Abstract
These experiments were designed to investigate illusions of movements of the fingers produced by combined feedback from muscle spindle receptors and receptors located in different regions of the skin of the hand.
Vibration (100 Hz) applied in cyclic bursts (4 s ‘on’, 4 s ‘off’) over the tendons of the finger extensors of the right wrist produced illusions of flexion-extension of the fingers. Cutaneous receptors were activated by local skin stretch and electrical stimulation. Illusory movements at the metacarpophalangeal (MCP) joints were measured from voluntary matching movements made with the left hand.
Localised stretch of the dorsal skin over specific MCP joints altered vibration-induced illusions in 8/10 subjects. For the group, this combined stimulation produced movement illusions at MCP joints under, adjacent to, and two joints away from the stretched region of skin that were 176 ± 33, 122 ± 9 and 67 ± 11 % of the size of those from vibration alone, respectively. Innocuous electrical stimulation over the same skin regions, but not at the digit tips, also ‘focused’ the sensation of movement to the stimulated digit.
Stretch of the dorsal skin and compression of the ventral skin around one MCP joint altered the vibration-induced illusions in all subjects. The illusions became more focused, being 295 ± 57, 116 ± 18 and 65 ± 7 % of the corresponding vibration-induced illusions at MCP joints that were under, adjacent to, and two joints away from the stimulated regions of skin, respectively.
These results show that feedback from cutaneous and muscle spindle receptors is continuously integrated for the perception of finger movements. The contribution from the skin was not simply a general facilitation of sensations produced by muscle receptors but, when the appropriate regions of skin were stimulated, movement illusions were focused to the joint under the stimulated skin. One role for cutaneous feedback from the hand may be to help identify which finger joint is moving.
The importance of feedback from muscle spindle receptors in kinaesthesia has been well established (Goodwin et al. 1972; Eklund, 1972; Craske, 1977; Clark et al. 1985; Gandevia, 1985; see McCloskey, 1978; Gandevia, 1996 for review). This has been demonstrated for the perception of movement at many joints including the fingers (Goodwin et al. 1972; Craske, 1977; Clark et al. 1985), elbow (Goodwin et al. 1972; Roll & Vedel, 1982), knee (Horch et al. 1975; Clark et al. 1979), ankle (Clark et al. 1985) and big toe (McCloskey et al. 1983). Accordingly, it is generally believed that muscle spindle feedback is the dominant source of kinaesthetic information. However, for the perception of finger movements evidence is accumulating that feedback from cutaneous receptors is also important (Edin & Johansson, 1995; Gandevia, 1995; Collins & Prochazka, 1996).
Microneurographic recordings have shown that signals from cutaneous receptors on the dorsal side of the hand (Edin & Abbs, 1991; Edin, 1992; Grill & Hallett, 1995), and to a lesser extent on the ventral side (Hulliger et al. 1979; Burke et al. 1988), provide information about finger position and movement. Evidence that such feedback may contribute to kinaesthesia comes from several studies in which the ability to detect passively applied movements was significantly decreased during anaesthesia of the hand (Provins, 1958; Goodwin et al. 1972; Gandevia & McCloskey, 1976; Refshauge et al. 1998). More direct evidence comes from recent studies in which stimulation of ensembles of cutaneous receptors in the hand generated illusions of finger movement (Edin & Johansson, 1995; Collins & Prochazka, 1996). The idea that a kinaesthetic role for cutaneous receptors may be unique to the hand arose in part, indirectly, because anaesthesia of the skin around the knee did not decrease detection of passive movements at the knee (Horch et al. 1975; Clark et al. 1979).
The nature of the contribution by cutaneous inputs to kinaesthesia is unclear. One view is that they provide a general facilitation of the other proprioceptive channels (Provins, 1958; Gandevia & McCloskey, 1976) although this need not preclude other contributions. The demonstration that movement illusions can be evoked by stimulation of receptors in the skin of the hand (Edin & Johansson, 1995; Collins & Prochazka, 1996) suggests that feedback from these receptors may also have a specific kinaesthetic role. In the present study we investigated how feedback from cutaneous and muscle spindle receptors combines in the perception of movements at the metacarpophalangeal (MCP) joints of the hand. We hypothesised that cutaneous inputs help localise which joint in the hand is moving. To test this, we compared the amplitudes of illusory flexion movements at the MCP joints, evoked by the activation of muscle spindle receptors by vibration of the tendons of the finger extensors, with illusions evoked by additional stimulation of various regions of skin which normally move during movements at specific MCP joints. Preliminary accounts of these results have been presented (Collins et al. 1999; Collins et al. 2000).
METHODS
Fourteen subjects (8 male, 6 female) participated, with 10 being studied for each skin stimulation protocol (see below). Six subjects participated in all four protocols; the other eight participated in two of the four protocols. All subjects provided informed written consent and none reported any history of neuromuscular disease. The experiments lasted 1–3 h. All procedures were conducted in accordance with the Declaration of Helsinki and were approved by the local ethics committee. Vibration was used to activate muscle spindle endings in the finger extensor musculature. Cutaneous receptors in localised regions on the hand were activated by skin stretch or electrical stimulation.
Subjects were seated with their arms supported to just distal to the wrist on a padded table (Fig. 1A and B). The hands hung relaxed over the edge of the table with the wrist and fingers slightly flexed. A screen prevented vision of the right arm distal to the mid-forearm. Subjects were informed that the experiments were designed to investigate sensations from the hand. The various experimental manipulations to be performed on the right hand were described. Subjects were asked to describe any sensations resulting from the different manipulations including touch, pressure, warmth, tingling or movement. They were also requested to match any perceived movements as accurately as possible with the contralateral (left) hand. Length gauges across the MCP joints of digits II-V of the left hand were used to monitor the matching movements. Each length gauge was calibrated before and after an experiment and provided an output which was linear across the range of movements studied (R2 typically between 0.98 and 0.99). These gauges permitted the matching movements to be performed relatively unimpeded due to their low compliance (∼2 mN deg−1). The left hand was in full view of the subjects and they could use vision to assist in the matching movements although this was not stressed.
Figure 1. Experimental protocol.
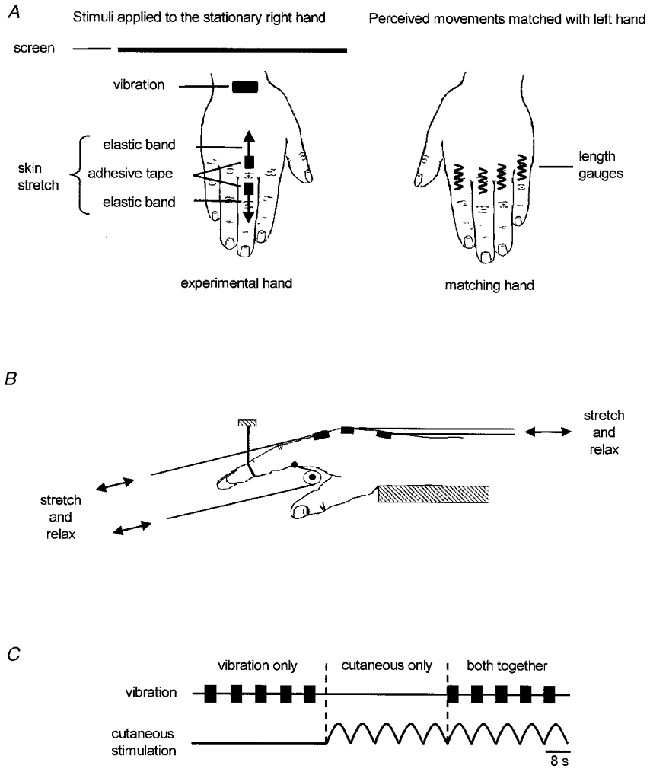
A, method used to apply the stimuli to the stationary right hand and to monitor the resulting perceived movements with matching movements of the left hand. B, method used to stretch the skin on the dorsal and ventral sides of the MCP joint of digit II. The same technique was used at digits III and IV (not shown). C, schematic representation of the timing of stimulus delivery within an experimental block. The order of presentation of the three different types of stimulation was randomised.
General methods
During a typical period of data collection subjects received 30 successive cycles of stimulation. These data are subsequently referred to as a ‘block’. The type of stimulation was varied so that subjects received sets of five consecutive cycles involving vibration alone, skin stimulation alone, or both vibration and skin stimulation together (Fig. 1C). The order of the sets of each type of stimulation was randomised between blocks. However, subjects never received the same type of stimulus for more than five consecutive cycles. Within each block subjects received 10 cycles of each stimulus type. When vibration and skin stimulation were applied together the skin stimulation was applied ‘in-phase’ with the vibration (Fig. 1C). The type of skin stimulation was the same within a block but varied between blocks. The order in which subjects received the different types of skin stimulation was randomised.
Vibration
Vibration (100 Hz) was applied at the wrist over the first three tendons (digits II-IV, Fig. 1A) of the extensor digitorum and extensor indicis muscles using a vibrator (Wahl, part no. 4013) with a custom-built tip (18 mm wide × 7 mm long). At the start of each experiment the location and pressure of application of the vibration was carefully adjusted based on two criteria. First, the vibration was adjusted to provide a clear illusion of flexion of all the fingers as indicated by matching movements of the contralateral hand. Occasionally, this criterion could not be met as the illusions were small, variable or restricted primarily to a single digit. When this occurred, the vibrator tip was adjusted to evoke clear movement illusions which involved as many fingers as possible and the experiment was continued. Second, the vibration was adjusted to avoid any visible finger movements associated with reflex muscle activation. In two subjects such movements could not be avoided and these subjects were not part of the study. During the experiments the vibrator was clamped in place and occasionally adjusted to maintain a consistent illusion based on the matching movements and subjective descriptions. The vibration was applied intermittently at 0.125 Hz (4 s on, 4 s off, see Fig. 1C) to evoke illusions of slow, cyclical flexion-extension of the MCP joints.
Skin stimulation protocols
Skin stretch
Stretch of the skin was used to mimic the skin movements that might occur during movement at specific MCP joints. The stretch was applied at different MCP joints using techniques which were variants of those described previously (Collins & Prochazka, 1996).
Dorsal skin over the MCP joint(s)
The first technique involved stretch of a small region of the dorsal skin over the MCP joints of digit III, digit IV, or digits III and IV together. Adhesive tape (∼7 mm wide × 10 mm long) attached to elastic bands was applied to the skin approximately 15 mm apart, one distal and one proximal to the MCP joint (shown for digit III in Fig. 1A). The bands were stretched and relaxed manually by the experimenter at approximately 0.125 Hz such that the skin was progressively stretched for 4 s then relaxed for 4 s (Fig. 1C). A strain gauge attached to one of the bands indicated the time course of the stretch. Care was taken to minimise any actual movements of the fingers associated with the skin stretch. Ten subjects participated. Three blocks were collected for eight subjects and for the other two subjects, the skin stretch was not applied over digit IV alone, and thus only two blocks were collected. Thus, 28 blocks of data were collected.
Dorsal and ventral skin over the MCP joint and over the dorsum of the corresponding metacarpal
These trials incorporated the dorsal skin stretch (as described above) with additional stretch of a portion of the skin on the dorsum of the hand over the corresponding metacarpal bone and compression of the skin on the ventral side of the MCP joint (Fig. 1B). Movements of the fingers on the experimental hand associated with the stretch were restricted by wires wrapped around the distal interphalangeal joint. For the additional dorsal stretch, adhesive tape (∼15 mm wide × 10 mm long) was stuck to the skin over the corresponding metacarpal, 30–40 mm proximal to the MCP joint. This was attached to an elastic band which was stretched in a proximal direction in time with stretch applied over the MCP joint. For the ventral stretch, short pieces of thread (∼18 mm), soaked in cyano-acrylate glue, were stuck to the ventral skin along the crease at the base of the MCP joints of digits II-IV extending slightly into the adjacent web space(s). A long thread tied to the middle of the shorter threads ran around a pulley under the hand, proximal to the MCP joint. This thread was attached to an elastic band and was pulled in time with the dorsal skin stretch resulting in compression of the skin pad at the base of the MCP joint and slight stretch of the skin in the web space similar to that which occurs during finger flexion. In a given experimental block this stretch was applied at digit II, III or IV, resulting in three blocks for each of the ten subjects (for a total of 30 blocks).
Electrical stimulation
Dorsal aspect of MCP joints
This technique was used to activate cutaneous receptors in a similar region of skin to the dorsal skin stretch technique (described above) without any extraneous movements of the experimental hand which may have accompanied the stretch. Two pairs of self-adhesive electrodes (3M 1180, cut to ∼7 mm × 10 mm) were applied to the skin over the MCP joints of digits III and IV in approximately the same locations as the tape used to apply the skin stretch. The electrical stimulation consisted of rectangular pulses (0.5 ms duration) at 200 Hz. Prior to data collection, stimulus intensity was increased slowly to identify the perceptual threshold and the maximal intensity which subjects perceived as strong but not painful and produced paraesthesia in the region between the electrodes. This intensity (2.0 ± 0.1 times perceptual threshold, mean ±s.e.m.) was then used for the maximal level in a block. Electrode placement was adjusted to ensure that the stimulation was not accompanied by radiating sensations or visible finger movements. Stimulus intensity was manually adjusted from just below perceptual threshold to ‘maximal’ intensity at approximately 0.125 Hz (4 s increasing, 4 s decreasing, see Fig. 1C). This stimulation was applied over digit III, digit IV or both digits resulting in three blocks for each of the 10 subjects. For two subjects, stimulation was not applied over digit IV alone, and thus only two blocks were collected for those subjects (for a total of 28 blocks).
Distal phalanx
To provide a cutaneous signal which would alert the subject to a particular digit but which did not arise from receptors activated by MCP movements, electrical stimulation was applied to the distal phalanx of digit II or IV using similar methods to those described above. Electrodes (∼3 mm wide × 10 mm long) were attached to the lateral aspect of the distal phalanx of digits II and IV. Two blocks were collected for each of the 10 subjects during which the stimulation (1.6 ± 0.1 times perceptual threshold) was applied to either digit II or digit IV (for a total of 20 blocks).
Control studies
In four subjects we investigated the effects of small passive movements at the MCP joint of the index finger on the magnitude of the vibration-induced illusions. These trials involved the same standardised protocol, but instead of skin stimulation, the MCP joint was moved cyclically (0.125 Hz) at each of two amplitudes (∼1.5 and ∼3.0 deg) in two experimental blocks. During these trials the vibration was applied during the flexion phase of the movement. In no case did these movements significantly alter the amplitude of the illusory movements.
Recordings and measurements
The time course of the application of vibration, skin stretch and electrical stimulation were recorded, along with the movements at the MCP joints for digits II-V on the matching hand. Data were sampled using Spike 2 software (Cambridge Electronic Design) and a CED 1401 interface. The amplitudes of the illusory movements were quantified by calculating the amplitude of the matching movements made with the contralateral hand. Movements of digits II-IV were analysed because the vibration was applied only over the extensor tendons to these digits, and consequently the illusory movements of digit V were often small and variable. Data from all cycles of stimulation were used. If no illusory movement occurred the amplitude was recorded as zero. Extension movements were assigned a negative value.
Statistical analysis
Statistical tests identified differences in the amplitude of illusory movements evoked by vibration alone compared with the amplitudes evoked by combined vibration and skin stimulation. Two-way analysis of variance (ANOVA) using digit (II, III, IV) and stimulus type (vibration or both together) as factors compared movement amplitudes within each block for each subject. Two-way repeated measures ANOVA was used to identify significant differences for each block across subjects. When tests for normality failed, the data were transformed by rank and the tests repeated. Student-Newman-Keuls post hoc tests were used to identify the detail of any significant effect revealed by the ANOVA. The effect of the skin stimulation was considered to be ‘non-specific’ or ‘generalised’ when there was a significant difference but no significant interaction (i.e. movements were either larger or smaller at all test digits). The skin stimulation was considered to ‘focus’ the movement illusions to a digit or digits when a significant interaction was identified and post hoc tests identified at which digit(s) the effect of the skin occurred. Focusing occurred if, during combined stimulation, illusory movements became relatively larger at the digit associated with the skin stimulation than at adjacent digits. This could arise because of an increase in size of the illusory movement at the digit associated with the skin stimulation and/or a decrease in size of the movement at the adjacent digits.
To test for the overall effect of each type of skin stimulation across subjects and blocks, the amplitudes of illusory movements during the combined stimulation were normalised to the amplitudes at the corresponding digit during vibration alone. The data were then grouped according to the proximity of the digit to the region of cutaneous stimulation (‘stimulated’ digit, adjacent to the stimulated digit, or 2 digits away from the stimulated digit). The correlation between the size of the perceived movements and proximity to the stimulated digit was determined using the Spearman rank correlation test. Analyses were performed using SigmaStat software (Jandel Scientific v. 2.0) with significance set at the 5 % level. All descriptive statistics are given as the mean ±s.e.m.
RESULTS
These experiments investigated the effect of stimulation of different regions of the skin of the hand on the amplitude of vibration-induced illusions of finger flexion. Stimulation of the skin altered the vibratory-evoked illusions at the MCP joint of at least one of the test digits in 64/106 (60 %) blocks (for definition see Methods). The most consistent effects (25/30 blocks, 83 %) occurred when skin stretch was applied to both the dorsal and ventral surfaces of the hand.
Skin stretch
Dorsal skin over the MCP joint(s)
An example of the effect of skin stretch over the dorsum of the digit IV MCP joint is shown for a single subject in Fig. 2, with raw data from part of an experimental block in Fig. 2A, and data pooled from that block in Fig. 2B. When given alone, the vibration induced similar illusory movements at all the test digits. The skin stretch alone induced clear illusions of movement at digits III and IV. Combined vibration and skin stretch resulted in illusory movements which were largest under the stretched region of skin and became progressively smaller at more remote digits.
Figure 2. Amplitudes of illusory movements evoked when skin stretch was applied over the dorsal aspect of the MCP joint of digit IV in one subject.
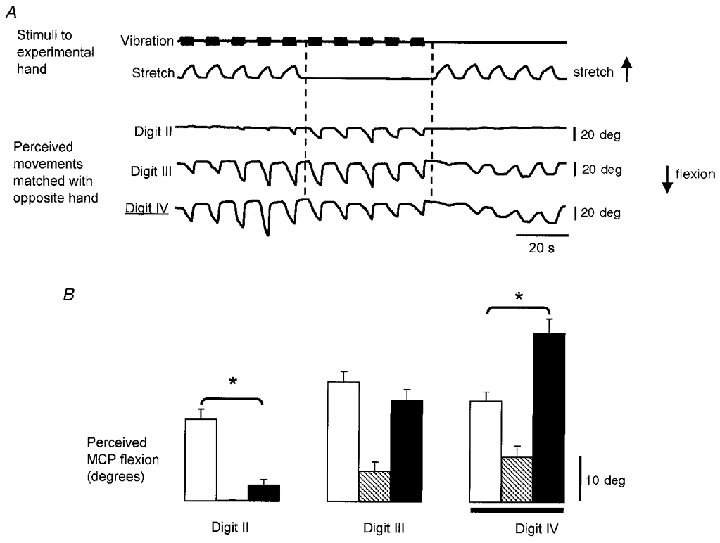
A, raw data from a single subject when skin stretch was applied over the dorsum of the MCP joint of digit IV. The top two traces show the time course of the application of the vibration to the finger extensor tendons at the wrist and the skin stretch over the MCP joint. The bottom three traces show the resulting illusory movements at the MCP joints as indicated by the voluntary movements of the matching hand. The digit at which the skin stimulation was applied is indicated with a double underline. Flexion at the MCP joint is shown as a downward deflection. B, mean amplitude (±s.e.m.) of the illusory movements from all cycles of each type of stimulation from the data shown in A (n = 10 for each mean). Data shown for vibration as open bars, skin stretch as hatched bars, and vibration combined with skin stretch as filled bars. The skin stimulation was applied at digit IV as indicated by the horizontal line. *Significant differences between vibration and the combined stimulation.
For the group (n = 10), stretch of the dorsal skin over the MCP joint altered the amplitude of the vibratory-evoked illusory movements of at least one of the test digits (digits II-IV) in 8/10 subjects and 14/28 blocks (50 %). In 10/28 blocks the stimulation significantly increased the amplitude of the vibration-induced illusory movements under the stretched skin but in three blocks movement amplitude decreased. In nine blocks the effect was ‘non-specific’ (for definition see Methods); illusory movements increased at all test digits in six blocks and decreased in three blocks. In a further three blocks, movement illusions were ‘focused’ to the MCP joint(s) underlying the stimulated region of skin (see Methods). In an additional block the sensation of movement was focused to the stimulated and one adjacent digit, and in another block this sensation was focused to a non-stimulated digit.
Although subjects often could not detect this skin stretch when it was applied during periods of vibration, in several blocks stretch of the skin still altered the amplitude of the vibration-induced illusory movements. When subjects detected the applied stretch they were often unable to identify the finger over which it was applied.
When data were pooled across subjects for each of the three regions of skin that were stretched (MCP III, MCP IV, MCP III and IV), although the mean size of illusory movements of the stimulated digit tended to be larger when both stimuli were applied together, this was significant only when the skin stretch was applied over the MCP joint of digit IV. In this situation there was a significant interaction (P < 0.001) and post hoc tests showed that the illusions became focused to digit IV as the skin stretch increased the size of the vibration-induced illusions of movement at that digit and decreased them at digit II.
The pooled mean size of the illusory movement at the MCP joint(s) under the stretched region of skin was 19.0 ± 2.5, 1.5 ± 1.0 and 23.0 ± 3.0 deg for vibration alone, skin stretch alone and both stimuli together, respectively. As shown in Fig. 2, skin stimulation alone was sufficient to evoke illusions of movement in some subjects (maximal mean amplitude 14.5 deg). To test across all subjects and blocks whether skin stretch focused the sensation of movement to the stimulated digit, movement amplitudes at each MCP joint during the combined vibration and skin stretch were normalised to those evoked by vibration alone. The normalised movement amplitudes were compared for the stimulated digit, the digit adjacent to the stimulated digit, and digits that were further away (filled circles in Fig. 5). Stretch of the dorsal skin over specific MCP joints had a significant focusing effect on the sensation of movement (Rs= 0.28, P = 0.01).
Figure 5. Summary of the effects of each type of skin stimulation.
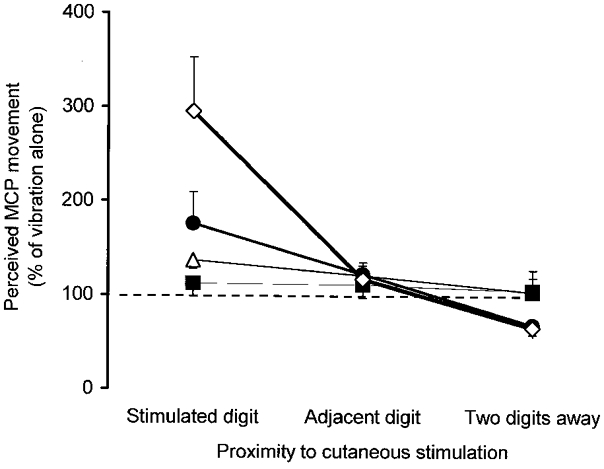
Grouped data for illusory movement amplitudes evoked by combined skin stimulation and vibration over the finger extensors, normalised to the corresponding amplitudes evoked by vibration alone. Data are grouped according to the proximity to the region of stimulated skin. Data are shown for skin stretch on the dorsal side of the MCP joint (•), skin stretch on the dorsal and ventral sides of the hand (⋄), electrical stimulation on the dorsal side of the MCP joint (▵), and electrical stimulation at the distal phalanx (▪).
Dorsal and ventral skin over the MCP joint and over the dorsum of the corresponding metacarpal
When the skin on the dorsal and ventral surface of an MCP joint was stimulated there was a large and consistent effect on the amplitude of the vibration-induced illusions of movement. The amplitudes of the vibration-induced illusory movements changed in all subjects and 25/30 (83 %) of the blocks. In 19 blocks illusory movements were larger at the stimulated digit and in one they were smaller. In only two blocks was the effect generalised to all the digits; in one block movements were larger at all the test digits and in the other they were smaller. In contrast, this skin stretch focused the illusion to the stimulated digit in 19 blocks and to the stimulated plus an adjacent digit in a further four blocks. It never focused the movement illusions to a non-stimulated digit only.
Subjects usually detected the ventral skin stretch, even during periods of vibration and were able to identify the location and the approximate direction of the applied stretch. However, detection of the dorsal component of this skin stretch was poor, similar to that described in the previous section.
Raw data from one subject in which the skin stretch was applied at digit IV are shown in Fig. 3A. Illusory movements arose when the vibration and skin stretch were applied separately. Furthermore, the combined dorsal and ventral stretch focused the sensation of movement to the stimulated digit; illusory movements were significantly larger at digit IV but were smaller at digit II, compared with those during vibration alone (Fig. 3B).
Figure 3. Amplitudes of illusory movements evoked when the skin stretch was applied over the dorsal aspect of the hand and the dorsal and ventral sides of the MCP joint of digit IV in one subject.
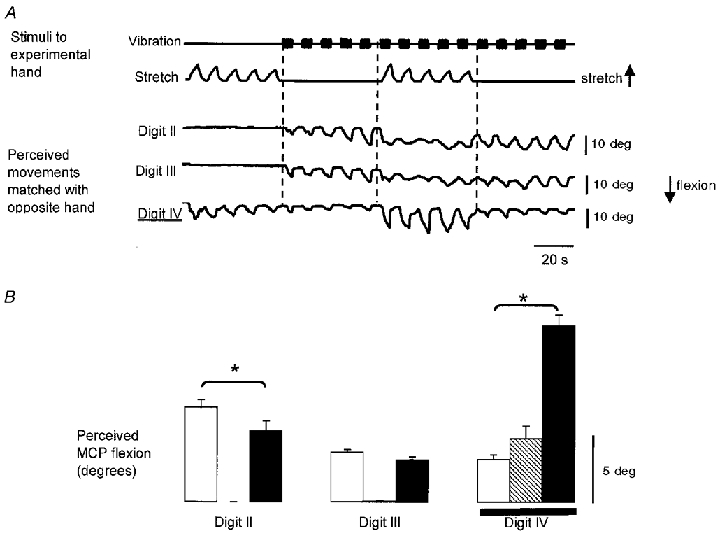
A, raw data from a single subject when the skin was stretched on the dorsal side of the hand and compressed on the ventral side of the MCP joint at digit IV. The top two traces show the time course of the application of the vibration and the skin stretch. The bottom three traces show the resulting illusory movements at the MCP joints as indicated by the voluntary movements of the matching hand. Flexion at the MCP joint is shown as a downward deflection. The digit at which the skin stimulation was applied is indicated with the double underline. B, mean amplitude (±s.e.m.) of the illusory movements from all cycles of each type of stimulation from the data shown in A (n = 10 for each mean). Data shown for vibration only (open bars), skin stretch only (hatched bars), and vibration combined with skin stretch (filled bars). The skin stimulation was applied at digit IV as indicated by the horizontal line. *Significant differences.
Mean data averaged across all subjects (n = 10) for each of the three regions of skin that were stretched are shown in Fig. 4. Illusory movements under the stretched region of skin were significantly larger during the combined stimulation, compared with vibration alone, for all three stimulated skin regions. In contrast, the skin stretch did not alter the magnitude of the vibration-induced illusions at three of the four digits which were adjacent to the skin stimulation, and reduced it at the other adjacent digit. Vibration-induced movement illusions were reduced at both MCP joints which were two digits away from the cutaneous stimulation. Thus, this skin stimulation technique focused the perception of movement to the joint under the stretched skin for each of the three stimulated skin regions (P < 0.001). When averaged across subjects, the combined stimulation produced movement illusions under the stimulated region of skin which were similar to those that might be predicted from illusions to each stimulus presented separately (Fig. 4). However, this apparent addition did not occur for all individual subjects, and simple addition cannot explain the occasional reductions seen at remote digits.
Figure 4. Effect of dorsal and ventral skin stimulation at each digit across subjects.
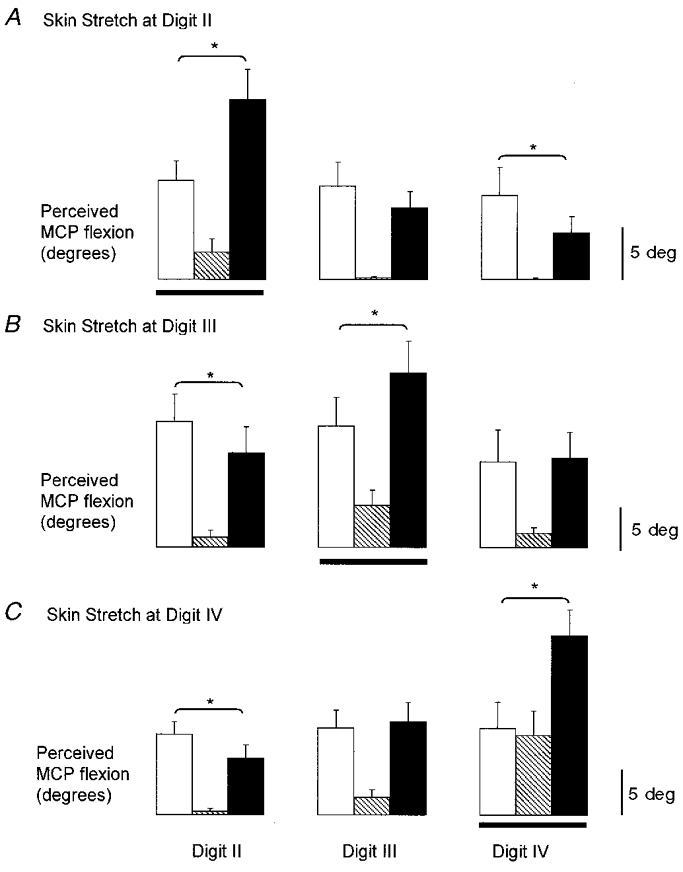
Mean amplitude of illusory movements from all cycles of stimulation during blocks in which the skin stretch to dorsal and ventral skin was applied at digit II (A), digit III (B) and digit IV (C). Horizontal lines indicate the digit at which the skin stretch was applied. Data shown for vibration only (open bars), skin stretch only (hatched bars), and vibration combined with skin stretch (filled bars). *Significant differences (n = 10 subjects for each mean).
Across subjects the mean size of the illusory movements at the MCP joint under the stimulated region of skin was 11.0 ± 1.5, 5.0 ± 1.0 and 18.5 ± 2.0 deg for vibration alone, skin stretch alone and both stimuli together, respectively. This skin stretch was the most consistent at evoking illusions of movement when delivered alone (maximal mean amplitude 21.0 deg). These movements tended to be localised to the stimulated digit, often with smaller movements at adjacent digits (hatched bars in Fig. 4). The pooled data in Fig. 5 (open diamonds) show how effective this type of skin stimulation was in focusing the sensation of movement to the joint under the region of stimulated skin (Rs= 0.66, P < 0.0001).
Electrical stimulation
Dorsal aspect of MCP joints
Innocuous electrical stimulation over the MCP joints altered the vibratory-evoked illusory movements of at least one digit in 17/28 (61 %) of the blocks. Movements at the stimulated digit were significantly larger in 12 blocks when both stimuli were delivered together compared with vibration alone, and were smaller in four blocks. In 14 blocks the effect was generalised to all the test digits. In 10 of these blocks movements at all the test digits were larger, and in the other four they were smaller. In three blocks this electrical stimulation focused the movement illusion to the stimulated digits(s) and never focused the illusion to a non-stimulated digit. Subjects (n = 10) could detect this type of stimulation during periods of vibration more easily than skin stretch over the same region, but often had difficulty correctly identifying the location. All subjects reported this stimulation as a local ‘pins and needles’ sensation and occasionally they felt that the skin was being stretched.
Of the three regions of stimulated skin (MCP III, MCP IV, MCP III and IV), stimulation over both MCP III and IV together significantly increased the size of the vibration-induced illusions at all the test digits. Across subjects the mean size of the illusory movements at the MCP joint(s) under the stimulated region of skin was 16.5 ± 1.5, 1.5 ± 1.0 and 21.0 ± 2.0 deg for vibration alone, electrical stimulation alone, and both stimuli together, respectively. Electrical stimulation evoked illusions of movement in some subjects when applied alone (maximal mean amplitude 19.5 deg). Across all subjects and blocks the effect of electrical stimulation when combined with vibration was largest at joints under the stimulated skin and progressively weaker at more ‘distant’ MCP joints (open triangles in Fig. 5). This effect was significant (Rs= 0.22, P = 0.04), but tended to be smaller than when the same region of skin was actually stretched.
Distal phalanx
Electrical stimulation was delivered to the distal phalanx of either digit II or IV to provide a cutaneous signal which did not arise from receptors normally activated by MCP movements. All subjects (n = 10) detected this cutaneous stimulation during vibration at the wrist and correctly identified the stimulated digit. This procedure altered the vibration-induced illusory movements in 8/20 (40 %) of the blocks. In three blocks illusory movements were larger at the stimulated digit but in four they were smaller. In six blocks this stimulation had a non-specific effect at all the test digits (2 larger movements, 4 smaller movements). In two blocks, the sensation of movement was focused to the stimulated digit and was never focused to a non-stimulated digit. However, across subjects there was no significant effect of the electrical stimulation at either digit tip on the amplitude of the vibratory-evoked illusion of movement and there was no significant focusing effect (Rs= 0.12, P = 0.36, filled squares in Fig. 5).
Across subjects the mean sizes of the illusory movements at the MCP joint under the stimulated region of skin were 8.0 ± 1.5, 0.0 ± 0.5 and 8.5 ± 2.0 deg for cycles of vibration alone, electrical stimulation alone and both stimuli together, respectively. This stimulation rarely evoked illusions of movement at the MCP joint when applied alone, and when movements were perceived they were small (maximal mean amplitude 1.0 deg).
DISCUSSION
Illusions of movements of the fingers, evoked by vibration of the finger extensor tendons, were altered when evoked at the same time as stimulation of localised regions of the skin of the hand. During this combined stimulation, kinaesthetic judgements were based on concurrent information from cutaneous and muscle spindle receptors. The skin stimulation did not simply provide a general facilitation of the vibration-induced illusions of movement but often focused the sensation of movement to the joint below the stimulated region of skin. The effect was strongest when cutaneous receptors on both the dorsal and ventral surface of the hand were activated.
Kinaesthetic integration of cutaneous and muscle spindle receptor feedback
Much of the evidence for the kinaesthetic importance of muscle spindles has come from studies of movement illusions during tendon vibration. Despite the artificial nature of this type of stimulation, the resulting illusions are consistent with a lengthening of the vibrated muscle, thus suggesting that muscle spindle afferents can be a major source of information about movement (e.g. Goodwin et al. 1972; Eklund, 1972; Craske, 1977; see McCloskey, 1978; Gandevia, 1996 for review). This idea is supported by the results of experiments which utilise a variety of experimental approaches (e.g. Gandevia et al. 1983; McCloskey et al. 1983; Gregory et al. 1988). It has recently been shown that movement illusions can also be evoked by activation of populations of cutaneous receptors, at least at the hand (Edin & Johansson, 1995; Collins & Prochazka, 1996). Therefore, it is likely that both receptor populations are involved in kinaesthesia of the hand, although it is still not clear how the CNS integrates the information. The kinaesthetic contribution from joint receptors, which are also activated during movement (Ferrell, 1980; Burke et al. 1988; Edin, 1990), is also not clear although illusions of movement evoked by microstimulation of single joint afferents suggest that they may play a role (Macefield et al. 1990; see also Ferrell et al. 1987; Clark et al. 1989). During natural movements, kinaesthetic judgements may be based on all the available sensory information or the information contained in a single, ‘dominant’ afferent channel. The decrement in the ability to detect passively applied movements during anaesthesia of the hand (Provins, 1958; Goodwin et al. 1972; Gandevia & McCloskey, 1976; Refshauge et al. 1998) suggests that some integration occurs. However, this decrement may be due to the removal of feedback from receptors located in the skin, joints or both. Our experiments provide evidence that cutaneous and muscle spindle feedback are used together in kinaesthesia at the MCP joints. In primates, potential sites for convergence of inputs from both classes of receptor include neurons in the sensorimotor cortex (Heath et al. 1976; Lemon, 1981) and thalamus (Maendly et al. 1981; Butler et al. 1992). Recent evidence suggests the possibility of similar convergence at the cuneate nucleus (Xu & Wall, 1999; cf. Hummelsheim et al. 1985). The data in Fig. 4 (pooled across subjects) might suggest that the contribution of cutaneous and muscle spindle feedback is additive. However, the interaction between the two inputs was probably more complex than this as there were many blocks in individual subjects in which the effect was clearly not additive, particularly at MCP joints remote from the skin stimulation where the illusion sometimes became smaller.
Roles for cutaneous receptors in kinaesthesia
Feedback from the skin of the hand may facilitate inputs from the other proprioceptive channels (Goodwin et al. 1972; Gandevia & McCloskey, 1976). There were blocks in the present experiments in which skin stimulation over a single MCP joint altered movement sensations at all digits equally. However, in many blocks the skin stimulation focused the movement illusions to the joint underlying the stimulated skin, suggesting that cutaneous receptors may also have a more specific role which may be to help identify which joint in the hand is moving. This may be particularly important for the perception of finger movements where cutaneous receptors are ideally situated to signal movements at individual joints. In contrast, deriving movement location from spindle discharge in the multiarticular muscles of the fingers would require some degree of computation. Modelling studies show that feedback from muscle spindles in the extrinsic muscles of the hand is a poor indicator of individual finger joint angles and more precisely reflects the location of the fingertip (Biggs et al. 1999). While it has been shown that kinaesthetic judgements at other joints in the upper limb are based on the integration of muscle spindle feedback from multiple muscle groups (Gandevia et al. 1983; Gilhodes et al. 1986; Verschueren et al. 1998; cf. Wise et al. 1996), such a computation for the fingers might be enhanced by feedback from the skin. This type of interaction between cutaneous and muscle spindle receptors was suggested to account for the differences in movement illusions observed when vibration was applied over the extensor tendons at the wrist compared with the dorsum of the hand (Verschueren et al. 1998). In a complimentary way, feedback from muscle spindles may help reduce ambiguities arising from cutaneous inputs. However, the fact that subjects can detect which finger is being moved passively when feedback from sensory receptors distal to the wrist are blocked by ischaemia (Goodwin et al. 1972) indicates that feedback from muscle spindles alone is sufficient to detect which is the moving digit.
The present experiments also show that the effect of the skin stimulation was strongest with stimulation of appropriate regions of the skin. Electrical stimulation of the distal phalanx, a region of skin not normally activated during MCP movements, altered the vibration-induced movement illusions in the fewest number of blocks and, across subjects, the effect was small and non-specific. In contrast, stretch of a small region of skin on the dorsal side of the MCP joints, a region clearly stretched during MCP movements, altered the illusions of movement more frequently and, across subjects, focused the sensation of movement to the joint under the stimulated region of skin (see Fig. 5). Electrical stimulation of the same region of skin had a weaker focusing effect on the movement sensations, perhaps because this stimulation is less specific to those cutaneous receptors which are activated by movement. The most consistent and largest effects were seen when the dorsal skin was stretched and the ventral skin was compressed (Fig. 5). In a previous study investigating movement illusions during skin stimulation, illusions could not be evoked unless both the dorsal and ventral skin were stimulated (Edin & Johansson, 1995), although in a different study, illusions were produced with stimulation of only the dorsal skin (Collins & Prochazka, 1996). In this latter study the direction of the perceived movements varied between subjects; in the former study all subjects perceived movements consistent with the applied stretch. This difference may reflect an important role for the ventral skin in kinaesthesia of the hand. Alternatively, in the study of Collins & Prochazka (1996) the skin on the dorsum of the hand was stretched in a proximal direction, opposite to that during natural movements, which may have provided conflicting signals about movement direction. A similar type of stretch was used in the present experiments which may have led to an underestimation of the contribution made from the skin. Clearly, use of a technique which activated cutaneous receptors in a more physiologically relevant manner, encompassing receptors remote from the moving joint, may have had a more powerful effect on the vibration-induced sensations of movement.
Experimental considerations
We used vibration to initiate movement illusions of all the fingers. Vibration of muscle tendons is a powerful stimulus to muscle spindle endings (Bianconi & Van Der Meulen, 1963; Brown et al. 1967; Burke et al. 1976; Roll & Vedel, 1982) and the resulting movement illusions are thought to depend primarily on that feedback (Goodwin et al. 1972; Roll & Vedel, 1982). Although the vibration will also activate some cutaneous receptors around the wrist and these may have contributed to the movement illusions evoked by the vibration (Verschueren et al. 1998), this would not account for the differences seen with the additional stimulation of localised regions of the skin.
Two techniques were used to stimulate ensembles of cutaneous receptors in localised regions of the hand. Electrical stimulation was applied using surface electrodes at intensities unlikely to activate a significant proportion of non-cutaneous afferents. We also stretched different regions of the skin of the hand to provide a stimulus which was more specific to the receptors activated during natural finger movements (Edin & Johansson, 1995; Collins & Prochazka, 1996). Although we minimised actual movements of the experimental hand, occasional small movements (<0.1 deg, see Collins & Prochazka, 1996) may have activated some muscle spindle receptors. This is unlikely to have confounded our results because in control experiments the addition of deliberate cyclical MCP movements (mean amplitude ∼1.5 and ∼3.0 deg) did not alter the amplitude of the vibration-induced illusions (see Methods). This suggests that the perceptual effect of the skin stretch resulted from the activation of cutaneous receptors rather than inadvertent activation of non-cutaneous receptors. The method used to assess the size of the illusory movement was flexion of the MCP joints of the contralateral hand. Because there are limits, both biomechanical and neural, to the degree of independent movement at the fingers (Kilbreath & Gandevia, 1994), it is likely that the measured differences in sizes of the illusions at the various fingers underestimates the true size of the focusing effects.
The effect of skin stimulation in focusing the sensation of movement to the joint under the stimulated skin did not simply reflect a shift in attention to that digit. First, the focusing effect sometimes occurred when the skin stretch could not be detected by the subject. Secondly, electrical stimulation of the skin at the distal phalanx did not have this focusing effect, despite the fact that all subjects could clearly identify which finger was being stimulated. However, when the vibration-induced illusions of MCP movement were increased in size by local skin stretch, often the perceived motion at ‘distant’ MCP joints was reduced, which may reflect some ‘attentional’ effects. Alternatively, this reduction may reflect a change in how the nervous system interpreted the vibration-induced spindle volley. When vibration was given alone, the spindle discharge may have been perceived as arising from all of the test digits. With the additional skin stimulation, the same spindle afferent volley may have been perceived as arising from the joint under the stimulated region of skin, resulting in larger perceived movements at that joint and proportionately smaller movements at more distant joints.
In summary, these experiments show that the CNS continuously integrates feedback from cutaneous and muscle spindle receptors when making kinaesthetic judgements at the MCP joints of the hand. Stimulation of the skin on the dorsal aspect of the MCP joint was sufficient to focus the sensation of movement, but stimulation of the dorsal and ventral skin together had the largest effect. One role for local cutaneous feedback may be to help localise which finger joint is moving. The extent to which these results can be generalised to other joints of the body is yet to be established and it may be that the skin of the hand is unique in its contribution to kinaesthesia.
Acknowledgments
This work was funded by the NH&MRC (Australia), NSERC (Canada) and AHFMR (Canada). We would like to thank Professor D. I. McCloskey and Dr R. Fitzpatrick for helpful comments during preparation of the manuscript.
References
- Bianconi R, Van Der Meulen JP. The response to vibration of the end organs of mammalian muscle spindles. Journal of Neurophysiology. 1963;26:177–190. doi: 10.1152/jn.1963.26.1.177. [DOI] [PubMed] [Google Scholar]
- Biggs J, Horch K, Clark FJ. Extrinsic muscles of the hand signal fingertip location more precisely than they signal the angles of individual finger joints. Experimental Brain Research. 1999;125:221–230. doi: 10.1007/s002210050677. [DOI] [PubMed] [Google Scholar]
- Brown MC, Engberg I, Matthews PBC. The relative sensitivity to vibration of muscle receptors of the cat. The Journal of Physiology. 1967;192:773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, Macefield G. Responses to passive movement of receptors in joint, skin and muscle of the human hand. The Journal of Physiology. 1988;402:347–361. doi: 10.1113/jphysiol.1988.sp017208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration of non-contracting muscles. The Journal of Physiology. 1976;261:673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EG, Horne MK, Rawson JA. Sensory characteristics of monkey thalamic and motor cortex neurones. The Journal of Physiology. 1992;445:1–24. doi: 10.1113/jphysiol.1992.sp018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark FJ, Burgess RC, Chapin JW, Lipscomb WT. Role of intramuscular receptors in the awareness of limb position. Journal of Neurophysiology. 1985;54:1529–1540. doi: 10.1152/jn.1985.54.6.1529. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Grigg P, Chapin JW. The contribution of articular receptors to proprioception with the fingers in humans. Journal of Neurophysiology. 1989;61:186–193. doi: 10.1152/jn.1989.61.1.186. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Horch KW, Bach SM, Larson GF. Contributions of cutaneous and joint receptors to static knee-position sense in man. Journal of Neurophysiology. 1979;42:877–888. doi: 10.1152/jn.1979.42.3.877. [DOI] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. The Journal of Physiology. 1996;496:857–871. doi: 10.1113/jphysiol.1996.sp021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Gandevia SC. Integration of kinesthetic signals from cutaneous and muscle receptors activated by hand movement. Proceedings of the Australian Physiological and Pharmacological Society. 1999;30:28. P. [Google Scholar]
- Collins DF, Refshauge KM, Gandevia SC. Localised stimulation of the dorsal and ventral skin of the hand focuses vibratory-evoked illusions of finger movement in humans. Proceedings of the Australian Neuroscience Society. 2000;11:49. [Google Scholar]
- Craske B. Perception of impossible limb positions induced by tendon vibration. Science. 1977;196:71–73. doi: 10.1126/science.841342. [DOI] [PubMed] [Google Scholar]
- Edin BB. Finger joint movement sensitivity of non-cutaneous mechanoreceptor afferents in the human radial nerve. Experimental Brain Research. 1990;82:417–422. doi: 10.1007/BF00231261. [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. Journal of Neurophysiology. 1992;67:1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. Journal of Neurophysiology. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. The Journal of Physiology. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund G. Position sense and state of contraction: the effects of vibration. Journal of Neurology, Neurosurgery, and Psychiatry. 1972;35:606–611. [Google Scholar]
- Ferrell WR. The adequacy of stretch receptors in the cat knee joint for signalling joint angle throughout a full range of movement. The Journal of Physiology. 1980;299:85–99. doi: 10.1113/jphysiol.1980.sp013112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell WR, Gandevia SC, McCloskey DI. The role of joint receptors in human kinaesthesia when intramuscular receptors cannot contribute. The Journal of Physiology. 1987;386:63–71. doi: 10.1113/jphysiol.1987.sp016522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Illusory movements produced by electrical stimulation of low-threshold muscle afferents from the hand. Brain. 1985;108:965–981. doi: 10.1093/brain/108.4.965. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Kinaesthetic illusions involving the hand which are not dependent on muscle afferents. Proceedings of the Australian Physiological and Pharmacological Society. 1995;25:31. P. [Google Scholar]
- Gandevia SC. Kinesthesia: roles for afferent signals and motor commands. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Exercise, Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 128–172. section 12. [Google Scholar]
- Gandevia SC, Hall LA, McCloskey DI, Potter EK. Proprioceptive sensation at the terminal joint of the middle finger. The Journal of Physiology. 1983;335:507–517. doi: 10.1113/jphysiol.1983.sp014547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI. Joint sense, muscle sense, and their combination as position sense, measured at the distal interphalangeal joint of the middle finger. The Journal of Physiology. 1976;260:387–407. doi: 10.1113/jphysiol.1976.sp011521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhodes JC, Roll JP, Tardy-Gervet MF. Perceptual and motor effects of agonist-antagonist muscle vibration in man. Experimental Brain Research. 1986;61:395–402. doi: 10.1007/BF00239528. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PBC. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. Journal of Neurophysiology. 1988;59:1220–1230. doi: 10.1152/jn.1988.59.4.1220. [DOI] [PubMed] [Google Scholar]
- Grill SE, Hallett M. Velocity sensitivity of human muscle spindle afferents and slowly adapting type II cutaneous mechanoreceptors. The Journal of Physiology. 1995;489:593–602. doi: 10.1113/jphysiol.1995.sp021075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, Hore J, Phillips CG. Inputs from low threshold muscle and cutaneous afferents of hand and forearm to areas 3a and 3b of baboon’s cerebral cortex. The Journal of Physiology. 1976;257:199–227. doi: 10.1113/jphysiol.1976.sp011364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch KW, Clark FJ, Burgess PR. Awareness of knee joint angle under static conditions. Journal of Neurophysiology. 1975;38:1436–1447. doi: 10.1152/jn.1975.38.6.1436. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Thelin A-E, Vallbo Å B. The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. The Journal of Physiology. 1979;291:233–249. doi: 10.1113/jphysiol.1979.sp012809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummelsheim H, Wiesendanger R, Wiesendanger M, Bianchetti M. The projection of low-threshold muscle afferents of the forelimb to the main and external cuneate nuclei of the monkey. Neuroscience. 1985;16:979–987. doi: 10.1016/0306-4522(85)90110-1. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. The Journal of Physiology. 1994;479:487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. The Journal of Physiology. 1981;311:497–519. doi: 10.1113/jphysiol.1981.sp013601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI. Kinesthetic sensibility. Physiological Reviews. 1978;58:763–820. doi: 10.1152/physrev.1978.58.4.763. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Cross MJ, Honner R, Potter EK. Sensory effects of pulling or vibrating exposed tendons in man. Brain. 1983;106:21–37. doi: 10.1093/brain/106.1.21. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. The Journal of Physiology. 1990;429:113–129. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maendly R, Ruegg DG, Wiesendanger M, Wiesendanger R, Lagowska J, Hess B. Thalamic relay for group I muscle afferents of forelimb nerves in the monkey. Journal of Neurophysiology. 1981;46:901–917. doi: 10.1152/jn.1981.46.5.901. [DOI] [PubMed] [Google Scholar]
- Provins KA. The effect of peripheral nerve block on the appreciation and execution of finger movements. The Journal of Physiology. 1958;143:55–67. doi: 10.1113/jphysiol.1958.sp006043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refshauge KM, Kilbreath SL, Gandevia SC. Movement detection at the distal joint of the human thumb and fingers. Experimental Brain Research. 1998;122:85–92. doi: 10.1007/s002210050494. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Experimental Brain Research. 1982;47:177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Cordo PJ, Swinnen SP. Representation of wrist joint kinematics by the ensemble of muscle spindles from synergistic muscles. Journal of Neurophysiology. 1998;79:2265–2276. doi: 10.1152/jn.1998.79.5.2265. [DOI] [PubMed] [Google Scholar]
- Wise AK, Gregory JE, Proske U. The effects of muscle conditioning on movement detection thresholds at the human forearm. Brain Research. 1996;735:125–130. doi: 10.1016/0006-8993(96)00603-8. [DOI] [PubMed] [Google Scholar]
- Xu J, Wall JT. Functional organization of tactile inputs from the hand in the cuneate nucleus and its relationship to organization in the somatosensory cortex. Journal of Comparative Neurology. 1999;411:369–389. [PubMed] [Google Scholar]


