Abstract
The neuronal nicotinic subunit β3 forms functional receptors when co-expressed with both an α and a β subunit, such as α3 and β4. We examined the subunit stoichiometry of these ‘triplet’α3β4β3 receptors by expression in Xenopus oocytes of the α3, β4 and β3 subunits, either in wild-type form or after insertion of a reporter mutation.
The mutation chosen was the substitution of a conserved hydrophobic residue in the second transmembrane domain of the subunits (leucine or valine 9′) with a hydrophilic threonine. In other ion channels within the nicotinic superfamily, this mutation type consistently increases the potency of agonists. In muscle-type nicotinic receptors, the magnitude of this effect is approximately constant for each mutant subunit incorporated.
In α3β4β3 receptors, the ACh EC50 was decreased by approximately 17-fold when this mutation was in α3 alone and only by fourfold when β3 alone was mutated. Mutating β4 was equivalent to mutating α3, suggesting that the ‘triplet’ receptor contains one copy of β3 and two copies each of α3 and β4.
Mutating β3 and α3 or β3 and β4 reduced the ACh EC50 further, to values two- to threefold lower than those seen when only α3 or β4 carried the mutation.
In ‘pair’α3β4 receptors (known to contain two α and three β subunits), mutating β4 had a greater effect on the ACh EC50 than mutating α3, in agreement with an α:β ratio of 2:3 and a constant and independent effect of each copy of the mutation.
Our results suggest that α3β4β3 neuronal nicotinic receptors contain one copy of β3 and two copies each of α3 and β4 and confirm that in pair α3β4 receptors the α/β subunits are present in a 2:3 ratio.
The muscle-type nicotinic acetylcholine receptor (nAChR) is the best characterised of all ligand-gated ion channels. For this receptor, we know both its subunit composition (α, β, δ and ε in adult muscle) and subunit stoichiometry (two copies of α and one each of β, δ and ε). Despite the homology between neuronal and muscle receptor subunits and the likely similarity in the pentameric structures of the assembled muscle and neuronal receptors, the stoichiometry and subunit composition of neuronal nAChRs are still open questions. This is because we have to deal not with one, but with several distinct types of native neuronal nAChRs, and also because the number of combinations theoretically possible (starting from the twelve types of neuronal nicotinic subunits cloned, α2 to α10 and β2 to β4) is very high (McGehee & Role, 1995).
Some simplifying assumptions can be made, as only some of the possible combinations can form functional channels when expressed in heterologous systems; for instance, in Xenopus oocytes some α subunits (α7 to α9) can form homomeric channels, whereas most of the other α subunits (namely α2-α4 and α6) can form functional channels only if co-expressed with a β subunit (either β2 or β4). The latter type of receptor (which we shall refer to as a ‘pair’ receptor) is a pentamer with an α:β stoichiometry of 2:3, as shown by radiolabelling and single channel studies on chick α4β2 receptors expressed in oocytes (Anand et al. 1991; Cooper et al. 1991).
Nevertheless, extrapolating the ‘either homomer or pair’ rule to native receptors may be a serious oversimplification; the fact that one (or two) subunits are sufficient to produce a functional nicotinic receptor does not mean that native receptors are necessarily made in this minimally functional fashion, by just one or two types of subunit. For instance, the main synaptic nAChR in chick parasympathetic ciliary ganglia is made up of three or four different subunits, α3, β4 and α5 (with or without β2; Vernallis et al. 1993; Conroy & Berg, 1995); the situation is even more complex in chick sympathetic ganglia (Listerud et al. 1991; Yu & Role, 1998a,b). In the central nervous system, an example of complexity comes from cerebellar nAChRs, most of which contain three different β subunits, including β3 (Forsayeth & Kobrin, 1997; for a review see Sivilotti et al. 2000). Furthermore, the simple homomer or pair scheme does not account for subunits such as α5 and β3, which can only be assembled into functional receptors when co-expressed with another two different subunits, for instance as α4β2α5 or α3β4β3 receptors (which we shall refer to as ‘triplet’ receptors). Incorporation of α5 into functional receptors may manifest itself by changes in the agonist sensitivity, single channel conductance or desensitisation properties of the pair nAChR upon co-expression of α5 in oocytes (Ramirez-Latorre et al. 1996; Sivilotti et al. 1997; Gerzanich et al. 1998; Nelson & Lindstrom, 1999). For β3, we have recently shown that insertion of a reporter mutation in β3 changes the sensitivity to ACh and the relative maximum response to nicotine of triplet α3β4β3 receptors (Groot-Kormelink et al. 1998).
Nothing is known of the stoichiometry of such triplet neuronal nAChRs. We now address this question for recombinant, oocyte-expressed α3β4 +β3 receptors by an extension of the reporter mutation technique that we used to show β3 incorporation. The residue chosen for the mutation is the highly conserved leucine (Leu) approximately in the middle of the sequence of the pore-lining second transmembrane domain (TM2) of receptor subunits in the nicotinic superfamily; in the TM2 numbering of Miller (1989), this is the 9th residue downstream from the NH2-terminal end of TM2 (i.e. 9′; see Fig. 1). The precise role of this residue is as yet controversial; it may provide a gate to the channel which would be kept shut by the hydrophobic interaction between the Leu residues (Unwin, 1993), or may be situated extracellularly to the gate (Akabas et al. 1994). Despite this uncertainty of the role of 9′ Leu, the effect of swapping the hydrophobic Leu with a more hydrophilic amino acid, such as serine (Ser) or threonine (Thr), is clear and remarkably consistent across the whole nicotinic superfamily, leading to a decrease in the agonist EC50 on the mutated receptor (Revah et al. 1991; Yakel et al. 1993). The magnitude of this increase in agonist potency depends on the number of mutated subunits incorporated into the muscle-type nAChR (Labarca et al. 1995; Filatov & White, 1995), each additional copy of the mutation making a similar contribution that is independent of which subunit carries the mutation. The independent and progressive effect of increasing the number of mutant subunits can be expected to make mutations in 9′ Leu useful for investigating subunit stoichiometry (see for instance Chang et al. 1996). As well as the decrease in agonist EC50, there are other changes in receptor properties, namely a slowing of desensitisation (possibly due to a desensitised state becoming conducting) and an increase in spontaneous openings. The magnitude of these effects is also directly related to the number of 9′ Leu mutations carried by the receptor and to the nature of the mutation, being greater for a Leu to Ser than for a Leu to Thr mutation (Revah et al. 1991; Chang & Weiss, 1998, 1999).
Figure 1. Sequences of the TM2 region for the human neuronal nicotinic α3, β4 and β3 subunits.

In this alignment of the TM2 region the conserved hydrophobic residue in 9 (Leu or Val) is boxed and marked by an arrow. The numbering of the TM2 domain follows Miller (1989).
We have examined the ACh sensitivity of α3β4 +β3 receptors containing mutated subunits and compared it with that of wild-type receptors. We chose to use a Leu to Thr mutation, as our previous work indicated that this mutation produced a sufficient shift in the dose-response curve (Groot-Kormelink et al. 1998). An additional advantage of using a Thr mutation is that it produced no observable increase in spontaneous openings. As the mutation was introduced in each of the different subunits or in combinations of more than one subunit, there was a marked, regular progression in the magnitude of the potency shift produced, suggesting that each copy of the mutation had an approximately constant and independent effect. Analysis of our results indicated that triplet receptors formed by α3 and β4 plus β3 contain one copy of β3 and two copies each of α3 and β4 and confirmed that in pair receptors the α/β subunits are present in a 2:3 ratio.
METHODS
Construction of cRNA for oocyte expression
cDNAs for the human α3, β3 and β4 (GenBank accession numbers Y08418, Y08417 and Y08416, respectively), containing only coding sequences and an added Kozak consensus sequence (GCCACC) immediately upstream of the start codon (Groot-Kormelink & Luyten, 1997), were subcloned into the pSP64GL vector, which contains 5′ and 3′ untranslated Xenopusβ-globin regions (Akopian et al. 1996). Constucts mutated in 9′ (α3Leu279Thr, β3Val273Leu, β3Val273Thr and β4Leu272Thr, where Val stands for valine) were created using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) and their full-length sequence verified. These mutants shall be referred to hereafter as α3LT, β3VL, β3VT and β4LT, respectively. All cDNA/pSP64GL plasmids were linearised immediately downstream of the 3′ untranslated β-globin sequence, and cRNA was transcribed using the SP6 Mmessage Mmachine kit (Ambion). cRNA quality and quantity were checked by gel-electrophoresis and comparison with RNA concentration and size markers.
Xenopus oocyte preparation and electrophysiological recording
Female Xenopus laevis frogs were anaesthetised by immersion in neutralised ethyl m-aminobenzoate solution (tricaine, methanesulphonate salt; 0.2% solution weight/volume; Sigma Chemical Co.), and killed by decapitation and destruction of the brain and spinal cord (in accordance with Home Office guidelines) before removing the ovarian lobes. Clumps of stage V-VI oocytes were dissected in a sterile modified Barth's solution of composition (mm): NaCl 88; KCl 1; MgCl2 0.82; CaCl2 0.77; NaHCO3 2.4; Tris-HCl 15; with 50 U ml−1 penicillin and 50 μg ml−1 streptomycin; pH 7.4 adjusted with NaOH. The dissected oocytes were treated with collagenase (type IA, Sigma Chemical Co; 65 min at 18°C, 245 collagen digestion units ml−1 in Barth's solution, 10-12 oocytes per ml), rinsed, stored at 4°C overnight, and manually defolliculated the following day before cRNA injection (46 nl per oocyte). The oocytes were incubated for approximately 60 h at 18°C in Barth's solution containing 5% heat-inactivated horse serum (Gibco BRL; Quick & Lester, 1994), then stored at 4°C. Experiments were carried out at a room temperature of 18-20°C between 2.5 and 14 days from injection.
cRNA was injected at a ratio of 1:1 in order to express α3β4 receptors, and at a ratio of 1:1:20 (α3:β4:β3) in order to express α3β4β3 receptors. We previously found (Groot-Kormelink et al. 1998) that when a 1:1:1 ratio of cRNA was used for the α3β4 and β3 subunits, the major part of the agonist-induced current was carried through β3-containing receptors; when a 1:1:20 ratio was used, the proportion of current carried through non-β3-containing receptors was too low to be quantified by fitting multiple-component Hill curves. Consequently, in the present study we chose a 1:1:20 ratio for triplet expression. The amount of cRNA to be injected (in 46 nl of RNase-free water) for each combination was determined empirically, with the aim of achieving a maximum ACh-evoked current of 1.5-2 μA. The maximum amount of cRNA injected per oocyte was 5-10 ng (wild-type combinations and triplet combinations containing more than one Thr-mutant subunit and the α3β4β3VT combination). Lower amounts were sufficient for other combinations (0.25-1 ng).
Oocytes, held in a 0.2 ml bath, were perfused at 4.5 ml min−1 with modified Ringer solution (mm: NaCl 150, KCl 2.8, Hepes 10, MgCl2 2, 0.5 μm atropine sulphate, Sigma Chemical Co.; pH 7.2 adjusted with NaOH) and voltage clamped at -70 mV, using the two-electrode clamp mode of an Axoclamp-2B amplifier (Axon Instruments). Electrodes were pulled from Clark borosilicate glass GC150TF (Warner Instrument Corp) and filled with 3 m KCl. The electrode resistance was 0.5-1 MΩ on the current-passing side. The bath was grounded via an Ag-AgCl pellet. Experiments were terminated if the total holding current exceeded 2 μA, in order to reduce series resistance errors; the typical grounding and access resistance of our recording conditions (< 1 kΩ) would cause a worst-case clamp error smaller than 2 mV. We chose a nominally Ca2+-free solution in order to minimise the contribution of Ca2+-gated chloride conductance which is endogenous to the Xenopus oocyte and may be activated by Ca2+ entry through the neuronal nicotinic channels (see for an example Sands et al. 1993).
The agonist solution (acetylcholine chloride, Sigma Chemical Co.; freshly prepared from frozen stock aliquots) was applied via the bath perfusion for a period sufficient to obtain a stable plateau response (at low concentrations) or the beginning of a sag after a peak (at the higher concentrations); the resulting inward current was recorded on a flat bed chart recorder (Kipp & Zonen) for later analysis. A minimum interval of 5 min was allowed between ACh applications, as this was found to be sufficient to ensure reproducible responses. In order to compensate for possible decreases in agonist sensitivity throughout the experiment, a standard concentration of ACh (approximately EC20 for the particular combination used) was applied every third response. The experiment was started only after checking that this standard concentration gave reproducible responses. The average changes in the response to this ACh standard concentration observed by the end of the experiment for the different combinations ranged from a 46 ± 6% decrease (α3LTβ4) to a 19 ± 11% increase (α3β4LTβ3VT; data expressed as a percentage of the initial response). All the data shown in the paper are compensated for the response rundown; however, we found that applying this compensation did not affect the conclusions of our work, as the results of analysing the original data (without compensation) were similar (data not shown).
Experimental controls
A series of control experiments showed that injection of one subunit alone (wild-type or mutant, namely α3, α3LT, β3, β3VL, β3VT, β4, β4LT) or of pair combinations of β3 with either α3 (α3β3, α3β3VL, α3β3VT, α3LTβ3, α3LTβ3VL, α3LTβ3VT) or β4 (β4β3, β4β3VL, β4β3VT, β4LTβ3, β4LTβ3VL, β4LTβ3VT) did not result in the expression of functional nAChRs (i.e. current responses to 1 mm ACh were less than 5 nA; n = 5-8 for each combination tested; total cRNA injected 10 ng; 1:1 ratio for the pairwise combinations).
Holding current in oocytes expressing mutant receptors
The introduction of 9′ hydrophilic mutations into receptors of the nicotinic superfamily can increase the rate of spontaneous openings, i.e. openings of the channel in the absence of agonist (Chang & Weiss, 1998, 1999). We failed to observe such an effect: there was no significant increase in the average oocyte holding current (IH) at the start of the experiment (Table 1) with the introduction of progressively higher numbers of mutant subunits. This lack of correlation between holding current and putative number of mutated subunits per receptor was maintained even if the holding current was normalised to the maximum agonist-induced current in each oocyte (data not shown). Clearly application of a selective channel blocker (or an inverse agonist if available) is the only reliable way to measure the proportion of holding current caused by spontaneous nAChR channel openings, especially against a background of variability in holding current between one oocyte and the next and between different batches of oocytes. Nevertheless, it would seem that any effect on spontaneous channel openings of the introduction of up to three 9′ hydrophilic substitutions is too small to be detected in the Xenopus oocyte system.
Table 1.
Effects of 9′ mutations in different subunits on the concentration-response curve to ACh
| EC50 (μm) | Imax(nA) | nH | Dose ratio | Square root of dose ratio | Cube root of dose ratio | IH (nA) | n | |
|---|---|---|---|---|---|---|---|---|
| α3β4β3wt | 242.7 ± 44.1 | 1333 ± 373 | 1.42 ± 0.1 | — | — | — | 300 ± 83.4 | 6 |
| α3β4β3VL | 163.1 ± 13.4 | 305.7 ± 69.3 | 1.47 ± 0.08 | 1 | — | — | 230 ± 63.1 | 4 |
| [159.5–189.8] | ||||||||
| α3β4β3VT | 43.4 ± 2.2 | 240.9 ± 41.3 | 1.28 ± 0.04 | 4.12 | — | — | 175 ± 23.5 | 10 |
| [3.77–4.52] | ||||||||
| α3LTβ4β3VL | 10.5 ± 1.4 | 993 ± 157 | 1.14 ± 0.06 | 17.1 | 4.14 | — | 148 ± 56.3 | 4 |
| [14.9–19.7] | [3.86–4.44] | |||||||
| α3β4LTβ3VL | 9.54 ± 2.1 | 963.6 ± 107.1 | 1.12 ± 0.06 | 21.9 | 4.68 | — | 90 ± 15.8 | 4 |
| [18.6–25.6] | [4.31–5.06] | |||||||
| α3LTβ4β3VT | 4.49 ± 0.37 | 1866 ± 276 | 1.13 ± 0.04 | 41.6 | — | 3.46 | 80 ± 20.4 | 4 |
| [37.6–46.0] | [3.35–3.58] | |||||||
| α3β4LTβ3VT | 3.22 ± 0.53 | 592 ± 177 | 0.94 ± 0.04 | 50.3 | — | 3.69 | 83 ± 23 | 4 |
| [43.7–58.0] | [3.52–3.87] | |||||||
| α3β4wt | 180 ± 17.0 | 1430 ± 425 | 1.81 ± 0.09 | 1 | — | — | 184 ± 57.2 | 7 |
| [163.6–193.9] | ||||||||
| α3Ltβ4 | 5.8 ± 1.0 | 1043 ± 411 | 1.15 ± 0.08 | 37.3 | 6.11 | — | 85 ± 28.7 | 6 |
| [31.2–44.6] | [5.58–6.68] | |||||||
| α3β4LT | 0.75 ± 0.05 | 562 ± 264 | 0.92 ± 0.08 | 292 | — | 6.63 | 200 ± 83 | 4 |
| [267–318] | [6.44–6.83] |
EC50, Imax and hH are the means (± s.d. of the mean) of parameter estimaes obtained by fitting separately each concentration-response curve with a Hill equation. Dose ratios were estimated from fits in which curves were constrained to have the same slope (Figs 2B, 3B and 4B) and are expressed in relation to receptors containing 5 leucines in 9′ (which onsequently have dose ratio values of 1). The same parallel fits allowed estimation of 2.01-unit likelihood intervals (in square brackets under the means of the parameters they refer to; see Methods): these are equivalent to 95% confidence intervals. The superscript wt stands for the wild-type.
Curve fitting
All dose-response curves were fitted with the Hill equation:
| (1) |
where I is the response, measured at its peak, [A] is the agonist concentration, Imax is the maximum response, EC50 is the agonist concentration for 50% maximum response and nH is the Hill coefficient. We used least squares fitting by the program CVFIT, (courtesy of D. Colquhoun and I. Vais, available from http://www.ucl.ac.uk/pharmacology/dc.html).
Fitting was done in stages, as follows. Each dose-response curve was fitted separately, individual responses being equally weighted, in order to obtain estimates for Imax, EC50 and nH. The means and standard deviation of the mean for each combination are shown in Table 1.
Each response in a particular oocyte was then normalised to the fitted Imax for that experiment; all the normalised responses for a given combination were then pooled, giving the data points shown by the symbols in B and C of Fig. 2–4. The pooled normalised data points were then fitted again with the Hill equation (with weight given by the reciprocal of their variance), either in a free fit (see Fig. 2C, 3C and 4C) or with the curves constrained to be parallel (i.e. to have equal Hill slopes, displayed in Fig. 2B, 3B and 4B). The constrained fits to the normalised pooled experiments were used to estimate the horizontal distance between the dose-response curves (i.e. the dose ratio) obtained from receptors carrying different 9′ mutations (Table 1). Dose ratios were expressed relative to either triplet or pair receptors which had only Leu in 9′ (which therefore had dose ratios of 1; as the dose-response curves for the mutant receptors were shifted to the left, dose ratios for the mutant receptors were greater than 1). These constrained fits allowed the estimation of 2.01-unit likelihood intervals for the dose ratios (or the EC50 for the two reference combinations, α3β4 and α3β4β3VL); these correspond to confidence limits (± 2 standard deviations) for a normally distributed variable (Colquhoun & Sigworth, 1995).
Figure 2. Agonist sensitivity of ‘triplet’α3β4β3 nicotinic receptors containing 9′ Thr mutations in α3 and/or β3.
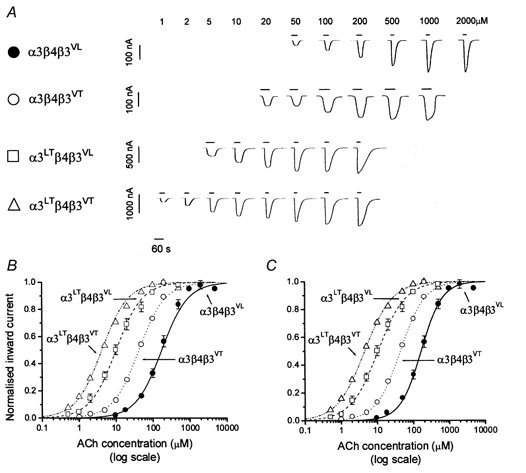
A, examples of inward currents elicited by a representative range of ACh concentrations (see values above the application bars) in oocytes expressing α3β4β3VL, α3β4β3VT, α3LTβ4β3VL or α3LTβ4β3VT. ACh was bath-applied to oocytes clamped to a holding potential of -70 mV. B and C show ACh concentration-response curves pooled from experiments such as the ones shown in A (n = 4-10 oocytes): each response was normalised to the maximum current evoked by ACh in that oocyte (see Methods). Pooled normalised results were fitted with the Hill equation either as a free fit (C) or under the constraint of equal slopes (B) in order to estimate the horizontal distance between the curves as a dose ratio (see Table 1). The concentration-response curves shown refer to oocytes injected with α3β4β3VL (•), α3β4β3VT (○), α3LTβ4β3VL (□) and α3LTβ4β3VT (▵); bars show standard deviation of the mean (when larger than the symbol). Note the progressive increase in the potency of ACh as the 9′ Thr mutation was introduced in β3, α3 alone or α3 and β3.
Figure 4. Agonist sensitivity of ‘pair’α3β4 nicotinic receptors containing 9′ Thr mutations in α3 or β4.
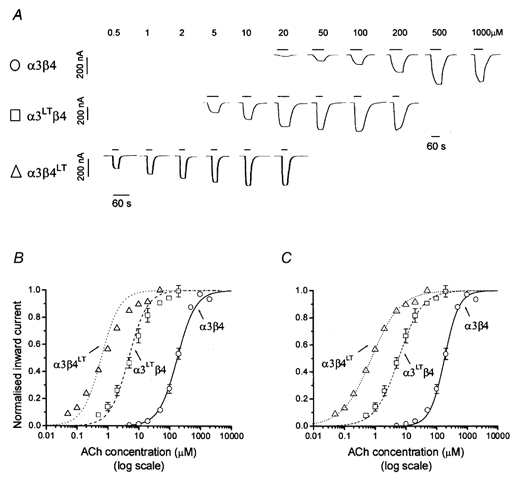
A, examples of inward currents elicited by bath-applied ACh in oocytes expressing α3β4, α3LTβ4 or α3β4LT. B and C show ACh concentration-response curves pooled from experiments such as the ones shown in A (n = 4-7 oocytes): each response was normalised to the maximum current evoked by ACh in that oocyte (see Methods). Pooled normalised results were fitted with the Hill equation either as free fit (C) or under the constraint of equal slopes (B) in order to estimate the horizontal distance between the curves as a dose ratio (see Table 1). The concentration-response curves refer to oocytes injected with α3β4 (○), α3LTβ4 (□) or α3β4LT (▵). Note that the greater increase in ACh sensitivity was produced by mutating β4.
Figure 3. Agonist sensitivity of ‘triplet’α3β4β3 nicotinic receptors containing 9′ Thr mutations in β4 and/or β3.
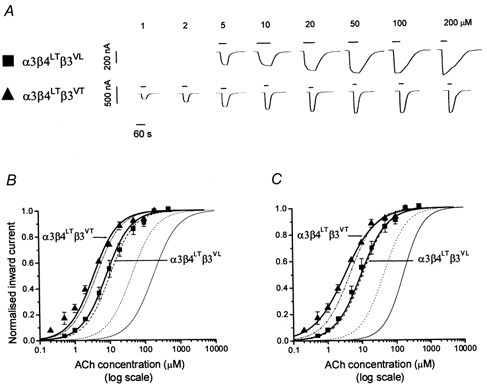
A, examples of inward currents elicited by a representative range of ACh concentrations in oocytes expressing α3β4LTβ3VL or α3β4LTβ3VT. ACh was bath-applied to oocytes clamped at -70 mV. B and C show ACh concentration-response curves pooled from experiments such as the ones shown in A (n = 4 oocytes): each response was normalised to the maximum current evoked by ACh in that oocyte (see Methods). Pooled normalised results were fitted with the Hill equation either as free fit (C) or under the constraint of equal slopes (B) in order to estimate the horizontal distance between the curves as a dose ratio (see Table 1). The concentration-response curves shown refer to oocytes injected with α3β4LTβ3VL (▪) or α3β4LTβ3VT (▴). B and C also show for reference (curves with no symbols, reproduced from Fig. 2) the concentration-response curves relating to 9′ Thr mutations in α3 and/or β3. Note that the effect of mutating β4 is similar to that of mutating α3.
RESULTS
α3β4β3: comparing β3VL with β3wt
In all subunits belonging to the nicotinic superfamily, the midpoint (9′) residue in the second transmembrane domain is highly conserved, being occupied by a hydrophobic amino acid. This is a Leu in all vertebrate nicotinic subunits, except for β3 and α5, which have a Val in 9′ (position 273 for β3; see Fig. 1). In homomeric receptors formed by subunits which have Leu in 9′, introducing a Val in this position increases agonist sensitivity, slows desensitisation (see for homomeric α7 nAChR, Revah et al. 1991) and increases spontaneous openings (for GABAC 1 receptors, see Chang & Weiss, 1998). Such effects of a Leu to Val mutation in 9′ are smaller, but similar to those of the Leu to Thr mutation we planned to use in our investigation. Additionally, the crucial assumption for the interpretation of our experiments in terms of stoichiometry is that the main determinant for the effect of the Thr mutations on agonist EC50 should be their copy number in the assembled receptor complex. This assumption is validated by data obtained for receptors that have only Leu in the 9′ position (such as muscle nAChR, see Labarca et al. 1995; see also our data on the α3β4 receptor below and the Discussion). It was therefore considered possible that the presence of one or more non-Leu residues in the 9′ position (which would be contributed by wild-type β3 subunits to the assembled triplet receptor) might confound the effect of progressively introducing 9′ Thr in the different subunits. This would reduce the sensitivity of our experimental design. In order to avoid this potential problem, we introduced a Val273Leu (i.e. V9′L) mutation in β3, and used the β3VL rather than β3wt in all the experiments (unless otherwise specified).
The ACh sensitivity of α3β4β3wt receptors (see Groot-Kormelink et al. 1998) was compared with that of α3β4β3VL receptors and – contrary to expectations – found to be consistently increased in α3β4β3VL receptors (see Table 1). As the decrease in the EC50 of ACh was relatively small (from 242.7 ± 44.1 to 163.1 ± 13.4 μm), it was still thought preferable to use β3VL rather than β3wt in the work described below, in order to introduce subunits carrying the 9′ Thr mutation into receptors which only had Leu in the 9′ position.
Mutating α3 has a greater effect than mutating β3 in α3β4β3 receptors
Figure 2 show the result of introducing the 9′ Thr mutation in either the β3 or the α3 subunits. Figure 2A shows representative responses elicited by a range of ACh concentrations in oocytes injected with the different combinations: the concentration-response curves are shown in Fig. 2B and C. Figure 2B shows fits in which the Hill slope was constrained to be the same for all curves: these were used to obtain estimates of the distance between the curves and the 2-unit likelihood intervals for the EC50 values (expressed in Table 1 as dose ratios, see Methods).
As we previously reported (Groot-Kormelink et al. 1998), the introduction of β3VT shifted the concentration- response curve of ACh for the triplet α3β4β3VT receptor to the left, relative to the receptor containing β3wt. When the triplet containing β3VT is compared to the triplet containing only Leu in 9′ (i.e. α3β4β3VL), the shift is by a factor of approximately 4 (see Table 1).
The effect of introducing the mutation in α3, rather than in β3, was considerably larger, producing a further fourfold increase in the potency of ACh on α3LTβ4β3VL receptors compared to α3β4β3VT receptors. When α3LTβ4β3VL receptors were compared to receptors with only Leu in 9′ (α3β4β3VL), they were found to be 17-fold more sensitive to ACh.
If both β3 and α3 carried the 9′ Thr mutation, the concentration-response curve for ACh shifted even further to the left, with an additional decrease in the ACh EC50 of about threefold. Thus the introduction of β3VT alone, α3LT alone and α3LT together with β3VT into the α3β4β3VL receptor produced a progressive change in ACh sensitivity, with stepwise three- to fourfold increases.
Mutating β4 is equivalent to mutating α3 in α3β4β3 receptors
If the α3β4β3 receptor contains two copies of α3 and one of β3, it follows that the receptor should also contain two copies of β4, in order to make up the total subunit number of five. This would predict that mutating β4 should have the same effect as mutating α3. This question was addressed by the next set of experiments, which are shown in Fig. 3.
Introducing β4LT (alone or with β3VT) in the α3β4β3VL receptor had the same effect as introducing α3LT; the concentration-response curves for the β4LT-containing receptors (Fig. 3B and C) were very close to those for the α3LT-containing receptors (shown for reference as dashed curves in Fig. 3B and C); indeed the 2-unit likelihood intervals for the EC50 values (Table 1) overlap both for α3LT or β4LT alone and for α3LT or β4LT together with β3VT. Again the simplest explanation for these results is that α3β4β3 receptors contain two copies of α3 and β4, but only one copy of the β3 subunit.
Mutating β4 is not equivalent to mutating α3 in α3β4 receptors
Further confirmation that the effect of the mutation on ACh sensitivity only depends on the number of mutant subunits in a nicotinic receptor comes from another series of experiments. Here the reporter mutation 9′ Thr was introduced in either subunit of pair α3β4 receptors. The rationale for this is that pair neuronal nicotinic receptors are known to contain α:β subunits in a 2:3 ratio (see for chick α4β2 receptors, Anand et al. 1991; Cooper et al. 1991). If our hypothesis is correct and the effect of the mutation is independent of which subunit type carries it, the increase in ACh potency (compared with the wild-type pair receptors) should be greater when the mutation is introduced in β4 than when the mutation is introduced in α3. This is indeed what was found, as shown in Fig. 4; α3β4LT receptors were about sixfold more sensitive to ACh than α3LTβ4 receptors, which in turn were 37-fold more sensitive than α3β4 receptors (see dose ratio values in Table 1). This is consistent with the receptor containing one more copy of β4 than α3, i.e. three copies of β4 to two copies of α3 in the pentameric α3β4 receptor.
If the effect of each hydrophobic to hydrophilic substitution in 9′ is independent of position and multiplicative on the ACh EC50 (i.e. additive in terms of free energy), then plotting the logarithm of the ACh EC50 against the presumed number of mutations in each pentamer channel should yield a linear plot. Figure 5 shows that this is indeed the case both for the α3β4 pair (Fig. 5B) and for the α3β4β3 triplet receptors (Fig. 5A), both of which show an approximately linear relation between log EC50 and the putative number of mutations in the receptor. Figure 5 also shows clearly that the effect of the mutation did not saturate up to a presumed mutation copy number of three (note, however, the apparent shallowing of the effect in the triplet receptor passing from two to three mutations).
Figure 5. Relation between the ACh EC50 of the different combinations and their putative number of 9′ Thr mutations.
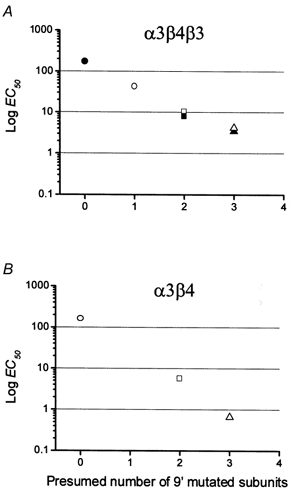
EC50 values are plotted on a log scale against the number of mutant subunits in each combination. This number was calculated on the basis of a subunit stoichiometry of 2:2:1 for the triplet α3β4β3 (A) and 2:3 for the pair α3β4 (B). Symbols as in Figs 2–4. Note the approximate linearity of the decline in EC50 for both types of receptor.
DISCUSSION
The data presented above show that introducing a hydrophobic to hydrophilic mutation in the 9′ position of TM2 of neuronal nicotinic subunits increases the agonist sensitivity of the resulting nAChR. When the receptor expressed is an α3β4β3 triplet, mutations in α3 or β4 are equivalent in effect, and they shift the ACh dose- response curve to the left by an amount log(r) (where r is the dose ratio of Table 1) that is approximately twice the shift observed if β3 is mutated. If the mutation is inserted in both the β3 and the α3 (or β4) subunits, the magnitude of the resulting log(r) shift is approximately the sum of the shifts caused by each mutation separately. These results suggest that, in the triplet nAChR α3β4β3, the α3, β4 and β3 subunits are present in a 2:2:1 ratio.
Assumptions of the reporter mutation approach
The first condition for the validity of our interpretation of the data is that the reporter mutation should not affect the correct assembly and therefore the subunit stoichiometry of the neuronal nAChR. The reporter mutation we chose is in TM2, i.e. in the pore-lining region. Mutations in this area are not expected to influence assembly directly, since the sequence determinants for subunit assembly are not in the pore-lining region, but in the NH2-terminal domain preceding the first transmembrane domain (Gu et al. 1991; Yu & Hall, 1991; Sumikawa, 1992; Kreienkamp et al. 1995). An indirect, long-range effect of a channel mutation on the assembly cassette cannot in principle be excluded, but would appear to be unlikely, as no such effect was reported by the numerous studies which have mutated TM2 residues in order to establish which amino acids are accessible from the open or closed pore and how these residues influence nAChR channel conductance and ionic permeability. For instance, if muscle nAChR carrying 9′ mutations had a stoichiometry different from the normal (2:1:1:1 for α:β:γ:δ, respectively), detectable changes in single-channel conductance would be expected, because of the differences in the TM2 domains of the α, β, γ, δ-subunits (see Imoto et al. 1988): these are not seen when 9′ is mutated to Ser, Thr or isomers of Leu (Labarca et al. 1995; Filatov & White, 1995; Kearney et al. 1996). Evidence that the 9′ Thr mutation does not significantly affect the ligand-binding site is provided by the observation that the binding of the competitive antagonist (+)-tubocurarine to recombinant muscle nAChRs is not affected by introducing the 9′ Thr mutation in the γ-subunit (Filatov & White, 1995).
In order to be able to interpret our results reliably, in terms of subunit stoichiometry, other conditions must also be verified. In particular, the overall effect of the reporter mutation must depend only on the number of mutations incorporated in the channel, not on the type of subunit that carries the mutation. Secondly, the relation between number of mutants and magnitude of the observed change in ACh potency must be a simple one, the ideal case being that the effect of each additional mutation is independent of the number of mutations already in the channel. Thirdly, the receptors considered must have uniform stoichiometry.
Comparison with other receptors in the nicotinic superfamily
These criteria have been shown to hold true for the muscle nAChR: in this receptor (which has a stoichiometry of 2:1:1:1 for α:β:γ:δ, respectively) the introduction of a progressively greater number of 9′ Ser or 9′ Thr mutations has a consistent effect on ACh EC50, an effect which is independent and similar for each additional mutation. Each Ser in 9′ produced roughly a 10-fold shift in EC50: there was no overlap between the range of EC50 observed for combinations with the different mutation copy numbers (Labarca et al. 1995). In a further analysis of muscle nAChR, Kearney et al. (1996) have investigated the lack of full subunit equivalence (probably due to asymmetry in the pore and interactions between adjacent subunits) by introducing in 9′ a wide range of unnatural amino acid residues with progressive increments in side-chain length or branching.
The situation appears quite different for the GABAA receptor. Introduction of 9′ Leu to Ser mutations in α1β2γ2 GABAA recombinant receptors is very effective in increasing the receptor sensitivity to GABA, but the effectiveness of each mutation depends on which subunit type carries it. Consequently, stoichiometry could not be deduced from the EC50 shifts directly, but had to be confirmed by ascertaining how many dose-response components result from injecting mixtures of cRNA for the wild-type and mutant subunits (Chang et al. 1996). In GABAA receptors, a further complication is that the shift in EC50 saturates when more than two subunits carry the mutation (Chang & Weiss, 1999).
There is another confounding factor, which in principle may impinge differently on different receptors (and perhaps explain some of the discrepancies observed). The linearity and multiplicativity of the effect of each mutated subunit that we observed would be expected if the mutation produces linearly additive changes in the free energy of one or more steps of channel activation. That is because free energy values are related to the logarithm of rate (and equilibrium) constants. However, 9′ hydrophilic mutations are also known to affect desensitisation: changes in desensitisation should also alter the observed agonist EC50 to an extent which may depend on the impact of desensitisation on the wild-type dose-response curve. Since the extent to which desensitisation affects an agonist dose-response curve is difficult to determine macroscopically, this additional action of the mutation could make the final effect of the mutation unpredictable and estimates of stoichiometry unreliable.
Neuronal nicotinic receptor stoichiometry
There is little evidence to tell us whether the effects of 9′ hydrophilic mutations on neuronal nAChR are predictable like those described for muscle nAChR or resemble those described for GABAA receptors. 9′ hydrophilic TM2 mutations in a neuronal nAChR have been extensively investigated (see Revah et al. 1991) and found to enhance the potency of ACh on α7 receptors. As recombinant α7 receptors are homomeric, these findings cannot address the relation between the magnitude of the shift and the number of mutated subunits. We have therefore ourselves obtained corroborating evidence that in neuronal nAChR the effect of each copy of the 9′ mutation can be approximated as being independent and equivalent. First of all, we have introduced the reporter mutation in pair nAChR; a similar pair combination (chick α4β2) is the only neuronal nicotinic receptor whose stoichiometry has been investigated and is known to contain α and β subunits in a 2:3 ratio (Anand et al. 1991; Cooper et al. 1991). It would seem reasonable to assume that pair combinations of related subunits have the same stoichiometry, irrespective of the species from which they were cloned. We found that in α3β4 receptors, the increase in ACh potency produced by mutating α (which we term rα3) is 37.3-fold (see dose ratio values in Table 1), whereas the increase in ACh potency produced by mutating β (rβ4) is 292-fold (see Table 1). It can therefore be seen that:
The actual values observed were (37.3)1/2= 6.11 and (292)1/3= 6.63, respectively, in good agreement with the prediction. This is what we expect if the effect of each copy of the reporter mutation is equivalent, irrespective of whether it is carried by the α or the β subunits. The graphical counterpart of the numerical example above is the near-linearity of Fig. 5B, which shows the plot of the log(EC50) for ACh against the putative number of mutant subunits, calculated on the basis of a subunit stoichiometry of 2:3 for α3β4.
Further evidence that each additional mutation has a similar effect in neuronal nAChR (irrespective of the number of mutations already incorporated in the pore) comes from another set of our experiments. In α3β4β3 triplets the combined effect of a mutation in β3 together with a mutation in either α3 or β4 is approximately equal to the product of the effect of the two mutations alone (again see the linearity of plot in Fig. 5A).
Neuronal nicotinic receptors commonly display multiple conductance levels (Papke, 1993). In native receptors different levels are linked by direct transitions and therefore are likely to be produced by different states of the same molecular complex (Sivilotti et al. 1997). An alternative explanation is that each level is produced by a receptor with a different stoichiometry; this could account for the differences in conductances observed for the rat α2β2 combination expressed in oocytes using 1:9 and 9:1 α to β ratios (Papke et al. 1989). It is not known whether such receptor mosaics are produced by the combinations we studied; conclusive evidence on this point could come from single-channel recording and the analysis of sublevel transitions within bursts or within clusters of openings separated by desensitised periods. We have, however, assessed the likely effect of the presence of different receptor stoichiometries in the pair receptor by modelling the dose-response relation of such complexes (assuming only that the EC50 is determined by the number of 9′ Thr mutations in the receptor complex). While modelling suggests that such receptor heterogeneity could explain the shallower Hill slope observed in mutant receptors (see below), it also predicted that in this case the EC50 shift produced by mutating α3 should be similar to that produced by mutating β4. This is in clear contrast with our findings that the α3β4LT receptor complex is approximately sixfold more sensitive to ACh than α3LTβ4 (Fig. 4B and C).
These observations substantiate our conclusions that the subunit stoichiometry in the α3β4β3 triplet is indeed 2:2:1. This stoichiometry fits in well with what is known of non-homomeric neuronal nAChRs, namely that their pentameric structure contains at least two agonist binding sites, each requiring the presence of an α subunit; as in the muscle receptor (Colquhoun & Sakmann, 1985), it is likely that both binding sites must be occupied for channel opening to occur effectively. The presence of at least two binding sites (which on average must be occupied for the channel to open) is in good accord with the observation of a Hill slope greater than 1 (1.42 for the wild-type triplet). It is unlikely that β3 can play the role of an α subunit with respect to agonist binding, given that the sequence of the β3 presumed binding domains is very similar to that of the other β subunits.
The slope of the ACh concentration-response curves in mutant receptors
The greatest shifts in agonist sensitivity (i.e. those observed in α3LTβ4β3VT, α3β4LTβ3VT and α3β4LT) were associated with a decrease in the slope of the concentration- response curves. Such changes often result from receptor heterogeneity (the EC50 values for two receptor types have to differ by something approaching 100-fold before the presence of two components becomes detectable in dose-response curves, if the Hill coefficient values are similar to those observed for this type of receptor). While this explanation cannot be discounted in principle for our data, it is difficult to see why receptor heterogeneity should increase with the increase in the number of mutant subunits in the receptor, particularly since the decrease in slope is not limited to the triplet receptors, but can be observed for the α3β4 pair receptors (see the α3β4LT curve, Fig. 4C). Another more likely explanation is that the slope decreases because monoliganded openings contribute more current as the proportion of mutant subunits in the receptor rises. Monoliganded openings are known to occur in muscle nicotinic receptors (although they are relatively rare at all but the lowest concentrations, Colquhoun & Sakmann, 1985); their contribution to the total current can be expected to increase with mutation number, if the reporter mutation does indeed facilitate gating. A receptor which needs the binding of only a single agonist molecule to open can be expected to give rise to an equilibrium dose-response curve with a slope of 1. Hence, a greater contribution of monoliganded openings can be expected to make the curve shallower. Similar effects were observed by Chang & Weiss (1999) in GABAA receptors.
Relevance to native nAChR
For our finding on the stoichiometry of recombinant β3-containing receptors to be relevant to native nAChR, two questions must be answered; firstly whether we can extrapolate from the oocyte expression system to a mammalian neurone and secondly whether the β3 subunit is incorporated into native nAChR together with the α3 and β4 subunits.
On the first point, oocytes do express muscle-type nAChR with an accurate native stoichiometry (Anand et al. 1991). No comparative data are available for neuronal nAChR; nevertheless, the fact that the α4β2 nAChR expressed in oocytes contains two copies of the α subunit (Cooper et al. 1991) does suggest that recombinant heteromeric nAChRs contain two agonist binding sites. The presence of two binding sites in the oocyte-expressed receptor is in agreement with the fact that Hill slope values measured for native nAChR indicate that on average at least two agonist molecules need to bind in order for the channel to open (Covernton et al. 1994). At single-channel level most of oocyte-expressed α3β4 nAChRs differ in conductance and kinetics from native ganglionic receptors (Sivilotti et al. 1997; Lewis et al. 1997; but see Nelson & Lindstrom, 1999). We assume here that whatever it may be that causes discrepancies in conductances and kinetics does not have a major effect on relative potencies or stoichiometry. In support of this view, we have found that, at whole-cell level, oocytes do reproduce the relative agonist potencies seen for native nicotinic receptors with reasonable accuracy (Covernton et al. 1994).
Receptors that contain the α3 and β4 subunits are thought to be mainly expressed in the peripheral nervous system; co-assembly of these two subunits into the same receptor complex has been proved by immunoprecipitation in the chick ciliary ganglion (Vernallis et al. 1993; Conroy & Berg, 1995) and in adult rat trigeminal ganglia (Flores et al. 1996). In rat trigeminal ganglia mRNA for the β3 subunit is also present (Liu et al. 1998; for a review see McGehee & Role, 1995). In the central nervous system, receptors containing the α4 and β2 subunits are likely to play a major role, but this is not exclusive. Indeed, mRNA for the α3, β4 and β3 subunits has been detected in the rat brainstem, i.e. interpeduncular nucleus, thalamus, habenula (in particular in the ventromedial medial habenula, Le Novère et al. 1996; for a pharmacological analysis of these receptors see Quick et al. 1999) and in the locus coeruleus (Léna et al. 1999). Coincidence of expression of the mRNA for these subunits doesn't necessarily imply that they co-assemble into functional receptors, but unfortunately there are few studies examining this issue at protein level. The best evidence comes from the recent demonstration by sequential immunopurification (Vailati et al. 2000) that, in chick retina, the β3 subunit does assemble with a subset of subunits which include α3 and β4. The expression of β3 protein has also been found to be widespread in the rat central nervous system (cerebral cortex, striatum, hippocampus, cerebellum and medulla, Forsayeth & Kobrin, 1997). In addition, immunoprecipitation of nicotinic receptors from the rat cerebellum reveals the presence of a receptor subpopulation containing four different subunits, including β3, namely α4β2β3β4 (Forsayeth & Kobrin, 1997). Note that receptors that contain four different subunits are known to form in the chick ciliary ganglion (Conroy et al. 1995).
Although there is good evidence that β3-containing nAChRs exist in native tissue, it is not yet known whether these native receptors could be triplets (as the recombinant receptors we investigated), i.e. whether they contain only three different subunits instead of, for instance, four (as is the case for muscle). Nevertheless, the presence in triplet receptors of only one copy of β3 (which replaces one of the three β4 subunits in the pair receptors), does argue for the existence of constraints to the stoichiometry of neuronal nAChR, which may extend to ‘quadruplet’ receptors, particularly with regard to the ratio of α to β subunits.
In summary, our results confirm a 2:3 ratio for α3β4 nAChR receptors and indicate that only one copy of β3 is present in α3β4β3 receptors. Bearing in mind the sequence similarity between β3 and α5, which has led to their inclusion in the same subunit tribe (Corringer et al. 2000), it will be important to test whether a 2:2:1 stoichiometry applies more generally, namely to all triplet neuronal nAChRs formed by the assembly of one subunit from tribe III-1 (i.e. α3, but also α2, α4 or α6), one subunit from tribe III-2 (such as β4 or β2) and one subunit from tribe III-3 (β3 or α5). While our data do not shed light on the topology of β3-containing receptors, knowledge of subunit stoichiometry is essential to the investigation of what other β3-containing combinations are possible and of how β3 contributes to the nAChR structure and function.
Acknowledgments
This work was supported by the Wellcome Trust (grant 055524), the Royal Society and the Millennium fund of the School of Pharmacy.
References
- Akabas MH, Kaufmann C, Archdeacon P, Karlin A. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the α subunit. Neuron. 1994;13:919–927. doi: 10.1016/0896-6273(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium-channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. Journal of Biological Chemistry. 1991;266:11192–11198. [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. Journal of Neuroscience. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Weiss DS. Substitutions of the highly conserved M2 leucine create spontaneously opening ρ1 γ-aminobutyric acid receptors. Molecular Pharmacology. 1998;53:511–523. doi: 10.1124/mol.53.3.511. [DOI] [PubMed] [Google Scholar]
- Chang YC, Weiss DS. Allosteric activation mechanism of the α1β2γ2 γ-aminobutyric acid type A receptor revealed by mutation of the conserved M2 leucine. Biophysical Journal. 1999;77:2542–2551. doi: 10.1016/s0006-3495(99)77089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. Journal of Physiology. 1985;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York: Plenum Press; 1995. pp. 483–587. [Google Scholar]
- Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic receptors distinguished by different subunit compositions. Journal of Biological Chemistry. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- Cooper E, Couturier S, Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991;350:235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Corringer P-J, Le Novère N, Changeux J-P. Nicotinic receptors at the amino acid level. Annual Review of Pharmacology and Toxicology. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- Covernton PJO, Kojima H, Sivilotti LG, Gibb AJ, Colquhoun D. Comparison of neuronal nicotinic receptors in rat sympathetic neurons with subunit pairs expressed in Xenopus oocytes. Journal of Physiology. 1994;481:27–34. doi: 10.1113/jphysiol.1994.sp020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov GN, White MM. The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Molecular Pharmacology. 1995;48:379–384. [PubMed] [Google Scholar]
- Flores CM, Decamp RM, Kilo S, Rogers SW, Hargreaves KM. Neuronal nicotinic receptor expression in sensory neurons of the rat trigeminal ganglion: demonstration of α3α4, a novel subtype in the mammalian nervous system. Journal of Neuroscience. 1996;16:7892–7901. doi: 10.1523/JNEUROSCI.16-24-07892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsayeth JR, Kobrin E. Formation of oligomers containing the β3 and β4 subunits of the rat nicotinic receptor. Journal of Neuroscience. 1997;17:1531–1538. doi: 10.1523/JNEUROSCI.17-05-01531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. α5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal α3 nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics. 1998;286:311–320. [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Luyten WHML. Cloning and sequence of full-length cDNAs encoding the human neuronal nicotinic acetylcholine receptor (nAChR) subunits β3 and β4 and expression of seven nAChR subunits in the human neuroblastoma cell line SH-SY5Y and/or IMR-32. FEBS Letters. 1997;400:309–314. doi: 10.1016/s0014-5793(96)01383-x. [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Luyten WHML, Colquhoun D, Sivilotti LG. A reporter mutation approach shows incorporation of the “orphan” subunit β3 into a functional nicotinic receptor. Journal of Biological Chemistry. 1998;273:15317–15320. doi: 10.1074/jbc.273.25.15317. [DOI] [PubMed] [Google Scholar]
- Gu Y, Camacho P, Gardner P, Hall ZW. Identification of two amino acid residues in the ε subunit that promote mammalian muscle acetylcholine receptor assembly in COS cells. Neuron. 1991;6:879–887. doi: 10.1016/0896-6273(91)90228-r. [DOI] [PubMed] [Google Scholar]
- Imoto K, Busch C, Sakmann B, Mishina N, Konno T, Nakai J, Bujo H, Mori Y, Fukuda K, Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988;335:645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Kearney PC, Zhang H, Zhong W, Dougherty DA, Lester HA. Determinants of nicotinic receptor gating in natural and unnatural side chain structures at the M2 9′ position. Neuron. 1996;17:1221–1229. doi: 10.1016/s0896-6273(00)80252-4. [DOI] [PubMed] [Google Scholar]
- Kreienkamp HJ, Maeda RK, Sine SM, Taylor P. Intersubunit contacts governing assembly of the mammalian nicotinic acetylcholine receptor. Neuron. 1995;14:635–644. doi: 10.1016/0896-6273(95)90320-8. [DOI] [PubMed] [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA. Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature. 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- Lena C, d'Exaerde AD, Cordero-Erausquin M, Le Novère N, Arroyo-Jimenez MDM, Changeux J-P. Diversity and distribution of nicotinic acetylcholine receptors in the locus ceruleus neurons. Proceedings of the National Academy of Sciences of the USA. 1999;96:12126–12131. doi: 10.1073/pnas.96.21.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novere N, Zoli M, Changeux J-P. Neuronal nicotinic receptor α6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. European Journal of Neuroscience. 1996;8:2428–2439. doi: 10.1111/j.1460-9568.1996.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Lewis TM, Harkness PC, Sivilotti LG, Colquhoun D, Millar N. Heterologous expression of a neuronal nicotinic receptor yields channels whose properties are dependent on host cell type. Journal of Physiology. 1997;505:299–306. doi: 10.1111/j.1469-7793.1997.299bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listerud M, Brussaard AB, Devay P, Colman DR, Role LW. Functional contribution of neuronal AChR subunits revealed by antisense oligonucleotides. Science. 1991;254:1518–1521. doi: 10.1126/science.1720573. published erratum appears in Science255, 12 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chang CQ, Jiao YQ, Simon SA. Neuronal nicotinic acetylcholine receptors in rat trigeminal ganglia. Brain Research. 1998;809:238–245. doi: 10.1016/s0006-8993(98)00862-2. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- Miller C. Genetic manipulation of ion channels: a new approach to structure and mechanism. Neuron. 1989;2:1195–1205. doi: 10.1016/0896-6273(89)90304-8. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Lindstrom J. Single channel properties of human α3 AChRs: impact of β2, β4 and α5 subunits. Journal of Physiology. 1999;516:657–678. doi: 10.1111/j.1469-7793.1999.0657u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RAJ. α3β4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester HA. Methods for expression of excitability proteins in Xenopus oocytes. Methods in Neurosciences. 1994;19:261–279. [Google Scholar]
- Papke RL. The kinetic properties of neuronal nicotinic receptors: genetic basis of functional diversity. Progress in Neurobiology. 1993;41:509–531. doi: 10.1016/0301-0082(93)90028-q. [DOI] [PubMed] [Google Scholar]
- Papke RL, Boulter J, Patrick J, Heinemann S. Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron. 1989;3:589–596. doi: 10.1016/0896-6273(89)90269-9. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi J-L, Devillers-Thiéry A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux J-P. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- Sands SB, Costa ACS, Patrick JW. Barium permeability of neuronal nicotinic receptor α7 expressed in Xenopus oocytes. Biophysical Journal. 1993;65:2614–2621. doi: 10.1016/S0006-3495(93)81296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti LG, Colquhoun D, Millar N. Comparison of native and recombinant neuronal nicotinic receptors: problems of measurement and expression. In: Clementi F, Gotti C, editors. Neuronal Nicotinic Receptors. Berlin, Heidelberg, New York: Springer-Verlag; 2000. pp. 379–416. [Google Scholar]
- Sivilotti LG, McNeil DK, Lewis TM, Nassar MA, Schoepfer R, Colquhoun D. Recombinant nicotinic receptors, expressed in Xenopus oocytes, do not resemble native rat sympathetic ganglion receptors in single-channel behaviour. Journal of Physiology. 1997;500:123–138. doi: 10.1113/jphysiol.1997.sp022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K. Sequences on the N-terminus of ACh receptor subunits regulate their assembly. Molecular Brain Research. 1992;13:349–353. doi: 10.1016/0169-328x(92)90218-z. [DOI] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 Å resolution. Journal of Molecular Biology. 1993;229:1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Vailati S, Moretti M, Balestra B, Mcintosh M, Clementi F, Gotti C. β3 subunit is present in different nicotinic receptor subtypes in chick retina. European Journal of Pharmacology. 2000;393:23–30. doi: 10.1016/s0014-2999(00)00067-4. [DOI] [PubMed] [Google Scholar]
- Vernallis AB, Conroy WG, Berg DK. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron. 1993;10:451–464. doi: 10.1016/0896-6273(93)90333-m. [DOI] [PubMed] [Google Scholar]
- Yakel JL, Lagrutta A, Adelman JP, North RA. Single amino acid substitution affects desensitization of the 5hydroxytryptamine type 3 receptor expressed in Xenopus oocytes. Proceedings of the National Academy of Sciences of the USA. 1993;90:5030–5033. doi: 10.1073/pnas.90.11.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Role LW. Functional contribution of the α7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. Journal of Physiology. 1998a;509:651–665. doi: 10.1111/j.1469-7793.1998.651bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Role LW. Functional contribution of the α5 subunit to neuronal nicotinic channels expressed by chick sympathetic ganglion neurones. Journal of Physiology. 1998b;509:667–681. doi: 10.1111/j.1469-7793.1998.667bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X-M, Hall ZW. Extracellular domains mediating ε subunit interactions of muscle acetylcholine receptor. Nature. 1991;352:64–67. doi: 10.1038/352064a0. [DOI] [PubMed] [Google Scholar]


