Abstract
Monocarboxylate transporter (MCT) 4 is the major monocarboxylate transporter isoform present in white skeletal muscle and is responsible for the efflux of lactic acid produced by glycolysis. Here we report the characterisation of MCT4 expressed in Xenopus oocytes.
The protein was correctly targeted to the plasma membrane and rates of substrate transport were determined from the rate of intracellular acidification monitored with the pH-sensitive dye 2′,7′-bis-(carboxyethyl)-5(6)-carboxyfluorescein (BCECF).
In order to validate the technique, the kinetics of monocarboxylate transport were measured in oocytes expressing MCT1. Km values determined for L-lactate, D-lactate and pyruvate of 4.4, > 60 and 2.1 mM, respectively, were similar to those determined previously in tumour cells.
Comparison of the time course of [14C]lactate accumulation with the rate of intracellular acidification monitored with BCECF suggests that the latter reflects pH changes close to the plasma membrane associated with transport, whilst the former may include diffusion-limited movement of lactate into the bulk cytosol.
Km values of MCT4 for these substrates were found to be 28, 519 and 153 mM, respectively, and for a range of other monocarboxylates values were at least an order of magnitude higher than for MCT1. Vmax values appeared to be similar for all substrates.
K0.5 values of MCT4 (determined at 30 mM L-lactate) for inhibition by α-cyano-4-hydroxycinnamate (991 μM), phloretin (41 μM), 5-nitro-2-(3-phenylpropylamino)benzoate (240 μM), p-chloromercuribenzene sulphonate (21 μM) and 3-isobutyl-1-methylxanthine (970 μM, partial inhibition) were also substantially higher than for MCT1. No inhibition of MCT4 by 2 mM 4,4′-diisothiocyanostilbene-2,2′-disulphonate was observed.
The properties of MCT4 are consistent with published data on giant sarcolemmal vesicles in which MCT4 is the dominant MCT isoform, and are appropriate for the proposed role of MCT4 in mediating the efflux from the cell of glycolytically derived lactic acid but not pyruvate.
Monocarboxylates such as lactate and pyruvate play a central role in cellular metabolism and metabolic communication between tissues (Denton & Halestrap, 1979; Poole & Halestrap, 1993). Essential to these roles is their rapid transport across the plasma membrane that is catalysed by a family of proton-linked monocarboxylate transporters (MCTs). Nine MCT-related sequences have so far been identified in mammals each having a different tissue distribution (Price et al. 1998; Juel & Halestrap, 1999; Halestrap & Price, 1999). MCT1 is ubiquitously expressed but is especially prominent in heart and red muscle where it is upregulated in response to increased work, suggesting an important role in lactic acid oxidation (McCullagh et al. 1997; Pilegaard & Asp, 1998; Pilegaard et al. 1999a; Juel & Halestrap, 1999). In contrast, MCT4 is most evident in white muscle and other cells with a high glycolytic rate such as tumour cells and white blood cells, suggesting an important role in lactic acid efflux (Wilson et al. 1998; Juel & Halestrap, 1999). MCT2 is expressed less widely and appears to be associated with tissues that demonstrate a high uptake affinity for lactate and pyruvate such as the kidney and liver (for gluconeogenesis) and neurons (for oxidation) (see Halestrap & Price, 1999). Indeed, expression of MCT2 in Xenopus oocytes has confirmed that its substrate affinity is much higher than MCT1, especially for pyruvate (Lin et al. 1998; Bröer et al. 1999).
Detailed analyses of substrate and inhibitor kinetics have only been described for MCT1 and MCT2 following heterologous expression in Xenopus oocytes (Lin et al. 1998; Bröer et al. 1998, 1999). In this paper we demonstrate that the kinetics of MCT1 and MCT4 can be readily determined in Xenopus oocytes by measurement of the rate of intracellular acidification monitored fluorimetrically using the pH-sensitive dye 2′,7′-bis-(carboxyethyl)-5(6)-carboxyfluorescein (BCECF). MCT4 was shown to have a Km for L-lactate of about 30 mM, three- to fivefold higher than found for MCT1, whilst for most other monocarboxylates, such as pyruvate, Km values were in excess of 100 mM. Affinities for a range of inhibitors were also less than for MCT1. These properties are consistent with those determined in giant sarcolemmal membrane vesicles from white skeletal muscle in which MCT4 is the predominant MCT isoform (see Juel, 1997; Juel & Halestrap, 1999). The lower affinity of MCT4 for pyruvate than for L-lactate contrasts with MCT1 and MCT2, whose affinities for pyruvate are greater than for L-lactate (Halestrap & Price, 1999). However, it is argued that this is appropriate for the key role that MCT4 plays in lactic acid efflux from muscle.
METHODS
Materials
Hepes-free acid was obtained from Melford laboratories Ltd (Chelsworth, Suffolk, UK). Trizma base, transport substrates and inhibitors were all supplied by Sigma Chemical (Poole, Dorset, UK). All other chemicals and biochemicals were obtained from BDH Laboratory Supplies (Poole, Dorset, UK) unless otherwise stated.
Synthesis of rat MCT1 and human MCT4 cRNA
Using a previously constructed MCT1-pCIneo construct, plasmid DNA was linearised with NotI (Boehringer-Mannheim, UK) and transcribed in vitro using a T7 RNA polymerase kit (mMESSAGE mMACHINE, Ambion Inc., Austin, TX, USA). Synthesis of human cRNA for MCT4 was initially performed in a similar manner but when microinjected into Xenopus oocytes gave inconsistent results, perhaps reflecting the instability of the message. To circumvent this problem, the human MCT4 coding sequence was cloned into the Xenopus oocyte expression vector pGEM-HeJuel (pGHJhMCT4, kindly provided by Dr Stefan Bröer, Physiologisches Institut, University of Tübingen, Germany). This vector contains the 5′- and 3′-untranslated regions of the Xenopusβ-globin flanking a multiple cloning site and the stability of the transcribed MCT4 mRNA sandwiched between the untranslated globin sequence is likely to be enhanced in the oocyte (Bröer et al. 1997). For expression, plasmid DNA was linearised with SalI (Helena BioSciences, Sunderland, UK) and transcribed in vitro as above.
Experimental procedures
Injection of oocytes
Oocytes were surgically removed from Xenopus laevis females under terminal anaesthesia. The oocytes were subjected to 10 washes in Barth's modified medium (88 mM NaCl, 1 mM KCl, 0.82 mM MgSO4, 2.4 mM NaHCO3, 0.42 mM CaCl2, 10 mM Hepes, 5 mM sodium pyruvate, 50 μg ml−1 gentamicin (Fluka, Poole, UK), adjusted to pH 7.6 with NaOH). The oocyte suspension (5 ml) was treated with 2 mg ml−1 collagenase A (Sigma) dissolved in Barth's modified medium for 3 h, before being thoroughly washed and allowed to recover overnight at 18°C. Healthy looking oocytes (stages V and VI) were then selected and half were injected with 12 ng MCT4 or MCT1 cRNA in water using a microinjection device. The other half were left uninjected or injected with water as a control group. Oocytes were then cultured in Barth's modified medium, which was changed daily, at 18°C for at least 48 h. Transport measurements were performed between 48 and 72 h after injection of oocytes.
Western blotting of oocyte membranes
Crude oocyte membranes were prepared using solubilisation buffer (1 % (w/v) Triton X-100, 0.1 % (w/v) SDS, 150 mM NaCl, 10 mM Tris-HCl, pH 7.2). Fifteen microlitres of buffer was added to five oocytes in an Eppendorf tube which was then gently tapped until the solution turned cloudy and incubated for 5 min at room temperature before centrifugation at 16000g for 1 min. The supernatant was transferred into a fresh tube and the centrifugation repeated. The supernatant was again placed in a fresh tube and a sample (10 μg protein as determined with Bradford reagent) separated by SDS-PAGE prior to Western blotting using a polyclonal anti-peptide antibody directed against the carboxy terminus of MCT4 (raised in a New Zealand White rabbit killed at the end of the experiment under terminal anaesthesia) and detection by enhanced chemiluminescence (ECL) as described previously (Wilson et al. 1998).
Immunofluorescence confocal microscopy
Fresh oocytes were placed on pieces of cork, covered in OCT embedding compound (Tissue-Tek, Sakura Finetek Europe B.V., The Netherlands) and frozen in liquid nitrogen-cooled isopentane. Frozen sections (5 μm) were cut, placed on silanised slides and air dried at room temperature for 1 h before fixing with ice-cold acetone for 10 min. Permeabilisation and staining were then carried out as previously described (Wilson et al. 1998) using a carboxy terminus MCT4 anti-peptide antibody and tetramethylrhodamine isothiocyanate (TRITC)-conjugated anti-rabbit IgG secondary antibody. Samples were mounted with Mowiol (Calbiochem) and examined with a Leica TCS-NT confocal scanning microscope (63 × 1.32 NA oil immersion objective lens).
Transport assays
Transport of substrates into Xenopus oocytes was determined by monitoring changes in intracellular pH (pHi) measured using BCECF. This indicator has been used successfully by others to measure pHi in Xenopus oocytes (Sasaki et al. 1992). Six to ten healthy oocytes were selected and placed in 1 ml uptake buffer (95 mM NaCl, 2 mM KCl, 0.82 mM MgCl2, 1 mM CaCl2, 20 mM Tris-Hepes, pH 7.4) containing 5 μM BCECF-AM and incubated at room temperature for 30 min. Samples loaded with BCECF were protected from light at all times. A single BCECF-loaded oocyte was placed dark-side (animal pole) up on a coverslip in a 50 μl Perspex incubation chamber, held in a thermostatically controlled incubation vessel attached to the stage of a Nikon Diaphot inverted microscope. The oocyte was located in the centre of the field and the objective lens (×10) focused on it. Medium containing the required concentration of monocarboxylate was superfused over the oocyte with rapid switching (< 1 s) between different media using remotely operated solenoid valves. The osmolarity of the media was kept constant by isosmotic replacement of NaCl with the required concentration of monocarboxylate (Na salt). Changes in BCECF fluorescence were measured using a spinning wheel fluorescence system (Cairn Instruments, Faversham, Kent, UK) with excitation at 440 and 490 nm and emission at 510 nm as described previously (Wang et al. 1996; Wilson et al. 1998). The filter wheel was rotated at 32 r.p.m. and data were averaged to display 1 data point per second.
Presentation of results
The rate of fluorescence change was determined by fitting the data to a first-order progress curve using FigP and Pfit software (Biosoft, Cambridge, UK). In order to correct for different levels of MCT expression in each oocyte, all rates of substrate transport were expressed as a percentage of the rate obtained with 30 mM L-lactate added to the same oocyte. Kinetic constants were calculated using the combined data from several oocytes fitted to the relevant equation by non-linear least-squares regression.
RESULTS
Expression of MCT4 in Xenopus oocytes
In the experiment depicted in Fig. 1 we used antibodies against MCT4 to demonstrate that microinjection of MCT4 cRNA caused expression of MCT4 protein at the plasma membrane of the oocyte. This was apparent in the Western blots of a crude membrane fraction (Fig. 1A) and by immunofluorescence confocal microscopy (Fig. 1B). To assess whether the expressed protein was active we loaded the oocytes with BCECF and measured the decrease in pHi that accompanied lactate-H+ transport. It should be noted that it was found that in order to obtain consistent traces it was important that the oocyte was placed on the coverslip with the animal pole (dark side) facing up (i.e. with the vegetal (light) pole exposed to the incident fluorescent light). Typical data are shown in Fig. 2 for transport of L-lactate, D-lactate and pyruvate. Non-injected oocytes or those injected with water alone showed no intracellular acidification in response to monocarboxylate addition (data not shown but see also Bröer et al. 1998). In contrast, oocytes injected with MCT4 cRNA showed rapid acidification clearly demonstrating that the expressed protein was active.
Figure 1. Detection of MCT4 protein in microinjected Xenopus oocytes.
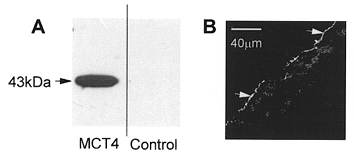
In A, 10 μg of crude oocyte membranes (see Methods) were separated by SDS-PAGE and subjected to Western blotting with MCT4 antibodies. In B, oocytes were sectioned and the location of MCT4 was revealed using confocal immunofluorescence microscopy. Arrowheads reveal the specific staining of the plasma membrane which was abolished in the presence of blocking peptide (data not shown).
Figure 2. Measurement of monocarboxylate transport by MCT1 and MCT4 in Xenopus oocytes using BCECF fluorescence.
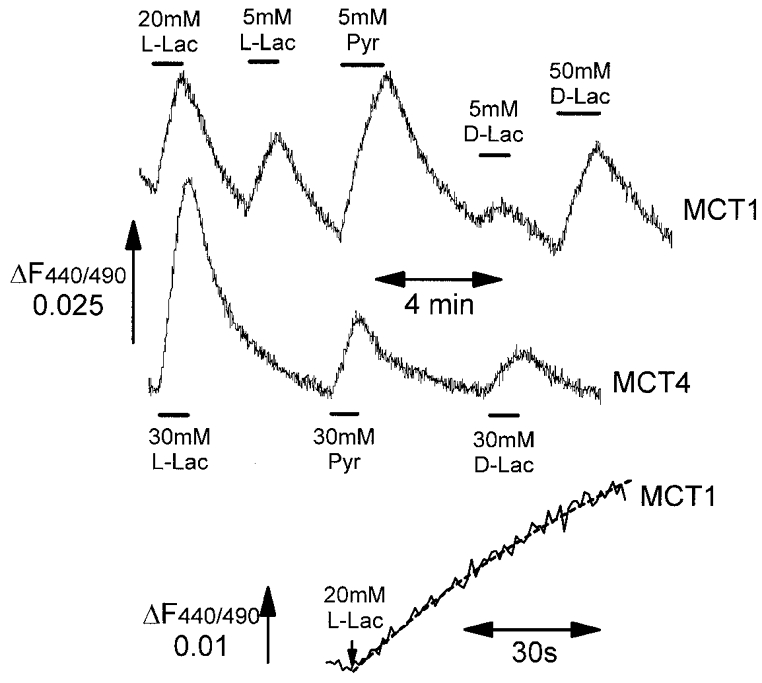
Xenopus ooctes were transfected by microinjection with MCT1 or MCT4 cRNA and changes in BCECF fluorescence ratio of a single oocyte were monitored in response to superfusion with L-lactate (L-Lac), D-lactate (D-Lac) or pyruvate (Pyr) as indicated. The bottom trace shows an expanded scale of the time course of fluorescence change (continuous trace) with the first-order fit by non-linear regression (dashed line) used to determine the initial rate of transport.
Validation of the use of BCECF to measure transport kinetics in Xenopus oocytes
We have previously demonstrated that in a range of mammalian cells the initial rate of decrease in pHi parallels the initial rate of monocarboxylate transport and can be used to characterise the kinetics of transport (Carpenter & Halestrap, 1994; Wang et al. 1994; Jackson & Halestrap, 1996; Wilson et al. 1998). Previous work from Bröer's laboratory has shown that for oocytes expressing MCT1, the Km values for lactate and pyruvate determined using radiotracer techniques are similar to those determined from pH changes measured using intracellular electrodes (Bröer et al. 1998, 1999). These data imply that pH changes may also be used to follow substrate transport in oocytes. However, in order to confirm that BCECF can be used in a similar manner, we expressed MCT1 in oocytes and measured the change in BCECF fluorescence in response to superfusion of the oocytes with L-lactate, pyruvate and D-lactate. Data are shown in Fig. 2. The initial rates of change in fluorescence ratio were calculated by first-order regression analysis of the time course as indicated in the expanded trace of Fig. 2. Absolute rates of transport are dependent on the levels of MCT expression and are thus not meaningful for direct comparison between oocytes. To circumvent this problem, for each oocyte rates of transport were expressed relative to the rate of response at 20 mM L-lactate. From these rates determined at different substrate concentrations we obtained Km values for pyruvate, L-lactate and D-lactate of 2.09 ± 0.37, 4.38 ± 0.74 and >60 mM, respectively. These values are very similar to those we determined previously for MCT1 in tumour cells and those obtained by Bröer et al. (1998) in oocytes (see Table 1). Thus we conclude that the BCECF technique is appropriate for measurement of the kinetic properties of MCTs expressed in oocytes. Using this technique it was found that Vmax values were similar for all three substrates.
Table 1.
Km values of different MCT isoforms for a range of monocarboxylates
| Substrate | MCT4 Km (Oocytes) (mm) | MCT1 Km (Tumour cells) (mm) | MCT1 Km (Oocytes) (mm) | MCT2 Km (Oocytes) (mm) |
|---|---|---|---|---|
| Formate | >500† | >100 | — | — |
| Bicarbonate | >500† | — | — | — |
| Oxamate | >500† | 49 | — | — |
| Glyoxylate | >500† | 63 | — | — |
| l-Lactate | 28 ± 4(6) | 4.5 (6.4) | 3.5 (4.4) | 0.74 |
| d-Lactate | 519 ± 35 (5) | 27.5 (46.6) | (> 60) | — |
| Pyruvate | 153 ± 6(8) | 0.7 (2.1) | 1.0 (2.1) | 0.08 |
| S-Chloropropionate | 46 ± 2.5 (5) | 0.7 | — | — |
| R-Chloropropionate | 51 ± 2.6 (3) | 0.7 | — | — |
| d, l-α-Hydroxybutyrate | 56 ± 3(4) | 2.6 | — | — |
| l-β-Hydroxybutyrate | 824 ± 64(4) | 11.4 | — | 1.2* |
| d-β-Hydroxybutyrate | 130 ± 9.6 (3) | 10.1 | — | 1.2* |
| γ-Hydroxybutyrate | >500† | 7.7 | — | — |
| Acetoacetate | 216 ± 27(4) | 5.5 | — | 0.8 |
| α-Ketobutyrate | 57 ± 3.3(4) | 0.2 | — | — |
| α-Ketoisocaproate | 95 ± 5.1(4) | — | 0.7 | 0.1 |
| α-Ketoisovalerate | 113 ± 9.6 (4) | — | 1.3 | 0.3 |
| β-Phenylpyruvate | >500† | — | — | — |
Data for MCT4 are from the present study and were determined as described in the legend to Fig. 3. Km values were derived by least-squares regression to the Michaelis-Menten equation. Combined data from the number of oocytes shown in parentheses were employed in the analysis and values are given ± S.E. (the standard error of the fitted parameter value). In view of the high Km value for most substrates, regression analysis did not allow accurate estimation of both Vmax and Km. Only for l-lactate was Kmsx calculated directly (2.5 ± 0.2 of the rate relative to that at 20 mm l-lactate, see Fig. 3) whilst for all other substrates Vmax was fixed at 2.5. Data for MCT1 in tumour cells in suspension and by fluorescence microscopy (in parentheses) were taken from Carpenter & Halestrap (1994) and Wilson et al. (1998), respectively, and for MCT1 in Xenopus oocytes from Bröer et al. (1998) or the present studies (in parentheses). Data for MCT2 are from Bröer et al. (1999).
d,l racemic mix used in these studies.
Uptake at 50 mm too low to measure.
Substrate and inhibitor specificity of MCT4
The kinetics of MCT4 were determined for a range of substrates using the same technique. Again rates of transport at different substrate concentrations were normalised against the rate observed at 20 mM L-lactate and data fitted by non-linear least-squares regression to the Michaelis-Menten equation. Data for L-lactate, pyruvate and D-lactate are shown in Fig. 3 and derived Km values are summarised in Table 1. This table also presents data for a range of other monocarboxylates. For the majority of substrates the Km values were substantially greater than 60 mM, the highest concentration employed. Osmotic constraints did not allow the use of substrate concentrations higher than 95 mM and this prevented accurate determination of both Km and Vmax by regression analysis. Thus the assumption was made that Vmax was the same for all substrates as appears to be the case for monocarboxylate transport in most cells (Poole & Halestrap, 1993; Halestrap & Price, 1999). For L-lactate the derived Vmax value (expressed relative to the rate at 20 mM L-lactate) was 2.5 ± 0.2 and this value was fixed in the calculation of the Km for all other substrates. As is apparent from Fig. 3, the data for pyruvate and D-lactate fit well when this assumption is made and allow an accurate estimation of Km. Furthermore, where substrates exhibited a Km value of less than 60 mM the data could be fitted without fixing Vmax. When this was done for S-chloropropionate, R-chloropropionate and d,l-α-hydroxybutyrate derived Vmax values (± s.e.) were 2.6 ± 0.6, 5.3 ± 2.0 and 2.8 ± 0.9, respectively. The high standard errors on these values reflect the difficulty of fitting Vmax when all substrate concentrations used were below the Km value. Nevertheless, the data suggest that the assumption of similar Vmax values is not unreasonable. Even if our Km values derived in this manner are not strictly valid, this approach does provide an accurate measure of the relative rates of transport of different substrates by MCT4 over a wide range of concentrations.
Figure 3. Substrate concentration dependence of lactate and pyruvate transport into MCT4-expressing Xenopus oocytes.
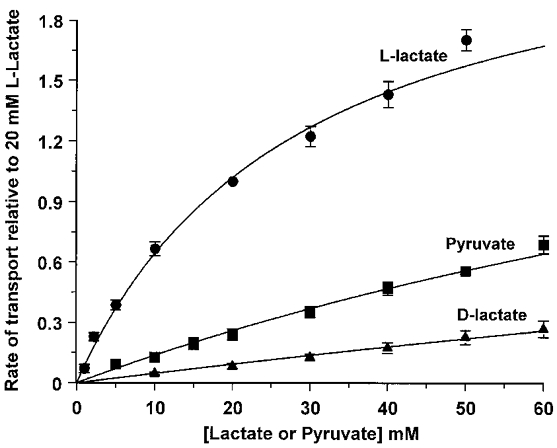
Individual oocytes were superfused with 20 mM L-lactate for 2 min followed by substrate-free buffer as shown in Fig. 2. When the 440/490 fluorescence ratio of BCECF had returned to basal level the cycle was repeated with increasing concentrations of the required substrate. Rates of transport were calculated from the initial rate of change in fluorescence ratio and expressed relative to the rate induced by 20 mM L-lactate on the same oocyte. Symbols and error bars indicate the means ±s.e.m. of rates obtained with 6, 8 or 5 oocytes for L-lactate, pyruvate and D-lactate, respectively. Data were fitted by non-linear regression to the Michaelis-Menten equation and derived Km values are given in Table 1. Vmax for L-lactate relative to the rate at 20 mM L-lactate was calculated (± s.e.) as 2.5 ± 0.2 and was fixed at this value for D-lactate and pyruvate as explained in the text.
In Fig. 4 and Table 2, data on the inhibition of transport by a range of well-established inhibitors of monocarboxylate transporters are shown. Transport was determined in the presence of 30 mM L-lactate (i.e. approximate Km value) and increasing concentrations of inhibitor were added simultaneously with the L-lactate. Rates in the presence of inhibitor were expressed as a percentage of the rate in its absence and fitted to the equation for non-competitive inhibition in order to derive a K0.5 value. Representative fits are shown in Fig. 4 whilst the derived K0.5 values are given in Table 2 where they are compared with values obtained previously for other MCT isoforms. It should be noted that for 3-isobutyl-1-methylxanthine (IBMX in Fig. 4A), the data could only be fitted to the equation for partial inhibition. p-Chloromercuribenzene sulphonate (pCMBS), a thiol reagent that inhibits MCT1 but not MCT2 was found to inhibit MCT4 totally, but the K0.5 varied substantially between oocytes as indicated by the large s.e. We have also observed substantial variation in the K0.5 value for pCMBS inhibition of lactate transport into tumour cells and hepatocytes (Carpenter & Halestrap, 1994; Wang et al. 1994; Jackson & Halestrap, 1996; Wilson et al. 1998). This variability probably reflects the non-specific nature of the inhibitor that may bind to other thiols on the cell surface in competition with those on MCTs.
Figure 4. Inhibition of lactate transport in MCT4-expressing oocytes.
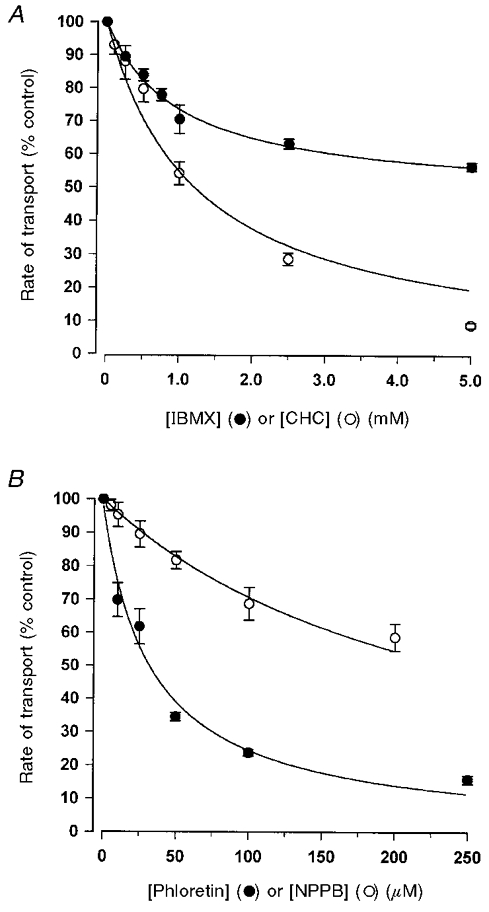
Rates of transport induced by 30 mM L-lactate were determined in the presence of increasing concentrations of the inhibitor (added simultaneously with the substrate) using the same protocol as described in Fig. 3. For α-cyano-4-hydroxycinnamate (CHC, n = 4), phloretin (n = 4) and 5-nitro-2-(3-phenylpropylaminobenzoate (NPPB, n = 5) data were fitted by non-linear regression to the equation for non-competitive inhibition, V = 100/(1 +[I]/K0.5), where V is the rate of transport in the presence of inhibitor (I) as a percentage of that in its absence. For 3-isobutyl-1-methylxanthine (IBMX, n = 3) the equation was modified to include a non-inhibitable component NI, i.e. V = (100 – NI)/(1 +[I]/K0.5) + NI. The calculated value for NI (± s.e.) was 48 ± 4 whilst values for K0.5 are given in Table 2.
Table 2.
K0.5 values of different MCT isoforms for a range of inhibitors
| Inhibitor | MCT4 K0.5 (Oocytes) (μM) | MCT1 K1 (Tumour cells) (μM) | MCT2 K1 (Oocytes) (μM) |
|---|---|---|---|
| α-Cyano-4-hydroxyeinnamate | 991 ± 148(4) | 166 | 24 |
| Phloretin | 41 ± 8.8 (4) | 5 | 14 |
| 3-Isobutyl-1-methylxanthine* | 970 ± 186 (3) | 288 | — |
| 5-Nitro-2(3-phenylpropylamino)benzoate | 240 ± 21 (5) | 9 | — |
| p-Chloromercuribenzene sulphonate | 21 ± 7(4) | 112 | NI |
| 4,4′-Diisothiocyanostilbene-2,2′-disulphonate | NI | 434 | — |
Data for MCT4 are from the present study and were determined as described in the legend to Fig. 4. K0.5 values were derived by least-squares regression to the equation for non-competitive inhibition. Combined data from the number of oocytes shown in parentheses were employed in the analysis and values are given ± S.E. Data for MCT1 in tumour cells and MCT2 in Xenopus oocytes are taken from Carpenter & Halestrap (1994) and Bröer et al. (1999), respectively.
Partial inhibition observed (52% maximum) as shown in Fig. 4A, NI, no inhibition observed at highest concentration used.
A potential weakness of the use of BCECF to determine the effects of inhibitors on lactate transport is that they might affect intracellular pH buffering capacity. Thus an agent that increased buffering capacity would cause a decrease in the initial rate of lactate induced acidification. Such a mechanism is unlikely to explain the results presented here, since the simultaneous addition of inhibitor with lactate circumvents any longer term effects that these reagents might have on intracellular buffering capacity. Furthermore, we have confirmed that these inhibitors have no effect on butyrate-induced acidification in a range of mammalian cells, and thus do not perturb cytosolic buffering capacity in these cells (Carpenter & Halestrap, 1994; Wang et al. 1994; Jackson & Halestrap, 1996; Wilson et al. 1998). Unfortunately, butyrate does not permeate the Xenopus oocyte plasma membrane rapidly at pH 7.4 in the absence of MCT expression and thus it is not possible to use this technique in our present experiments.
DISCUSSION
The use of BCECF to determine transport kinetics
The Km values obtained for MCT1 using BCECF closely match those obtained in tumour cells which only express MCT1 (Carpenter & Halestrap, 1994; Wilson et al. 1998) and suggest that this technique can be used with confidence in Xenopus oocytes, despite their much larger size. The time course of the change in pHi detected with BCECF in response to lactate transport reaches equilibrium within about 2 min (Fig. 2). This is similar to the time course measured by Bröer et al. (Bröer et al. 1998) when they used a pH electrode placed close to the membrane of oocytes, and also to time courses of transport determined in a range of mammalian cells at the same temperature using either BCECF or radiotracer techniques (Carpenter & Halestrap, 1994; Wang et al. 1994; Jackson & Halestrap, 1996; Wilson et al. 1998). However, when a radiotracer technique was used in oocytes, much slower time courses of transport were obtained that did not reach equilibrium even after 30 min of incubation (Bröer et al. 1997). Similarly, the use of a pH electrode placed in the cytosol further from the plasma membrane also gave much slower rates of transport (Bröer et al. 1998). It is well established that transporter kinetics can be affected by unstirred layer effects (deHemptine et al. 1983; Walter & Gutknecht, 1984) and thus the kinetics determined for uptake of a metabolite via a transporter may vary depending on the surface to volume ratio and geometry of the cell. These data suggest that rates of substrate transport into oocytes determined with either the radiotracer technique or intracellular pH electrodes deep in the cytosol may be prone to error due to limitations of diffusion of lactate and protons from the submembrane compartment into the bulk cytosol. As such, they do not necessarily give an accurate picture of the transport kinetics found in most cells with much smaller diameters and therefore less diffusion limitation. In contrast, our data suggest that changes in BCECF fluorescence are detecting pH changes close to the plasma membrane, with the signal from deeper within the cytosol being quenched. Further evidence to suggest that this may be the case comes from comparisons of the time course of α-ketoisocaproate uptake into oocytes measured with a pH electrode (Bröer et al. 1998) and into tumour cells and hepatocytes with BCECF (Carpenter & Halestrap, 1994; Jackson & Halestrap, 1996). In the latter case rapid uptake is followed by inhibition of transport, most probably as the hydrophobic side-chain of the substrate becomes bound to a hydrophobic pocket on the inner face of the transporter (Poole & Halestrap, 1993; Halestrap & Price, 1999). In oocytes such a biphasic time course is not observed (Bröer et al. 1998), and this could be explained if transport becomes limited by diffusion of transported substrate into the bulk cytosol rather than the transporter itself. We conclude that the use of the BCECF technique in oocytes may provide a measurement of transporter activity that more accurately reflects the kinetics observed in a normal cell than do radiotracer or pH electrode experiments.
Further evidence to suggest that our technique provides an accurate assessment of transporter kinetics comes from a comparison of the substrate and inhibitor specificity of MCT4 derived from our oocyte experiments with those found for monocarboxylate transport into giant sarcolemmal vesicles (Juel, 1997; Bonen et al. 1997; Juel & Halestrap, 1999). These vesicles contain large amounts of MCT4 but also some MCT1. Thus Km values for L-lactate in such preparations have been reported to lie somewhere between 13 and 40 mM whilst the Km values for D-lactate, pyruvate, α-ketoisovalerate and ketone bodies were found to be very high (> 50 mM) in agreement with the results presented here. Similarly, L-lactate transport into sarcolemmal vesicles was also found to be sensitive to α-cyano-4-hydroxycinnamate (K0.5, 4 mM) and pCMBS but not DIDS (see Juel, 1997; Juel & Halestrap, 1999).
Comparison of MCT4 with other MCT isoforms
Tables 1 and 2 allow comparison of the Km and K0.5 values of MCT1, MCT2 and MCT4 for a range of substrates and inhibitors and this suggests that the affinity of MCT4 for its substrates and inhibitors is much less than that of either MCT1 or MCT2. Thus the Km of MCT4 for L-lactate is about sixfold higher that of MCT1 whilst for other substrates Km values are 20–100 times greater. Most dramatic is the difference in affinity of the MCT isoforms for the α-keto acids such as pyruvate and α-ketobutyrate. A surprising feature that MCT1 and MCT4 share is their lack of stereoselectivity for D- and L-2-chloropropionate despite their strong selectivity for L- over D-2-hydroxypropionate (lactate). MCT4 also exhibits a substantially lower affinity for most inhibitors than does MCT1, with the exception of pCMBS. In contrast the substrate and inhibitor affinity of MCT2 is much higher than for MCT1, although pCMBS is an exception and does not inhibit MCT2 (Garcia et al. 1995; Lin et al. 1998; Bröer et al. 1999). Since pCMBS is impermeant it must react with an extrafacial cysteine group of MCT1 and MCT4, and comparison of the sequences of the isoforms (Halestrap & Price, 1999) suggests which residues may be involved. Thus there is a cysteine present in the extracellular loop between transmembrane domains 3 and 4 of both MCT4 (Cys108) and MCT1 (Cys106) that is lacking in MCT2 (Ser106). Similarly, inhibition of MCT1 by stilbene disulphonates such as DIDS is believed to involve binding to extracellular lysine or arginine groups close to the substrate binding site (Halestrap & Price, 1999). Since stilbene disulphonates also inhibit MCT2 but not MCT4, it seems probable that MCT4 may lack an extracellular lysine or arginine residue present in the other two isoforms. The obvious candidate is Lys413 in MCT1 and Lys404 in MCT2 which is replaced by a methionine (Met385) in MCT4. This conclusion is further strengthened by our demonstration that changing Lys413 to a glutamine in MCT1 prevents covalent modification and irreversible inhibition of MCT1 by DIDS (Meredith et al. 1999).
The properties of MCT4 are appropriate for its proposed role in lactic acid efflux from muscle
MCT1 and MCT4 are the major MCT isoforms in skeletal muscle, with the expression of MCT1 correlating with the oxidative capacity of the muscle whilst MCT4 is dominant in glycolytic fibres (McCullagh et al. 1996; Wilson et al. 1998; Pilegaard et al. 1999b; Bonen et al. 2000). Since MCT4 is also found in other glycolytic tissues such as white blood cells (Price et al. 1998; Wilson et al. 1998), we have suggested that its expression may be regulated in concert with that of other enzymes of the glycolytic pathway (Juel & Halestrap, 1999; Halestrap & Price, 1999). If MCT4 plays a critical role in lactic acid efflux from white muscle, it would be predicted that its kinetic properties should reflect this. This would seem to be the case. The very low affinity of MCT4 for pyruvate (Km, 150 mM) may be particularly important since it prevents the loss of pyruvate from the muscle. This is critical since high rates of glycolysis require that the NADH produced by glyceraldehyde-3-phosphate dehydrogenase is re-oxidised. This is achieved by pyruvate, the end product of glycolysis, being converted to lactate (Denton & Halestrap, 1979). If pyruvate were to be lost from the cell by MCT-mediated transport, this would not be possible and glycolysis and ATP production would be impaired. The low affinity of MCT4 for other substrates such as ketone bodies is also consistent with its presence in glycolytic tissues which do not oxidise or produce these metabolites. In this respect MCT4 contrasts strongly with MCT2 which has a very high affinity for pyruvate (Km < 0.1 mM) and other monocarboxylates (Lin et al. 1998; Bröer et al. 1999). However, MCT2 is found in tissues for which monocarboxylate influx is important, including liver and kidney (lactate and pyruvate for gluconeogenesis) and neurons (lactate, pyruvate and ketone bodies for oxidation). In contrast, MCT1 has a ubiquitous distribution and can serve a role in either lactic acid efflux or influx depending on the required balance between oxidative metabolism and glycolysis (see Halestrap & Price, 1999). MCT3 is found exclusively in the basolateral membrane of the retinal pigment epithelium and is most closely related to MCT4 (Yoon et al. 1997; Philp et al. 1998). No detailed kinetics of MCT3 have been described, but in view of its role in enabling the efflux of lactic acid derived from retinal metabolism into the choroidal blood supply, properties similar to its closest relative, MCT4, would seem likely.
The relatively low affinity of MCT4 for L-lactate (Km, 28 mM) might not have been predicted since it will lead to less efficient loss of lactic acid from the muscle and thus cause lactic acid accumulation. However, this is known to occur as exercise progresses and probably plays a critical role in the development of fatigue as the muscle contractile machinery and glycolysis become inhibited by the decrease in pHi. Such accumulation of lactic acid within the muscle and consequent impairment of glycolysis may have an important physiological function. It may limit the maximum rate at which the muscle produces lactic acid to that which can be cleared by those organs utilising it such as liver and heart. If muscles were able to export lactic acid in an unrestrained fashion, such that it never accumulated within the myocytes even under extreme conditions, such a feedback loop might not be possible. As a result lactic acid concentrations within the blood might accumulate to the extent that there would be a risk of lactic acidosis occurring.
Note added in proof
Since submission of this paper K. S. Dimmer, B. Friedrich, F. Lang, J. W. Deitmer & S. Bröer have published a characterisation of rat MCT4 expressed in Xenopus oocytes determined using radiotracers (Biochemical Journal350, 219–227 (2000)). The results of these authors are in broad agreement with those presented here, although the substrate affinities they report are generally higher than those determined in the present study.
Acknowledgments
This work was supported by grants from the Wellcome Trust, the Royal Society and the Medical Research Council (MRC Cell Imaging Facility and MRC Studentship to J.E.M.F.). We thank Dr Stefan Bröer for the provision of the pGHJhMCT4 plasmid and both Dr Bröer and Dr Helen Christian for helpful discussions.
References
- Bonen A, Baker SK, Hatta H. Lactate transport and lactate transporters in skeletal muscle. Canadian Journal of Applied Physiology. 1997;22:531–552. doi: 10.1139/h97-034. [DOI] [PubMed] [Google Scholar]
- Bonen A, Miskovic D, Tonouchi H, Lemieux K, Wilson MC, Marette A, Halestrap AP. Abundance and subcellular distribution of MCT1 and MCT4 in heart and fast-twitch skeletal muscles. American Journal of Physiology – Endocrinology and Metabolism. 2000;278:E1067–1077. doi: 10.1152/ajpendo.2000.278.6.E1067. [DOI] [PubMed] [Google Scholar]
- Bröer S, Bröer A, Schneider H-P, Stegen C, Halestrap AP, Deitmer JW. Characterisation of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochemical Journal. 1999;341:529–535. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, Hamprecht B, Magistretti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes – Expression of two different monocarboxylate transporters in astroglial cells and neurons. Journal of Biological Chemistry. 1997;272:30096–30102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- Bröer S, Schneider HP, Bröer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochemical Journal. 1998;333:167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter L, Halestrap AP. The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells studied with the intracellular pH indicator BCECF. Biochemical Journal. 1994;304:751–760. doi: 10.1042/bj3040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deHemptine A, Marrannes R, Vanheel B. Influence of organic acids on intracellular pH. American Journal of Physiology. 1983;245:C178–183. doi: 10.1152/ajpcell.1983.245.3.C178. [DOI] [PubMed] [Google Scholar]
- Denton RM, Halestrap AP. Regulation of pyruvate metabolism in mammalian tissues. Essays in Biochemistry. 1979;15:37–77. [PubMed] [Google Scholar]
- Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. Journal of Biological Chemistry. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochemical Journal. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- Jackson VN, Halestrap AP. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein. Journal of Biological Chemistry. 1996;271:861–868. doi: 10.1074/jbc.271.2.861. [DOI] [PubMed] [Google Scholar]
- Juel C. Lactate-proton cotransport in skeletal muscle. Physiological Reviews. 1997;77:321–358. doi: 10.1152/physrev.1997.77.2.321. [DOI] [PubMed] [Google Scholar]
- Juel C, Halestrap AP. Lactate transport in skeletal muscle – role and regulation of the monocarboxylate transporter. The Journal of Physiology. 1999;517:633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RY, Vera JC, Chaganti RSK, Golde DW. Human monocarboxylate transporter 2 (MCT2) is a high affinity pyruvate transporter. Journal of Biological Chemistry. 1998;273:28959–28965. doi: 10.1074/jbc.273.44.28959. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Poole RC, Halestrap AP, O'Brien M, Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. American Journal of Physiology. 1996;271:E143–150. doi: 10.1152/ajpendo.1996.271.1.E143. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Poole RC, Halestrap AP, Tipton KF, O'Brien M, Bonen A. Chronic electrical stimulation increases MCT1 and lactate uptake in red and white skeletal muscle. American Journal of Physiology. 1997;273:E239–246. doi: 10.1152/ajpendo.1997.273.2.E239. [DOI] [PubMed] [Google Scholar]
- Meredith D, Roberts M, Halestrap AP. Both K290 and K413 are essential for DIDS covalent modification of the rat proton-linked monocarboxylate (lactate) transporter MCT1 expressed in Xenopus laevis oocytes. The Journal of Physiology. 1999;517.P:25P. [Google Scholar]
- Philp NJ, Yoon H, Grollman EF. Monocarboxylate transporter MCT1 is located in the apical membrane and MCT3 in the basal membrane of rat RPE. American Journal of Physiology. 1998;274:R1824–1828. doi: 10.1152/ajpregu.1998.274.6.R1824. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Asp S. Effect of prior eccentric contractions on lactate/H+ transport in rat skeletal muscle. American Journal of Physiology. 1998;276:E554–559. doi: 10.1152/ajpendo.1998.274.3.E554. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Domino K, Noland T, Juel C, Hellsten Y, Halestrap AP, Bangsbo J. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. American Journal of Physiology. 1999a;276:E255–261. doi: 10.1152/ajpendo.1999.276.2.E255. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Terzis G, Halestrap A, Juel C. Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. American Journal of Physiology. 1999b;276:E843–848. doi: 10.1152/ajpendo.1999.276.5.E843. [DOI] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. American Journal of Physiology. 1993;264:C761–782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochemical Journal. 1998;329:321–328. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Ishibashi K, Nagai T, Marumo F. Regulation mechanisms of intracellular pH of Xenopus laevis oocyte. Biochimica et Biophysica Acta. 1992;1137:45–51. doi: 10.1016/0167-4889(92)90098-v. [DOI] [PubMed] [Google Scholar]
- Walter A, Gutknecht J. Monocarboxylic acid permeation through lipid bilayer membranes. Journal of Membrane Biology. 1984;77:255–264. doi: 10.1007/BF01870573. [DOI] [PubMed] [Google Scholar]
- Wang X, Levi AJ, Halestrap AP. Substrate and inhibitor specificities of the monocarboxylate transporters of single rat heart cells. American Journal of Physiology. 1996;270:H476–484. doi: 10.1152/ajpheart.1996.270.2.H476. [DOI] [PubMed] [Google Scholar]
- Wang XM, Levi AJ, Halestrap AP. Kinetics of the sarcolemmal lactate carrier in single heart cells using BCECF to measure pH(i) American Journal of Physiology. 1994;267:H1759–1769. doi: 10.1152/ajpheart.1994.267.5.H1759. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Heddle C, Kirk P, Roberts M, Meredith D, Barclay AN, Brown MH, Halestrap AP. Co-localization of immunoglobulin superfamily member CD147 with a monocarboxylate transporter (MCT1) The Journal of Physiology. 1999;517.P:58P. [Google Scholar]
- Wilson MC, Jackson VN, Heddle C, Price NT, Pilegaard H, Juel C, Bonen A, Montgomery I, Hutter OF, Halestrap AP. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. Journal of Biological Chemistry. 1998;273:15920–15926. doi: 10.1074/jbc.273.26.15920. [DOI] [PubMed] [Google Scholar]
- Yoon HY, Fanelli A, Grollman EF, Philp NJ. Identification of a unique monocarboxylate transporter (MCT3) in retinal pigment epithelium. Biochemical and Biophysical Research Communications. 1997;234:90–94. doi: 10.1006/bbrc.1997.6588. [DOI] [PubMed] [Google Scholar]


