Abstract
The role of skeletal muscle ergoreceptors (afferents sensitive to muscle contraction, differentiated into metaboreceptors, sensitive to metabolic changes, and mechanoreceptors, sensitive to mechanical changes) in the genesis of the increased ventilatory drive in chronic heart failure is controversial. We have aimed to clarify the contribution of muscle metaboreceptors in the leg to ventilation and to compare this with the contribution of mechanoreceptors.
Eighteen heart failure patients and 12 controls were studied. Metaboreceptor and mechanoreceptor responses were measured in the leg by bicycle exercise with and without regional circulatory occlusion during recovery, and by active and equivalent passive limb movement, respectively.
Patients, in comparison with controls, had a lower peak V̇O2 (Oxygen uptake) (18.1 ± 1.6 vs. 24.5 ± 2.5 ml min−1 kg−1, P < 0.05), and an evident metaboreceptor contribution to the ventilatory response (3.5 ± 1.6 vs. −4.0 ± 1.3 l min−1, P < 0.001). Passive limb movement increased ventilation in both patients and controls (+3.7 ± 0.4 and +2.9 ± 0.5 l min−1 from baseline, P < 0.003), but this was associated with an increase in V̇O2 (+0.1 ± 0.01 and +0.1 ± 0.02 l min−1 from baseline, P < 0.001). The ratio of the increase in ventilation to the increase in V̇O2 during passive movement was not significantly higher than that during active exercise for either patients or controls, suggesting a limited contribution from the mechanoreceptors.
In chronic heart failure the presence of a muscle metaboreceptor reflex is also demonstrated in the leg, while mechanoreceptors exhibited a non-significant contribution in both patients and controls. The hypothesis of a peripheral origin of symptoms of exertional intolerance in this syndrome is confirmed as being mainly due to metabolic stimulation of the muscle metaboreceptors.
Muscle fatigue and breathlessness are major causes of morbidity in patients with chronic heart failure (Grieve et al. 1999). These symptoms are associated with reduced exercise tolerance (Mancini et al. 1992) as well as an increased ventilatory response to exercise (Lipkin et al. 1985; Buller & Poole-Wilson, 1990), the causes of which are still poorly understood. The disordered physiology of exercise seen in patients with chronic heart failure is not necessarily directly attributable to disordered central haemodynamics. In fact, no correlation exists between the indices of exercise tolerance and left ventricular ejection fraction or pulmonary capillary wedge pressure; an intervention acting on peripheral metabolism such as physical training can improve the exercise tolerance without affecting the cardiac output (Coats et al. 1992), while therapeutic interventions such as cardiac transplantation can improve the central haemodynamics without benefiting the exercise capacity (Franciosa & Cohn, 1979; Wilson et al. 1984).
A better understanding of the pathophysiology of these symptoms could be of benefit in patient management and in the identification of the most appropriate therapy to improve quality of life and survival. These symptoms may cause patients to avoid physical activity, which may adversely affect not only the quality of life but also its prognosis (Belardinelli et al. 1999).
Two classes of neural mechanisms have been postulated in the origin of the increase in ventilation, cardiac rate, sympathetic nerve discharge and cardiac contractility during dynamic exercise. The first is the central command system which involves the direct activation of brainstem locomotion, autonomic and ventilatory circuits by the cerebral processes occurring at the onset of exercise (Krogh & Lindhard, 1913; Goodwin et al. 1972; Eldridge et al. 1985). The second class of mechanism is the reflex network, which can activate brainstem autonomic and ventilatory circuits by signals from the periphery, such as III and IV muscle afferents, whose receptors are uncapsulated nerve endings which are triggered during exercise (Alam & Smirk, 1937; Coote et al. 1971; McCloskey & Mitchell, 1972). Evidence supports roles for both neural mechanisms in causing cardiovascular and respiratory responses to exercise (Kaufman & Forster, 1996; Waldrop et al. 1996; Adreani et al. 1997).
The group III and IV muscle afferents, defined as ergoreceptors because they are stimulated by muscle work (Kao et al. 1963), have been classified as mechanoreceptors, which sense physical movement, and metaboreceptors which sense chemical stimuli relating to muscle work. Activation of these afferents may affect heart rate, blood pressure, cardiac output, stroke volume, ventilation and microneurographic sympathetic nerve activity (McCloskey & Mitchell, 1972; Tibes, 1977; Mark et al. 1985; Piepoli et al. 1995). Of the two reflexes, the metaboreflex has received more research attention. During and after exercise, due to the build-up of metabolites, these receptors are stimulated, causing an increase in ventilation (the metaboreflex) (Piepoli et al. 1995). Early acidification and depletion of high energy metabolites (e.g. phosphocreatinine) observed in chronic heart failure may over-activate this reflex, contributing to the disordered exercise physiology of chronic heart failure. Abnormally large metaboreflex responses in chronic heart failure have been demonstrated in forearm muscles (Piepoli et al. 1996) and in small muscles used in dorsiflexing the foot (Grieve et al. 1999). However, controversial evidence has been published on metaboreceptor activation in the leg (Clark et al. 1995; Francis et al. 1999). A possible contribution of muscle mechanoreceptors in exercise limitation in heart failure has been proposed, but, to our knowledge no further investigations have been performed in this area. (McClain et al. 1993).
To further investigate the causes of exercise intolerance in chronic heart failure, we therefore set out to study the contribution of the metaboreflex and the mechanoreflex in the leg to the ventilatory response to exercise in patients with chronic heart failure. We have turned our attention to the larger muscles of the calf which perform plantarflexion to raise the body's weight against gravity whilst walking: these muscles may be clinically more important in patients with chronic heart failure who frequently experience weakness and discomfort of the calf muscles during exercise. Furthermore we have attempted to assess the contribution of mechanoreceptors, by comparing passive and active leg movements.
METHODS
Study population
We studied 18 patients with stable chronic heart failure and 12 age-matched, normal subjects. Baseline characteristics are shown in Table 1. The study was performed according to the Declaration of Helsinki and was approved by the local ethics committee. All subjects gave written informed consent.
Table 1.
Clinical charactersitics and baseline values of the study population
| CHP patients | Normal controls | ||
|---|---|---|---|
| n | 18 | 12 | |
| Ischaemic/dilated (n) | 11/7 | ||
| Sex: male/female | 14/4 | 8/4 | |
| Age (years) | 62.1 ± 3.18 | 60.3 ± 3.65 | |
| Weight (kg) | 78.2 ± 2.92 | 75.4 ± 4.2 | |
| Exercise duration (min) | 6.9 ± 0.8 | 10.8 ± 0.9* | |
| LVEF (%) | 30.8 ± 3.24 | ||
| Peak VO2 (ml min−1 kg−1) | 18.1 ± 1.64 | 25.6 ± 2.51* | |
| VE at rest (1 min−1) | 13.9 ± 1.08 | 14.5 ± 1.77 | |
| VO2 at rest (1 min−1) | 0.4 ± 0.03 | 0.37 ± 0.03 | |
| VCO2 at rest (1 min−1) | 0.34 ± 0.280 | .33 ± 0.03 | |
| HYHA class (n) | |||
| II | 15 | n.a. | |
| III | 3 | ||
| Medications (n) | None | ||
| ACE-inhibitors | 15 | ||
| Diuretic | Loop | 13 | |
| Thiazide | 2 | ||
| K spare | 3 | ||
| β-Blockers | 5 | ||
| Aspirin | 9 | ||
| Warfarin | 4 | ||
| Digoxin | 3 | ||
| Statin | 7 | ||
| Amiodarone | 5 | ||
| Calcium antagonist | 1 | ||
Values are given as means ± S.E.M.; CHF, chronic heart failure; LVEF, left ventricular ejection fraction; NYHA, functional classification of the cardiovascular disability according to the New York Heart Association
P < 0.05 vs. CHF patients.
All patients were clinically stable and had no change of medication in the 3 months prior to the study. They were not involved in any exercise-training programme or had significant chronic lung disease, valvular heart disease, or neuromuscular disorders. These patients had no evidence of exercise-induced myocardial ischaemia, arrhythmias, or claudication. Patients with peripheral vascular disease, myopathy, or thyroid dysfunction were also excluded. The patients were all symptomatic on exercise and limited by breathlessness or muscle fatigue. All patients underwent a maximal treadmill, symptom-limited cardiopulmonary exercise test using a modified Bruce protocol (stage 0: 1.0 m.p.h. at 5.0% gradient) on a treadmill (Marquette Case 15).
Ventilatory data
Subjects breathed through a mouthpiece and wore a nose clip. Ventilation was measured continuously on-line using a calibrated heated pneumotachograph. Oxygen uptake (VO2) and carbon dioxide production (VCO2) was measured breath-by-breath using a respiratory mass spectrometer (Amis, Innovision, Denmark).
Protocol
All experimental sessions were carried out in a temperature-controlled, air-conditioned room. After clinical screening the subjects performed at least two routine cardiopulmonary exercise tests, to determine their exercise capacity and to familiarise them with the laboratory environment. The mechanoreflex and metaboreflex studies were performed on separate days. The subjects were asked to avoid strenuous physical activity for 24 h before the mechanoreflex and metaboreflex tests and to refrain from eating and smoking 3 h before the tests began. The subject rested for 30 min in a quiet environment before participating in the tests.
Metaboreceptor test
The evaluation of the metaboreflex activity in the lower limb included two exercise protocols performed in random order: (i) a 6 min session of cycling on an cycle ergometer (ERG 601, Bosch, Germany). During the first minute the workload was increased from unloaded (0 W) to a load that produced 60-70% (30-70 W) of the previously determined peak VO2. This was then maintained for 5 min. Gas exchange was recorded throughout the test, during exercise and for the 5 min of recovery period; (ii) the same protocol followed by, immediately from the cessation of exercise, 3 min of venous and arterial regional circulatory occlusion (RCO), by inflation of bilateral upper thigh tourniquets to 30 mmHg above peak exercise arm systolic pressure (Grieve et al. 1999) (Hokanson E20 Rapid Cuff Inflator and AG101 Cuff Inflator Air Source, Bellevue, WA, USA). Thus, the contribution of the muscle metaboreceptors was evaluated by trapping the metabolites in the exercising muscle after exercise. This protocol has been shown to fix the metabolic state of the muscle and to maintain the activation of the metaboreceptors (Piepoli et al. 1996). The metaboreflex contribution to the exercise changes, calculated as the difference in ventilation between the 2nd and 3rd minute of post-exercise RCO and the difference in ventilation between the 2nd and 3rd minute of recovery without RCO.
Eight minutes separated each bout of leg exercise. A control RCO by tourniquet inflation at 30 mmHg above systolic pressure at rest was also performed without prior exercise to study the possibility that occlusion alone might engender a reflex response.
Figure 1 schematically represents the patient set-up and how the equipment was positioned on the body.
Figure 1.
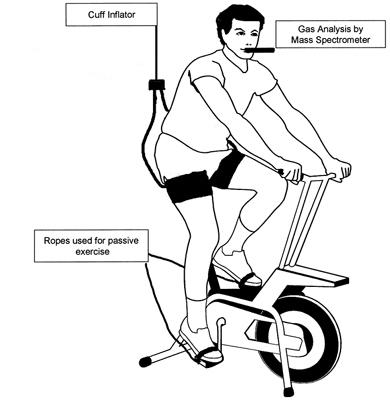
Schematic representation of the set-up and the position of the equipment on the body of the subject.
Mechanoreceptor test
In a subgroup of 10 patients, (7 males, 3 females, 63.3 ± 2.7 years) and eight male controls (59.7 ± 2.8 years), we evaluated the mechanoreflex activity in the lower limb. This involved one episode of 3 min of passive movement followed by one episode of 3 min of active exercise. The passive movement and active exercise were separated by a 3 min rest period. The passive movement was performed by attaching a rope around each pedal of the cycle ergometer and securing the subject's feet to the pedal via toe clips. Each patient was instructed to relax and not to use any muscles while their legs were being revolved. The pedals were rotated by the investigator at a rate of 45 r.p.m. For active exercise, the patient cycled at 45 r.p.m. at a workload of 10 W for 3 min. A 10 W load was applied during the active exercise so that the subject could not over-rotate the pedals leading to a compensatory shift in posture (Fig. 1).
Ventilatory parameters were measured during passive movement and active exercise. The increase in ventilation (VE) per unit increase in VO2 (dVE/dVO2 ratio) between passive movement and active exercise was assessed to quantify the ventilation response due to muscle work during the two tests. The mechanoreflex contribution to exercise was quantified as the difference in elevation of the dVE/dVO2 ratio between passive movement and active exercise.
Statistical analysis
Means for groups are presented with the standard errors of the means. Comparison between groups were made using Student's paired or unpaired t test as appropriate, with P < 0.05 considered to be significant. For multivariate analysis ANOVA was used.
RESULTS
The control group matched the patient group in age and weight. Compared with controls, patients had a lower peak VO2 and exercise duration. There was no significant difference in ventilation at rest between patients and controls (Table 1).
Metaboreceptor test
All subjects completed the metaboreceptor protocol without complication. The ventilatory variables increased similarly during the bouts of exercise without and with post-exercise RCO: in patients (ventilation, 55.31 ± 3.36 and 54.15 ± 3.42 l min−1; VO2, 1.28 ± 0.12 and 1.29 ± 0.11 l min−1; VCO2, 1.32 ± 0.12 and 1.44 ± 0.12 l min−1, P = n.s. for each comparison) and controls (ventilation, 63.04 ± 9.77 and 57.89 ± 8.56 l min−1; VO2, 1.46 ± 0.09 and 1.40 ± 0.12 l min−1; VCO2, 1.50 ± 0.11 and 1.52 ± 0.15 l min−1, P = n.s.). During recovery, ventilation in patients was higher during RCO than without RCO and the size of this response increased sequentially from the 1st to 3rd minute (Table 2). In controls, the ventilation was lower during RCO than without RCO; as a consequence a significant difference in ventilation was observed between the two groups of subjects during the 2nd and 3rd minute (Table 2). A typical experimental result in one heart failure patient and one control subject is expressed graphically in Fig. 2.
Table 2.
Ventilatory variables during recovery (1st, 2nd, 3rd minute and the mean of the 2nd and 3rd minute) during the run without regional circulatory occlusion (No RCO) and the run with regional circulatory occlusion (RCO) in controls and patients with heart failure
| Controls | Patients | ||||||
|---|---|---|---|---|---|---|---|
| No RCO | RCO | Δ | No RCO | RCO | Δ | ||
| Recovery 1st minute | V̇E | 43.14 ± 4.17 | 40.41 ± 4.92 | −2.76 ± 1.83 | 36.43 ± 2.20 | 37.02 ± 2.89 | 0.59 ± 1.75 |
| V̇O2 | 1.03 ± 0.05 | 0.89 ± 0.05 | −0.14 ± 0.03 | 0.90 ± 0.05 | 0.86 ± 0.05 | −0.04 ± 0.26 | |
| V̇CO2 | 1.12 ± 0.06 | 1.04 ± 0.07 | −0.08 ± 0.03 | 0.92 ± 0.05 | 0.95 ± 0.07 | 0.03 ± 0.04 | |
| Recovery 2nd minute | V̇E | 31.42 ± 3.48 | 27.66 ± 2.85† | −3.76 ± 1.12 | 25.47 ± 1.76 | 28.08 ± 2.37† | 2.61 ± 0.91* |
| V̇O2 | 0.58 ± 0.04 | 0.51 ± 0.03 | −0.06 ± 0.03 | 0.58 ± 0.03 | 0.56 ± 0.04 | −0.03 ± 0.01 | |
| V̇CO2 | 0.71 ± 0.05 | 0.63 ± 0.03 | −0.08 ± 0.04 | 0.62 ± 0.04 | 0.65 ± 0.05 | 0.03 ± 0.02 | |
| Recovery 3rd minute | V̇E | 25.80 ± 2.52 | 20.96 ± 1.84† | −4.84 ± 1.50 | 20.50 ± 1.45 | 24.83 ±2.94*† | 4.33 ± 2.23* |
| V̇O2 | 0.46 ± 0.02 | 0.41 ± 0.04 | −0.05 ± 0.03 | 0.47 ± 0.03 | 0.47 ± 0.04 | 0.00 ± 0.03 | |
| V̇CO2 | 0.55 ± 0.03 | 0.46 ± 0.04 | −0.09 ± 0.04 | 0.49 ± 0.04 | 0.53 ± 0.05 | 0.05 ± 0.04 | |
| Recovery 2nd and 3rd minute | V̇E | 28.61 ± 3.00 | 24.31 ± 2.34† | −4.30 ± 1.30 | 22.99 ± 1.61 | 26.45 ± 2.66*† | 3.47 ± 1.57* |
| V̇O2 | 0.52 ± 0.03 | 0.46 ± 0.03 | −0.06 ± 0.03 | 0.53 ± 0.03 | 0.51 ± 0.04 | −0.01 ± 0.02 | |
| V̇CO2 | 0.63 ± 0.04 | 0.55 ± 0.04 | −0.08 ± 0.04 | 0.55 ± 0.04 | 0.59 ± 0.05 | 0.04 ± 2.27 | |
Values are given as mean ± S.E.M., in 1 min−1; δ, difference
P < 0.05 vs controls
P < 0.05 vs. No RCO.
Figure 2.
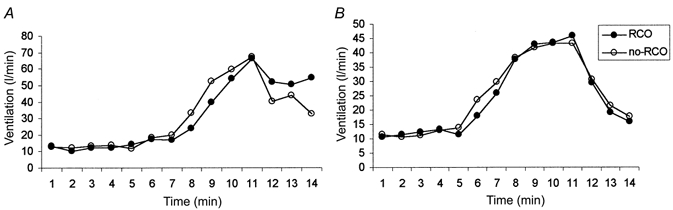
Experimental results in a representative heart failure patient and a control subject are expressed graphically. We compared the leg exercise with control recovery (○) vs. exercise run with RCO (regional circulatory occlusion) recovery (•): i.e. the metaboreflex effect. Note the persistent elevation in ventilation during RCO recovery in heart failure patient (A), absent in control subject (B).
There was no significant difference between VO2 or VCO2 during RCO in either subject group (Table 2).
RCO without previous exercise had no significant effect on ventilation in patients (rest: 14.7 ± 1.3 l min−1; RCO:14.3 ± 1.2 l min−1, P = n.s.) and controls (rest: 15.7 ± 1.7 l min−1; RCO: 17.0 ± 2.4 l min−1, P = n.s.).
Mechanoreceptor test
Table 3 shows the ventilatory variables values during the mechanoreflex test including passive and active limb movements.
Table 3.
Comparison of passive movement and active exercise in patients and controls
| Patients | Controls | ||
|---|---|---|---|
| Rest | V̇E | 12.64 ± 0.43 | 11.94 ± 0.61 |
| V̇O2 | 0.37 ± 0.04 | 0.4 ± 0.03 | |
| V̇CO2 | 0.3 ± 0.03 | 0.34 ± 0.03 | |
| Passive | V̇E | 16.33 ± 0.6 | 14.86 ± 1.1 |
| V̇02 | 0.47 ± 0.04 | 0.5 ± 0.03 | |
| V̇CO2 | 0.41 ± 0.04 | 0.45 ± 0.02 | |
| Rest | V̇E | 13.27 ± 0.47 | 12.82 ± 0.74 |
| V̇O2 | 0.39 ± 0.04 | 0.36 ± 0.03 | |
| V̇CO2 | 0.34 ± 0.03 | 0.33 ± 0.03 | |
| Active | V̇E | 22.53 ± 1.92 | 22.34 ± 3.64 |
| V̇O2 | 0.64 ± 0.05 | 0.71 ± 0.05 | |
| V̇CO2 | 0.57 ± 0.04 | 0.64 ± 0.05 |
Values are given as mean ± S.E.M., in min l−1. Passive, passive movement; Active, active exercise.
Passive limb movement caused a significant increase in ventilation in both patients and controls with respect to the baseline (+3.7 ± 0.4 l min−1 (P < 0.003) and +2.9 ± 0.5 l min−1 (P < 0.003), respectively). In both groups of subjects, this was associated with an increase in metabolism (VO2: +0.1 ± 0.01 l min−1 (P < 0.002) and +0.11 ± 0.02 l min−1 (P < 0.001) vs. baseline).
Active limb movement also caused a significant increase in ventilation in both patients and controls with respect to the baseline (+9.9 ± 1.9 l min−1 (P < 0.05) and +10.4 ± 3.3 l min−1 (P < 0.001) respectively), associated with an increase in metabolism (VO2 0.26 ± 0.04 l min−1 (P < 0.001) and +0.31 ± 0.07 l min−1 (P < 0.004), respectively). Consequently the increase in ventilation per unit increase in oxygen uptake (dVE/dVO2 slope) was not statistically significant between passive movement and active exercise in either patients (43.7 ± 7.1 and 35.6 ± 4.9, P = n.s.) or controls (33.3 ± 8.0 and 25.8 ± 4.8, P = n.s.). However, during both passive movement and active exercise the relationship between the change in ventilation and increased workload (dVE/dVO2) was steeper in patients than in the controls, although this difference did not reach significance (P = n.s. by ANOVA).
DISCUSSION
Fundamental studies were performed by Kao in establishing the role of peripheral factors in the control of ventilation during exercise. Cross-perfusion techniques were developed to identify the neural and humoral pathways in exercise hyperpnoea. He concluded that the primary exercise stimulus is neural (Kao, 1963). The neural pathway originating in the muscles requires the existence of local peripheral receptors for its action, which respond to chemical, thermal or mechanical stimuli. Kao referred to them as ‘ergoreceptors’, to mean the receptors activated by innoxious stimuli (low-threshold units) such as muscle contraction. This is in contrast to the receptors activated by noxious stimuli (high-threshold units) which are called nociceptors. Ergoreceptors were differentiated into mechanoreceptors and chemoreceptors (Kao, 1963). The work of McCloskey & Mitchell (1972) and Kaufman & Forster (1996) have demonstrated that the thinly myelinated group III (Aδ) and the unmyelinated group IV (C) muscle afferents can be activated by the mechanical and/or metabolic changes that occur in a muscle during contraction. We preferred to refer to muscle receptors using the original term of ergoreceptors proposed by Kao and differentiated them into metaboreceptors (sensitive to the metabolic, chemical factors of muscle work) (Piepoli et al. 1995; Clark et al. 1995) and mechanoreceptors (sensitive to muscle movements, i.e. muscle spindles, Golgi tendon organs) (Goodwin et al. 1972). It is well known that the large afferent fibres (Ia, Ib or II) are not involved in the cardiovascular and respiratory changes of exercise; anodal blockade or chemical activation of these large fibres have no effect on the expression of the exercise pressor reflex (Waldrop et al. 1996).
This paper reports two major findings. Firstly, a significant metaboreceptor response in the lower limb of patients with chronic heart failure was observed. This response was absent in healthy controls. The negative effect of circulatory occlusion in the control group suggests that in physiological conditions factors other than neural mechanisms play a major role in ventilatory control during leg exercise, such as humoral mechanisms.
Secondly, the role of mechanoreceptors in the ventilatory control of both controls and patients seemed to be small because the changes seen in ventilation during passive movement as well as during active exercise were proportional to the VO2 increase, i.e. explicable in terms of a metaboreceptor response, since the increase in VO2 during passive movement indicates that active muscular work is occurring. Nevertheless, the presence of mechanoreceptor activity cannot be ruled out entirely as the methodology used here does not completely isolate the mechanoreflex contribution from the metaboreflex one in the ventilatory response. However heart failure patients still showed a significantly higher ventilatory response during the mechanoreflex testing.
Metaboreceptor contribution to physiological responses to exercise
Patients with chronic heart failure have both exercise intolerance and an increased ventilatory response to exercise; the causes of these abnormal responses to exercise are still poorly understood. A key role for muscle afferents has been proposed. When work is performed, metabolites are released from the exercising muscle. These metabolites may stimulate nerve endings situated within unmyelinated and small myelinated afferents in skeletal muscle (Kao, 1963). Stimulation of these nerves leads to a reflex increase in blood pressure, sympathetic activity and ventilation (Piepoli et al. 1995). Patients with chronic heart failure and abnormalities in skeletal muscle metabolism have earlier acidification and build-up of metabolic products, and so activate these reflexes; this results in an abnormal, steep ventilatory response to exercise.
Previous clinical studies of arm exercises have shown large metaboreflex responses (Piepoli et al. 1996) in patients with chronic heart failure, which correlate with the abnormal over-ventilation (VE/VCO2 slope) and reduced tolerance to effort (peak VO2). Both are hallmarks of this syndrome (Piepoli et al. 1999).
Within the lower limb, studies concentrating on the muscles that dorsiflex the foot have also shown a distinct metaboreflex response (Grieve et al. 1999), but work with larger muscle groups has been unsuccessful in demonstrating this effect (Francis et al. 1999). A clear metaboreflex response originating from the large muscle group of the lower limb is demonstrated in the present study. In the 1st min of recovery there was no significant difference in the metaboreflex component of the ventilatory response between patients with chronic heart failure and controls (the presence or absence of RCO has no effect on the return to resting ventilation). However during the 2nd and 3rd minute of recovery, ventilation was significantly higher in the presence of RCO. This implies that as time passes metabolites are washed out of the limb if uncuffed but are retained if cuffed, so that the difference increases with time.
There are several possible reasons why the present results conflict with previous work (Francis et al. 1999) which did not observe any role of metaboreflexes in the ventilatory control during work of large muscle groups of the lower limb. Firstly, it is well known that patients with lower exercise tolerance have more markedly enhanced ventilatory responses to exercise (Chua et al. 1997). Our patients probably had a more severe degree of heart failure than those in the study of Francis et al. (1999) (peak VO2 of 18 ± 2 ml min−1 kg−1vs. 22 ± 5 ml min−1 kg−1). If metaboreceptor hypersensitivity is linked to severity of heart failure, as has been reported by Piepoli et al. (1999), then it is possible that the effect was too weak in their patient group to be measurable. Secondly, our study involved two identical exercise protocols (which were sub-maximal) performed on the same day in random order. In the current study, VO2 attained in the two exercise episodes, before RCO and no-RCO, did not differ significantly between patients (15.5 ± 1.5 vs. 16.1 ± 1.6 ml min−1 kg−1, P = 0.07) or controls (19.1 ± 1.7 vs. 19.5 ± 1.6 ml min−1 kg−1, P = 0.49). The study of Francis et al. (1999) used symptom-limited maximal exercise tests, which were performed on separate days. Both factors may have increased random variation in the level of exercise and ventilation which may have contributed to obscure any metaboreflex effect. It is not possible to estimate the size of this random variation in workload between the tests because the standard deviation of the signed difference between the peak VO2 of the two tests was not presented. A third difference between our two studies is the method of analysing the metaboreflex: Francis et al. (1999) considered the half-life of ventilation during RCO. In contrast we have measured the magnitude of the metaboreflex response as the absolute ventilation level at different time points during recovery. The result from the present study supports our previous observations that the magnitude of the metaboreflex response increases as time passes during cuff occlusion. This would be consistent with the concept of both experiments (i.e. with and without RCO) being equivalent for the first few seconds but becoming more different from each other as time passes because metabolites build up in the RCO state but are washed out in the no-RCO state. Ventilation returns to half its normal value within a short period of time and therefore the half-life concentrates on the early part of recovery; unfortunately this period is one in which the metaboreflex is the least prominent. When we applied the half-life method used by Francis et al (1999), no significant metaboreceptor response was obtained, because of the large standard deviation during RCO and no-RCO test within the patient group (123 ± 14 vs. 111 ± 9 s, P = 0.27) and the control group (103 ± 11 vs. 113 ± 13 s, P = 0.56). The metaboreceptor response is more readily observed using the approach of the present study.
In a previous study from our laboratory we observed a large metaboreflex effect to arm exercise (Piepoli et al. 1996), while a smaller effect has been observed during leg exercise. An explanation for this difference is that venous return may not have been completely blocked from the exercising muscle of the leg. During the RCO period, cuff inflation was 30 mmHg above peak exercise arm blood pressure. The cuff inflation was briefly tested before each test to observe the subjects’ response. When the cuff was inflated more than 30 mmHg above peak exercise arm blood pressure it was too painful for subjects to withstand for 3 min. With the artery in the lower limb running deep in the muscle, total circulatory occlusion may not have occurred. The quadriceps muscle group is made up of four muscles which offer a large resistance to the occluding cuff. Slight leakage of metabolites into the systemic circulation may have an affect on the ventilation reducing the observed metaboreceptor response. Alternatively, arm exercise involves a smaller muscle mass which may show a predominant neural reflex control of ventilation in respect to leg exercise where the larger muscle mass causes greater release of blood-borne metabolic products. Humoral control of ventilation may be more important than reflexes during leg exercise in normal individuals.
The underlying mechanism of the metaboreflex remains incompletely elucidated. There may be an increase in concentration of metabolites in patients vs. controls or an increase in the sensitivity to the same level of metabolites. Further studies are needed to establish this phenomenon and to elucidate the factors that are responsible for metaboreflex stimulation.
Mechanoreceptor contribution to physiological responses to exercise
A heterogeneous group of receptors with different properties have been shown to modulate cardiovascular effector activity. The majority of group III fibres appear to be activated by deformation changes occurring in the contracting muscle and are termed mechanoreceptors (Mitchell, 1985). It has been shown by Mitchell (1985) that mechanoreceptors initiate a cardiovascular response, but their role is uncertain. De Meersman et al. (1998) have shown that passive movement of the leg and/or joint can stimulate cardiovascular autonomic reflexes. Their study was particularly interesting as it indicated that passive lower limb movement was shown to cause an increase in VO2 of almost 90% of the size of the increase produced by active unloaded exercise. However, the conclusion of De Meersman et al. (1998) should be treated with caution because the study was performed over a period of only 1 min. This short time period may be a limitation to the sensitivity of the study in detecting small changes.
Our results showed that changes in ventilation, VO2 and VCO2 occurred with passive limb movements which were quite large, even though the ventilatory increase during passive movement was proportional to the increase in VO2 (dVE/dVO2 slope) in both controls and heart failure patients. This suggested no significant role of muscle mechanoreflex in either group.
However, this may also be a limitation of the protocol used here to assess the mechanoreflex effect on ventilation. Limb movement could not be completely passive in a subject with muscle tone. Although the muscles are not being deliberately exercised, in any muscle possessing tone, passive movements with stretching and postural changes of muscle during leg rotation are necessarily associated with work done by the myofilaments and therefore with changes in metabolism. This increase in metabolism engenders a rise in ventilation via the metaboreceptor reflex. With coexisting metaboreceptor and mechanoreceptor ventilatory reflexes, both are expected to be activated (to some extent) in all forms of movement, active or passive.
Metaboreceptor responses can be expected to be proportional to the work done, while mechanoreceptor responses relate to the extent of movement. Thus if mechanoreceptor responses were responsible for the majority of the overall ventilatory increment in active exercise, the ventilatory increment to passive movement would be expected to be just as large. In contrast, we see only a relatively small increase in ventilation with passive movement, and that this increment is in keeping with the small increase in energy consumption of the muscles. We therefore conclude that metaboreceptors rather than mechanoreceptors are responsible for the majority of the ventilatory respiration to exercise.
However, in comparison with controls, during passive movement a non-significant larger increase in ventilation was observed in chronic heart failure patients, with comparable metabolic consumption (dVE/dVO2 slope). This suggests an over-activation of another stimulus, which is not a metaboreflex, because it is independent of muscle work. Thus a possible contribution of receptors, sensitive to passive movement (mechanoreflex), in the ventilatory response to exercise in this syndrome could not be neglected. Further studies are necessary to investigate the possible contribution of mechanoreceptors in this aspect.
In conclusion, during leg exercise using the muscle groups involved in ambulating, patients with moderate to severe chronic heart failure manifest a prominent component of skeletal muscle afferents (metaboreflex) in the ventilatory response to exercise that is not seen in normal subjects. A small mechanoreceptor response was envisaged in heart failure; however, more elaborate and sensitive testing is required to examine this reflex further. The ergoreflex overactivation may explain the origin of breathlessness and fatigue in exercise as both may have a common origin in metabolic abnormalities. Our findings support the hypothesis that an intervention able to improve skeletal muscle metabolism (such as physical training) can contribute to reversing the abnormal ventilatory and haemodynamic response to exercise by reducing the ergoreflex activity.
Acknowledgments
A.C.S. and M.F.P. are supported by the Wellcome Foundation. D.P.F. is supported by the British Heart Foundation (FS 98005). L.C.D. is supported by the Robert Luff Fellowship. A.J.S.C is supported by the Viscount Royston Trust.
References
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. Journal of Applied Physiology. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Observation in man upon a blood pressure raising reflex arising from the voluntary muscles. Journal of Physiology. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- Buller NB, Poole-Wilson PA. Mechanism of the increased ventilatory response to exercise in patients with chronic heart failure. British Heart Journal. 1990;63:281–283. doi: 10.1136/hrt.63.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua TP, Ponikowski P, Harrington D, Anker SD, Webb-Peploe K, Clark AL, Poole-Wilson PA, Coats AJ. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. Journal of American College of Cardiology. 1997;7:1585–1590. doi: 10.1016/s0735-1097(97)00078-8. [DOI] [PubMed] [Google Scholar]
- Clark AL, Piepoli M, Coats AJS. Skeletal muscle and the control of ventilation on exercise: evidence for metabolic receptors. European Journal of Clinical Investigation. 1995;25:299–305. doi: 10.1111/j.1365-2362.1995.tb01705.x. [DOI] [PubMed] [Google Scholar]
- Coats AJS, Adamopoulos S, Radaelli A, Mccance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C. Controlled trial of physical training in chronic heart failure. Circulation. 1992;85:2119–2131. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzales JF. The reflex nature of the pressor response to muscular exercise. Journal of Physiology. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meersman RE, Zion AS, Weir JP, Lieberman JS, Downey JA. Mechanoreceptors and autonomic responses to movement in humans. Clinical Autonomic Research. 1998;8:201–205. doi: 10.1007/BF02267782. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respiratory Physiology. 1985;59:313–317. doi: 10.1016/0034-5687(85)90136-7. [DOI] [PubMed] [Google Scholar]
- Franciosa JA, Cohn JN. Effect of isosorbide dinitrate on responses to submaximal and maximal exercise in patients with congestive heart failure. American Journal of Cardiology. 1979;43:1009–1014. doi: 10.1016/0002-9149(79)90368-0. [DOI] [PubMed] [Google Scholar]
- Francis N, Cohen-Solal A, Logeart D. Peripheral muscle ergoreceptors and ventilatory response during exercise recovery in heart failure. American Journal of Physiology. 1999;276:H913–917. doi: 10.1152/ajpheart.1999.276.3.H913. [DOI] [PubMed] [Google Scholar]
- Goodwin GE, MacCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. Journal of Physiology. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve DA, Clark AL, MacCann GP, Hillis WS. The ergoreflex in patients with chronic stable heart failure. International Journal of Cardiology. 1999;68:157–164. doi: 10.1016/s0167-5273(98)00349-0. [DOI] [PubMed] [Google Scholar]
- Kao FF. An experimental study of the pathways involved in exercise hypernea employing cross-circulation techniques. In: Cunningham DC, Lloyd BB, editors. The Regulation of Human Respiration. Oxford, UK: Blackwell; 1963. pp. 461–502. [Google Scholar]
- Kaufman MP, Forster HV. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD, USA: American Physiological Society; 1996. Reflexes controlling circulatory, ventilatory and airway responses to exercise; pp. 381–447. section 12, chap. 10. [Google Scholar]
- Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. Journal of Physiology. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin DP, Perrins J, Poole-Wilson PA. Respiratory gas exchange in the assessment of patients with impaired ventricular function. British Heart Journal. 1985;54:321–328. doi: 10.1136/hrt.54.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacClain J, Hardy C, Enders B, Smith M, Sinoway L. Limb congestion and sympathoexcitation during exercise. Journal of Clinical Investigation. 1993;92:2353–2359. doi: 10.1172/JCI116840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCloskey DI, Mitchell JH. The regulation of respiration and circulation during the initial stages of muscular work. Journal of Physiology. 1972;224:173–186. [Google Scholar]
- Mancini DM, Walter G, Reichek N, Lenkinski R, MacCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of the sympathetic nerve response to static exercise in humans. Circulation Research. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Mitchell JH. Cardiovascular control during exercise: Central and reflex neural mechanisms. American Journal of Cardiology. 1985;55:34–41D. doi: 10.1016/0002-9149(85)91053-7. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Coats AJS. Muscle metaboreceptors in hemodynamic, autonomic and ventilatory response to exercise in man. American Journal of Physiology. 1995;269:1428–1436. doi: 10.1152/ajpheart.1995.269.4.H1428. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJS. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJS. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. American Heart Journal. 1999;137:1050–1056. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- Tibes U. Reflex inputs to the cardiovascular and respiratory centers from dynamically working canine muscles. Circulation Research. 1977;42:332–341. doi: 10.1161/01.res.41.3.332. [DOI] [PubMed] [Google Scholar]
- Waldrop TGF, Eldridge FL, Iwamoto GA, Mitchell JH. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD, USA: American Physiological Society; 1996. Central neural control of respiration and circulation during exercise; pp. 333–380. section 12, chap 9. [Google Scholar]
- Wilson JR, Martin JL, Ferraro N. Impaired skeletal muscle nutritive flow during exercise in patients with congestive heart failure-role of cardiac pump dysfunction as determined by the effect of dobutamine. American Journal of Cardiology. 1984;53:1308–1315. doi: 10.1016/0002-9149(84)90085-7. [DOI] [PubMed] [Google Scholar]


