs, the greater was the facilitation of the monosynaptic reflexes. This facilitation was maximal when the MSRs were evoked at the time of the peak of the spontaneous nCDP and decayed gradually thereafter, but was still detectable at a C-T interval of 30 ms (Fig. 8B).
Figure 8. Facilitation of monosynaptic reflexes during the occurrence of spontaneous nCDPs.
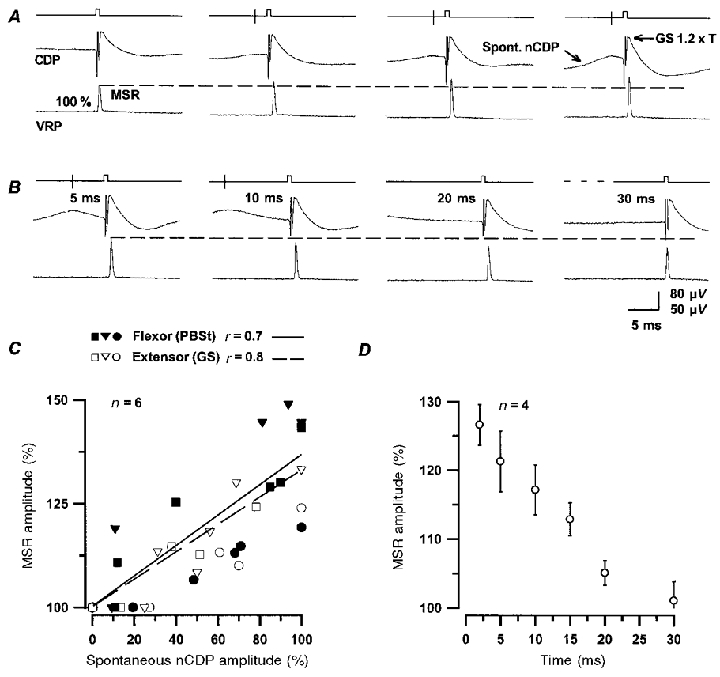
A, changes in GS monosynaptic reflexes preceded at a fixed time interval by spontaneous nCDPs of increasing amplitudes. First set of traces are reflexes that were not preceded by spontaneous nCDPs for at least 2 s (control). B, same, but MSRs were generated at different time intervals after the occurrence of spontaneous nCDPs of fixed amplitude, as indicated. In all cases the GS nerve was stimulated with pulses of 1.2T strength. Note facilitation of MSRs. C, facilitation of flexor (PBSt, filled symbols) and extensor (GS, open symbols) MSRs versus amplitude of the ‘conditioning’ spontaneous nCDPs in 6 experiments (indicated by different symbols). Abscissa, amplitude of the spontaneous nCDPs, expressed as a fraction of the largest averaged potential in each particular series (100 %). Ordinates, amplitude of MSRs as a percentage of control (100 %). Continuous and interrupted lines, best linear fits for flexors and extensors, respectively. Coefficients of correlation (r) are shown above the panel. D, time course of facilitation of GS MSRs produced during the occurrence of the spontaneous nCDPs. Data taken from 4 different experiments. Abscissa, time relative to the peak of the ‘conditioning’ spontaneous nCDPs. Vertical bars, s.d. Trigger signal (vertical line) and the stimulating pulse are illustrated by the upper traces in A and B.
Figure 8C shows data obtained from six different experiments where we examined the relation between the amplitude of the ‘conditioning’ spontaneous nCDPs, expressed as a percentage of the largest nCDPs recorded in each series, and the amplitude of the MSRs, as indicated. In all cases the magnitude of the facilitation of the MSRs, both of flexors and extensors, increased with increasing sizes of the ‘conditioning’ spontaneous nCDPs. The time course of the facilitation of the MSRs resembled that of the falling phase of the spontaneous nCDPs during the first 25–30 ms (Fig. 8D).
Influence of spontaneous activity of dorsal horn neurones on the variability of monosynaptic reflexes
Activation of the Ia afferents at a constant frequency, as done when studying MSRs (Rudomin & Dutton, 1969a; Gossard et al. 1994), will occur in relation to spontaneous nCDPs of variable amplitudes. It is therefore possible that the fluctuations of the MSRs observed in these studies were introduced by variations in the spontaneous activity of dorsal horn neurones.
Figure 9A shows superimposed traces of the CDPs and of the MSRs evoked by PBSt stimulation at a constant frequency (0.5 Hz). Note that the stimulus to the PBSt nerve (two pulses of 1.7T) was applied during spontaneous nCDPs of variable amplitudes, and that the MSRs elicited under these conditions showed considerable amplitude fluctuations (see expanded traces in Fig. 9B). The graph in Fig. 9B shows the mean amplitude of the MSRs produced by stimulation of the PBSt nerve with varying strengths and the filled circles in Fig. 9C and F show their respective variances and coefficients of variation (standard deviation divided by mean), plotted against the mean amplitude of the MSRs. Since previous studies have indicated that the variance versus MSR amplitude has a parabolic shape (Rudomin & Dutton, 1969a), we have also drawn in Fig. 9C the best parabolic fit.
Figure 9. Changes in mean and variance of monosynaptic reflexes during the occurrence of spontaneous nCDPs.
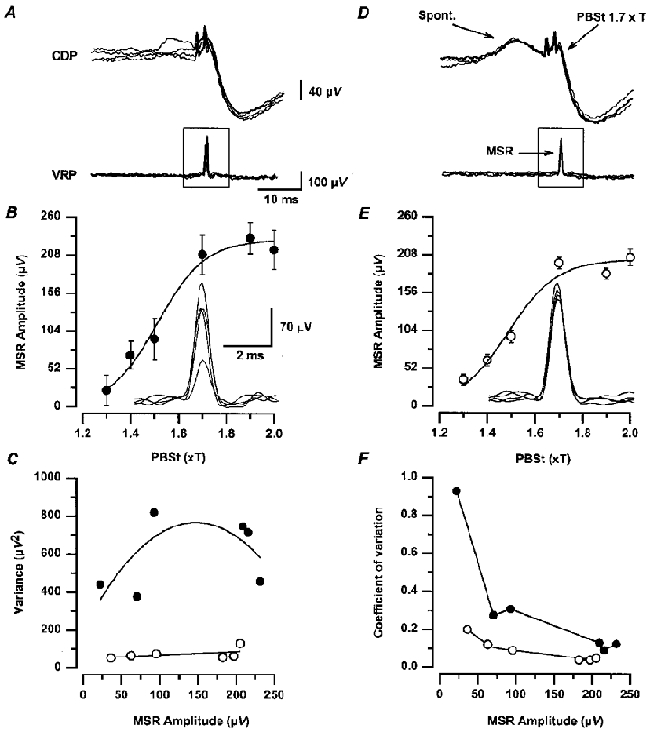
A, 5 superposed recordings of cord dorsum (CDP) and ventral root potentials (VRP) produced by PBSt nerve stimulation with 2 pulses of 1.7T delivered at 0.5 Hz. Note monosynaptic reflexes in VRP recordings. D, the same, but nerve stimuli were applied at a fixed time interval after the peak of spontaneous nCDPs of the same size (see upper traces). Insets in B and E show the MSRs illustrated in A and D, with higher amplification and faster time base. B and E, graphs of mean and s.d. of MSRs produced by PBSt stimuli of different strengths, evoked as in A and D, respectively. C and F, variance and coefficient of variation versus mean amplitude of the series of MSRs illustrated in B and E. •, MSRs produced at a constant frequency (0.5 Hz) that were preceded by spontaneous nCDPs of different amplitudes as in A. ^, MSRs preceded by spontaneous nCDPs of a fixed amplitude as in D. Further explanations in text.
We compared the MSRs preceded by spontaneous nCDPs of variable amplitudes with those reflexes preceded by spontaneous nCDPs of the same amplitude, as shown in Fig. 9D. Under these conditions the MSRs had a significantly smaller variability (see inset of Fig. 9E), throughout the whole amplitude range (Fig. 9C and F, open circles). Similar results were obtained in two other experiments. It thus seems likely that the variability of MSRs analysed in previous studies (Rudomin & Dutton, 1967, 1969a; Gossard et al. 1994) was to a great extent due to fluctuations in the spontaneous activity of neurones located in the dorsal horn.
Stimulation of cutaneous afferents transiently reduces the probability of occurrence of spontaneous nCDPs
Previous studies have indicated that stimulation of muscle and cutaneous nerves reduces the excitability fluctuations of Ia afferents and the variability of MSRs with a time course that resembles that of PAD and of presynaptic inhibition (Rudomin & Dutton, 1967, 1969a,b; Rudomin et al. 1969). In view of the results described in the previous section, it seemed possible that these effects were due to a transient reduction of the activity of the dorsal horn neurones involved in the generation of the spontaneous nCDPs. To this end we examined the changes in spontaneous nCDPs that followed stimulation of low-threshold cutaneous afferents.
Figure 10 illustrates data obtained in one of three experiments where this question was examined. Figure 10A shows the nCDPs generated in the absence of any purposeful stimulation. The raster display was constructed by selection of the spontaneous nCDPs exceeding a predetermined amplitude (as in Fig. 2A). The histogram was obtained by adding data from 91 sweeps. Figure 10B and C shows the changes produced by stimulation of the SU and SP nerves, respectively, with single pulses of 1.2T strength. It may be seen that following these stimuli, the probability of occurrence of spontaneous nCDPs was decreased for 100–150 ms, which is precisely the time during which stimulation of cutaneous nerves reduces the variance of the MSRs (Rudomin & Dutton, 1969a; Rudomin et al. 1969, 1975). It thus seems reasonable to propose that intermittent activity of the neurones producing spontaneous nCDPs determines, to a great extent, the variability of the MSRs. Stimulation of cutaneous nerves will decrease the spontaneous activity of these neurones and also the fluctuations of the MSRs.
Figure 10. The probability of occurrence of spontaneous nCDPs is transiently reduced after stimulation of low-threshold cutaneous afferents.
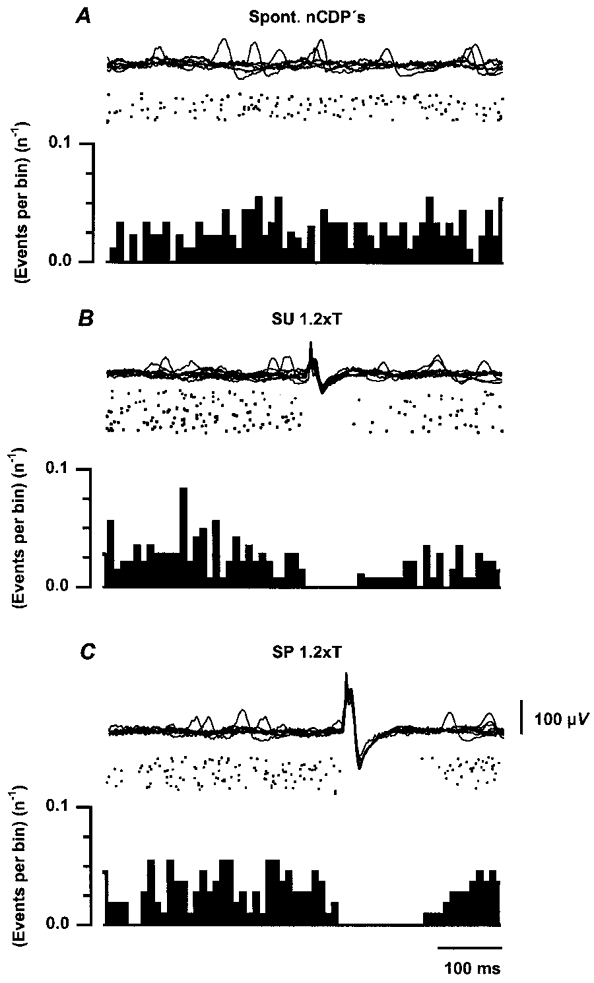
A, from above downwards, superimposed traces of cord dorsum potentials, raster displays and histograms showing occurrence of spontaneous nCDPs. Ordinates in histograms are events per bin divided by the number of sweeps (n). B and C, same but during stimulation of the SU and SP nerves with single pulses of 1.2T. Number of sweeps was 91, 145 and 111 in A, B and C, respectively. Further explanations in text.
DISCUSSION
Origin of the spontaneous nCDPs
The present study has indicated that in the lumbar L6-L7 dorsal horn of the anaesthetised cat, there is a set of neurones which are sporadically active and produce the spontaneous nCDPs. These neurones appear to be located in the same region as neurones receiving monosynaptic inputs from low-threshold cutaneous afferents. As shown in Fig. 2, this region corresponds to laminae III-VI, in confirmation of electrophysiological (Eccles et al. 1963b; Tapper et al. 1973; Brown et al. 1973; Willis & Coggeshall, 1991) and anatomical studies (Brown, 1981).
Our data also indicate that the changes produced in various spinal reflex pathways during the generation of the spontaneous nCDPs are similar to those produced by electrical stimulation of low-threshold cutaneous afferents (Figs 4–6). This suggests very strongly that the dorsal horn neurones that produce the spontaneous nCDPs are the same as those neurones which respond to stimulation of low-threshold cutaneous afferents.
This proposal agrees well with the findings of Tapper et al. (1973) and Brown et al. (1973) in the decerebrated spinal cat, who found that most of the dorsal horn neurones in laminae III-VI responding mono- or oligosynaptically to hair stimulation in the hindlimb were also spontaneously active. Further evidence supporting this proposal arises from the finding that during the occurrence of the spontaneous nCDPs, there is a concurrent, highly synchronised increase in the activity of single interneurones located in laminae III-VI that respond mono- or oligosynaptically to stimulation of low-threshold cutaneous afferents (Brown et al. 1973; Manjarrez et al. 1996, 1997, 1998).
The above results are also in agreement with those reported by Wall & Lidierth (1997), Lidierth & Wall (1998) and Eblen-Zajjur & Sandkuhler (1997) in the spinal cord of the anaesthetised rat. Wall & Lidierth (1997) and Lidierth & Wall (1998) showed, by means of spike-triggered averaging, that the discharge of cells as deep as lamina III and a few cells even deeper is correlated to the DRP, while Eblen-Zajjur & Sandkuhler (1997) have shown in addition that neurones in layers III-VI are also spontaneously active. It thus seems that there are several functionally distinct groups of spontaneously active neurones whose activity can be correlated with DRPs and CDPs. This would explain the different profiles observed in the power spectra of the CDPs recorded from individual experiments (Fig. 1C-J). As mentioned in Results, the experimental protocol used in the present study allowed the location and functional connections of the spinal neurones responsible for the largest spontaneous nCDPs generated with frequencies below 2 Hz. The present approach is now being used to disclose the connectivity of the interneuronal populations contributing to the higher-frequency components of the cord dorsum potentials.
Sources of variability of monosynaptic reflexes
Rudomin & Dutton (1969a) made a thorough analysis of the fluctuations of the MSRs produced by constant afferent volleys. They found that these fluctuations were reduced by conditioning stimulation of muscle and cutaneous nerves with a time course resembling that of PAD and presynaptic inhibition. They also found that the excitability of Ia terminals in the spinal cord had considerable fluctuations that were reduced by the same conditioning stimuli that reduced the fluctuations of the MSRs (Rudomin & Dutton, 1969b). This led to the proposal that the fluctuations of the MSRs were mostly of presynaptic origin (see also Rudomin et al. 1975). More recently, Gossard et al. (1994) have shown that fluctuations of postsynaptic origin also contribute to the variability of the MSRs.
Rudomin et al. (1969) showed in addition that the fluctuations of the MSRs of the same or of synergistic motor pools were highly correlated and suggested that this correlation was due to the synchronised activity of interneurones acting on the pathways leading to PAD of Ia afferents (see also Rudomin & Madrid, 1972; Rudomin et al. 1987).
Our observations now indicate that in the anaesthetised cat with the intact neuroaxis, the fluctuations of the MSRs are introduced, at least in part, by the spontaneous activity of the ensemble of dorsal horn neurones that generates the spontaneous nCDPs (Fig. 11). These interneurones appear to have inhibitory actions on the pathways that produce PAD and presynaptic inhibition of Ia afferents (Figs 6A-D and 7A, B and D) and excitatory actions (probably monosynaptic) on motoneurones (see Rosenberg, 1970; Pinter et al. 1982; Fleshman et al. 1988), as illustrated in Fig. 11, based on information derived from previous studies (see Rudomin et al. 1983).
Figure 11. Diagram of possible connections of dorsal horn neurones with segmental pathways producing PAD of muscle and cutaneous afferents.
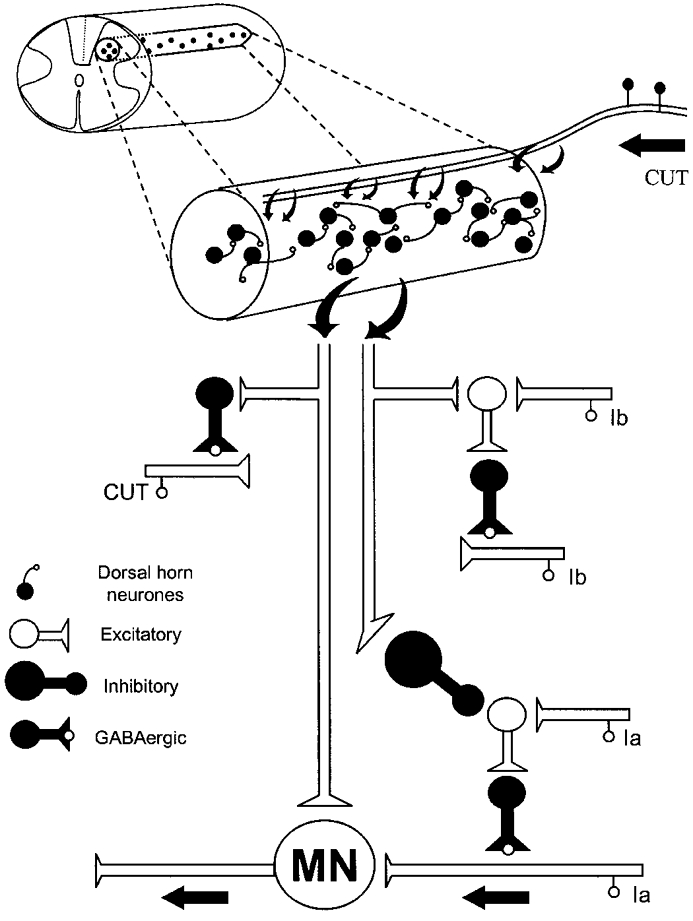
The set of spontaneously active dorsal horn neurones is represented as a fine cylinder extending rostro-caudally for at least one spinal segment. These neurones receive monosynaptic connections from low-threshold cutaneous afferents and have mutual excitatory (mono- or oligosynaptic) connections. They also have excitatory connections with spinal motoneurones (MN) and with the pathways producing PAD of cutaneous (CUT) and Ib afferents, as well as inhibitory connections with the first-order intermediate nucleus interneurones mediating the PAD of Ia afferents (see Rudomin et al. 1983). Further explanations in text.
The inability of Gasteiger & Brust-Carmona (1964) and of Molt & Gasteiger (1976) to disclose the correlation between the fluctuations of the spontaneous nCDPs and the fluctuations of the MSRs appears to be due to the approach used by them to examine this issue. Namely, they examined the possible correlation between MSRs that were preceded by spontaneous nCDPs larger that 125 μV and the MSRs produced by randomly applied stimuli. In contrast, our observations were made by measuring the relation between spontaneous nCDPs of different amplitudes and the amplitude of the MSRs evoked at a constant C-T time interval.
Our results further indicate that electrical stimulation of cutaneous nerves transiently reduces the activity of the dorsal horn neurones involved in the generation of the spontaneous nCDPs (Fig. 10). This is what should be expected on the basis of the observations of Brown et al. (1973), who found that stimulation of some spots in the hairy skin of the cat hindlimb may produce monosynaptic excitation in a given dorsal horn neurone, while stimulation of other spots may produce inhibition or excitation followed by inhibition in the same dorsal horn neurone. It then follows that electrical stimulation of low-threshold afferents in a cutaneous nerve will produce excitation followed by inhibition that leads to a transient reduction of the spontaneous activity of the dorsal horn neurones and also of the spontaneous nCDPs (Fig. 10). This will in turn reduce the fluctuations of the MSRs (Fig. 9).
Some functional implications
The present investigation documents the existence of a set of dorsal horn neurones that are spontaneously active in the anaesthetised cat. As shown by Manjarrez et al. (1997, 1998), simultaneous recording from pairs of interneurones in these laminae has further indicated that during the spontaneous nCDPs, these interneurones fire in a highly synchronised manner (see also Eblen-Zajjur & Sandkuhler, 1997). Synchronisation of the spontaneous activity of the dorsal horn neurones could result from activity in common sensory, propriospinal or descending inputs, or because of mutual excitatory connections between individual dorsal horn neurones in the ensemble (Brown et al. 1973, 1979).
During the occurrence of the spontaneous nCDPs, there is a concomitant facilitation of the monosynaptic responses of laminae III-VI neurones. This suggests that firing of any neurone in this ensemble, either spontaneously because of intrinsic membrane properties, or synaptically induced by peripheral or central sources, will recruit other neurones in the population. This will produce a burst of activity that will be probably curtailed by coactivation of inhibitory pathways (see also Brown et al. 1973, 1979; Manjarrez et al. 1996, 1997).
It is very tempting to propose that the spontaneously active synchronising system of dorsal horn neurones is geared to provide a basal level of activity in sensory and motor pathways, which can be adjusted by segmental, propriospinal and descending inputs received by the ensemble (see Gibbs et al. 1995). In this context, a relevant question would be the extent to which this synchronising system is activated during the execution of specific sensory and motor tasks in intact, non-anaesthetised behaving animals, and whether or not it can be fractionated in several subsets, each controlling restricted sensory and motor domains.
The information available to answer the first question is rather limited. In his study of spinal dorsal horn neurones in intact non-anaesthetised, behaving cats, Collins (1987) found that low-threshold responsive neurones in laminae III-VI had significantly lower spontaneous firing rates than low-threshold neurones in the acute, barbiturate-anaesthetised preparations, but was increased by mechanical stimulation of the neurones’ receptive fields in the skin. Since no simultaneous recordings of pairs of dorsal horn neurones have been made in the behaving cat, either in the absence or during intentional skin stimulation, it is not clear if the spontaneous activity of the different neurones was correlated, and how this correlation was modified during walking or other motor tasks, where, at least in humans, there is an increased correlation between the EMG activity of functionally related muscles (see Gibbs et al. 1995).
Pertaining to the second question, we have found (Manjarrez et al. 1997, 1998) that in the barbiturate-anaesthetised cat, the spontaneous nCDPs recorded at one site in the lumbar spinal cord are positively correlated with spontaneous nCDPs recorded in neighbouring sites. As distance between the two recording sites is increased, there is a drop in the correlation between the spontaneous nCDP, but significant correlations are still observed one segment rostral and one segment caudal from the reference recording site (placed at L6). Based on the observations of Collins (1987), it is tempting to suggest that in the non-anaesthetised behaving cat, the correlated activity between pairs of dorsal horn neurones with overlapping receptive fields will have a more restricted rostro-caudal distribution, and probably a higher local influence on the spinal reflex pathways. A more detailed knowledge of the interconnections between the different functional groups of neurones in the dorsal horn, including those with ascending projections via the spino-cervical tract (Bryan et al. 1973), and their connections with segmental and supraspinal neuronal ensembles (see Brown et al. 1981; Willis & Coggeshall, 1991), seems essential for a more complete understanding of the role of this subset of spontaneously active neurones in sensory discrimination and motor control.
Disclosure of synaptic connections of class II neurones with afferent fibres and motoneurones
The present study has indicated that the neuronal ensembles involved in the generation of the spontaneous nCDPs produce synchronous activation of interneurones mediating PAD of cutaneous and tendon organ afferents, as well as synchronous inhibition of the interneurones mediating the PAD of muscle spindle afferents. This implies that demonstration of short-latency dorsal root potentials which are time-locked to the activity of class II interneurones (assumed to mediate PAD of Ib afferents; see Rudomin et al. 1987), or of interneurones which are activated by group II muscle and by cutaneous afferents (Jankowska & Ridell, 1995) is a necessary, but not sufficient, condition to support the contention that these interneurones have mono- or oligosynaptic connections with the afferent fibres.
An additional requirement would be that the spike-triggered DRPs appear without the concurrent generation of spontaneous nCDPs. This requirement has been achieved for the glycinergic iVRPs produced by class I neurones, but not for the DRPs and iVRPs produced by class II neurones (Rudomin et al. 1987, 1990). This suggests that the set of dorsal horn neurones responsible of the spontaneous nCDPs is strongly coupled with class II interneurones. Attempts to overcome this coupling by direct activation of class II neurones, by means of glutamate diffusion through the recording electrode, have not been successful (see Rudomin et al. 1987). An alternative approach to disclose the synaptic connections of class II neurones with afferent fibres has been to directly activate these neurones, by means of electrical microstimulation, while measuring the resulting PAD in individual afferents (see Quevedo et al. 1997).
Acknowledgments
We thank Dr David McCrea for his critical comments of the manuscript and to A. Rivera and D. Chávez for technical assistance. This work was partly supported by NIH grant NS 09196, CONACyT 41739, 3908N and by Sistema Nacional de Investigadores, México. E.M. had support from CONACyT, FOMES-BUAP and from the National Polytechnic Institute.
References
- Bremer F. L'activitéélectrique ‘spontanée' de la moelle épiniere. Archives International Physiologie. 1941;51:51–84. [Google Scholar]
- Brown AG. Organization in the Spinal Cord. Anatomy and Physiology of Identified Neurones. Berlin Heidelberg: Springer-Verlag; 1981. [Google Scholar]
- Brown PB, Koerber HR, Yezierski RP. Cross-correlation analysis of connectivities among cat lumbosacral dorsal horn cells. Journal of Neurophysiology. 1979;42:1199–1211. doi: 10.1152/jn.1979.42.5.1199. [DOI] [PubMed] [Google Scholar]
- Brown PB, Moraff H, Tapper DN. Functional organisation of the cat's dorsal horn: spontaneous activity and central cell response to single impulses in single type I fibres. Journal of Neurophysiology. 1973;36:827–839. doi: 10.1152/jn.1973.36.5.827. [DOI] [PubMed] [Google Scholar]
- Bryan RN, Treviño DL, Coulter JD, Willis WD. Location and somatotopic organization of the cells of origin of the spino-cervical tract. Experimental Brain Research. 1973;17:177–189. doi: 10.1007/BF00235027. [DOI] [PubMed] [Google Scholar]
- Collins JG. A descriptive study of spinal dorsal horn neurones in the physiologically intact, awake, drug-free cat. Brain Research. 1987;416:34–42. doi: 10.1016/0006-8993(87)91493-4. [DOI] [PubMed] [Google Scholar]
- Eblen-Zajjur AA, Sandkuhler J. Synchronicity of nociceptive and non-nociceptive adjacent neurons in the spinal dorsal horn of the rat: stimulus-induced plasticity. Neuroscience. 1997;76:39–54. doi: 10.1016/s0306-4522(96)00286-2. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmit RF, Willis WD. Depolarization of the central terminals of group Ib afferent fibers of muscle. Journal of Neurophysiology. 1963a;26:1–27. doi: 10.1152/jn.1963.26.1.44. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis WD. Depolarization of the central terminals of cutaneous afferent fibers. Journal of Neurophysiology. 1963b;26:646–661. doi: 10.1152/jn.1963.26.1.44. [DOI] [PubMed] [Google Scholar]
- Enríquez M, Jiménez I, Rudomin P. Segmental and supraspinal control of synaptic effectiveness of functionally identified muscle afferents in the cat. Experimental Brain Research. 1996;107:391–404. doi: 10.1007/BF00230421. [DOI] [PubMed] [Google Scholar]
- Fleshman JW, Rudomin P, Burke RE. Supraspinal control of a short latency cutaneous pathway to hindlimb motoneurons. Experimental Brain Research. 1988;69:449–459. doi: 10.1007/BF00247299. [DOI] [PubMed] [Google Scholar]
- Gasteiger EL. Beyond homeostasis and cybernetics. A speculation from studies of spinal noise. Atti della Accademia Medica Lombarda. 1969;24:31–43. [Google Scholar]
- Gasteiger EL, Brust-Carmona H. On the relation of spinal reflex variability to internal noise. Transactions of the New York Academy of Sciences. 1964;26:688–696. doi: 10.1111/j.2164-0947.1964.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Gasteiger EL, Ichikawa S. The relation of the spinal electrogram of the cat to intrinsic and extrinsic factors. Boletín del Instituto de Estudios Médicos y Biológicos de México. 1963;21:223–234. [PubMed] [Google Scholar]
- Gibbs J, Harrison LM, Stephens JA. Organization of inputs to motoneurone pools in man. The Journal of Physiology. 1995;485:245–256. doi: 10.1113/jphysiol.1995.sp020727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard JP, Floeter MK, Kawai Y, Burke RE, Chang T, Schiff SJ. Fluctuations of excitability in the monosynaptic reflex pathway to lumbar motoneurons in the cat. Journal of Neurophysiology. 1994;72:1227–1239. doi: 10.1152/jn.1994.72.3.1227. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. The Journal of Physiology. 1985;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Ridell JS. Interneurons mediating presynaptic inhibition of group II muscle afferents in the cat spinal cord. The Journal of Physiology. 1995;483:473–479. doi: 10.1113/jphysiol.1995.sp020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzak H, Gasteiger EL. Spinal electrogram of freely moving cat: supraspinal and segmental influences. Brain Research. 1970;22:207–220. doi: 10.1016/0006-8993(70)90005-3. [DOI] [PubMed] [Google Scholar]
- Khan T, Myklebust J, Swiontek T, Sayers S, Dauzvardis M. Electrical field distribution within the injured spinal cord: injury potentials and field distribution. Journal of Neurotrauma. 1994;11:699–710. doi: 10.1089/neu.1994.11.699. [DOI] [PubMed] [Google Scholar]
- Lidierth M, Wall PD. Dorsal horn cells connected to the Lissauer tract and their relation to the dorsal root potential in the rat. Journal of Neurophysiology. 1998;80:667–679. doi: 10.1152/jn.1998.80.2.667. [DOI] [PubMed] [Google Scholar]
- Lund S, Lundberg A, Vyklicky L. Inhibitory action from flexor reflex afferents on transmission to Ia afferents. Acta Physiologica Scandinavica. 1965;64:345–355. doi: 10.1111/j.1748-1716.1965.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Manjarrez E, Jiménez I, Rudomin P. Functional organization of neuronal ensembles associated with spontaneous cord dorsum potentials in the cat spinal cord. Society for Neuroscience Abstracts. 1996;340:1. [Google Scholar]
- Manjarrez E, Jiménez I, Rudomin P. Functional relationships between the neuronal ensembles generating spontaneous negative cord dorsum potentials and single dorsal horn interneurons. Society for Neuroscience Abstracts. 1997;912:13. [Google Scholar]
- Manjarrez E, Jiménez I, Sánchez J, Rudomin P. Lumbar spontaneous cord dorsum potentials are originated by synchronized activity of dorsal horn neurons. Society for Neuroscience Abstracts. 1998;453:11. [Google Scholar]
- Mark VH, Gasteiger EL. Observations on the role of afferent and descending impulses on the spontaneous potentials of the spinal cord. Electroencephalography and Clinical Neurophysiology. 1953;5:251–258. doi: 10.1016/0013-4694(53)90012-1. [DOI] [PubMed] [Google Scholar]
- Molt JT, Gasteiger EL. Variability in spinal reflexes: a correlation with spontaneous slow wave activity in cat spinal cord. Experimental Neurology. 1976;52:132–145. doi: 10.1016/0014-4886(76)90206-5. [DOI] [PubMed] [Google Scholar]
- Pinter MJ, Burke RE, O'Donovan MJ, Dum RP. Supraspinal facilitation of cutaneous polysynaptic EPSPs in cat medial gastrocnemius motoneurons. Experimental Brain Research. 1982;45:133–143. doi: 10.1007/BF00235772. [DOI] [PubMed] [Google Scholar]
- Polo A, Zanette G, Manganotti P, Bertolassi L, De Grandis D, Rizzuto N. Spinal somatosensory evoked potentials in patients with tethered cord syndrome. Canadian Journal of the Neurological Sciences. 1994;21:325–330. doi: 10.1017/s0317167100040907. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Eguibar JR, Lomelí J, Rudomin P. Patterns of connectivity of spinal interneurons with single muscle afferents. Experimental Brain Research. 1997;115:387–402. doi: 10.1007/pl00005709. [DOI] [PubMed] [Google Scholar]
- Rojas-Piloni JG, Manjarrez E, Jiménez I, Rudomin P. Modulation of monosynaptic reflexes by spontaneous synchronized activity of spinal dorsal horn neurons. Society for Neuroscience Abstracts. 1999;562:9. [Google Scholar]
- Rosenberg ME. Synaptic connections of alpha extensor motoneurones with ipsilateral and contralateral cutaneous nerves. The Journal of Physiology. 1970;207:231–255. doi: 10.1113/jphysiol.1970.sp009058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Burke RE, Nuñez R, Madrid J, Dutton H. Control by presynaptic correlation: a mechanism affecting information transmission from Ia fibers to motoneurons. Journal of Neurophysiology. 1975;38:267–284. doi: 10.1152/jn.1975.38.2.267. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Dutton H. Effects of presynaptic and postsynaptic inhibition on the variability of the monosynaptic reflex. Nature. 1967;216:292–293. doi: 10.1038/216292a0. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Dutton H. Effects of conditioning afferent volleys on variability of monosynaptic responses of extensor motoneurons. Journal of Neurophysiology. 1969a;32:140–157. doi: 10.1152/jn.1969.32.2.140. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Dutton H. Effects of muscle and cutaneous afferent nerve volleys on excitability fluctuations of Ia terminals. Journal of Neurophysiology. 1969b;32:158–169. doi: 10.1152/jn.1969.32.2.158. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Dutton H, Muñoz-Martinez J. Changes in correlation between monosynaptic reflexes produced by conditioning afferent volleys. Journal of Neurophysiology. 1969;32:759–772. doi: 10.1152/jn.1969.32.5.759. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Jiménez I, Quevedo J, Solodkin M. Pharmacologic analysis of inhibition produced by last-order intermediate nucleus interneurons mediating nonreciprocal inhibition of motoneurons in cat spinal cord. Journal of Neurophysiology. 1990;63:147–160. doi: 10.1152/jn.1990.63.1.147. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Jiménez I, Solodkin M, Dueñas S. Sites of action of segmental and descending control of transmission on pathways mediating PAD of Ia and Ib-afferent fibers in cat spinal cord. Journal of Neurophysiology. 1983;50:743–769. doi: 10.1152/jn.1983.50.4.743. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Madrid J. Changes in correlation between monosynaptic responses of single motoneurons and information transmission produced by conditioning volleys to cutaneous nerves. Journal of Neurophysiology. 1972;35:44–64. doi: 10.1152/jn.1972.35.1.44. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Núñez R, Madrid J, Burke RE. Primary afferent hyperpolarization and presynaptic facilitation of Ia afferent terminals induced by large cutaneous fibers. Journal of Neurophysiology. 1974;37:413–429. doi: 10.1152/jn.1974.37.3.413. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Solodkin M, Jiménez I. PAD and PAH response patterns of group Ia- and Ib-fibers to cutaneous and descending inputs in the cat spinal cord. Journal of Neurophysiology. 1986;56:987–1005. doi: 10.1152/jn.1986.56.4.987. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Solodkin M, Jiménez I. Synaptic potentials of primary afferent fibers and motoneurones evoked by single intermediate nucleus interneurons in the cat spinal cord. Journal of Neurophysiology. 1987;57:1288–1313. doi: 10.1152/jn.1987.57.5.1288. [DOI] [PubMed] [Google Scholar]
- Sindou M, Turano G, Pantieri R, Mertens P, Mauguiere F. Intraoperative monitoring of spinal cord SEPs during microsurgical DREZotomy (MDT) for pain, spasticity and hyperactive bladder. Stereotactic Functional Neurosurgery. 1994;62:164–170. doi: 10.1159/000098613. [DOI] [PubMed] [Google Scholar]
- Stuhmeier KD, Grabitz K, Mainzer B, Sandmann W, Tarnow J. Use of the electrospinogram for predicting harmful spinal cord ischemia during repair of thoracic or thoracoabdominal aortic aneurysms. Anesthesiology. 1993;79:27A–28A. [PubMed] [Google Scholar]
- Tapper DN, Brown PB, Moraff H. Functional organization of the cats dorsal horn: connectivity of myelinated fiber systems of hairy skin. Journal of Neurophysiology. 1973;36:817–826. doi: 10.1152/jn.1973.36.5.817. [DOI] [PubMed] [Google Scholar]
- ten Cate J. Spontaneous electrical activity of the spinal cord. Electroencephalography and Clinical Neurophysiology. 1950;2:445–451. doi: 10.1016/0013-4694(50)90080-0. [DOI] [PubMed] [Google Scholar]
- Wall PD, Lidierth M. Five sources of a dorsal root potential: their interactions and origins in the superficial dorsal horn. Journal of Neurophysiology. 1997;78:860–871. doi: 10.1152/jn.1997.78.2.860. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. New York: Plenum Press; 1991. [Google Scholar]
- Willis WD, Weir MA, Skinner RD, Bryan RN. Differential distribution of spinal cord field potentials. Experimental Brain Research. 1973;17:169–176. doi: 10.1007/BF00235026. [DOI] [PubMed] [Google Scholar]


