Abstract
Simultaneous recordings were made from gamma (γ) motor axons and from muscle spindle afferents of the medial gastrocnemius (MG) muscle during locomotion in decerebrate cats. The γ-neurons were identified as static or dynamic (γs or γd) by correlating their behaviour during midbrain stimulation with changes in muscle spindle afferent responses to muscle stretch.
On the basis of their behaviour during locomotion, γs neurons could be divided into two groups. One group (type-1) showed strongly and smoothly modulated discharge increasing in parallel with the active muscle shortening in ankle extension, but with phase advance. The other group (type-2) also showed a modulated pattern, but with increased firing centred on the flexion phase. The proportions of the two were 13 type-1 and 7 type-2.
The type-1 firing pattern accurately predicted the difference in firing frequency for secondary afferents obtained by subtracting from the recordings made during active movements the response of the same units to the movements repeated passively in the absence of fusimotor activity.
The type-2 pattern also became consistent with the difference signal, when operated on by a phase lag appropriate to the effects of bag2 intrafusal fibres. These results suggest that there may be some degree of separate control of chain and bag2 intrafusal fibres.
The discharge of γd axons was also found to fluctuate with the locomotor cycle, with a pattern very distinct from that of the γs records. The γd firing frequency rose very suddenly from zero to a maximum at the onset of muscle shortening and continued into the beginning of lengthening. The term ‘interrupted’ discharge is suggested as a useful description. The timing of this discharge was shown to be appropriate for sensitising the primary afferents to detect the onset of stretch.
The primary and secondary sensory axons from mammalian muscle spindles feed back to the central nervous system information regarding length changes of muscles, but in turn act under a complex system of efferent control through the γ-motor nerve fibres. The γ-motoneurons are conventionally divided into two groups, static (γs) or dynamic (γd) according to whether they have, respectively, a biasing action on primary and secondary afferent endings or a sensitising action on the dynamic response of primary endings alone (Matthews, 1962; Boyd, 1981). Because so far it has not been possible to record from such small axons in normally moving animals, attempts to find what patterns of activity occur naturally in γs and γd motor fibres have depended largely on deductions from spindle afferent recordings (Taylor & Cody, 1974; Prochazka et al. 1977). However, the complexity of the interactions between γs and γd activity and movement has made interpretation of the results difficult and no definitive description has been achieved. Some direct γ recordings have been made in reduced preparations, but in these cases there has been difficulty in identifying the individual γ-axons as static or dynamic (Lund et al. 1979; Appenteng et al. 1980; Murphy et al. 1984). We have now achieved simultaneous recordings from several single γ-axons and from a number of spindle afferents from the medial gastrocnemius (MG) muscle in the decerebrate cat during locomotion. The consequent clear identification of the γ-axons and new insights into their effects on spindle afferent firing have enabled us to identify hitherto unexpected patterns of γ-motor activity and to clarify ideas about the normal working of the static and dynamic γ-motor systems. Preliminary data have been published in abstract form (Taylor et al. 1999b, 2000b).
METHODS
A full description of the preparation of the animals and the procedures for recording signals, applying stimuli and inducing locomotion have been published recently (Taylor et al. 2000a). In the main series of experiments, which involved decerebration, anaesthesia was induced by means of 5 % halothane vapour in equal parts of nitrous oxide and oxygen passed into a 30 l box at a rate of 10 l min−1. Anaesthesia was then continued with 2 % halothane in the N2O-O2 mixture via a face mask during cannulation of the trachea. Thereafter the same mixture was administered via the cannula. All the surgical procedures were carried out under this anaesthesia with monitoring of arterial blood pressure, end-tidal PCO2 and rectal temperature. Adequate depth of anaesthesia was also confirmed by loss of corneal reflex and flexion withdrawal reflex of the forelimb. After surgical preparation animals were mounted in a stereotaxic frame and supported above a treadmill belt. Pressure points were infiltrated with local anaesthetic. A urethral catheter was inserted to allow free drainage of the bladder. Fluid replacement was effected by intravenous infusion of 10 ml doses of 5 % dextran in saline as required. The left hindlimb, which was denervated except for MG and tibialis anterior (TA) muscles, was kept clear of the belt by fixation at mid-femur and at the lower end of the tibia, leaving the foot free to rotate about the ankle through the action of MG and TA. Six single muscle spindle afferents from MG were recorded from dorsal root filaments exposed by a unilateral laminectomy, leaving most of the roots intact. To facilitate recording without movement artefacts, silver hook electrodes were mounted in a floating plastic holder sutured to the interspinous ligament. At this point pre-mammillary decerebration was carried out, with complete removal of the brain rostral to the plane of section, in order to render the animal totally insentient. The administration of the halothane-N2O-O2 mixture was discontinued and the activity of several single γ-motor axons recorded from small fascicles separated from the MG nerve, leaving the rest of the nerve intact. Efferent axons which fired spontaneously or in response to cutaneous or brainstem stimulation were accepted as γ-motor if their conduction velocity (measured by backward spike triggered averaging from a nerve cuff electrode) lay between 12 and 45 m s−1. Well co-ordinated locomotor movements of three legs occurred on running the treadmill alone or with the addition of stimulation in the mesencephalic locomotor region (MLR). Following Shik et al. (1966) the location of the MLR was taken to be that of the cuneiform nucleus and stimulus trains (20 Hz, 0.2 ms, 0.2-2.0 V) were applied in that region contralaterally. In the left hindlimb, rhythmic movements at the ankle alternated with those on the other side. The movements could be transduced without significant loading and recorded on magnetic tape. The recording was subsequently played back through a servo mechanism to reproduce the active movements passively. Afferent and efferent recordings were made simultaneously, first during periods of walking on the treadmill. Subsequently, fusimotor activity was suppressed with two or three intravenous doses of 12 mg kg−1 of sodium pentobarbitone and recording continued while the previously recorded ankle movements were repeated passively. Data were recorded through a CED 1401-plus interface and a 486 computer running Spike2 software (CED Ltd, Cambridge, UK). The afferents were characterised by their response to muscle stretch (ankle rotation), muscle twitch, conduction velocity and, at the end of the experiment, by the effects of intravenous succinylcholine (200 μg kg−1) to determine the functional contacts of each afferent on bag1 and bag2 intrafusal muscle fibres (Taylor et al. 1992a,b). The ramp and hold muscle stretches were applied by the servo mechanism rotating the ankle in order to stretch the MG by 5 mm in 1 s, hold for 1.5 s and return in 1 s. The stretches were repeated continuously every 6 s during the succinylcholine test. Ventilation was maintained artificially during the short period following succinylcholine administration during which respiratory muscles were paralysed.
Additional observations were made in three cats, fully anaesthetised with sodium pentobarbitone (60 mg kg−1 intraperitoneal) to explore the effects on spindle primary afferent responses of γd stimulation applied at various phases of cyclic stretching. The methods were as described in detail previously (Taylor et al. 1998). Anaesthesia was monitored by observation of arterial blood pressure and supplements of sodium pentobarbitone (12 mg kg−1 in 1 ml saline intravenous), given as necessary. Note that the use of succinylcholine was accompanied by artificial ventilation, as described above. At the end of all experiments death was ensured by the administration of a large overdose of sodium pentobarbitone (5 ml of 60 mg ml−1).
RESULTS
Identification of γs and γd axons
The results are based on recordings from 68 spindle afferents and 50 γ-axons of MG in 9 cats. The characterisation of the spindle afferents included testing with succinylcholine (SCh), as described above in Methods. The purpose of this testing was to determine for each afferent the presence or absence of functional contacts on bag1 and bag2 intrafusal fibres, i.e. to classify them as b1c, b1b2c, b2c or c type (Price & Dutia, 1989; Taylor et al. 1992a,b). This was essential for the subsequent interpretation of fusimotor effects (Taylor et al. 1992c). The first requirement in studying the γ-motor recordings was to identify them as from static or dynamic efferents. This was done by electrical stimulation of the midbrain at regions which produced marked static or dynamic fusimotor effects, which could be monitored by simultaneous recordings from spindle afferents. In Fig. 1 the firing of three single γ-axons is shown before and during stimulation in the midbrain at a point dorsal to the midbrain locomotor region (MLR). The upper two units showed an immediate marked increase in impulse frequency and in irregularity during stimulation, but the third, after a delay of 14 s, showed a decrease in discharge rate. The bottom two records are from a b1b2c primary and a b2c secondary afferent, respectively, chosen from the six muscle spindle afferents recorded in that experiment. Throughout the simultaneous recording of afferent and efferent discharges, continuous ramp and hold muscle stretches were applied every 6 s. Since both afferents (as well as the four not shown) showed a marked increase in bias during stimulation, there must have been an increase in γs output. There was evidently no increase in γd firing because the primary afferent showed no increase in dynamic sensitivity to muscle lengthening. Later in the record there was a small increase in dynamic response in the primary afferent at the end of stimulation and this corresponded with the restoration of firing in the third γ-axon. It was concluded that the first two γ-axons were static and the third was dynamic. This conclusion was based on the assumption that the γ-axons recorded were representative of their respective populations which supplied the MG muscle through the uninterrupted part of its nerve. This assumption seems to be justified because all the spindle afferents recorded were affected in the same way.
Figure 1. Identification of γ-axons by reciprocal activation of γs and inhibition of γd.
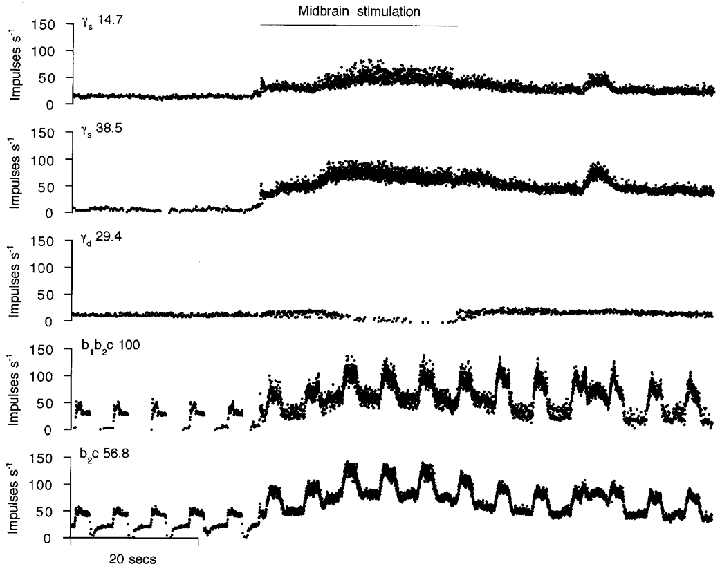
The upper three traces show the firing of three single γ-axons from a small filament of the MG nerve, which was otherwise intact (type and conduction velocity, in m s−1, are shown above each trace). The bottom two traces show the firing of two MG spindle afferents; their intrafusal fibre contacts and conduction velocity (in m s−1) are shown to the left, above the traces. All records are instantaneous frequency plots. Ramp and hold stretches of MG (not shown) were applied continuously every 6 s. The spindle records show that midbrain stimulation (20 Hz, 0.2 ms, 1.3 V, signalled by the horizontal bar) increases static and decreases dynamic fusimotor activity.
A more specific identification of γd units is shown in another animal in Fig. 2. Here, stimulation at the lateral border of the cuneiform nucleus caused a strong excitation of the upper two units and inhibition of the third. At the same time the static component of spindle firing is seen to be reduced, both for b2c and b1b2c afferent types. There is also a striking increase in the dynamic responsiveness of the b1b2c spindle primary afferent. It is concluded that the upper two γ-axons were dynamic and the third one static. The total numbers of the two types identified unequivocally as above were 28 γs and 13 γd.
Figure 2. Identification of γ-axons by reciprocal activation of γd and inhibition of γs.
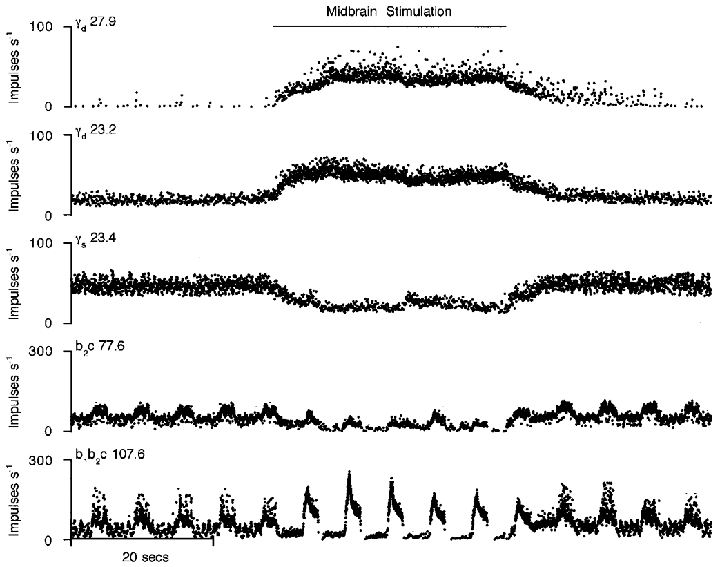
The layout is the same as for Fig. 1, but the data are from a different experiment. Midbrain stimulation at a different site from Fig. 1 now enhances the dynamic fusimotor activity and reduces the static.
It has been stated that the resting firing frequency of γ-motoneurons permits their distinction as static or dynamic (Murphy et al. 1984). However, as illustrated in Figs 1 and 2, in the present experiments either type may have high or low frequencies of firing in periods before the onset of locomotion. Also, the resting rate of each unit varied from time to time spontaneously. This has not therefore been considered further as a reliable means of identification.
Static fusimotor firing patterns
Following identification, the firing of the two γs axons of Fig. 1 was recorded during a period of locomotion with the ankle free to move without loading (Fig. 3). In the real time display in Fig. 3A the lower unit is seen to modulate smoothly from 35 to 145 impulses s−1 with each step cycle. The upper unit fired with a mean frequency of 50 impulses s−1 and was also smoothly modulated, but with lower amplitude than the other. The relationship of the γs firing to the movement is best displayed in cycle averages as shown in Fig. 3B, taking the point of maximum ankle flexion as time zero. To facilitate comparison between steps of slightly varying duration, the time axis has been normalised with respect to cycle duration, before computing averages. There is seen to be a clear contrast between the behaviour of the two γs units. In the more strongly modulated one the average frequency started to rise abruptly 15 % of a cycle before the onset of muscle shortening (ankle extension), was elevated throughout the shortening phase and fell rapidly at the beginning of the lengthening phase. The other unit showed a slow, steady rise in frequency, starting at the peak of extension and continuing throughout the flexion phase. Its frequency then fell during the extension (muscle shortening) phase.
Figure 3. Behaviour of the two γs motor axons from Fig. 1 during rhythmic stepping movements.
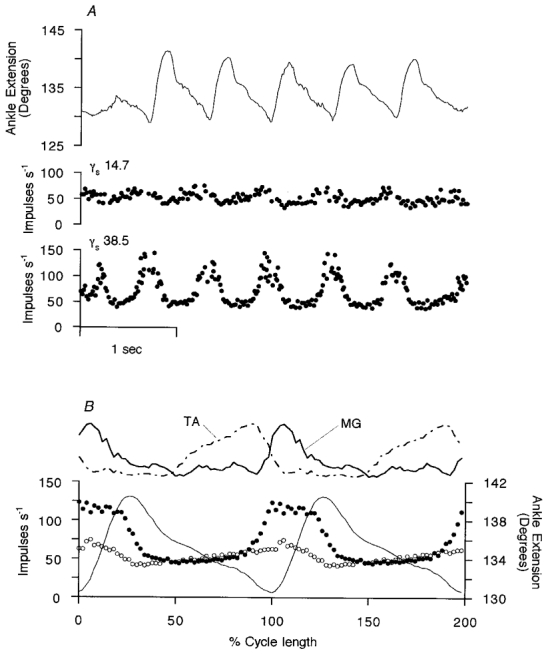
In A the instantaneous γ-axon firing frequencies and ankle rotation are shown in real time (same γs axons as in Fig. 1). The γs motor axon conduction velocities are shown in m s−1. The upper trace is ankle rotation with extension (plantar flexion) upwards. The angle is measured between the front of the tibia and the dorsum of the foot. In B, cycle averages are computed for 14 cycles of regular amplitude of ankle movement from the data illustrated in A. The two top traces are the smoothed, rectified EMG signals from MG and TA. The lower three records are the averaged frequency records for a type-1 γs axon (•) and a type-2 γs axon (○) and the averaged ankle angle (continuous line). In the horizontal scale, time is normalised with respect to the individual stepping cycle durations. The mean cycle period was 0.65 s.
The mean EMG records in the upper part of Fig. 3B show that the modulation of the first unit is approximately in phase with MG EMG activity, whilst the increased firing of the other coincided mainly with tibialis anterior (TA) activity. In order to compare the patterns of modulation seen on different occasions it is convenient to take the records normalised with respect to the stepping period, to subtract the minimum or background firing frequency and to make polar plots. This is shown in Fig. 4A for the pair of γs axons from Fig. 3 and in Fig. 4B for a pair from another experiment. Different patterns for the two units in each case are now emphasised and more clearly related to certain phases of the step cycle and patterns of flexor and extensor EMG. Of the 20 MG γs axons recorded in the presence of strong and regular locomotor movements, 13 consistently increased their firing during extension and will be designated as type-1 γs neurons. The remaining 7 increased their firing during flexion and will be referred to as type-2. Ensemble cycle average responses have been computed separately for all the available type-1 and type-2 units after normalising with respect to cycle period and are shown in Fig. 5. The mean patterns of γs modulation confirm the descriptions given above. For type-1 units the mean firing rate was 50.3 impulses s−1 and the mean (± s.d.) modulation depth 34.4 ± 17.5 impulses s−1. For the type-2 units the values were 62.0 and 23.1 ± 6.9 impulses s−1, respectively. The modulation depths were not significantly different (Student’s t test, P = 0.124). Mean conduction velocity for the two groups were 27.0 ± 8.6 and 21.5 ± 5.3 m s−1, respectively, which were not significantly different (t test, P = 0.139).
Figure 4. Polar plots of the timing of mean γs firing frequency and mean rectified EMG.
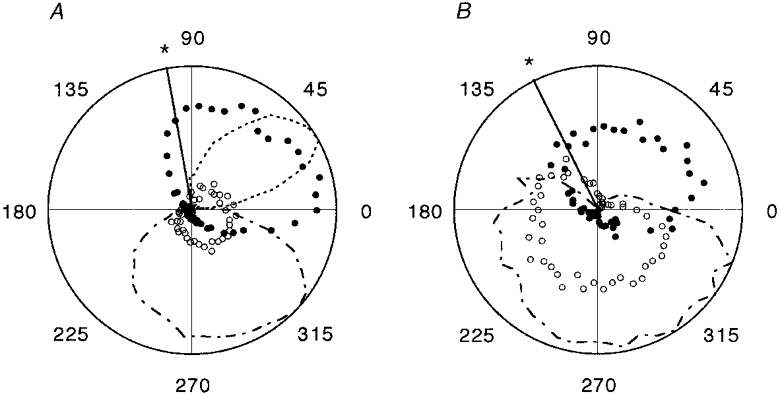
In results from two experiments, A and B, averages are shown for 14 normalised cycles of locomotion in each case, with time running anticlockwise and the radius of the circle representing 60 impulses s−1 for the γs units. •, mean firing frequencies for type-1 γs units; ○, type-2. Mean rectified EMG activity (arbitrary units) is shown by the fine dotted line for MG and dashed and dotted lines for TA. The heavy radial line (*) indicates the moment of transition from ankle extension to flexion. The position in the normalised cycles is shown in degrees. Zero degrees is the point of maximum flexion.
Figure 5. Ensemble mean patterns of firing of type-1 and type-2 γs neurons.
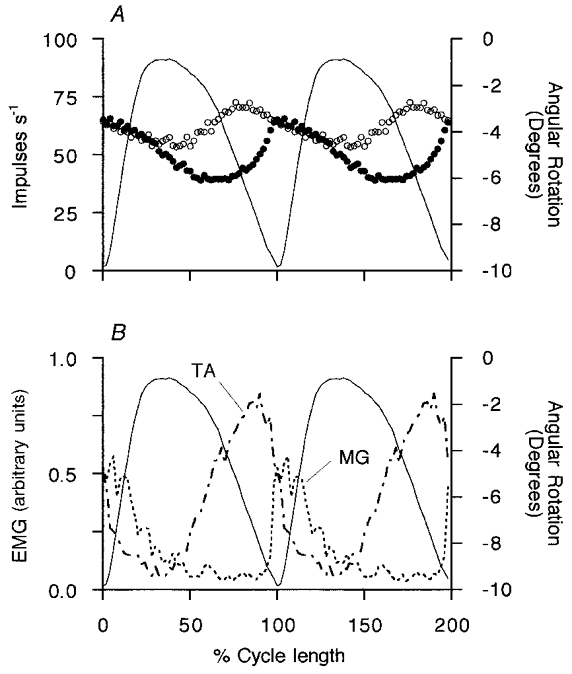
A shows the mean of 13 type-1 units (•) and of 7 type-2 units (○). B shows mean rectified EMG from MG (dotted) and TA (dashed and dotted) in arbitrary units. In both panels the mean ankle movement is shown as continuous thin lines, calibrated in degrees with ankle extension upward.
Effects of the two γs patterns on spindle afferent firing
It has been proposed recently (Taylor et al. 2000a) that variations in the γs output during locomotion can be estimated by recording spindle secondary afferent firing during active movements and then subtracting from it the firing caused by applying the same movements passively, after suppressing fusimotor output with intravenous sodium pentobarbitone. The resulting so-called ‘secondary difference signal’ was argued to be close to a scaled version of the γs firing frequency profile. The present experiments provided the opportunity to test this proposition for MG by comparing the cycle-averaged firing of γs units with the cycle-averaged secondary difference signal (normalising time with respect to cycle period). In Fig. 6 it is seen that the time course of the secondary difference signal is indeed closely similar to that of the type-1 γs unit. Figure 6A–C shows three periods of progressively larger movements in the same experiment. In all cases the two signals are generally similar, although differences are more obvious with the largest movements. Thus, in the largest movement (panel C) the secondary difference signal collapses at the time of most rapid muscle shortening. This may be explained as due to the inability of intrafusal shortening, induced by γs activation, to keep up with extrafusal shortening. Figure 6B and C also shows that the final fall in the difference signal lags behind the fall in the type-1 γs unit firing. The firing profile of a type-2 γs unit is also shown in these records, but this does not, at first sight, appear to contribute to the secondary difference signal. However, if the type-2 record in Fig. 6B is delayed by an amount corresponding to 72 deg of phase lag and then averaged with the type-1 signal as shown in Fig. 6D, then the new profile matches the difference signal almost perfectly. The proposed justification for this procedure is that if the type-2 γs activity exerted its effect via contraction of bag2 fibres, then a substantial phase lag is to be expected. The reasons for the choice of this value of phase shift are considered in the Discussion below. The observed match implies that the type-2 γs pattern may be making a contribution to spindle firing in this way. Figure 7 shows data from another experiment in which the secondary difference signal was computed from an ensemble of five single secondary afferents. In this instance a type-1 γs unit was recorded and its firing profile is shown superimposed. It is clear that the general outline of the type-1 γs firing is reproduced in the secondary difference signal, except for the period of rapid shortening during which the latter fell sharply. Evidently, here again the very large and rapid extrafusal shortening is likely to have caused the sensory region of the intrafusal fibres to go slack. No record of type-2 γs firing was available, but it seems possible that the slow rise in the secondary difference signal extending from 50 to 75 % of the cycle, unaccounted for by the type-1 firing, could have been due to the type-2 pattern, as seen in Fig. 3. In a total of six experiments in which appropriate recordings were made, the main part of the secondary difference signal could be explained in this way as being principally determined by the type-1 γs firing pattern, with an additional phase lagging contribution from type-2.
Figure 6. Prediction of the secondary difference signal from the mean γs firing patterns recorded in MG nerve filaments.
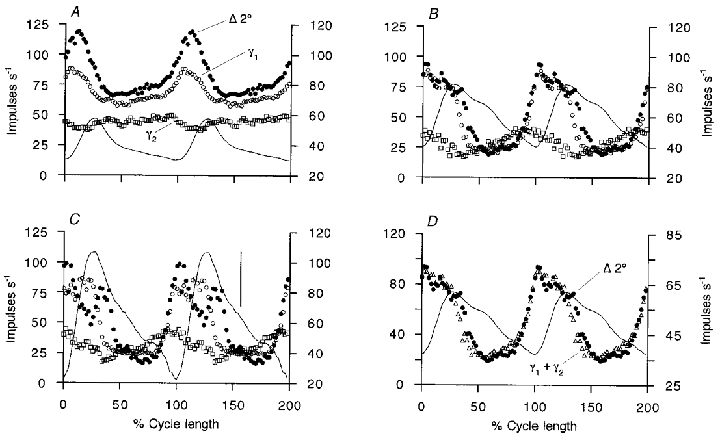
The mean secondary difference signals derived from a single secondary afferent (Δ 2° (•) and left frequency scales) are shown in A–C for three progressively larger movement sequences. From the same experiment, the behaviour of a type-1 γs unit (γ1, ○) and a type-2 γs unit (γ2, □) are shown superimposed (right frequency scales). Mean ankle angle records are continuous thin lines, extension upward. D shows the secondary difference signal (Δ 2°, •) and the ankle angle record (thin line) from B. Superimposed as open triangles is the mean of the type-1 record and the type-2 record in which the latter has been shifted to approximate 72 deg phase lag (right frequency scale). In C the vertical bar represents 5 deg of ankle rotation. The mean cycle period was 0.95 s in A, 0.83 s in B and D, and 0.65 s in C.
Figure 7. Comparison of ensemble secondary difference signal with type-1 γs firing pattern during a sequence of large amplitude movements.
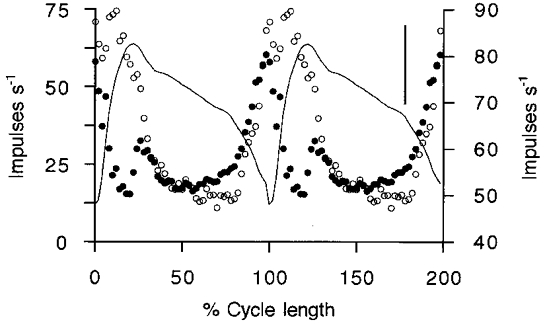
The secondary difference signal (•, left frequency scale) is derived from an ensemble of 5 single secondary afferents and the γs record (○, right frequency scale) is from a single type-1 unit recorded in the same experiment. Ankle angle is the continuous line with extension upward. The vertical bar represents 10 deg of ankle rotation. The mean cycle period was 0.96 s.
Dynamic fusimotor firing patterns
Dynamic fusimotor neurons were identified as illustrated in Figs 1 and 2. The discharge patterns of two γd axons recorded simultaneously are shown as raster displays in Fig. 8B and C. The firing fluctuated in time with each locomotor cycle, but this fluctuation is seen to be different from the smoothly modulated patterns occurring in the γs axons. The γd units are silent during the latter part of the flexion phase and start firing suddenly immediately before the start of muscle shortening and cease again during the early part of flexion. The firing frequency profiles are best seen in the ensemble mean in Fig. 8D. The frequency rose very rapidly to a maximum, fell slowly at first during extension and then rapidly to zero with the onset of flexion. The rising phase was much faster than the falling phase. This pattern was typical of all the 13 dynamic units recorded, save for one in which the interruption was variable and less marked. The peak frequencies of the 13 γd units ranged from 18 to 90 impulses s−1 with a mean of 42.6 ± 23.5 impulses s−1. Their mean conduction velocity was 28.6 ± 7.8 m s−1.
Figure 8. Timing of γd firing.
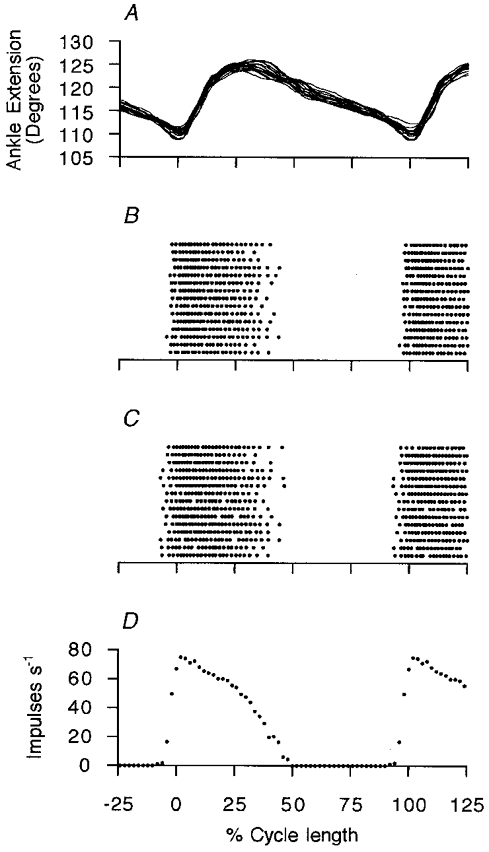
Data from an experiment in which 2 γd units were recorded simultaneously. A shows 15 superimposed ankle movement traces, time-normalised with respect to cycle length. Below, in B and C, the firing of the two γd units is shown in dot raster form, to emphasise the interrupted nature of their firing. D shows the ensemble mean for these two units in terms of frequency as a function of normalised cycle position. The mean cycle period was 0.96 s.
Since the γd firing reaches its maximum frequency suddenly and is then maintained relatively constant for a part of the cycle before returning to silence, it appears to be qualitatively different from the smoothly modulated γs pattern. It therefore seems possible that it is the timing of the γd burst which is of greatest importance. Three separate experiments were performed to examine the consequences of placing a burst of γd stimulation in various phases of a stretch cycle. Fully anaesthetised animals were used as described previously (Taylor et al. 1998; see Methods). The responses to muscle stretch of primary spindle afferents from soleus were recorded during stimulation of single γd axons isolated in cut ventral rootlets. In Fig. 9 an example is shown in which a strong dynamic effect of continuous stimulation at 75 Hz is seen during the ramp and hold (panel A) and during 1 Hz sinusoidal stretches (panel B). When the stimulation was restricted to 50 % of the sinusoidal stretch cycle, its effect depended critically on its timing. Thus, Fig. 9D shows that with the burst starting 50 ms after the onset of shortening and extending 50 ms into lengthening its dynamic effect was lost. However, when the stimulation period was delayed to extend 150 ms into lengthening (panel E) then there was a prominent dynamic response. It should also be noted that the afferent discharge frequency collapsed very rapidly with the cessation of the stimulation despite the increasingly rapid lengthening occurring at that time. In the latter part of lengthening the response was actually less than during control. This result has been confirmed in 22 individual γd effects on primary afferents in soleus muscle. Similar experiments have been reported by Appenteng et al. (1982) and by Morgan et al. (1985), but in those cases the emphasis was on the possibility of supporting Ia afferent firing by γd stimulation during muscle shortening.
Figure 9. The importance of timing for the effect of γd stimulation on primary afferent response to cyclic stretches.
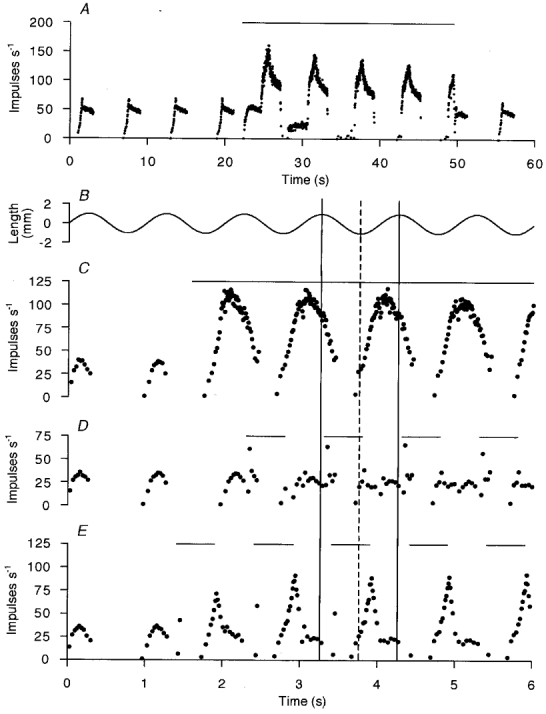
A shows the instantaneous frequency plot of the response of a primary afferent in soleus muscle to continuous stimulation of a γd axon at 75 Hz (horizontal bar) during ramp and hold stretches. The ramps (not shown) were of 5 mm in 1 s held for 1.5 s and released in 1 s, repeating every 6 s. C, D and E show responses to sinusoidal stretch at 1 Hz (B, stretch upwards). In C the γd stimulation is continuous (horizontal bar) whilst in D and E stimulation is for 50 % of the cycles (interrupted bars). The two continuous vertical guidelines in B–E are drawn at the peak of stretch, whilst the dashed line is drawn at the point of minimum stretch.
DISCUSSION
Behaviour of static γ-motor activity
Previous recordings of spindle activity during locomotion in decorticate cats (Perret & Buser, 1972; Perret & Berthoz, 1973) have shown that the γs discharge must be fluctuating rhythmically, approximately in parallel with α-motor activity. Subsequently, direct recordings in locomoting decerebrate cats (Murphy et al. 1984) confirmed the existence of such a pattern in γ-motoneurons identified as static. Because their fluctuating firing was superimposed on a tonic background, they were described as ‘tonically modulated’. The present experiments, using a different method of identifying static and dynamic γ-motoneurons, have also found that the γs units are modulated in time with the locomotor cycle, but have gone further in showing they may be divided into two sub-populations. The majority (65 %), which are designated as type-1, fire in a smoothly modulated pattern, which closely resembles the time course of the difference of active minus passive firing of the spindle secondary afferents. Such a γs pattern was predicted recently from an analysis of spindle afferent recordings alone, both primary and secondary (Taylor et al. 2000a). The present results also confirm that under the conditions of these experiments the relationship between modulated γs firing frequency and secondary difference frequency is essentially linear. It happens also that the range of frequency fluctuation of the γs neurons is approximately the same as that of the secondary difference signal. The recent analysis of spindle recordings (Taylor et al. 2000a), from TA and MG, predicted that the varying γs firing pattern should have a time course very similar to that of the active muscle shortening, but that in the case of MG the γs discharge would be phase advanced relative to the unloaded movement. This phase advance in the case of MG has been confirmed by the present direct γ recordings. The spindle recordings suggested that the MG γs modulation was superimposed on a tonically raised level of static activity and this also is confirmed.
The new and unexpected finding is that a sub-group (35 %) of γs neurons show a modulation which rises to a peak frequency more aligned with TA contraction. This subgroup has been designated as type-2. The two types of discharge pattern appear to be qualitatively different and it is appropriate to speculate on the possible functional significance of such a specialisation. It is suggested that the type-2 γs axons are those which are directed preferentially to the bag2 fibres, whilst type-1 γs axons are those innervating chain alone or bag2 and chain fibres together. In support of this it was found (Fig. 6D) that a combination of the type-1 signal and the type-2 signal with a phase lag of 72 deg gave a very good match of the secondary difference signal. The choice of this relatively large phase shift arose from experiments in which single γs axons were stimulated with pulse trains sinusoidally modulated in frequency at 1 Hz (Ellaway et al. 1996; Taylor et al. 1999a; R. Durbaba, A. Taylor, P. H. Ellaway & S. Rawlinson, unpublished observations). It was shown that those axons which contact only bag2 intrafusal muscle fibres pass on much less of this modulation to the primary spindle afferents than do those acting on chain alone or jointly on bag2 and chain fibres, presumably because of the slow contractile properties of bag2 fibres. The observed afferent modulation by bag2 fibres also lags the stimulus modulation by 25-85 deg at 1 Hz. The possiblity of a large value of phase shift was suggested by the observations of Appenteng et al. (1982) in which sinusoidal length changes were adjusted in phase to compensate for γs stimulation modulated at the same frequency. It was found that at 2 Hz the best compensation of Ia firing was achieved with a 72 deg phase shift. The study by Hulliger (1979) found a mean time constant of 58.5 ms for the effects on Ia afferents of step changes in γs stimulation rate and this would have given a phase lag of only 20.2 deg at 1 Hz. However, in that work there was no recognition of the different effects of chain, bag2 or mixed activation and it seems likely that the slow effect due to bag2 fibres was missed.
The question of the degree of specificity of innervation of bag2 and chain fibres by distinct axons is still unsettled. Some studies have found no evidence for such specificity (Celichowski et al. 1994), but others indicate that there may be enough separate innervation to permit a degree of independent control (Taylor et al. 1998). The present evidence for two different γs firing patterns suggests that the distinct properties of the bag2 and chain fibres may indeed be exploited by the CNS, as suggested by Gladden & McWilliam (1977) and Gladden (1981). Further testing of this hypothesis would require some means of determining which intrafusal fibres each type-1 or type-2 γs axon actually supplies.
Behaviour of dynamic γ-motor activity
With regard to the pattern of activity in γd motoneurons, the earlier experiments (Perret & Buser, 1972; Perret & Berthoz, 1973), though indicating some increased activity, could not be specific as to its pattern. Recently, more detailed analyses of spindle recordings have shown much greater sensitivity in the lengthening phase during active movements than during the same movements repeated passively after suppressing fusimotor output (Taylor et al. 1997, 2000a). This is characteristic of the effects of bag1 fibre activation and thus of γd firing during active movements. In the absence of direct recordings, this increased γd firing might have been expected to have been maintained tonically throughout the movements as a part of the ‘fusimotor set’ phenomenon (Prochazka et al. 1985) to adjust the spindle primary afferent sensitivity to a level generally appropriate to the task. However, the direct recordings of Murphy et al. (1984) had indicated that γd firing (as well as γs) fluctuated in frequency with the locomotor cycle. Because the γd frequency was observed to dip from a high value to zero or near zero, they were described as ‘phasically modulated’. The findings of the present experiments are essentially in agreement, but because of the use of instantaneous firing frequency recording rather than the low-pass filtered ratemeter used by Murphy et al. (1984), the very sudden onset and rapid fall to zero of the firing in each cycle are more obvious. These abrupt changes seem to be qualitatively different from the smoothly and continuously modulated firing of both types of γs motoneurons. In seeking for a term to describe the behaviour of γd units and to distinguish it from that of the γs motoneurons we have suggested the term ‘interrupted’ rather than ‘phasically modulated’ (Taylor et al. 2000b). A problem is that ‘interrupted’ implies the existence of a high background firing rate and in the present data γd neurons did not necessarily fire at a high rate at the onset of locomotion. Perhaps a more satisfactory term will emerge as these studies are continued. The observed timing of the γd discharge suggests that its function may be to sensitise the primary afferents to the initial rapid component of the flexion phase (Durbaba et al. 2000), but to allow the sensitivity to stretch to fall immediately afterwards. This proposal is supported by the additional observations of the effects of varying the timing of a period of stimulation during sinusoidal stretches. During locomotion, the γd firing appears to be timed so as to cause a vigorous afferent burst at the onset of muscle lengthening, which could then serve as a timing signal fed back to ensure that the central pattern generator should be entrained to the natural motion of the limb (Whelan & Pearson, 1997). The presence of γd firing during the muscle shortening phase would additionally make the spindle primary afferents sensitive to any unexpected lengthening which might result from obstruction of the limb’s trajectory.
Comparison of natural locomotor pattern with behaviour in the decerebrate cat
An important issue is the relevance of data obtained from the decerebrate cat to truly natural behaviour in the intact animal. This has been discussed previously (Taylor et al. 2000a), but some additional points should be considered. Various constraints inherent in the present experimental techniques may well have disturbed the normal neural output during locomotion. Thus, fixation and denervation of the hindlimb inevitably reduce normal afferent input, particularly that due to foot contact with the ground. Some reflex effects on γ-motoneurones of non-nociceptive afferents are indeed known to exist (Appelberg, 1985; Ellaway, 1985; Gladden et al. 1998), but at the present it is not known how effective they would be during locomotion. The remaining input from the other limbs would be expected to compensate for the denervation to some extent. The persistence of apparently normal gait and posture in the other limbs and the effective way in which the pace adapts to the treadmill speed also imply that the disturbance is not very great. Abnormal nociceptive inputs may arise from the fluctuating forces at the supporting clamps used here and it has been suggested that this may cause γ-motor reflexes, phasically linked to the locomotor cycle. However, local anaesthetic was infiltrated to reduce the possibility of this and it was found that forces deliberately applied did not cause obvious disturbance of γ-motor output. The effects of decerebration itself are not certain, though some disturbance to muscle tone and posture must be expected. The level of transection was approximately 6 mm rostral to the cuneiform nucleus. Consequently, the lesion of itself is not likely to have directly affected the structures in that region, which have such strong effects on γ-motor outflow, though there would have been a loss of descending influences. The best basis for comparison of locomotion in the decerebrated cat with normal is probably to be found in the profiles of spindle afferent firing. In normal cats these have been well summarised by Prochazka (1996) and more recently by Prochazka & Gorrasini (1998). In comparison with these published records it is clear that the cycle times for the decerebrate walking are similar and the muscle length changes cover the same range. However, because in the present preparation there is no opportunity for foot contact with the ground, there is no equivalent to the E2 and E3 phases, the extensor EMG burst is shortened and flexion is relatively prolonged. If the γd firing in MG extends throughout E1 in normal cats as seen in the decerebrate cat, then this would be expected to prime the spindles to give a vigorous Ia response on foot contact when there is a rapid but brief muscle stretch. This is precisely what has been reported in normal locomotion (Prochazka, 1996). The normal records also show excess afferent discharge during active muscle shortening over that expected without γ-motor support (see Fig. 1 of Prochazka et al. 1977) and this could be explained by the increased γs firing in this phase predicted from the present data. Thus, although there are significant departures from normal in the movement profiles in the hindlimb in our preparation, it seems likely that the observed timing of the γd and γs firing relative to E1 and the extensor EMG onset is a reliable indication of what occurs normally. What is not clear at the present is whether the γd firing in natural conditions continues on into E2 and E3.
Understanding the overall strategy of the use of the fusimotor system must await the availability of appropriate data from other muscles of the hindlimb and from other systems. It will also be important to develop recording methods suitable for more natural conditions. However, it is already clear that the recognition of different activity patterns in the dynamic and the two types of static fusimotor neurons argues strongly against the usefulness of the classical α-γ coactivation principle. It appears rather that full use may be made by the CNS of the different properties of the three different kinds of intrafusal muscle fibres to adapt the properties of the muscle spindle to suit many different functional situations.
Acknowledgments
This research was supported by a grant from the UK Medical Research Council. We thank Mrs O. D. Taylor for technical assistance.
References
- Appelberg B. Group II (muscle spindle?) afferent action on different classes of γ-motoneurones. In: Boyd IA, Gladden MH, editors. The Muscle Spindle. London: Macmillan; 1985. pp. 261–265. [Google Scholar]
- Appenteng K, Morimoto T, Taylor A. Fusimotor activity in masseter nerve of the cat during reflex movements. The Journal of Physiology. 1980;305:415–431. doi: 10.1113/jphysiol.1980.sp013373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenteng K, Prochazka A, Proske U, Wand P. Effect of fusimotor stimulation on Ia discharge during shortening of cat soleus muscles at different speeds. The Journal of Physiology. 1982;329:509–526. doi: 10.1113/jphysiol.1982.sp014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd IA. The action of the three types of intrafusal fibre in isolated cat muscle spindles of the dynamic and length sensitivities of primary and secondary sensory endings. In: Taylor A, Prochazka A, editors. Muscle Receptors and Movement. London: Macmillan; 1981. pp. 17–32. [Google Scholar]
- Celichowski J, EmonetDenand F, Laporte Y, Petit J. Distribution of static γ-axons in cat peronius tertius spindles determined by exclusively physiological criteria. Journal of Neurophysiology. 1994;71:722–732. doi: 10.1152/jn.1994.71.2.722. [DOI] [PubMed] [Google Scholar]
- Durbaba R, Taylor A, Ellaway PH, Rawlinson S. The importance of the timing of dynamic gamma discharge for its effects on the primary muscle spindle afferent response to stretch. The Journal of Physiology. 2000;527.P:136. [Google Scholar]
- Ellaway PH. The influence of muscle mechano-receptors on homonymous gamma motoneurones. In: Boyd IA, Gladden MH, editors. The Muscle Spindle. London: Macmillan; 1985. pp. 255–260. [Google Scholar]
- Ellaway PH, Taylor A, Durbaba R. Modulation of muscle spindle afferent discharge by bag1, bag2 and chain intrafusal fibres. The Journal of Physiology. 1996;497.P:104. doi: 10.1113/jphysiol.2002.031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden MH. The activity of intrafusal muscle fibres during central stimulation in the cat. In: Taylor A, Prochazka A, editors. Muscle Receptors and Movement. London: Macmillan; 1981. pp. 109–122. [Google Scholar]
- Gladden MH, Jankowska E, CzarkowskaBauch J. New observations on coupling between group II muscle afferents and feline γ-motoneurones. The Journal of Physiology. 1998;512:507–520. doi: 10.1111/j.1469-7793.1998.507be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden MH, McWilliam PN. The activity of intrafusal muscle fibres during cortical stimulation in the cat. The Journal of Physiology. 1977;273.P:28–29. [PubMed] [Google Scholar]
- Hulliger M. The response of primary spindle afferents to fusimotor stimulation at constant and abruptly changing rates. The Journal of Physiology. 1979;294:461–482. doi: 10.1113/jphysiol.1979.sp012941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JP, Smith AM, Sessle BJ, Murakami T. Activity of trigeminal alpha and gamma motoneurones and muscle afferents during performance of a biting task. Journal of Neurophysiology. 1979;42:710–725. doi: 10.1152/jn.1979.42.3.710. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. The differentiation of two types of fusimotor fibres by their effects on the dynamic response of muscle spindle primary endings. Quarterly Journal of Experimental Physiology. 1962;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Prochazka A, Proske U. Action of single dynamic fusimotor neurones on cat soleus Ia afferents during muscle shortening. Experimental Brain Research. 1985;58:56–61. doi: 10.1007/BF00238953. [DOI] [PubMed] [Google Scholar]
- Murphy PR, Stein RB, Taylor J. Phasic and tonic modulation of impulse rates in γ-motoneurones during locomotion in premammillary cats. Journal of Neurophysiology. 1984;52:228–243. doi: 10.1152/jn.1984.52.2.228. [DOI] [PubMed] [Google Scholar]
- Perret C, Berthoz A. Evidence of static and dynamic fusimotor actions on the spindle response to sinusoidal stretch during locomotor activities in the cat. Experimental Brain Research. 1973;18:178–188. doi: 10.1007/BF00234722. [DOI] [PubMed] [Google Scholar]
- Perret C, Buser P. Static and dynamic fusimotor activity during locomotor movements in the cat. Brain Research. 1972;40:165–169. doi: 10.1016/0006-8993(72)90123-0. [DOI] [PubMed] [Google Scholar]
- Price RF, Dutia MB. Physiological properties of tandem muscle spindles in neck and hindlimb muscles. Progress in Brain Research. 1989;80:47–56. doi: 10.1016/s0079-6123(08)62198-6. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell LB, Sheperd JT, editors. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. pp. 89–127. section 12. [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. The Journal of Physiology. 1998;507:293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M, Zangger P, Appenteng K. ‘Fusimotor set’: new evidence for α-independent control of γ-motoneurones during movement in the awake cat. Brain Research. 1985;339:136–140. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Westerman R, Ziccone SP. Ia afferent activity during a variety of voluntary movements in the cat. The Journal of Physiology. 1977;268:423–448. doi: 10.1113/jphysiol.1977.sp011864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN. Control of walking and running by means of electrical stimulation of the mid-brain. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Taylor A, Cody FWJ. Jaw muscle spindle activity in the cat during normal movements of eating and drinking. Brain Research. 1974;71:521–530. doi: 10.1016/0006-8993(74)90996-2. [DOI] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Ellaway PH, Rawlinson S. Patterns of fusimotor activity during locomotion in the decerebrate cat deduced from recordings from hindlimb muscle spindles. The Journal of Physiology. 2000a;522:515–532. doi: 10.1111/j.1469-7793.2000.t01-3-00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Ellaway PH, Rawlinson S. Fusimotor action during locomotion in the decerebrate cat. The Journal of Physiology. 2000b;525.P:6–7S. doi: 10.1111/j.1469-7793.2000.t01-3-00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Rodgers JF. The classification of afferents from muscle spindles of the jaw-closing muscles of the cat. The Journal of Physiology. 1992a;456:609–628. doi: 10.1113/jphysiol.1992.sp019356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Ellaway PH, Durbaba R. Physiological signs of the activation of bag2 and chain intrafusal muscle fibers of gastrocnemius muscle spindles in the cat. Journal of Neurophysiology. 1998;80:130–142. doi: 10.1152/jn.1998.80.1.130. [DOI] [PubMed] [Google Scholar]
- Taylor A, Ellaway PH, Durbaba R. Why are there three types of intrafusal muscle fibers? Progress in Brain Research. 1999a;123:121–131. doi: 10.1016/s0079-6123(08)62849-6. [DOI] [PubMed] [Google Scholar]
- Taylor A, Ellaway PH, Durbaba R, Rawlinson S. Static and dynamic γ-motoneurone discharge patterns recorded directly during locomotion. The Journal of Physiology. 1999b;521.P:42. [Google Scholar]
- Taylor A, Hidaka O, Durbaba R, Ellaway PH. Fusimotor influence on jaw muscle spindle activity during swallowing-related movements in the cat. The Journal of Physiology. 1997;503:157–167. doi: 10.1111/j.1469-7793.1997.157bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Rodgers JF, Fowle AJ, Durbaba R. The effect of succinylcholine on cat gastrocnemius muscle spindle afferents of different type. The Journal of Physiology. 1992b;456:629–644. doi: 10.1113/jphysiol.1992.sp019357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Rodgers JF, Fowle AJ, Durbaba R. Interpretation of spindle afferent recordings according to intrafusal fibre influence. In: Jami L, PierrotDeseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford: Pergamon Press Ltd; 1992c. pp. 105–111. [Google Scholar]
- Whelan PJ, Pearson KG. Comparison of the effects of stimulating group I afferents on cycle period during walking in conscious and decerebrate cats. Experimental Brain Research. 1997;117:444–452. doi: 10.1007/s002210050239. [DOI] [PubMed] [Google Scholar]


