Abstract
This study examined the effect of ingesting caffeine (6 mg kg−1) on muscle carbohydrate and fat metabolism during steady-state exercise in humans. Young male subjects (n = 10) performed 1 h of exercise (70 % maximal oxygen consumption (V̇O2,max)) on two occasions (after ingestion of placebo and caffeine) and leg metabolism was quantified by the combination of direct Fick measures and muscle biopsies.
Following caffeine ingestion serum fatty acid and glycerol concentration increased (P ≤ 0.05) at rest, suggesting enhanced adipose tissue lipolysis.
In addition circulating adrenaline concentration was increased (P ≤ 0.05) at rest following caffeine ingestion and this, as well as leg noradrenaline spillover, was elevated (P ≤ 0.05) above placebo values during exercise.
Caffeine resulted in a modest increase (P ≤ 0.05) in leg vascular resistance, but no difference was found in leg blood flow.
Arterial lactate and glucose concentrations were increased (P ≤ 0.05) by caffeine, while the rise in plasma potassium was dampened (P ≤ 0.05).
There were no differences in respiratory exchange ratio or in leg glucose uptake, net muscle glycogenolysis, leg lactate release or muscle lactate, or glucose 6-phosphate concentration. Similarly there were no differences between treatments in leg fatty acid uptake, glycerol release or muscle acetyl CoA concentration.
These findings indicate that caffeine ingestion stimulated the sympathetic nervous system but did not alter the carbohydrate or fat metabolism in the monitored leg. Other tissues must have been involved in the changes in circulating potassium, fatty acids, glucose and lactate.
It is clear that caffeine can be employed as an ergogenic agent for a wide range of exercise conditions (Graham, 1997) and it is often proposed that this effect is mediated by enhancing fat oxidation and decreasing carbohydrate use in the active muscles. However, not only does caffeine enhance exercise capacity when endogenous carbohydrates are not limiting metabolism (Anselme et al. 1992; MacIntosh & Wright, 1995; Jackman et al. 1996), but measurement of the respiratory exchange ratio (RER) has also commonly failed to support the theory of enhanced fat oxidation (Tarnopolsky et al. 1989; Graham & Spriet, 1991; Spriet et al. 1992; Greer et al. 2000a).
Caffeine can directly antagonize adenosine receptors in many tissues including tissues in the central nervous and cardiovascular systems and skeletal muscle and adipose tissue. This could result in a multitude of responses including adrenaline secretion (Spriet et al. 1992), altered blood flow (Daniels et al. 1998) and lipolysis. These in turn can interact and thus the ultimate metabolic consequences may be the result of such direct and/or indirect actions. One major reason for the uncertainty about the impact of caffeine on fat/carbohydrate metabolism in active muscle may be that muscle metabolism has not been directly assessed in human subjects. Previous studies (Tarnopolsky et al. 1989; Graham & Spriet, 1991; Spriet et al. 1992; Greer et al. 2000a) have relied on pulmonary measurements combined with assessments of metabolite concentrations in mixed venous blood.
The finding that caffeine ingestion does not change the RER during exercise means that the combined amount of muscle glycogen and blood glucose oxidized is unaltered. However, it is possible that the proportions of these two metabolic fuels oxidized, and/or that the amount of carbohydrate converted to lactate, is changed. For example, there are several reports that administration of caffeine or the dimethylxanthine theophylline increases venous blood lactate concentration (Anselme et al. 1992; Spriet et al. 1992; Jackman et al. 1996; Greer et al. 2000a) or muscle lactate concentration (Jackman et al. 1996; Greer et al. 2000a) during exercise. This has led to speculation that muscle lactate production could be increased (Prefaut et al. 1992; Jackman et al. 1996; Raguso et al. 1996). Also, there are reports (Vergauwen et al. 1994, 1997; Han et al. 1998; Derave & Hespel, 1999) that adenosine antagonism can reduce glucose uptake by contracting rodent muscle. In addition, Verguawen et al. (1997) demonstrated that caffeine and other adenosine antagonists actually enhanced glycogenolysis in contracting rat hindlimb musculature in the presence of isoproterenol. In contrast, muscle glycogen catabolism in exercising humans has been reported to be reduced (Essig et al. 1980; Erickson et al. 1987; Spriet et al. 1992) or to be unaltered (Jackman et al. 1996; Chesley et al. 1998; Greer et al. 2000a) after caffeine ingestion. However, there have been no direct assessments of the impact of caffeine on glucose uptake or lactate exchange for active, human muscle. While these various studies demonstrate that adenosine may be an important regulator of carbohydrate metabolism, the results are inconsistent and the mechanisms poorly understood.
Knowledge of the impact of caffeine on fat metabolism in humans during exercise is even more limited. One of the more consistent findings with caffeine administration is an increased plasma fatty acid (FA) concentration during rest (Robertson et al. 1981; van Soeren et al. 1996; Graham, 1997; Chesley et al. 1998) and this has been attributed to a stimulation of lipolysis as a result of caffeine directly antagonizing adenosine A1 receptors on adipocytes (van Soeren et al. 1996; Mohr et al. 1998; Greer et al. 2000a). While the lack of change in RER noted above signifies that total fat oxidation is unaltered by caffeine during exercise, it is unknown whether the proportion of FA and triglycerides oxidized is altered. The only two studies to examine these aspects in detail, are inconsistent. Essig et al. (1980) found that caffeine ingestion resulted in a 1.5-fold greater use of muscle triglycerides during exercise despite there being only a 50 % increase in fat oxidation. Raguso et al. (1996) reported no impact of theophylline on the RER or the rates of appearance or disappearance of plasma glycerol or FA at rest or during exercise.
This area of study has been very descriptive in nature and is lacking quantitative measurements of human muscle metabolism. Thus this study was performed to assess directly muscle carbohydrate and fat metabolism during exercise in humans following caffeine ingestion using both direct Fick measures and muscle biopsies. These data provide a detailed assessment of the systemic and local effects of caffeine ingestion on carbohydrate and fat metabolism as well as an evaluation of potential changes in skeletal muscle metabolic intermediates associated with this metabolism.
METHODS
Ten healthy male volunteers gave their written consent to participate in the study after being informed of the purposes of the study and the risks involved. They ranged in age from 20 to 28 years (mean, 25.7 years), weighed 68-111 kg (mean, 84.9 kg), and were 179-193 cm tall (mean, 184.9 cm). The protocol was approved by the Ethical Committee for Copenhagen and Frederiksberg and conformed to the Declaration of Helsinki.
Pre-experimental protocol
Each subject reported to the laboratory twice before the actual experiments. On the first visit he performed an incremental maximal oxygen consumption (V̇O2,max) test on a Krogh cycle ergometer. On a separate day the subject performed exercise at a power output predicted to require 70 % of his V̇O2,max to both confirm that the power output was correctly selected and habituate him to the exercise task.
Experimental protocol
Each subject completed two trials, administered double blind, one with caffeine ingestion and one with placebo, separated by 1 week. The subject abstained from all caffeine-containing foods and beverages for 48 h before the tests. The subject reported to the laboratory after an overnight fast; each test began between 08.00 and 09.00 h. Teflon catheters were inserted below the inguinal ligament into a femoral artery and vein of one leg and advanced so that the tips of the arterial and femoral catheters were approximately 2 cm proximal and distal, respectively, to the inguinal ligament. To allow for the measurement of blood flow by the constant infusion thermodilution technique (Andersen & Saltin, 1985), a thermistor (Edslab probe 94-030-2.5F) was inserted through the venous catheter and advanced 8 cm proximal to the catheter tip. The thermistor was connected to a cardiac output computer (American Edwards Laboratory) to record changes in temperature during the infusion of saline. Blood pressure was continuously monitored via the arterial catheter which was connected to a Statham blood pressure transducer.
After approximately 30 min of supine rest (designated -60 min) an arterial blood sample was taken and the subject ingested gelatin capsules containing either caffeine (6 mg (kg body wt)−1) or placebo (dextrose) with approximately 250 ml water. He then rested supine for a further 60 min before exercising for 60 min on a Krogh cycle ergometer at a power output requiring approximately 70 % of his V̇O2,max. At 0, 10, 30, 45 and 60 min heart rate and blood pressure were recorded, leg blood flow was determined, using a modification of the thermodilution technique as previously described (Andersen & Saltin, 1985), and arterial and venous blood samples were withdrawn. The total blood volume sampled during the experiment was approximately 250 ml. Immediately after blood sampling at 0, 10 and 60 min, a needle biopsy of the vastus lateralis muscle was taken with suction and immediately frozen in liquid nitrogen while still within the needle. During the trial muscle biopsies were taken from the same leg, the opposite leg was used for muscle biopsies in the second trial.
Pulmonary V̇O2 and RER were measured at 8, 28, 43 and 58 min by collecting expired air in Douglas bags for a 1 min interval.
Analysis
The Douglas bag volume was determined using a bell spirometer (Collins, Braintree, MA, USA) and the expired air O2 and CO2 contents were determined by paramagnetic (Servomex) and infrared (Beckman LB-2) systems, respectively. The arterial blood samples were analysed for haematocrit, and plasma insulin (radioimmunoassay kit, Novo-Nordisk, Copenhagen, Denmark). All blood samples were analysed for blood haemoglobin and O2 content (OSM 3 hemoximeter, Radiometer, Copenhagen, Denmark), blood lactate and glucose (glucose-lactate analyser, Yellow Springs, OH, USA), plasma catecholamines (by HPLC, Waters, Milford, MA, USA; Weiker et al. 1984), plasma potassium (flame photometry, Radiometer FLM3, Copenhagen, Denmark), plasma FA (NEFA C Kit Wako Bioproducts, Richmond VA, USA), plasma glycerol (Lowry & Passonneau, 1993) and plasma β-hydroxybutyrate (B-OH) (Williamson & Mellander, 1974) (n = 7 for the latter determination).
Muscle biopsies were freeze dried and any visible connective tissue, fat and blood were removed prior to analysis. The samples were analysed in duplicate for glycogen (Harris et al. 1974), glucose 6-phosphate (G6P) (Bergmeyer et al. 1974), total creatine (Bernt et al. 1974), lactate (Gutmann & Wahlefeld, 1974), citrate (Passonneau & Brown, 1974), acetyl CoA (Cederblad et al. 1990), and cAMP (cAMP-3H assay kit, Amersham Pharmacia Biotech Uppsala, Sweden).
Calculations
The net uptake or release of metabolites or substrates by the leg was calculated by multiplying the blood or plasma flow by the arterial – venous difference in concentration. The mean blood pressure was calculated as 33 % of the diastolic and 67 % of the systolic pressure at rest and as 50 % of each measure during exercise. Leg peripheral resistance was calculated as mean arterial pressure divided by leg blood flow. The net release or spillover of noradrenaline by the leg was calculated as described by Savard et al. (1987). Basically, the muscle extracts both noradrenaline and adrenaline from the arterial blood, but it simultaneously also releases noradrenaline that is ‘washed out’ of sympathetic nerve endings. The rate of extraction of adrenaline by the leg is used as an estimate of the noradrenaline extraction and is added to the net noradrenaline exchange in order to calculate the noradrenaline spillover. The muscle metabolite data for a given subject were corrected with the total creatine data based on the assumption that the total creatine should be constant during the time course of the experiment.
Statistics
The data were analysed using a two factor repeated measures analysis of variance for treatment and time effects. Significant interactions and time effects were further analysed using the Student-Newman-Keuls procedure. Statistical significance was accepted at the 0.05 confidence level. Data are expressed as means ± s.e.m.
RESULTS
Cardiopulmonary results
There were no significant differences between treatments in the RER data and the values reflect that the activity resulted in carbohydrate oxidation being the dominant substrate (Table 1). Similarly there were no significant differences between placebo and caffeine for either pulmonary or leg V̇O2 (Table 1). There were no significant differences between treatments for heart rate, but mean blood pressure (Table 1) was consistently elevated following caffeine ingestion by 4-8 mmHg (P < 0.05). Leg blood flow was not significantly different between treatments despite the blood pressure increase, reflecting that the peripheral resistance in the leg circulation was elevated by 10-15 % (P < 0.05) both at rest and exercise (Table 1).
Table 1.
A summary of the cardiopulmonary data
| Time during exercise trial | |||||
|---|---|---|---|---|---|
| Parameter | 0 min | 10 min | 30 min | 45 min | 60 min |
| Mean BP (mm Hg) | |||||
| Placebo | 99.3* (4.9) | 109.2* (3.8) | 106.4* (3.80 | 104.8* (5.3) | 101.4* (5.2) |
| Caffeine | 104.1 (3.6) | 115.7 (4.3) | 109.6 (3.3) | 111 (3.1) | 109.3 (4.1) |
| Leg V̇O2 (ml min-1) | |||||
| Placebo | 27.1 (3.6) | 1267.8 (75.5) | 1233.1 (60.1) | 1257.0 (60.3) | 1289.1 (57.1) |
| Caffeine | 20.9 (3.0) | 1248.7 (80.2) | 1278.7 (82.8) | 1264.3 (45.6) | 1268.4 (40.3) |
| Pulmonary V̇O2 (1 min-1) | |||||
| Placebo | — | 2.97 (0.0) | 3.11 (0.09) | 3.12 (0.08) | 3.13 (0.10) |
| Caffeine | — | 3.07 (0.12) | 3.14 (0.08) | 3.22 (0.09) | 3.21 (0.11) |
| HR (beats min−1) | |||||
| Placebo | — | 141(4) | 154(4) | 159(4) | 163(3) |
| Caffeine | — | 146(4) | 158(4) | 165(3) | 169(3) |
| LR (mmHg min 1−1) | |||||
| Placebo | 257* (32.22) | 16* (0.61) | 16* (0.65) | 16* (1.00) | 15* (0.83) |
| Caffeine | 312 (49.18) | 18 (1.41) | 17 (1.05) | 17 (1.04) | 17 (1.160 |
| RER | |||||
| Placebo | — | 0.95 (0.03) | 0.94 (0.04) | 0.94 (0.03) | 0.95 (0.03) |
| Caffeine | — | 0.94 (0.03) | 0.93 (0.03) | 0.93 (0.03) | 0.93 (0.03) |
The data are means with S.E.M. in parentheses. LR, leg vescular resistance.
Significant (P < 0.05) difference between caffeine and placebo treatments for a given time period.
Fat metabolism
Following caffeine ingestion, arterial plasma FA concentration increased significantly (P < 0.05) during the 1 h rest and remained greater (P < 0.05) than in the placebo trial at 10 and 30 min of exercise (Fig. 1A). The FA concentration declined sharply in both treatments during the initial phase of the exercise. In contrast, the arterial glycerol concentration (Fig. 1B) rose during exercise (P < 0.05) and was not significantly different (P > 0.05) between treatments. However, the change in concentration of both FA and glycerol during 1 h rest was significantly greater (P < 0.05) following caffeine ingestion. The changes in arterial FA concentrations at rest were 140 ± 40 and 441 ± 138 μM for placebo and caffeine, respectively, and the corresponding data for arterial glycerol were 3 ± 5 and 34 ± 12 μM. The leg exchange of FA was very similar between treatments and increased (P < 0.05) during exercise. There were no time or treatment effects for leg glycerol exchange (Table 2). As noted above, the RER data indicated that net oxidation of fat during exercise was not affected by caffeine ingestion. Arterial B-OH concentration (n = 7) was not significantly different between treatments (Fig. 1C) and although the changes during rest were 22 ± 12 and 102 ± 52 μM for placebo and caffeine, respectively, the differences were not significant (P > 0.05). Leg B-OH exchange was not changed significantly during exercise (Table 2).
Figure 1. A summary of the arterial fatty acid (A), glycerol (B) and β-hydroxybutyrate (C) responses to caffeine and exercise.
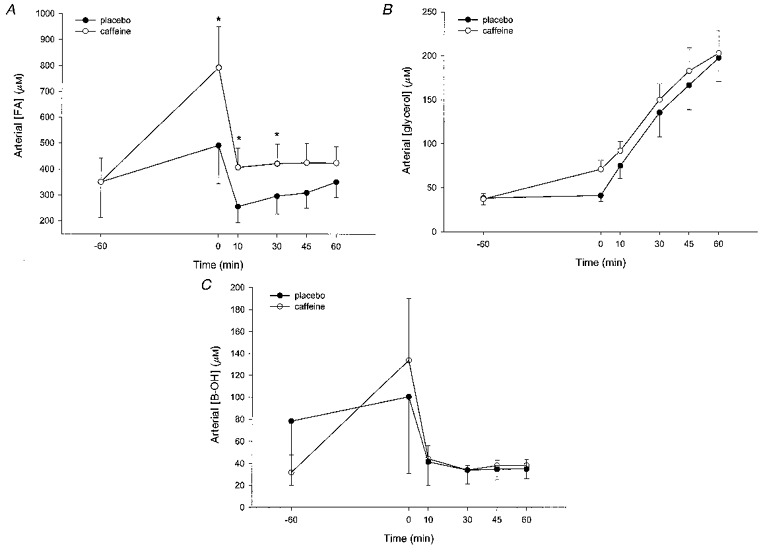
The data are means with the vertical bars representing one s.e.m. *P < 0.05, significant difference between treatments (○, caffeine; •, placebo).
Table 2.
A summary of the net flux across the leg for selected metabolites and potassium
| Time during exercise trial | |||||
|---|---|---|---|---|---|
| Parameter | 0 min | 10 min | 30 min | 45 min | 60 min |
| FA | |||||
| Placebo | −9.7 (5.6) | 68.5 (25.9) | 77.2 (15.6) | 43.6 (64.6) | 126.3 (37.3) |
| Caffeine | −10.6 (9.4) | 68.6 (34.2) | 111.0 (40.8) | 82.9 (31.2) | 109.8 (25.8) |
| Glycerol | |||||
| Placebo | −10.7 (3.0) | 12.8 (27.6) | −41.6 (44.3) | −15.9 (22.8) | −56.6 (54.6) |
| Caffeine | −2.8 (1.9) | −41.8 (28.3) | −11.6 (34.0) | −3.1 (14.6) | 6.7 (17.1) |
| B-OH | |||||
| Placebo | 8.7 (4.5) | 35.5 (14.8) | 39.8 (13.7) | 48.5 (13.4) | 11.3 (8.1) |
| Caffeine | 17.0 (7.4) | 16.9 (12.8) | 30.4 (8.6) | 29.5 (8.0) | 11.9 (24.4) |
| K+ | |||||
| Placebo | −9(6) | −49(148) | −25(217) | −292(237) | −324(197) |
| Caffeine | 15(15) | −384(197) | 188(213) | −456(112) | −227(94) |
| Lactate | |||||
| Placebo | −22(13) | −1204(371) | −877(232) | −759(372) | −692(429) |
| Caffeine | −33(11) | −1134(202) | −1796(853) | 131(846) | 499(775) |
| Glucose | |||||
| Placebo | 49(21) | 917(214) | 1248(176) | 1551(136) | 1653(148) |
| Caffeine | 33(21) | 889(173) | 1335(165) | 2227(568) | 1471(241) |
The data are the net exchange for one leg based on the product of arterial-venous concentration and blood or plasma flow. The data are means with S.E.M. in parentheses. All units are μmol min−1 and a negatve value reflects a net release.
Carbohydrate metabolism
Arterial blood glucose was modestly but significantly increased (P < 0.05) in the caffeine trial throughout the experiment (Fig. 2A). Arterial insulin concentration was low throughout the study (Table 3) and decreased over time (P < 0.05). In addition, there was a slight but significant increase (P < 0.05) in insulin during the caffeine experiments (Table 3). Glucose uptake by the leg increased during exercise (P < 0.05), but there was no significant difference (P > 0.05) between treatments (Table 2) despite the greater glucose delivery during the caffeine experiments. Intramuscular glycogen decreased (P < 0.05) by approximately 50 % of the resting level during the exercise, but no significant effect of caffeine could be detected (P > 0.05) (Fig. 3A).
Figure 2. A summary of the blood glucose (A) and lactate (B) responses to caffeine and exercise.
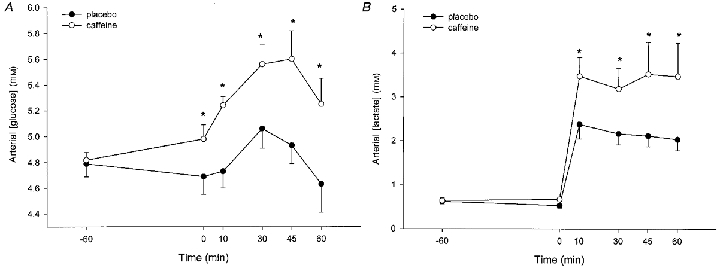
The data are means with the vertical bars representing one s.e.m. *Significant (P < 0.05) difference between treatments (○, caffeine; •, placebo).
Table 3.
A summary of arterial insulin, catecholamine and potassium data
| Time during exercise trial | ||||||
|---|---|---|---|---|---|---|
| Parameter | 60 min | 0 min | 10 min | 30 min | 45 min | 60 min |
| Insulin (μIU ml−1) | ||||||
| Placebo | 7*(1.1) | 7*(1.1) | 6*(1.4) | 7*(1.3) | 6*(1.0) | 4* (0.8) |
| Caffeine | 9(1.4) | 8(0.8) | 9(1.0) | 8(0.9) | 7(0.6) | 6(0.6) |
| Adrenaline (pg ml−1) | ||||||
| Placebo | 88(10) | 123(12) | 303*(35) | 368*(50) | 646(89) | 804(154) |
| Caffine | 108(13) | 267(32) | 611(106) | 427*(65) | 761*(207) | 1087(277) |
| Noradrenaline (pg ml−1) | ||||||
| Placebo | 197(20) | 235(26) | 1619(243) | 2212*(341) | 2571*(373) | 2929*(480) |
| Caffeine | 222(29) | 408(36) | 2018(284) | 2980(472) | 3534(545) | 3928(522) |
| K+ (mM) | ||||||
| Placebo | 3.93(0.06) | 3.90(0.08) | 4.85*(0.11) | 4.83*(0.09) | 4.85*(0.10) | 4.94*(0.10) |
| Caffeine | 3.92(0.07) | 3.82(0.07) | 4.55(0.09) | 4.62(0.08) | 4.71(0.09) | 4.78(0.10) |
All data are means with S.E.M. in parentheses.
Sinificant (P < 0.05) difference between caffeine and placebo treatments for a given time period.
Figure 3. A summary of the intramuscular concentrations of glycogen (A) and lactate (B) in response to caffeine and exercise.
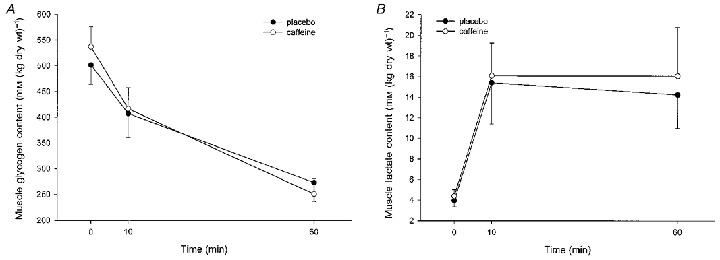
The data are means with the vertical bars representing one s.e.m. *Significant (P < 0.05) difference between treatments (○, caffeine; •, placebo).
Arterial blood lactate concentration was greater following caffeine ingestion throughout the exercise period (P < 0.05) (Fig. 2B) and the modest change during the 1 h rest was greater (P < 0.05) during the caffeine trial (the changes were -89 ± 23 and +32 ± 64 μM for placebo and caffeine, respectively). In marked contrast, there were no differences in either the increase in muscle lactate during the exercise (Fig. 3B) or in the lactate exchange across the monitored leg (Table 2).
Plasma catecholamines
The increase in arterial plasma adrenaline concentration at rest following caffeine ingestion (158 ± 39 pg ml−1) was significantly (P < 0.05) greater than that for placebo (36 ± 12 pg ml−1). During exercise, the caffeine treatment was associated with higher (P < 0.05) circulating adrenaline levels (Table 3). Similarly the plasma noradrenaline concentration rose more (P < 0.05) during rest following caffeine ingestion (186 ± 37 pg ml−1) compared with placebo (38 ± 13 pg ml−1) and was also increased (P < 0.05) at 30, 45 and 60 min of exercise. In addition, caffeine ingestion resulted in greater (P < 0.05) noradrenaline spillover at these time points (Fig. 4).
Figure 4. A summary of the response of noradrenaline spillover by the leg in response to caffeine and exercise.
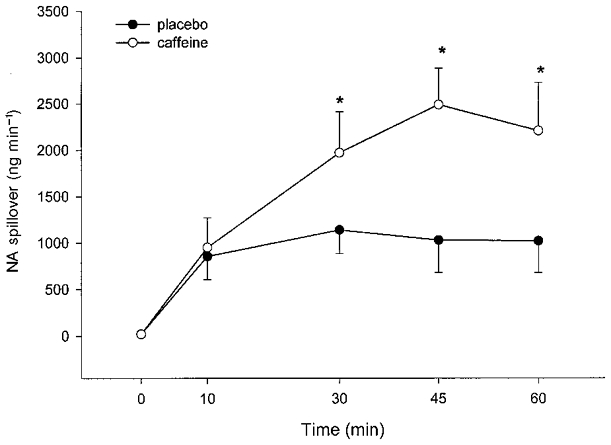
The data are means with the vertical bars representing one s.e.m. *Significant (P < 0.05) difference between treatments (○, caffeine; •, placebo).
Muscle metabolites
The data for intramuscular G6P, cAMP, and acetyl CoA are summarized in Table 4. While these data increased with exercise and the mean values were generally greater for the caffeine treatment, none of the differences between treatments was significant (P > 0.05). However, muscle citrate concentration increased (P < 0.05) during exercise and it was greater (P < 0.05) with caffeine treatment both at rest and during exercise (Table 4).
Table 4.
A summary of data for selected muscle metabolites
| Time during exercise trial | |||
|---|---|---|---|
| Parameter | 0 min | 10 min | 60 min |
| Acetyl CoA (mmol (kg dry wt)−1) | |||
| Placebo | 8.84(1.90) | 21.03(3.35) | 27.99(4.89) |
| Caffeine | 13.80(2.49) | 25.33(4.70) | 28.93(4.53) |
| cAMP (μmol (kg dry wt)−1) | |||
| Placebo | 2.99(0.42) | 3.77(0.43) | 3.41(0.39) |
| Caffeine | 2.89(0.42) | 4.03(0.57) | 4.27(0.61) |
| Citrate (mmol (kg dry wt)−1) | |||
| Placebo | 0.30*(0.05) | 0.66*(0.11) | 0.78*(0.13) |
| Caffeine | 0.46(0.06) | 0.91(0.15) | 0.96(0.18) |
| G6P (mmol (kg dry wt)−1) | |||
| Placebo | 1.00(0.32) | 2.37(0.37) | 1.83(0.33) |
| Caffeine | 1.42(0.39) | 2.72(0.54) | 2.87(0.44) |
All data are means with S.E.M. in parentheses.
Significant (P < 0.05) difference between caffine and placebo treatments for a given time period.
Plasma potassium
Arterial plasma potassium increased (P < 0.05) during exercise and during this period the concentration was significantly less (P < 0.05) in the caffeine treatment (Table 3). The leg release of potassium is increased (P < 0.05) with exercise and was not different (P > 0.05) with caffeine (Table 2).
DISCUSSION
The purpose of this study was to quantify the effects of caffeine ingestion on aspects of carbohydrate and fat metabolism in the exercising leg of human subjects. Caffeine ingestion increased mobilization of FAs into the plasma at rest and increased vascular resistance throughout the trial. In addition, caffeine increased circulating catecholamines and leg noradrenaline spillover during exercise. However, there was no change in RER during exercise and very few changes were observed in muscle substrate use. Most of the caffeine-related responses were not directly associated with the active muscle. These included increases in both arterial lactate and glucose concentrations and a moderation of the rise of plasma potassium. These findings suggest that the carbohydrate and fat metabolism in active muscle was generally unaltered, while other tissues, such as liver, adipose and resting muscle tissue, and tissues of the nervous system contributed significantly to the caffeine-related responses. Since caffeine is an adenosine antagonist, the present findings suggest that adenosine may be a more critical metabolic regulator in non-exercising tissues than in exercising muscle in humans.
Caffeine and the dimethylxanthines are known to be adenosine receptor antagonists that show little or no selectivity for receptor subtypes. A dose of 5-6 mg kg−1 has been shown (Graham & Spriet, 1995; Greer et al. 2000a) to result in plasma caffeine concentrations of approximately 30 μM which is not far short of the Ki of 40-44 μM for A1 and A2 receptors (Daly, 1993). In contrast, the paraxanthine concentration would be less than 5 μM, which is well below its Ki of 32-33 μM (Daly, 1993), and theobromine and theophylline concentrations would be barely detectable. Thus any effects via adenosine receptors are most likely to be precipitated by caffeine and not one of its metabolites. Since the caffeine concentration is only approaching the Ki it is likely that more marked physiological effects could be elicited by larger caffeine doses. Furthermore, Hellsten et al. (1998) demonstrated that muscle interstitial adenosine concentration increased from approximately 0.2 μM at rest to 1.1 and 2.0 μM during light and heavy exercise, respectively. This could favour the ability of adenosine to compete with caffeine for adenosine receptors in the active muscle compared with resting tissues.
Caffeine ingestion resulted in a modestly increased vasoconstrictor tone throughout the protocol; vascular conductance decreased and mean blood pressure increased, resulting in no change in leg blood flow. The increase in leg vascular resistance is in agreement with Daniels et al. (1998). They reported that caffeine increased resistance and reduced blood flow in the resting forearm vascular bed during leg exercise. This is consistent with a smooth muscle A1 receptor inhibition negating some of the vasodilatation effects of adenosine. However, Daniels et al. (1998) also found that caffeine accentuated the exercise-associated increase in angiotensin II and this may also contribute to the increased vascular resistance. Furthermore, caffeine resulted in an increase in arterial catecholamines and this could have a cardiovascular influence. Only rarely has caffeine been found to increase noradrenaline concentration (Graham & Spriet, 1995; Graham, 1997; Greer et al. 2000a). However, previously only mixed venous blood from the forearm has been analysed. This is a less sensitive indicator of changes in the sympathetic stimulation of selected tissues. The present finding of an increase in leg noradrenaline spillover during exercise is the first report that caffeine results in an increase in sympathetic nerve activity to active muscle. The increased spillover is similar to the doubling observed (Savard et al. 1989; Richter et al. 1992) when arm cranking was performed together with leg cycling. In the present study, the impact of the increased sympathetic drive is uncertain as it was only evident for the last 30 min of the exercise while the changes in conductance and pressure occurred at rest and throughout the exercise. Potassium is a local vasodilatory factor and the lower arterial potassium concentration during the caffeine trials could have contributed to this vascular response (Savard et al. 1989).
The increase in circulating adrenaline in response to caffeine ingestion is consistent with the literature (Graham & Spriet, 1995; Graham, 1997; Greer et al. 2000a), but its physiological importance is not clear. Previous studies (van Soeren et al. 1996; Mohr et al. 1998) found that tetraplegic patients had no plasma adrenaline response to caffeine, but had a normal elevation in plasma FAs both at rest (van Soeren et al. 1996) and during exercise (Mohr et al. 1998). Based on studies in rats an increase in circulating adrenaline would be predicted to increase muscle glycogenolysis during exercise (Richter et al. 1981, 1982). However, when Chesley et al. (1995) infused adrenaline into healthy subjects while they exercised for 15 min at 85 % V̇O2,max in order to generate a circulating adrenaline concentration similar to that associated with caffeine ingestion they found no changes in muscle glycogenolysis or whole body or muscle metabolism. Moreover, several studies did not show any changes in exercise-stimulated muscle glycogen breakdown after caffeine administration (Jackman et al. 1996; Chesley et al. 1998; Greer et al. 2000a). In fact several earlier studies showed a paradoxical decrease in muscle glycogen breakdown related to caffeine ingestion (Essig et al. 1980; Erickson et al. 1987; Spriet et al. 1992). Adding to the confusion is a study of perfused rat skeletal muscle, in which caffeine actually increased glycogen breakdown in contracting, oxidative muscle (Vergauwen et al. 1997). Thus there is a marked inconsistency between studies; even within investigations using only human subjects there is no consensus as to whether glycogen breakdown during exercise is affected by caffeine. We can offer no explanation for this; it does not appear to be due to differences in exercise intensity, the level of training of the subjects or caffeine dose.
The rise in arterial FA and glycerol at rest suggests that caffeine resulted in an increased adipose tissue lipolysis. This could be due either to the increased catecholamine stimulation or a direct adenosine antagonism by caffeine. As noted above, previous studies (van Soeren et al. 1996; Mohr et al. 1998; Greer et al. 2000a) suggest that caffeine can have direct effects on the A1 receptors of adipose tissue. Despite the caffeine-induced rise in plasma FA at rest, there were no significant differences between treatments for the net uptake of FA by the leg either at rest or during exercise. We observed no change in net glycerol release from the leg, no indication of a decrease in pulmonary RER, and no increase in leg V̇O2. Thus there is no indication that any aspect of fat oxidation was enhanced in the active muscle.
Caffeine was associated with a consistent, modest elevation in arterial glucose both at rest and during exercise. Raguso et al. (1996) found an increase of similar magnitude in arterial blood glucose following administration of the dimethylxanthine theophylline. In the present investigation, despite an increased glucose delivery to the active muscle there was no detectable difference in glucose uptake, suggesting a decrease in glucose clearance. In accordance with this notion there have been several reports (Vergauwen et al. 1994, 1997; Han et al. 1998; Derave & Hespel, 1999) that adenosine antagonism can inhibit glucose uptake by contracting, rodent muscle. Generally this has been observed when there was also stimulation by insulin and/or when adenosine receptor blockers that are more potent than caffeine were applied or when endogenous adenosine was degraded with adenosine deaminase. In the present study the combination of a very low insulin concentration and moderate levels of methylxanthines as well as the possibility of increased interstitial adenosine during exercise may have restricted our ability to observe such putative effects.
The one study that our data appear to be in conflict with is that by Raguso and coworkers (Raguso et al. 1996), however, close examination suggests that the two data sets are quite consistent and when combined, an alternative interpretation to that presented by the previous authors can be proposed. They infused theophylline, had subjects exercise at 75 % V̇O2,max and studied their metabolism by indirect calorimetry and by stable isotopic labelling of glycerol, palmitate and glucose. We administered caffeine orally, had subjects exercise at 70 % V̇O2,max for 60 min and studied metabolism by indirect calorimetry and with direct Fick and muscle biopsy measurements. In both studies RER remained unchanged, arterial glucose was elevated, insulin levels were low and blood lactate concentration was elevated in the methylxanthine trials. Raguso et al. (1996) observed no affect on FA disappearance and we found no change in muscle FA uptake or in glycerol release. Thus there is a great deal of similarity in the data, but the interpretation of the findings is different.
Raguso et al. (1996) reported a decreased glucose disappearance rate with theophylline infusion and assumed it was due to a decrease in glucose clearance by active muscles. Since there was no change in RER, they therefore also concluded that there was an increased muscle glycogenolysis. (This latter conclusion stands out as the only report of a methylxanthine-enhanced muscle glycogenolysis in human subjects.) However, our direct measures of glucose uptake and muscle glycogen concentration fail to confirm their assumptions regarding carbohydrate metabolism. Similarly, Greer et al. (2000a) found no evidence of increased muscle glycogenolysis when theophylline was ingested prior to exercise, findings very similar to those of the present study and of Raguso et al. (1996). It is possible that tissues other than the active muscle could account for their observation of a decreased glucose disappearance and that the glycogenolytic rate and glucose uptake by the active muscle were unaltered, as they were in the present study. Supporting such a contention, studies have shown that adenosine antagonism will decrease the insulin-induced glucose uptake of adipose tissue (Kather et al. 1985; Vannucci et al. 1989; Palmer et al. 1992; Crist et al. 1998). Preliminary results suggest a similar effect of caffeine on non-exercising tissues. After caffeine ingestion whole body glucose clearance is decreased by 25 % in resting subjects during a euglycaemic hyperinsulinaemic clamp (Greer et al. 2000b) and we have recently observed that leg glucose uptake during an euglycaemic hyperinsulinaemic clamp is also decreased by a similar magnitude following caffeine ingestion (F. S. L. Thong, T. E. Graham, B. Kiens & E. A. Richter, unpublished observations). Thus it is possible that adipose tissue and/or resting skeletal muscle reduced their glucose uptake which would account for the increased circulating glucose concentration in the present study and the decreased glucose disappearance rate reported by Raguso et al. (1996).
We cannot discount the possibility that the elevation in arterial glucose concentration could be due to increased hepatic production. Little is known about hepatic glucose production and adenosine. Adenosine and its agonists have been shown (McLane et al. 1990; Buxton et al. 1997) to increase hepatic glucose release, thus any antagonism should result in a decrease in glucose appearance. Furthermore, Raguso et al. (1996) found that theophylline infusion did not alter the rate of glucose appearance. Crist et al. (1998) reported that administration of an A1 receptor antagonist to lean rats during a hyperinsulinaemic clamp decreased glucose uptake in skeletal muscle and adipose tissue, but did not alter the glucose exchange at the liver. It is possible that the caffeine-induced increase in adrenaline increased hepatic glycogenolysis.
Several studies (Anselme et al. 1992; Spriet et al. 1992; Jackman et al. 1996; Greer et al. 2000a) have observed that caffeine ingestion resulted in an increase in blood lactate. The previously discussed work by Raguso et al. (1996) observed this with theophylline and attributed it to increased lactate production by active muscle. This would be complementary to their theory that there was increased glycogenolysis but no increase in carbohydrate oxidation. While we found a similar increase in arterial lactate concentration, there was no difference in either release or accumulation of lactate by the active muscle. Thus we conclude that the clearance of lactate by the liver or resting muscle must be inhibited or the release from other tissues is increased.
Consistent with the lack of evidence for enhanced fat oxidation we observed no differences between treatments in intramuscular G6P, acetyl CoA and cAMP. The only significant change in intramuscular metabolic intermediates was a consistent elevation in citrate. Previously Spriet et al. (1992) found a similar result, but only at rest, while Greer et al. (2000a) found that that neither caffeine or theophylline increased G6P, citrate or acetyl CoA in exercising muscle above that of placebo, but there was an increase in muscle cAMP. While we cannot explain these discrepancies, the majority of the findings among the investigations do not reflect a difference in putative regulators of fat and carbohydrate oxidation.
The finding that the exercise-induced increase in plasma potassium is less with caffeine ingestion is consistent with previous reports (MacIntosh & Wright, 1995; Lindinger et al. 1996). The fact that we do not see a decreased potassium release from the monitored leg is consistent with the proposal by Lindinger et al. (1996) that caffeine and/or catecholamines enhance potassium clearance by resting muscle through stimulation of Na+,K+-ATPase.
In conclusion, the present study showed that ingestion of the adenosine receptor antagonist, caffeine, before exercise has minimal effects on the metabolism and circulation in working muscle. In contrast, our study indirectly indicates that caffeine has distinct effects on the sympathetic nervous system, vascular resistance, adipose tissue lipolysis, and lactate, potassium and glucose metabolism in non-exercising tissues. These findings imply that adenosine is an important metabolic signal in some situations, but that the frequently reported ergogenic effects of caffeine are unlikely to be due to changes in the metabolism of active muscle or in its circulation. Unfortunately, caffeine is a non-selective adenosine receptor blocker and it can only be used in humans at concentrations that approach the Ki for these receptors. Thus the definitive study of the metabolic and cardiovascular roles of adenosine receptors in humans awaits the development of specific, high affinity blockers of the receptors.
Acknowledgments
The authors gratefully acknowledge the excellent assistance of Betina Bolmgreen, Irene Beck Nielsen, Felicia Greer, Danielle Battram and Premila Sathasivam. The research was supported by the Danish National Research Foundation, grant 504-14 and by the Natural Sciences and Engineering Research Council of Canada.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. Journal Physiology. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselme F, Collomp K, Mercier B, Ahmaidi S, Prefaut Ch. Caffeine increases maximal anaerobic power and blood lactate concentration. European Journal of Applied Physiology. 1992;65:188–191. doi: 10.1007/BF00705079. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Bernt E, Schmidt F, Stork H. D-glucose determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 3. New York: Academic Press; 1974. pp. 1196–1201. [Google Scholar]
- Bernt E, Bermeyer HU, Mollering H. Creatine. In: Bermeyer HU, editor. Methods of Enzymatic Analysis. Vol. 4. New York: Academic Press; 1974. pp. 1772–1776. [Google Scholar]
- Buxton DB, Fisher RA, Robertson SM, Olson MS. Stimulation of glycogenolysis and vasoconstriction by adenosine analogs in the perfused rat liver. Biochemical Journal. 1997;248:35–41. doi: 10.1042/bj2480035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederblad G, Carlin D, Constantin-Teodosiu P, Humphrey LD. Radioisotopic assays of CoASH and carnitine and their acetylated forms in human skeletal muscle. Analytical Biochemistry. 1990;185:274–278. doi: 10.1016/0003-2697(90)90292-h. [DOI] [PubMed] [Google Scholar]
- Chesley A, Howlett RA, Heigenhauser JF, Hultman E, Spriet LL. Regulation of muscle glycogenolytic flux during intense aerobic exercise following caffeine ingestion. American Journal of Physiology. 1998;275:R596–603. doi: 10.1152/ajpregu.1998.275.2.R596. [DOI] [PubMed] [Google Scholar]
- Chesley A, Hultman E, Spriet LL. Effects of epinephrine infusion on muscle glycogenolysis during intense aerobic exercise. American Journal of Physiology. 1995;268:E127–134. doi: 10.1152/ajpendo.1995.268.1.E127. [DOI] [PubMed] [Google Scholar]
- Crist GH, Xu B, Lanoue KFLCH. Tissue-specific effects of in vivo adenosine receptor blockade on glucose uptake by Zucker rats. FASEB Journal. 1998;12:1301–1308. doi: 10.1096/fasebj.12.13.1301. [DOI] [PubMed] [Google Scholar]
- Daly JW. Mechanism of action of caffeine. In: Garattini S, editor. Caffeine, Coffee, and Health. New York: Raven Press; 1993. pp. 97–150. [Google Scholar]
- Daniels JW, Mole PA, Shaffrath JD, Steele PM. Effects of caffeine on blood pressure, heart rate, and forearm blood flow during dynamic leg exercise. Journal of Applied Physiology. 1998;85:154–159. doi: 10.1152/jappl.1998.85.1.154. [DOI] [PubMed] [Google Scholar]
- Derave W, Hespel P. Role of adenosine in regulating glucose uptake during contractions and hypoxia in rat skeletal muscle. The Journal of Physiology. 1999;515:255–263. doi: 10.1111/j.1469-7793.1999.255ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Schwarzkopf RJ, McKenzie RD. Effects of caffeine, fructose, and glucose ingestion on muscle glycogen utilization during exercise. Medicine and Science in Sports and Exercise. 1987;19:579–583. [PubMed] [Google Scholar]
- Essig D, Costill DL, van Handel PJ. Effects of caffeine ingestion on utilization of muscle glycogen and lipid during leg ergometer cycling. International Journal of Sports Medicine. 1980;1:86–90. [Google Scholar]
- Graham TE. The possible actions of methylxanthines on various tissues. In: Reilly T, Orme M, editors. The Clinical Pharmacology of Sport and Exercise. Amsterdam: Elsvier Science B. V.; 1997. pp. 257–270. [Google Scholar]
- Graham TE, Spriet LL. Performance and metabolic responses to a high caffeine dose during prolonged exercise. Journal of Applied Physiology. 1991;71:2292–2298. doi: 10.1152/jappl.1991.71.6.2292. [DOI] [PubMed] [Google Scholar]
- Graham TE, Spriet LL. Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. Journal of Applied Physiology. 1995;78:867–874. doi: 10.1152/jappl.1995.78.3.867. [DOI] [PubMed] [Google Scholar]
- Greer F, Friars D, Graham TE. Comparison of caffeine and theophylline ingestion: exercise metabolism and endurance. Journal of Applied Physiology. 2000a;89:1837–1844. doi: 10.1152/jappl.2000.89.5.1837. [DOI] [PubMed] [Google Scholar]
- Greer F, Hudson R, Ross R, Graham TE. Adenosine receptor antagonism decreases glucose disposal in humans. Medicine and Science in Sports and Exercise. 2000b;32:S291. [Google Scholar]
- Gutmann I, Wahlefeld AW. L-(+)-Lactate determination with lactate dehydrogenase and NAD. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 3. New York: Academic Press; 1974. pp. 1464–1468. [Google Scholar]
- Han D-H, Hansen PA, Nolte LA, Holloszy JO. Removal of adenosine decreases the responsiveness of muscle glucose transport to insulin and contractions. Diabetes. 1998;47:1671–1675. doi: 10.2337/diabetes.47.11.1671. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo L-O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scandinavian Journal of Clinical Laboratory Investigation. 1974;33:109–120. [PubMed] [Google Scholar]
- Hellsten Y, MacLean D, Radergran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- Jackman M, Wendling P, Friars D, Graham T. Metabolic, catecholamine, and endurance responses to caffeine during intense exercise. Journal of Applied Physiology. 1996;81:1658–1663. doi: 10.1152/jappl.1996.81.4.1658. [DOI] [PubMed] [Google Scholar]
- Kather H, Wieland E, Fischer B, Schlierf G. Antilipolytic effects of N6-phenylisopropyladenosine and prostaglandin E2 in fat-cells of obese volunteers before and during energy restriction. Biochemical Journal. 1985;231:531–535. doi: 10.1042/bj2310531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindinger MI, Willmets RG, Hawke TJ. Stimulation of Na+, K+-pump activity in skeletal muscle by methylxanthines: evidence and proposed mechanisms. Acta Physiologica Scandinavica. 1996;156:347–353. doi: 10.1046/j.1365-201X.1996.200000.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. Towowa, NJ, USA: Humana Press; 1993. Enzymatic Analysis: A Practical Guide. [Google Scholar]
- MacIntosh BR, Wright BM. Caffeine ingestion and performance of a 1500 meter swim. Canadian Journal of Applied Physiology. 1995;20:168–177. doi: 10.1139/h95-012. [DOI] [PubMed] [Google Scholar]
- McLane MP, Black PR, Law WR, Raymond RM. Adenosine reversal of in vivo hepatic responsiveness to insulin. Diabetes. 1990;39:62–69. doi: 10.2337/diacare.39.1.62. [DOI] [PubMed] [Google Scholar]
- Mohr T, van Soeren M, Graham TE, Kjaer M. Caffeine ingestion and metabolic responses of tetraplegic humans during electrical cycling. Journal of Applied Physiology. 1998;85:979–985. doi: 10.1152/jappl.1998.85.3.979. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Taberner PV, Houslay MD. Alterations in G-protein expression, Gi function and stimulatory receptor-mediated regulation of adipocyte adenylyl cyclase in a model of insulin-resistant diabetes with obesity. Cellular Signalling. 1992;4:365–377. doi: 10.1016/0898-6568(92)90031-3. [DOI] [PubMed] [Google Scholar]
- Passonneau JV, Brown JG. Citrate: fluorimetric determination. In: Bermeyer HU, editor. Methods of Enzymatic Analysis. Vol. 3. New York: Academic Press; 1974. pp. 1565–1569. [Google Scholar]
- Prefaut C, Collomp K, Audran M, Ahmaidi S, Chatard JC. Benefits of caffeine ingestion on sprint performance in trained and untrained swimmers. European Journal of Applied Physiology. 1992;64:377–380. doi: 10.1007/BF00636227. [DOI] [PubMed] [Google Scholar]
- Raguso CA, Coggan AR, Sidossis LS, Gastaldelli A, Wolfe RR. Effect of theophylline on substrate metabolism during exercise. Metabolism. 1996;45:1153–1160. doi: 10.1016/s0026-0495(96)90016-5. [DOI] [PubMed] [Google Scholar]
- Richter EA, Aagaard T, Hargreaves M, Kjaer M. Effect of arm-cranking on leg blood flow and noradrenaline spillover during leg exercise in man. Acta Physiologica Scandinavica. 2000;144:9–14. doi: 10.1111/j.1748-1716.1992.tb09261.x. [DOI] [PubMed] [Google Scholar]
- Richter EA, Ruderman NB, Gavras H, Belur ER, Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. American Journal of Physiology. 1982;242:E25–32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- Richter EA, Sonne B, Christensen NJ, Galbo H. Role of epinephrine for muscular glycogenolysis and pacncreatic hormonal secretion in running rats. American Journal of Physiology. 1981;240:E526–532. doi: 10.1152/ajpendo.1981.240.5.E526. [DOI] [PubMed] [Google Scholar]
- Robertson D, Wade D, Workman R, Woosley RL, Oates JA. Tolerance to the humoral and hemodynamic effects of caffeine in man. Journal of Clinical Investigation. 1981;67:1111–1117. doi: 10.1172/JCI110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard G, Strange S, Kiens B, Richter EA, Christensen NJ, Saltin B. Noradrenaline spillover during exercise in active versus resting skeletal muscle in man. Acta Physiologica Scandinavica. 1987;131:507–515. doi: 10.1111/j.1748-1716.1987.tb08270.x. [DOI] [PubMed] [Google Scholar]
- Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: role of muscle mass. American Journal of Physiology. 1989;257:H1812–1818. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- Spriet LL, MacLean DA, Dyck DJ, Hultman E, Cederblad G, Graham TE. Caffeine ingestion and muscle metabolism during prolonged exercise in humans. American Journal of Physiology. 1992;262:E891–898. doi: 10.1152/ajpendo.1992.262.6.E891. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky MA, Atkinson SA, Macdougall JD, Sale DG, Sutton JR. Physiological responses to caffeine during endurance running in habitual caffeine users. Medicine and Science in Sports and Exercise. 1989;21:418–424. [PubMed] [Google Scholar]
- van Soeren M, Mohr T, Kjaer M, Graham TE. Acute effects of caffeine ingestion at rest in humans with impaired epinephrine responses. Journal of Applied Physiology. 1996;80:999–1005. doi: 10.1152/jappl.1996.80.3.999. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Klim CM, Martin LF, Lanoue KF. A1-adenosine receptor-mediated inhibition of adipocyte adenylate cyclase and lipolysis in Zucker rats. American Journal of Physiology. 1989;257:E871–878. doi: 10.1152/ajpendo.1989.257.6.E871. [DOI] [PubMed] [Google Scholar]
- Vergauwen L, Hespel P, Richter EA. Adenosine receptors mediate synergistic stimulation of glucose uptake and transport by insulin and by contractions in rat skeletal muscle. Journal of Clinical Investigation. 1994;93:974–981. doi: 10.1172/JCI117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergauwen L, Richter EA, Hespel P. Adenosine exerts a glycogen-sparing action in contracting rat skeletal muscle. American Journal of Physiology. 1997;272:E762–768. doi: 10.1152/ajpendo.1997.272.5.E762. [DOI] [PubMed] [Google Scholar]
- Weiker H, Feraudi M, Hagele H, Pluto R. Electrochemical determination of catecholamines in urine and plasma separations with HPLC. Clinical Chemistry Acta. 1984;141:17–25. doi: 10.1016/0009-8981(84)90162-1. [DOI] [PubMed] [Google Scholar]
- Williamson DH, Mellander S. D-(-)-3-Hydroxybutyrate. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 4. New York: Academic Press; 1974. pp. 1836–1839. [Google Scholar]


