Abstract
Experiments were performed on anaesthetized cats to test the hypothesis that fluid flow through dentinal tubules is part of the mechanism involved in the transduction of pain-producing stimuli in teeth.
In 11 animals, fluid flow through dentine and single- and multi-unit activity in intradental nerves were recorded simultaneously during the application of changes in hydrostatic pressure (-500 to +500 mmHg) to exposed dentine.
Seventeen A-fibres (conduction velocity (CV), 10.6-55.1 m s−1) were isolated that were pressure sensitive. The thresholds of these units in terms of dentinal fluid flow were in the range 0.3-2.1 nl s−1 mm−2 during outward flow from the pulp and 2.0-3.5 nl s−1 mm−2 during inward flow. All the units were more sensitive to outward than inward flow. Twenty-eight units (CV, 0.6-48.8 m s−1) were not pressure sensitive, and 12 of these had conduction velocities in the C-fibre range (< 2.5 m s−1). The velocities of the tubular contents were calculated by estimating the number and diameters of dentinal tubules exposed. At the threshold of single-fibre responses these velocities were in the range 31.7-222.9 μm s−1 during outward flow, and 211.4-369.6 μm s−1 during inward flow.
Repetitive pressure stimulation of dentine resulted in a progressive reduction in the evoked discharge, which was probably due to pulp damage.
In seven animals, 10 single intradental nerve fibres were selected that responded to hydrostatic pressure stimuli and their responses to the application of hot, cold, osmotic, mechanical and drying stimuli to exposed dentine were investigated. With these stimuli dentinal fluid flow could not be recorded in vivo for technical reasons and was therefore recorded in vitro after completion of the electrophysiological recordings.
With each form of stimulus, the discharge evoked in vivo was closely related to the flow predicted from the in vitro measurements. The results were therefore consistent with the hypothesis that the stimuli act through a common transduction mechanism that involves fluid flow through dentine.
Stimulation of dentine or dental pulp produces pain and probably no other sensation under most conditions (Carter & Matthews, 1989; Närhi et al. 1994). Dentine is sensitive to a range of different stimuli including heating, cooling, probing, drying, changes in hydrostatic pressure and the application of solutions of high osmotic pressure. It has a sparse innervation, which is provided by the terminals of nerves that penetrate a short distance into the tubules of the dentine from the underlying pulp. Several receptor mechanisms have been proposed to account for the sensitivity of dentine (reviewed in Matthews, 1985). According to one of these, the hydrodynamic hypothesis (Gysi, 1900; Bränströmm, 1963), the contents of the dentinal tubules act as hydraulic linkages between the external environment and the pulpal nerve endings. Stimuli applied to an exposed dentine surface cause displacement of the tubular contents, which in turn excites the nerve terminals. Enamel, which is an acellular tissue, normally protects the dentine of the crown from stimulation during function, although thermal stimulation of enamel can cause pain. In this case, the pain is due to the stimulus affecting the underlying dentine.
Ultrastructural studies (for review see Holland, 1985) have shown that only the inner (pulpal) ends of the dentinal tubules contain cellular material, yet dentine is sensitive throughout its thickness. All dentinal tubules contain the process of an odontoblast (which is involved in dentinogenesis) and some, especially those immediately above the tip of the coronal pulp (pulp cornu), also contain one or more non-myelinated terminals of sensory nerves (Fearnhead, 1961; Holland, 1981; Fried & Erélyi, 1982; Byers, 1984; Wakisaka et al. 1984; Holland et al. 1987; Maeda et al. 1994). These extend approximately 100 μm into the tubules from the pulp.
The hydrodynamic hypothesis is also supported by electrophysiological evidence that individual pulpal nerve fibres respond to many of the different forms of stimulus that cause pain in man. In both cats (Kollmann et al. 1982) and dogs (Närhi et al. 1982) recordings have been made from a group of pulpal nerve fibres in which each fibre responds to thermal, osmotic, mechanical and hydrostatic pressure stimulation, and to drying of exposed dentine. All of these stimuli would be expected to produce some displacement of the contents of the dentinal tubules and have been shown to produce fluid flow through dentine in extracted human teeth (Horiuchi & Matthews, 1973). Not all tooth pulp afferents respond in this way; others appear to be excited more directly by heating and by chemical stimuli (Matthews, 1977; Närhi et al. 1982).
A method has been devised that enables fluid flow through dentine to be recorded in vivo (Vongsavan & Matthews, 1992a). However, the only form of pain-producing stimulus that can be applied to the exposed dentine whilst using this technique is a change in hydrostatic pressure. This is because the dentine surface is inaccessible to mechanical and evaporative stimuli, and the recording system has to be maintained at a constant temperature, which precludes thermal stimulation.
The current experiments were carried out to investigate the proposed hydrodynamic mechanism by determining the relationship between the rate of fluid flow through dentine and the discharge evoked in intradental nerves by a range of different stimuli. In one series of experiments, hydrostatic pressure stimuli were applied to exposed dentine while fluid flow through dentine and both single- and multi-unit nerve activity were recorded simultaneously in vivo. In a second series of experiments, single- and multi-unit activity were recorded during the application of a variety of stimuli to dentine, then the fluid flow through the dentine produced by the same stimuli was recorded in vitro after the electrophysiological recordings were completed.
METHODS
Young adult cats were anaesthetized with sodium pentobarbitone (Sagatal; Rhöne Mérieux, Harlow, UK; induction 42 mg kg−1i.p., maintenance 4 mg kg−1 h−1 I. V.). In each animal, cannulae were inserted into a saphenous vein for the administration of further anaesthetic, and into a femoral artery to record arterial blood pressure; the trachea was cannulated to maintain the airway. Body temperature was maintained at 38.0 ± 0.5°C with an electric blanket controlled from a rectal thermistor. Each experiment was carried out on the mandibular canine tooth of one side. The cat was placed on its side and the head supported with a short rod that was attached to the skull over the frontal sinus with self-tapping screws and self-curing acrylic resin. The mandible was fixed to the maxilla with acrylic resin and hypodermic tubing inserted through holes drilled into the crowns of the molar teeth. A second rod was fixed to the acrylic resin linking the molar teeth and both rods were clamped to a heavy metal table. This provided the mechanical stability that was required to record dentinal fluid flow. Following completion of the experiment the animal was killed with an overdose of anaesthetic.
Neural responses and dentinal fluid flow recorded in vivo
Experiments were performed on 11 cats of either sex aged 7-12 months (2.2-4.6 kg).
Fluid flow recording
Dentine was exposed by removing 1 mm from the tip of the mandibular canine tooth with a rotating diamond disc irrigated with Ringer solution. The cut dentine surface was etched with 46 mmol l−1 ortho-phosphoric acid for 30 s to ensure that the ends of the dentinal tubules were open. Full details of the flow recording technique have been published elsewhere (Vongsavan & Matthews, 1992a; Matthews & Vongsavan, 1994) and will be described in outline only. The method involved sealing a small acrylic cap attached to a pulled-down glass capillary (internal diameter, 50 μm at its narrowest point) over the exposed dentine (Fig. 1). The cap and capillary were filled with milk diluted in Ringer solution (0.3% v/v). Fluid flow through dentine was detected by observing the resultant movement of the fat droplets of the milk in the capillary. This was done using a microscope with dark-ground illumination. A continuous record of the flow was also obtained by recording the pressure drop along the capillary with a low compliance, differential pressure transducer. The flow recording system was maintained at 30°C with a thermostatically controlled air jacket that also enclosed most of the crown of the tooth. After completion of the experiment the area of exposed dentine at the tip of the tooth was estimated by measuring its average diameter under a microscope.
Figure 1. Preparation used for recording fluid flow through dentine and the discharge evoked in intradental nerves during application of hydrostatic pressure stimuli to dentine in the cat.
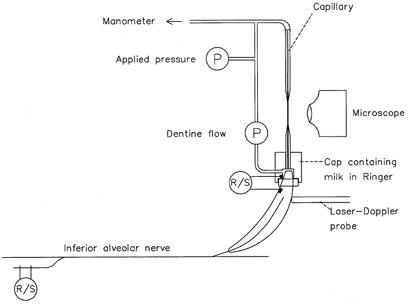
The flow of fluid through dentine was monitored with a low compliance pressure transducer that recorded the pressure drop along a capillary filled with Ringer solution, one end of which was sealed to the tooth. Hydrostatic pressure stimuli were applied from manometers connected to the free end of the capillary and were monitored with a second pressure transducer. Evoked discharges were recorded from filaments dissected from the inferior alveolar nerve and, as multi-fibre records, from nerve terminals in the tooth crown. A laser-Doppler probe was used to record pulpal blood flow. P, pressure transducer; R/S, electrodes used for recording and for stimulation.
Stimulation and recording from nerves in teeth
A pair of Ag-AgCl electrodes, one in the flow recording cap and one in a cervical cavity prepared 1 mm above the gingival margin, was used to electrically stimulate intradental nerves or obtain multi-fibre records from them (Horiuchi & Matthews, 1974; Cadden et al. 1983). The ipsilateral inferior alveolar nerve was exposed beneath the molar teeth and sectioned centrally. Single-unit recordings were made from fine filaments dissected from the nerve. Filaments were placed on a pair of platinum wire (diameter, 0.125 mm) electrodes in a pool of liquid paraffin. Pulpal fibres were identified either by recording from the filament during bipolar electrical stimulation of the tooth, or by stimulating the filament and recording from the tooth. Filaments were repeatedly divided until, ideally, only a single pulpal unit remained. Recordings were made with more than one unit in a filament provided their action potentials were sufficiently different to allow reliable discrimination. Conduction distances were measured from the cervical electrode in the tooth to the closest nerve electrode to it. To reduce the number of functional units from other tissues in each filament, which obscure the responses of pulpal fibres, the ipsilateral mental nerves were cut and tied. Also, the periodontal ligament of the tooth was partially removed with a slowly rotating bur irrigated with Ringer solution.
Pressure stimulation of dentine
Positive and negative hydrostatic pressure in the range -500 to +500 mmHg was applied to dentine in steps of 50 mmHg from two manometers via solenoid valves. The pressure stimuli were applied to dentine through the capillary of the flow recording system. The stimuli were applied in pairs of equal magnitude, with each stimulus pair consisting of 5 s at a positive pressure and 5 s at a negative pressure separated by 5 s at atmospheric pressure. Stimuli were applied in this way in an attempt to minimize damage to the odontoblasts and underlying pulp. It has been shown previously that the order in which such stimuli are applied has little effect on the responses to either positive or negative pressure. The interval between pairs of stimuli was at least 3 min. The magnitudes of the neural responses evoked during pressure stimulation of dentine were calculated by counting the number of evoked action potentials per 5 s pressure stimulus.
Microscopy
Sections of teeth were examined to determine the effects of pressure stimulation on the structure of the odontoblasts and pulp. Teeth from four experiments were analysed at the light level. The contralateral tooth was used as an unoperated control. Fixation of the teeth was with 4% formaldehyde, 4% glutaraldehyde and 5% picric acid in 0.1 mol l−1 phosphate buffer; either by immersion of the tooth crown in fixative or by intravascular perfusion via the carotid arteries.
Transverse slices of enamel, dentine and pulp (thickness, 1 mm) were prepared by sectioning the tooth with a diamond disc under irrigation with Ringer solution. They were decalcified and prepared for microscopy (Matthews & Hughes, 1988) then embedded in resin (Epon; Taab Laboratories Equipment Ltd, Aldermarston, Berks, UK). Longitudinal, semi-thin (1 μm) sections were cut and stained with Toluidine Blue. Teeth from five other experiments were examined in a scanning electronmicroscope to determine the number and diameters of the dentinal tubules exposed at the cut dentine surface (Vongsavan & Matthews, 1992a).
Neural responses recorded in vivo and dentinal fluid flow recorded in vitro
Experiments were carried out on seven male cats aged 8-10 months (2.4-4 kg). The animals were prepared as described above apart from the following details.
Tooth cap
The cap on the tooth functioned as a simple water jacket with inlet and outlet pipes and incorporated a glass-coated thermocouple to record the temperature of its contents. It was similar to that described by Kollmann & Matthews (1982). Approximately 4-5 mm of the tooth tip projected into the cap.
Non-electrical stimulation of the tooth
Recordings were made from each isolated unit during the application of hot, cold, osmotic, hydrostatic pressure, mechanical and drying stimuli to the tip of the tooth in the cap. For thermal stimulation, Ringer solution at 55°C then 5°C was passed through the cap for 3 s each from reservoirs (Kollmann & Matthews, 1982). The osmotic stimulus was 4 mol l−1 dextrose solution at 30°C and lasted 10 s. Hydrostatic pressure stimuli were applied by changing the pressure of the Ringer solution in the cap with paired stimuli (as described above) in the range ± 200 to ± 400 mmHg. The exposed dentine surface was stimulated mechanically by gently stroking it with the fire-polished tip of a glass probe (tip diameter, 0.5 mm) under Ringer solution for 30 s, and was dried with a stream of air for 10 s.
Recording fluid flow through dentine in vitro
Fluid flow through dentine was recorded in vitro after completion of the electrophysiological recordings. The method used was based on that described above but with the flow recorded from the root instead of the crown of the tooth, so that measurements could be made during stimulation of the crown (Vongsavan & Matthews, 1992b). The same stimuli were applied to the exposed dentine at the tooth tip as used in vivo. Before attaching the recording system, the terminal 3-4 mm of the root was removed with a diamond disc and the normal outward flow of fluid that occurs following exposure of dentine in vivo (Vongsavan & Matthews, 1992a) was reproduced by applying a constant pressure of 15 cmH2O (10 mmHg) to the root, through the capillary, with a water manometer. The area of exposed dentine was measured as described above.
Recording of pulpal blood flow
In all animals pulpal blood flow was recorded from the experimental tooth with a laser-Doppler flow meter (Moor Instruments Ltd, Axminster, Devon, UK; type MBF3D). The laser probe was held in contact with the enamel, with its centre 2 mm above the gingival margin, by a length of stainless steel tubing that was fixed to the tooth with cyanoacrylate cement. High intensity electrical stimulation of the tooth (necessary to identify C-fibres) evokes pulpal vasodilatation (Vongsavan & Matthews, 1992c). This increases pulpal tissue fluid pressure and the rate of fluid flow through exposed dentine. Non-electrical stimulation of dentine to determine the responses of a unit was therefore delayed until pulpal blood flow had returned to resting levels.
Data collection
Neural recordings were made with low noise, differential amplifiers (bandpass 10 or 100 Hz to 1 kHz), displayed on an oscilloscope, and digitized with a computer interface for storage and analysis (CED 1401; Cambridge Electronic Design, Cambridge, UK; each channel sampled at 5 kHz with 12-bit accuracy). Records of dentinal fluid flow, pressure, temperature and blood flow were also digitized and stored in the same way.
RESULTS
Neural responses and dentinal fluid flow recorded in vivo using hydrostatic pressure stimuli
In all preparations a continuous outward flow of fluid from dentine was recorded. The mean flow was 30.9 pl s−1 mm−2 (range, 11.9-52.9 pl s−1 mm−2; s.d., 13.8 pl s−1 mm−2).
Fluid flow through dentine during hydrostatic pressure stimulation
The flow recordings made by measuring the pressure drop along the capillary mirrored changes in the velocity of the fat droplets of the milk in the capillary. When pressure stimuli were applied, a maintained flow of fluid through dentine was recorded. There were also transients at the beginning and end of a pressure stimulus that lasted approximately 0.5 s (Fig. 3). These were due to compliance in the flow recording system. All measurements of flow were made 1 s after the application of pressure stimuli to avoid the transients.
Figure 3. Multi-unit and single-fibre responses of intradental nerves and records of fluid flow through dentine during hydrostatic pressure stimulation.
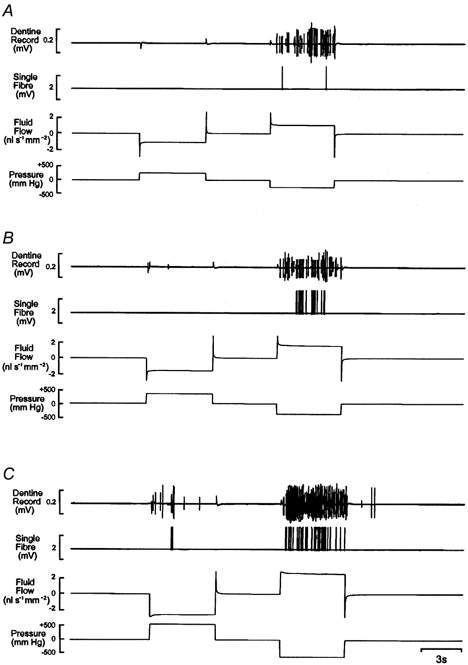
The hydrostatic pressure stimuli were ± 200 mmHg in A, ± 300 mmHg in B and ± 500 mmHg in C. The conduction velocity (CV) of the single pulpal afferent was 28.7 m s−1.
Responses of single fibres to hydrostatic pressure stimulation of dentine
Recordings were made from 45 single pulpal afferents with conduction velocities (CV) in the range 0.6-55.1 m s−1. Of these, 12 had conduction velocities in the C-fibre range (< 2.5 m s−1). Twenty-eight units (CV, 0.6-48.8 m s−1) were not pressure sensitive within the range tested (Fig. 2). Of the 17 fibres that responded to hydrostatic pressure stimuli, all were A-fibres (CV, 10.6-55.1 m s−1; mean, 26.1 m s−1; s.d., 13.2 m s−1; Fig. 2). Ten of these 17 units responded to negative pressure only; the remaining seven responded to both positive and negative pressure. None responded to positive pressure alone.
Figure 2. A-fibres but not C-fibres respond to hydrostatic pressure stimulation of exposed, etched dentine in the cat.
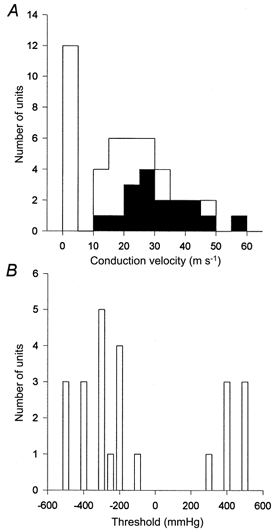
A, total number and conduction velocities of single fibres isolated. Those that responded to pressure stimuli are shown as filled columns. B, thresholds of single units to pressure stimuli.
The thresholds of the pressure-sensitive units to negative pressure were in the range -100 to -500 mmHg (median, -300 mmHg; s.d., -129 mmHg); and to positive pressure were +300 to +500 mmHg (median, 400 mmHg; s.d., 70 mmHg). All of the units were more sensitive to negative than positive pressure. All of the fibres that responded to positive and negative pressure did so in a similar manner: during the application of negative pressure, there was a progressive increase in discharge frequency with increasing stimulus intensity (Fig. 3). In contrast, positive pressure stimuli evoked few impulses, although there was a tendency for activity to increase with the magnitude of the stimulus (Fig. 3). Some pressure-sensitive units had high thresholds and responded with one or two action potentials only at the most negative pressure tested. In all cases, the firing rate of a unit during pressure stimulation tended to be irregular despite constant fluid flow (Fig. 3B and C).
The thresholds, in terms of flow, of units that responded to positive or negative pressure ranged from 0.3 to 2.1 nl s−1 mm−2 (mean, 1.3 nl s−1 mm−2; s.d., 0.4 nl s−1 mm−2) for outward flow, and from 2.0 to 3.5 nl s−1 mm−2 (mean, 2.4 nl s−1 mm−2; s.d., 0.8 nl s−1 mm−2) for inward flow. The thresholds to outward flow of units that responded to negative pressure only were not significantly different from those of units that responded to both positive and negative pressure (P > 0.05, Student’s unpaired t test). The relationships between flow and evoked discharge for a multi-fibre record from dentine and for a single unit are shown in Fig. 4. Other pressure-sensitive units showed a similar stimulus- response relationship.
Figure 4. The relationship between the number of action potentials evoked and the flow through dentine produced by hydrostatic pressure stimuli.
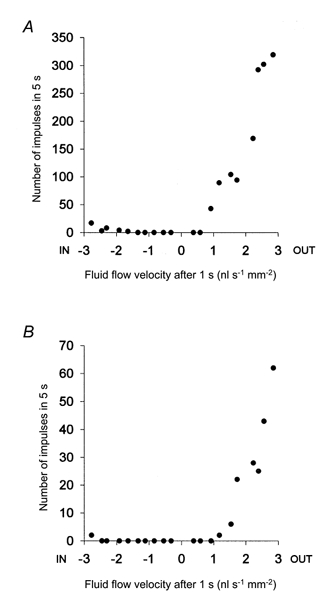
The number of action potentials evoked per 5 s stimulus was counted in both the multi-fibre records from dentine (A) and the single-unit records (B). Flow was measured 1 s after the application of a pressure stimulus. The data are from the same preparation as the records in Fig. 3.
Estimated flow velocities in individual dentinal tubules during hydrostatic pressure stimulation
The estimated number of dentinal tubules opened by removing 1 mm from the tip of a cat’s canine tooth was in the range 14675 to 28098 (mean, 22490). Their diameters at the exposed dentine surface ranged from 0.49 to 0.97 μm (mean, 0.73 μm). Based on these data, the responses of single units had thresholds, in terms of flow per tubule, in the range 13.3-93.3 fl s−1 during outward flow and between 88.5 and 154.7 fl s−1 during inward flow. The thresholds in terms of mean flow velocity at the outer ends of the tubules were 31.7-222.9 μm s−1 during outward flow and between 211.4 and 369.6 μm s−1 during inward flow.
The effects of repetitive pressure stimulation of dentine on intradental nerve activity, hydraulic conductance of dentine and pulpal histology
The number of action potentials recorded from dentine during repeated application of pressure stimuli always declined during an experiment. The decline observed with stimuli of a particular intensity with time during one experiment is shown in Fig. 5. In some cases the response decreased to the point where even the most intense stimulus (± 500 mmHg) evoked no response.
Figure 5. Effect of repetitive stimulation on the discharge evoked by hydrostatic pressure stimuli.
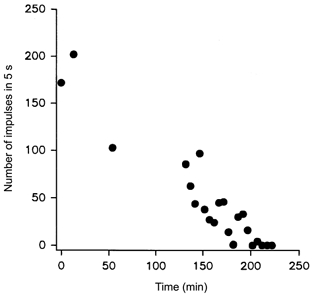
A ± 300 mmHg stimulus sequence was applied to dentine and the number of action potentials evoked in the multi-fibre record from dentine was counted. The stimuli were applied once for each single unit isolated during an experiment and thereafter every 5 min for a total of almost 4 h. The number of impulses evoked by each -300 mmHg stimulus is plotted against time.
The hydraulic conductance of the exposed dentine was calculated from the slope of the relationship between pressure and flow. Between the beginning and end of an experiment the hydraulic conductance increased significantly by 14.7% (s.d., 5.7%) from a mean of 0.33 × 10−8 m s−1 kPa−1 (s.d., 0.06 × 10−8 m s−1 kPa−1; n = 7) to 0.44 × 10−8 m s−1 kPa−1 (s.d., 0.13 × 10−8 m s−1 kPa−1; P < 0.02, Wilcoxon matched pairs test).
Histological examination of the teeth at the light level generally showed little damage to the pulp or odontoblasts in teeth that had been subjected to repeated application of hydrostatic pressure stimuli. There was, however, some evidence of disruption of the cytoarchitecture of the pulp that was absent from control teeth. Structures similar to cell nuclei were displaced a short distance into some dentinal tubules that terminated at the exposed dentine surface (Fig. 6A). These appeared to be the nuclei of odontoblasts and extended as far as the predentine. In some sections there was evidence of spaces or vesicles (diameter approximately 10 μm) in the tubules in the predentine and around the odontoblasts (Fig 6B). These were confined to the opened tubules (Fig. 6C).
Figure 6. The effects of repetitive hydrostatic pressure stimulation of dentine on the structure of dentine and pulp.
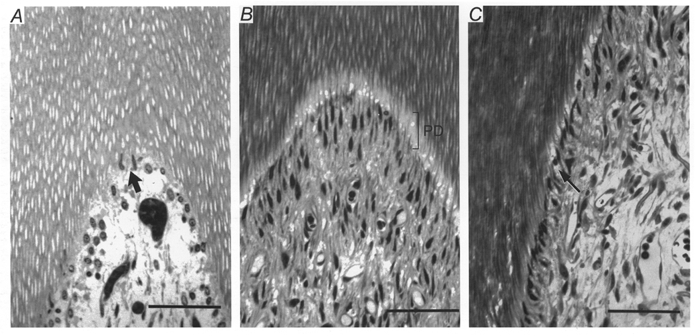
A, light micrograph of a longitudinal, semi-thin section taken from the cornu region of a decalcified tooth. Aspirated odontoblast nuclei (arrow) are visible in the pulpal ends of dentinal tubules that terminated in the area of exposed dentine to which pressure stimuli were applied. Tooth fixed by immersion. Scale bar, 200 μm. B, light micrograph of a section taken from a similar area to that in A of another tooth in which the odontoblast layer shows less evidence of disruption. There appear to be spaces or vesicles in the tubules in the predentine (PD) and around some of the odontoblasts. Scale bar, 200 μm. C, light micrograph from the same tooth as that shown in B but lower down the crown where the opened and unopened dentinal tubules are adjacent to each other (arrow). The spaces or vesicles in the predentine and around the odontoblasts, similar to those in B, are confined to the opened tubules. Scale bar, 200 μm.
Neural responses to a range of different stimuli recorded in vivo, and their relationship to dentinal flow recorded with the same stimuli in vitro
Responses of single units in vivo
Recordings were made from 55 single pulpal fibres (CV, 0.5-54.8 m s−1) in seven teeth. Thirty of these units responded to some form of non-electrical stimulus applied to dentine.
Eleven units (CV, 0.5-24.8 m s−1) responded to only one or two different forms of stimulus and were not investigated further. Of these, three responded to heating alone (CV, 0.5-1.4 m s−1) with a mean latency of 1.6 s (range, 1.3-1.8 s; s.d., 0.2 s). The remaining eight units (CV, 5.9-24.8 m s−1; mean, 16.7 m s−1; s.d., 5.5 m s−1) responded either to cooling, or to cooling and mechanical stimulation of dentine.
Ten units (CV, 12.8-54.8 m s−1; mean, 35.9 m s−1; s.d., 13.1 m s−1) responded to hydrostatic pressure, cold, mechanical and drying stimuli. Some responded also to a hot stimulus but none responded to an osmotic stimulus. These 10 units were selected for detailed study, including the relationship between fluid flow and evoked discharge. Figure 7 shows an example of the responses of one unit. All units responded to both positive and negative pressure. They were tested with only a limited range of hydrostatic pressure stimuli (± 200, 300 and 400 mmHg) to minimize desensitization of the preparation. The median threshold to positive pressure stimuli was +300 mmHg (range, +200 to +400 mmHg; s.d., 66 mmHg) and all responded to -200 mmHg. Each unit exhibited a similar relationship between hydrostatic pressure intensity and evoked discharge to those described earlier. Two of the units responded to both heating and cooling of the tooth and eight responded to cooling alone. Both units that responded to heating did so at the end of the hot stimulus (mean latency, 2.2 s). The responses to cooling were characterized by an initial high frequency burst of impulses (mean latency, 0.6 s; range, 0.3-1.5 s; s.d., 0.4 s), after which impulse frequency declined. There was a second shorter burst of impulses when the tooth was re-warmed to 30°C (Fig. 7B).
Figure 7. Responses of pressure-sensitive intradental nerves to other forms of stimulus.
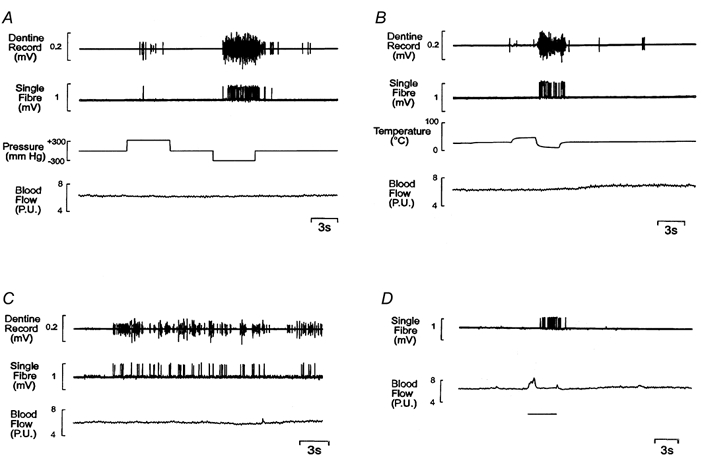
Responses recorded from a single unit (CV, 37.5 m s−1) and from dentine during stimulation of exposed dentine with hydrostatic pressure (± 300 mmHg, A); hot (55°C) and cold (5°C) Ringer solution (B); the fire-polished tip of a glass probe throughout the recording (C); and a stream of air at room temperature to dry the dentine surface (indicated by the bar, D). It was not possible to record from dentine during drying. The deflection of the blood flow trace in this record was due to inadvertent movement of the laser-Doppler probe while aspirating the Ringer solution from the tooth cap. The unit did not respond to an osmotic stimulus (4 mol l−1 dextrose solution).
Mechanical stimulation of the exposed dentine by drawing a glass probe across the surface evoked an irregular discharge (Fig. 7C). This pattern of response was due to the receptive field being restricted to only part of the exposed dentine surface, so the unit was stimulated only intermittently. Drying the exposed dentine surface evoked a high frequency discharge of impulses (Fig. 7D). The mean latency of the response was 1.9 s (range, 1.2-4.2 s; s.d., 0.9 s).
None of the non-electrical stimuli employed in this or the first series of experiments produced a consistent change in pulpal blood flow.
Relationship to dentinal flow recorded in vitro
When recordings of flow were made from the same teeth in vitro, using the same stimuli as in vivo, heating, positive pressure stimuli and mechanical stimulation of dentine all caused inward flow through the dentine, and cooling, negative pressure stimuli and osmotic stimuli caused outward flow.
The thresholds of each unit to hot and cold stimuli expressed in terms of dentinal flow were estimated by measuring the flow in vitro after a delay corresponding to the latency of the first action potential recorded in vivo. The onset of a thermal stimulus was taken as the time where the temperature of the cap contents had changed by 0.2°C. The mean delay after this of the resultant change in dentinal flow was 0.3 s (range, 0.1-0.4 s; s.d., 0.1 s) with both heating and cooling. At the threshold of the neural responses, the mean flow during heating was 0.4 nl s−1 mm−2 inwards (range, 0.3-0.5 nl s−1 mm−2; s.d., 0.1 nl s−1 mm−2), whereas mean flow at the threshold for cooling was 1.4 nl s−1 mm−2 outwards (range, 0.8- 1.6 nl s−1 mm−2; s.d., 0.3 nl s−1 mm−2). The mean peak flow with heating was 1.4 nl s−1 mm−2 (range, 1.0-2.2 nl s−1 mm−2; s.d., 0.4 nl s−1 mm−2) inwards, and with cooling was 2.0 nl s−1 mm−2 (range, 1.5-2.4 nl s−1 mm−2; s.d., 0.3 nl s−1 mm−2) outwards. The greater effect of the cold stimulus can be attributed to the fact that it was always preceded by the hot stimulus, which would have resulted in a greater temperature gradient across the dentine. No consistent effect of osmotic stimulation with 4 mol l−1 dextrose was recorded, although in some preparations a small outward flow (35-81 pl s−1 mm−2) was observed.
The onset of the flow caused by the application of hydrostatic pressure was much more rapid (mean latency, 0.09 s; s.d., 0.02 s). Peak flow during positive pressure stimuli was in the range 0.3-0.8 nl s−1 mm−2 inwards (mean, 0.5 nl s−1 mm−2; s.d., 0.2 nl s−1 mm−2); the corresponding flow evoked during negative pressure stimuli was 0.3-0.8 nl s−1 mm−2 outwards (mean 0.5 nl s−1 mm−2; s.d., 0.1 nl s−1 mm−2).
Mechanical stimulation produced a slowing of the normal outward flow and sometimes reversal of flow. Peak flow evoked by such stimuli was in the range 2.8 pl s−1 mm−2 outwards to 800 pl s−1 mm−2 inwards (mean, 300 pl s−1 mm−2 inwards; s.d., 300 pl s−1 mm−2). It was not possible to record the latency of the responses to mechanical stimulation of dentine as the time at which the stimulus was applied could not be recorded precisely.
The mean latency of the response to drying was 0.7 s (range, 0.5-0.9 s; s.d., 0.2 s), which included the time taken to suck out the contents of the cap and dry the exposed dentine surface. Drying evoked mean peak outward flows of 0.4 nl s−1 mm−2 (range, 0.2-0.6 nl s−1 mm−2; s.d., 0.2 nl s−1 mm−2).
During cooling, the peak outward flow occurred at the same time as the peak discharge rate and the flow decreased when the discharge frequency declined (Fig. 8). The burst of impulses that usually occurred when the tooth was re-warmed to 30°C coincided with the sudden reversal in the direction of flow, inwards towards the pulp (Fig. 8).
Figure 8. Effects of thermal stimulation on the discharge of a single pulpal afferent and fluid flow through dentine.
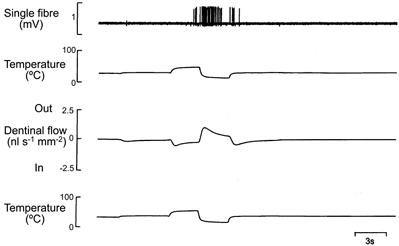
The upper pair of traces was recorded in vivo and shows the responses of a single pulpal fibre (CV, 26.4 m s−1). The lower pair of traces was recorded in vitro.
The relationship between the discharge evoked from the unit shown in Fig. 7 by different stimuli and the flow of fluid evoked by the same stimuli in vitro is shown in Fig. 9. The responses evoked by heating, osmotic stimulation and drying followed the same trend as that with hydrostatic pressure stimuli: a curve drawn through the hydrostatic pressure data points would pass close to the points for the other stimuli. A similar relationship was found in all the units tested, although the relationship between evoked discharge and flow with the cold stimulus was more variable than that obtained with the other stimuli.
Figure 9. The relationship between the mean frequency of impulses evoked in one pulpal afferent by different forms of stimulus and fluid flow through dentine.
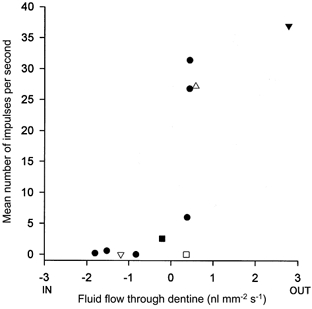
The impulse discharge was recorded in vivo and the associated dentinal flow, from the same tooth, was recorded in vitro. The unit is the same as that shown in Fig. 7. The flow data are the mean flows during the periods of stimulation. The stimuli were as follows: Ringer solution at 55°C (▿); Ringer solution at 5°C (▾); 4 mol l−1 dextrose solution (□); hydrostatic pressure in the range ± 200 to ± 400 mmHg in steps of 100 mmHg (•); mechanical stimulation of exposed dentine (▵); and drying of the dentine surface with an air stream (▵).
DISCUSSION
The relationship observed between fluid flow through dentine and the discharge evoked in a group of intradental A-fibres during the application of a range of different types of stimulus is consistent with the hydrodynamic hypothesis. The effects of hydrostatic pressure stimuli demonstrate the basic relationship between dentinal tubular flow and evoked impulse discharge. All the pressure-sensitive fibres increased their discharge progressively as outward flow increased above threshold, as found previously (Matthews et al. 1996). The same fibres were much less sensitive to inward flow, again confirming previous findings (Matthews et al. 1996). The fact that the impulse discharge was irregular during negative pressure stimulation, despite the total flow through the tubules being constant, may have been because the flow in individual tubules was non-uniform, due perhaps to temporary plugging of the tubules by the odontoblasts.
The relationship between flow and evoked discharge obtained with the cold stimulus did not follow the same trend as that obtained with other stimuli (e.g. Fig. 9). In all the units tested the neural response to the cold stimulus was equivalent to that evoked by substantially lower rates of outward flow with other forms of stimulus. This observation does not contradict the hydrodynamic hypothesis since the cold stimulus, which was applied to both the exposed dentine and some surrounding enamel, would have caused outward flow in some unopened tubules that would not have been affected by the non-thermal stimuli. The flow produced by the cold stimulus through the opened tubules may have been similar to that caused by a hydrostatic pressure stimulus that evoked the same discharge. Only units that responded to hydrostatic pressure stimuli were selected for study. The hot stimulus would similarly have affected more tubules than the hydrostatic pressure stimuli but in this case the inward flow produced was too small for the effect to be detectable.
None of the units tested in the current experiments responded to the application of an osmotic stimulus to dentine and this is consistent with the observation that this form of stimulus produced much smaller flows than the other stimuli. When compared with the flow produced by hydrostatic pressure stimuli, 4 mol l−1 dextrose produced less flow in the recently extracted cat teeth used here than was observed in human teeth (Horiuchi & Matthews, 1973). This may be due to cat dentine having a lower reflection coefficient for dextrose.
The results obtained with mechanical stimulation are not as readily explained. They appear to follow the trend of other data in Fig. 9 but this is misleading because not all the tubules contributing to the record would be affected in the same way with this form of stimulation. There would have been inward flow in the tubules under the probe tip but a slow outward flow in the remainder. For the flow record to show an overall inward flow there must have been a huge inward flow in the stimulated tubules, much greater than that suggested by the record. The fact that the discharge evoked was not correspondingly greater may have been because the high flow rate was very transient, limited by the compliance of the etched dentine surface, or because the responses evoked by other forms of stimulation such as the application of hydrostatic pressure, involved summation of the effects of flow in many branches of a single afferent in adjacent tubules. Such summation might occur if the site of impulse generation was at the first node of Ranvier central to a series of branched terminals. The underlying transduction mechanism operating during excitation of the receptors by probing exposed dentine may therefore be the same as with the other forms of stimulus.
Repeated application of pressure stimuli caused a gradual desensitization of the preparation. A similar effect has been observed with cold stimuli (Kollmann & Matthews, 1982). The observations on the hydraulic conductance of the exposed dentine show that this effect was not due to an increase in the resistance to flow in the dentinal tubules. Also, the effect does not appear to be time dependent or due to deterioration of the preparation as it was observed following repeated stimulation irrespective of the time after setting up the preparation. Furthermore, in other experiments when similar 5 s hydrostatic pressure stimuli were repeated at 3 min intervals, the number of action potentials evoked decreased progressively (T. Amess & B. Matthews, unpublished observations). In this case the decay was exponential and the evoked impulse count reached 50% of its initial value after 5-10 cycles of stimulus repetition. The sensitivity of the preparation did not recover when it was left unstimulated for several hours.
These observations suggest that the desensitization was due to cumulative structural damage that affected the transduction mechanism. The histological data confirmed that the structure of the pulp-dentine interface might be disrupted during an experiment. Also, Byers et al. (1988) showed that nerve terminals in dentinal tubules of rat molars were damaged when the overlying dentine was dried.
While it is clear that the stimuli employed in the present experiments are capable of causing tissue damage, in addition to producing pain in man, it is not known whether such damage, either to the nerve terminals themselves or to adjacent cells, is the adequate stimulus for the receptors. The estimated velocity of flow in the stimulated tubules, which is similar to earlier estimates (Matthews & Vongsavan, 1994), would seem to be sufficient to create shear forces of the order necessary to damage the odontoblast processes and associated nerve terminals (Matthews & Vongsavan, 1994). The greater sensitivity to outward than inward flow could be due to the cells being more resistant to the compression resulting from inward flow than to the tension caused by outward flow. Such mechanisms would be expected of nociceptors. Alternatively, the receptors might be mechanoreceptors sensitive to non-damaging deformation resulting from flow in the dentinal tubules. In the present work, only pressure-sensitive A-fibres were selected for detailed study. Other pulpal afferents, with conduction velocities in the C and Aδ ranges, respond to heating and chemical stimuli (Matthews, 1985; Nähri, 1985) but generally do not respond to hydrostatic pressure stimuli (Närhi & Haegerstam, 1983). They therefore appear to have a different transduction mechanism.
In conclusion, it appears that displacement of the contents of dentinal tubules can account for the discharge evoked in myelinated nerve fibres in tooth pulp by a range of different stimuli that cause pain from dentine in man. The associated receptors are not equally sensitive to inward and outward flow. The precise transduction mechanism remains unknown although the evidence suggests that the discharge may be evoked as a result of damage to the nerve terminals or odontoblasts caused by fluid flow through the tubules.
Acknowledgments
This work was supported by the Wellcome Trust.
References
- Branstromm M. A hydrodynamic mechanism in the transmission of pain-producing stimuli through the dentine. In: Anderson DJ, editor. Sensory Mechanisms in Dentine. Oxford: Pergammon Press; 1963. pp. 73–79. [Google Scholar]
- Byers MR. Dental sensory receptors. International Reviews of Neurobiology. 1984;25:39–94. doi: 10.1016/s0074-7742(08)60677-7. [DOI] [PubMed] [Google Scholar]
- Byers MR, Narhi MVO, Mecifi KB. Acute and chronic reactions of dental sensory nerve fibres to cavities and desiccation in rat molars. Anatomical Record. 1988;221:872–883. doi: 10.1002/ar.1092210412. [DOI] [PubMed] [Google Scholar]
- Cadden SW, Lisney SJW, Matthews B. Thresholds to electrical stimulation of nerves in cat canine tooth-pulp with Aβ-, Aδ- and C-fibre conduction velocities. Brain Research. 1983;261:31–41. doi: 10.1016/0006-8993(83)91280-5. [DOI] [PubMed] [Google Scholar]
- Carter GM, Matthews B. Responses of jaw muscles to electrical stimulation of tooth-pulp in rat, cat and man. In: Van Steenberghe D, De Laat A, editors. Electromyography of Jaw Reflexes in Man. Leuven: Leuven University Press; 1989. pp. 205–236. [Google Scholar]
- Fearnhead RW. The neurohistology of human dentine. Proceedings of the Royal Society of Medicine. 1961;54:877–884. [PMC free article] [PubMed] [Google Scholar]
- Fried K, Erdelyi G. Inferior alveolar nerve regeneration and incisor pulpal reinnervation following intramandibular neurotomy in the cat. Brain Research. 1982;244:259–268. doi: 10.1016/0006-8993(82)90084-1. [DOI] [PubMed] [Google Scholar]
- Gysi A. An attempt to explain the sensitiveness of dentine. British Journal of Dental Science. 1900;43:865–868. [Google Scholar]
- Holland GR. The incidence of dentinal tubules containing more than one process in the cuspal dentine of cat canine teeth. Anatomical Record. 1981;200:437–442. doi: 10.1002/ar.1092000406. [DOI] [PubMed] [Google Scholar]
- Holland GR. The odontoblast process: Form and function. Journal of Dental Research. 1985;64:499–514. doi: 10.1177/002203458506400403. (special issue) [DOI] [PubMed] [Google Scholar]
- Holland GR, Matthews B, Robinson PP. An electrophysiological and morphological study of the innervation and reinnervation of cat dentine. Journal of Physiology. 1987;386:31–43. doi: 10.1113/jphysiol.1987.sp016520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H, Matthews B. In-vitro observations of fluid flow through human dentine caused by pain-producing stimuli. Archives of Oral Biology. 1973;18:275–294. doi: 10.1016/0003-9969(73)90147-7. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Matthews B. Evidence on the origin of impulses recorded from dentine in the cat. Journal of Physiology. 1974;243:797–829. doi: 10.1113/jphysiol.1974.sp010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann W, Matthews B. Responses of intradental nerves to thermal stimulation of teeth in the cat. In: Matthews B, Hill RG, editors. Anatomical, Physiological and Pharmacological Aspects of Trigeminal Pain. Amsterdam: Excerpta Medica; 1982. pp. 51–65. [Google Scholar]
- Kollmann W, Matthews B, Suda H. Responses of intradental nerves to stimulation of dentine in the cat. Journal of Physiology. 1982;332:63. [Google Scholar]
- Maeda T, Honma S, Takano Y. Dense innervation of human radicular dental pulp as revealed by immunocytochemistry for protein gene-product 9. 5. Archives of Oral Biology. 1994;39:563–568. doi: 10.1016/0003-9969(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Matthews B. Responses of intradental nerves to electrical and thermal stimulation of teeth in dogs. Journal of Physiology. 1977;264:641–664. doi: 10.1113/jphysiol.1977.sp011687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. Peripheral and central aspects of trigeminal nociceptive systems. Philosophical Transactions of the Royal Society. 1985;B 308:313–324. doi: 10.1098/rstb.1985.0032. [DOI] [PubMed] [Google Scholar]
- Matthews B, Andrew D, Amess TR, Ikeda H, Vongsavan N. The functional properties of intradental nerves. In: Shimono M, Maeda T, Suda H, Takahashi H, editors. Dentin/Pulp Complex. Tokyo: Quintessence; 1996. pp. 146–153. [Google Scholar]
- Matthews B, Hughes SHS. The ultrastructure and receptor transduction mechanisms of dentine. Progress in Brain Research. 1988;74:69–76. doi: 10.1016/s0079-6123(08)63000-9. [DOI] [PubMed] [Google Scholar]
- Matthews B, Vongsavan N. Fluid flow through dentine: Interactions between neural and hydrodynamic mechanisms. Archives of Oral Biology. 1994;39:87S–95S. doi: 10.1016/0003-9969(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Närhi M. The characteristics of intradental sensory units and their responses to stimulation. Journal of Dental Research. 1985;64:564–571. doi: 10.1177/002203458506400411. (special issue) [DOI] [PubMed] [Google Scholar]
- Narhi M, Haegerstam G. Intradental nerve activity induced by reduced pressure applied to exposed dentine in the cat. Acta Physiologica Scandinavica. 1983;119:381–386. doi: 10.1111/j.1748-1716.1983.tb07353.x. [DOI] [PubMed] [Google Scholar]
- Närhi M, Hirvonen TJ, Hakumäki MOK. Responses of intradental nerve fibres to stimulation of dentine and pulp. Acta Physiologica Scandinavica. 1982;115:173–178. doi: 10.1111/j.1748-1716.1982.tb07062.x. [DOI] [PubMed] [Google Scholar]
- Närhi M, Yamamoto H, Ngassapa D, Hirvonen T. The neurophysiological basis and the role of inflammatory reactions in dentine hypersensitivity. Archives of Oral Biology. 1994;39:23S–30S. doi: 10.1016/0003-9969(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Vongsavan N, Matthews B. Fluid flow through cat dentine in vivo. Archives of Oral Biology. 1992a;37:175–185. doi: 10.1016/0003-9969(92)90087-o. [DOI] [PubMed] [Google Scholar]
- Vongsavan N, Matthews B. Hydrodynamic effects in human dentine in vitro. Journal of Dental Research. 1992b;71:153. (special issue) [Google Scholar]
- Vongsavan N, Matthews B. Changes in pulpal blood flow and in fluid flow through dentine produced by autonomic and sensory nerve stimulation in the cat. Proceedings of the Finnish Dental Society. 1992c;88(suppl. 1):491–497. [PubMed] [Google Scholar]
- Wakisaka S, Ichikawa H, Nishimoto T, Matsuo S, Yamamoto K, Nakata T, Akai M. Substance P-like immunoreactivity in the pulp dentine zone of human molar teeth demonstrated by indirect immunofluorescence. Archives of Oral Biology. 1984;29:73–75. doi: 10.1016/0003-9969(84)90046-3. [DOI] [PubMed] [Google Scholar]


