Abstract
We have investigated adrenal mRNA expression of the catecholamine synthetic enzymes tyrosine hydroxylase (TH) and phenylethanolamine N-methyltransferase (PNMT) following acute hypoxia in fetal sheep before (< 105 days gestation, n = 20) and after (> 125 days gestation, n = 20) the development of adrenal innervation and following pretreatment with the nicotinic receptor anatgonist hexamethonium (n = 12).
Total RNA was extracted from fetal adrenal glands collected at specific time points at 3-20 h after the onset of either hypoxia (∼50% reduction in fetal arterial oxygen saturation (SO2) for 30 min), or normoxia.
Before 105 days, there was a decrease in adrenal TH mRNA expression at 20 h after hypoxia and adrenal TH mRNA expression was directly related to the changes in arterial PO2 measured during normoxia and hypoxia. After 125 days, adrenal TH mRNA levels were suppressed for up to 12 h following hypoxia.
In both age groups, adrenal PNMT mRNA expression increased at 3-5 h after hypoxia and was inversely related to the changes in fetal arterial PO2 during normoxia or hypoxia.
After 125 days, the administration of hexamethonium (25 mg kg−1, I. V.) reduced TH mRNA but not PNMT mRNA expression after normoxia. After hexamethonium pretreatment, there was no significant change in either adrenal TH or PNMT mRNA expression following hypoxia.
We conclude that acute hypoxia differentially regulates adrenal TH and PNMT mRNA expression in the fetal sheep both before and after the development of adrenal innervation. After the development of adrenal innervation, however, the effect of acute hypoxia upon adrenal TH and PNMT mRNA expression is dependent upon neurogenic input acting via nicotinic receptors.
It is well established that acute hypoxia stimulates catecholamine secretion from the fetal sheep adrenal medulla and that this response is essential for the generation of appropriate fetal metabolic and cardio-vascular adaptions to hypoxia (Jones et al. 1988). The fetal catecholamine response to hypoxia is substantially greater than that which occurs in the adult and increases in magnitude towards term (Jones & Robinson, 1975; Widmark et al. 1989). Whilst complete functional innervation of the adrenal medulla does not occur until after 120 days gestation in the sheep (term = 147 ± 3 days gestation), it has been demonstrated that the adrenal gland secretes catecholamines in response to acute hypoxia prior to the development of adrenal innervation (Comline & Silver, 1961; Cheung, 1990). The specific sequence of cellular events underlying the non-neurogenic secretion of catecholamines from the immature adrenal medulla in response to hypoxia is unknown, although we have recently shown that oxygen-sensitive potassium channels are present on the chromaffin cell membranes in the fetal sheep adrenal gland (Rychkov et al. 1998). Furthermore, we have also shown that the non-neurogenic catecholamine response to hypoxia is dependent on the influx of calcium into the chromaffin cells via voltage-dependent L-type calcium channels (Adams et al. 1996). The development of adrenal innervation is associated with the loss of the non-neurogenic catecholamine response to hypoxia in vivo, and secretion becomes entirely dependent upon reflex stimulation of the preganglionic, cholinergic, splanchnic nerve (Cheung, 1990).
In the adult animal, it is clear that there is functional coupling of catecholamine secretion and synthesis within the adrenal chromaffin cell which maintains catecholamine stores after periods of increased adrenal stimulation (Bygdeman & von Euler, 1958; Wakade et al. 1988). Catecholamine secretion-synthesis coupling is mediated by trans-synaptic stimulation of the levels, activity, and gene expression of the catecholamine synthetic enzymes tyrosine hydroxylase (TH; tyrosine 3-monooxygenase; l-tyrosine, tetrahydropteridine: oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2) and phenylethanolamine N-methyltransferase (PNMT; S-adenosyl-l-methionine: phenylethanolamine N-methyltransferase, EC 2.1.1.28; Fluharty et al. 1985; Wong et al. 1993).
Whilst it is important that the fetal adrenal gland maintains its capacity to secrete catecholamines in response to repeated episodes of acute intrauterine hypoxia, it is not known whether there is coupling of catecholamine secretion and synthesis in the chromaffin cells of the fetal adrenal, or indeed whether such coupling is dependent on the development of adrenal innervation.
In the present study, we have investigated the time course of the responses of adrenal TH and PNMT mRNA expression to an acute episode of hypoxia, before (< 105 days gestation) and after (> 125 days gestation) the development of functional splanchnic innervation in the sheep fetus. The adrenal TH and PNMT mRNA responses to acute hypoxia after the development of functional splanchnic innervation were also measured following pretreatment with the nicotinic cholinergic receptor antagonist hexamethonium.
METHODS
Animals and surgery
All procedures were approved by the University of Adelaide Standing Committee on Ethics in Animal Experimentation.
Fifty-two fetal sheep from dated pregnant Border Leicester × Merino ewes were used in this study. Surgery was performed on ewes at either 88-92 days or at 116-120 days gestation under general anaesthesia induced by intravenous injection of sodium thiopentone (1.25 g; Pentothal, Rhone Merieux, Pinkenba, QLD, Australia) and maintained with inhalational halothane (3-4% in oxygen; Fluothane, ICI, Melbourne, Victoria, Australia). Vascular catheters were inserted into a maternal jugular vein, a fetal carotid artery and external jugular vein and the amniotic sac as previously described (Robinson et al. 1979). All catheters were flushed and filled with heparinised saline and exteriorised through an incision in the ewe's flank.
Ewes were housed under a 12 h light-dark cycle and fed once daily between 09.00 and 13.00 h, with water ad libitum. A post-operative recovery period of at least a week was allowed before any experiment was conducted.
Experimental protocols
Separate groups of fetuses at 96-105 days (n = 15) and at 129-144 days (n = 15) were exposed to a 30 min episode of hypoxia by altering the maternal fractional inspired oxygen content. Following a 60 min baseline period a large, transparent, polypropylene breathing bag was tied loosely over the ewe's head and a mixture of air (39%), N2 (58%), and CO2 (3%) was infused into the bag at a flow rate of 58 l min−1. The gas mixture was titrated over the 30 min period by adjusting the balance of air and nitrogen to maintain fetal arterial SO2 at approximately 50% of the baseline values. In control experiments, pregnant ewes at 96-105 days (n = 5) and 129-144 days (n = 5) were administered a normoxic gas mixture which consisted of air (97%) and CO2 (3%) at a flow rate of 58 l min−1.
In a separate group of fetuses at 129-144 days (n = 12), hexamethonium (hexamethonium bromide dihydrate; ICN Biomedicals, Cleveland, OH, USA) was administered as an I. V. bolus (25 mg kg−1 in 0.9% saline; 3 ml) at -30 min relative to fetal exposure to either hypoxia (n = 6) or normoxia (n = 6). This dose of hexamethonium has previously been shown to block sympathetic function in the sheep fetus for a period of several hours (Brace & Brittingham, 1986).
Fetal arterial blood samples (0.5 ml) were collected once daily on the first 4 days following surgery and on alternate days thereafter for measurement of fetal blood gases using an ABL 520 blood gas analyser (Radiometer, Copenhagen, Denmark) to assess fetal well being. During hypoxia or normoxia experiments, fetal arterial blood samples (3.5 ml) were obtained at the following time points relative to the onset of the regulation of maternal fractional inspired oxygen content: -60, -35, -25, -5, 5, 10, 30 and 60 min. An equivalent volume of isotonic saline was infused into the fetus following the withdrawal of each sample. A fetal arterial blood sample (0.5 ml) was used for blood gas analysis, and the remainder was placed into chilled heparinised tubes. Tubes were centrifuged at 1800 g for 10 min at 4°C and the plasma was separated, snap frozen in liquid N2 and stored at -20°C until assayed for catecholamines.
Tissue collection
Ewes were killed with an overdose of sodium pentobarbitone (25 ml at 325 mg ml−1; Lethabarb, Virbac, Peakhurst, NSW, Australia) at 3-5 h (96-105 days, n = 5; 129-144 days, n = 5), 12 h (96-105 days, n = 5; 129-144 days, n = 5), and 20 h (96-105 days, n = 5; 129-144 days, n = 5) after the onset of hypoxia (Table 1). In control experiments, ewes were also killed between 3 and 20 h after the onset of normoxia (96-105 days, n = 5; 129-144 days, n = 5; Table 1). In the hexamethonium-treated groups, ewes were killed at 3-5 h after the onset of either normoxia (n = 6) or hypoxia (n = 6; Table 1). Fetal sheep were delivered via laparotomy and killed by decapitation. Adrenal glands were dissected from each fetus, weighed, snap frozen in liquid N2, and stored at -70°C until total RNA was extracted.
Table 1.
Distribution of individual fetal sheep on the basis of age and treatment amongst designated post-mortem groups following exposure to experimental hypoxia.
| Post-hypoxia | ||||
|---|---|---|---|---|
| Control (normoxia) | 3–5h | 12h | 20h | |
| 96–105 days | n = 5 | n = 5 | n = 5 | n = 5 |
| 12 h, n = 5 | ||||
| 129–144 days | n = 5 | n = 5 | n = 5 | n = 5 |
| 3–5 h, n = 2 | ||||
| 12 h, n = 2 | ||||
| 20 h, n = 1 | ||||
| 129–144 days + Hex | n = 6 | n = 6 | — | — |
| 3–5 h, n = 6 | ||||
n, total number of individual fetal sheep without each group. Values in bold indicaet the subdistribution of fetal sheep in the control normoxia groups amongst the different post-mortem time points. Hex, hexamethonium (25 mg kg−1).
Extraction and measurement of plasma catecholamines
Plasma samples collected at -25 and -5 min (basal) or at 5 and 10 min (hypoxia) were combined in equivalent portions for catecholamine measurement. Plasma samples were also collected at -60 and -35 min and combined for extraction in the hexamethonium-treated groups.
Plasma catecholamines were partially purified by alumina absorption using a BAS plasma catecholamine kit (MF-9017; Bioanalytical Systems, West Lafayette, IN, USA) essentially as per the manufacturer's instructions with minor modifications. Briefly 500 μl of fetal plasma was used and made up to a volume of 2 ml with distilled water and the sample pretreatment step was omitted. 3,4-dihydroxybenzylamine (DHBA; Bioanalytical Systems) was added to each sample (50 μl at 80 pmol l−1 in 0.1 m perchloric acid (PCA)) as an internal standard to monitor the recovery of catecholamines from plasma. The efficiency of catecholamine recovery averaged 67.4 ± 1.8% and the catecholamine values were corrected for recovery. Aliquots (50 μl) of the extracted samples were injected onto a BAS 200A HPLC system (Bioanalytical Systems) using a refrigerated microsampler (CMA/Microdialysis, Stockholm, Sweden) at 4°C. Separation of the catecholamines was achieved using a 3 μm ODS, 100 × 3.2 mm, Brownlee Velosep RP-18 cartridge column (Perkin Elmer, Norwalk, CT, USA) and MP-2 catecholamine mobile phase (Bioanalytical Systems). The column temperature was maintained at 40°C and the mobile phase flow rate was 1 ml min−1. The potential of the glassy carbon electrode was set at +700 mV vs. a Ag-AgCl reference electrode whilst the gain was set to 0.5 nA. Catecholamines were automatically quantified by peak height integration using an IBM-compatible personal computer equipped with Barspec Data System chromatographic software (Barspec, Rehovot, Israel). Peak heights were referenced to standard curves generated for authentic catecholamine standards (noradrenaline bitartrate, adrenaline bitartrate, and DHBA hydrobromide; Bioanalytical Systems; 0.3125-20 nmol l−1 in 0.1 m PCA) to determine the catecholamine content of the samples. The sensitivity of the assay was approximately 0.05 pmol ml−1 and the intra- and inter-assay coefficients of variation were less than 10% for both noradrenaline (NA) and adrenaline.
Total RNA extraction
Total adrenal RNA was extracted from each adrenal gland using Tri-reagent (Sigma Chemical Co., St Louis, MO, USA). Briefly, adrenal glands were homogenised separately in Tri-reagent (1 ml) using a Polytron PT3000 homogeniser (Kinematica, Littau, Switzerland) at 30000 r.p.m. Bromo-3-chloropropane (0.1 ml) was added to the homogenate and the samples were then mixed and allowed to stand at room temperature for 5 min. The samples were centrifuged at 12000 g for 10 min at 4°C and the upper aqueous phase was transferred to separate sterile Eppendorf tubes. Total RNA was precipitated from the aqueous phase by adding isopropanol (0.5 ml) and allowing the samples to stand at room temperature. After 10 min, the samples were centrifuged (12000 g for 10 min at 4°C) and the supernatant was subsequently removed. The RNA pellets were washed by adding 75 % ethanol (1 ml) and centrifuging at 12000 g for 10 min at 4°C. Following the removal of the ethanol solution, the RNA pellets were air dried and resuspended in an appropriate volume of sterile water. Nucleic acid concentration, yield and purity were quantified by spectrophotometric measurement at 260 and 280 nm. Total RNA solutions were stored at -70°C prior to Northern blot analysis.
Probes and probe labelling
Oligonucleotide antisense probes complementary to nucleotides 361-389 of the peptide coding region of bovine PNMT mRNA sequence (29-mer; Wan et al. 1989) and nucleotides 151-180 of rat 18 S rRNA (30-mer; Chan et al. 1984) sequence were synthesised (Bresatec, Thebarton, SA, Australia). A full length (1.73 kb) bovine TH cDNA (D'Mello et al. 1988) was used to detect TH mRNA. The oligonucleotides were end-labelled using T4 polynucleotide kinase (7.9 U μl−1, Pharmacia, North Ryde, NSW, Australia) and γ-[32P]ATP (4000 Ci mmol−1, Bresatec). The cDNA probe was labelled by the random priming method with α-[32P]dCTP (3000 Ci mmol−1, Bresatec) and Klenow fragment (6.4 U μl−1) using an oligolabelling kit (Pharmacia).
Northern blot analysis
For each fetus, 20 μg of total adrenal RNA was denatured in 2.2 m formaldehyde and 50% (v/v) formamide at 55°C for 10 min. Separation of the total adrenal RNA was achieved by electrophoresis on a 1% agarose gel containing 2.2 m formaldehyde. The separated total adrenal RNA was subsequently transferred to zetaprobe nylon membranes (Biorad, Richmond, CA, USA) via downward capillary transfer using 10 × SSC as the transfer buffer.
Adrenal RNA from the post-normoxia and post-hypoxia time points from both gestational age groups was loaded on to the same gel. Similarly adrenal RNA from the fetal sheep in late gestation which was or was not pretreated with hexamethonium before exposure to normoxia or hypoxia was also loaded on to the same gel for each of two Northern blot analyses.
Following transfer, the membranes were washed in 10 × SSC for 10 min at room temperature and baked for 1 h at 80°C to fix the RNA to the membrane. Prior to hybridisation with oligonucleotide probes the membranes were initially prehybridised at 50°C for 18 h in a solution consisting of 5 × SSC, 20 mm NaH2PO4 (pH 7.2), 7% SDS, 5 × Denhardt's solution and 100 μg ml−1 of heat-denatured salmon sperm DNA. Membranes were then hybridised for 20 h at 50°C in 15 ml of fresh hybridisation solution containing 1-2 × 106 c.p.m. ml−1 of labelled oligonucleotide. For the TH cDNA probe, equivalent prehybridisation and hybridisation times were used, but the hybridisation solution consisted of 50% (v/v) deionised formamide, 5 × SSPE, 7% SDS (pH 7.2) and 100 μg ml−1 of heat-denatured salmon sperm DNA, with hybridisation being carried out at 42°C in the presence of 1-2 × 106 c.p.m. ml−1 of labelled probe.
Following hybridisation, membranes were washed in 1 × SSC and 0.1% SDS (1 × 30 min) and then subsequently in 0.1 × SSC and 0.1% SDS (1 × 30 min) at their hybridisation temperatures before being briefly air dried and exposed to a Fuji BAS-IIIs Phosphorimager Plate (Fuji Photo Co., Tokyo, Japan) for 24-48 h. Autoradiographs were obtained using a Fuji BAS 1000 Phosphorimager (Fuji Photo Co.) and quantitated using Fuji MacBAS software (Fuji Photo Co.).
Statistical analysis
All data are presented as the mean ±s.e.m. Logarithmic transformation of data was carried out where required to reduce heterogeneity of variance. One-way analysis of variance (ANOVA) was used to compare fetal body and adrenal weights across experimental groups. Within each age group the effects of normoxia or hypoxia on fetal arterial blood gas variables were determined using ANOVA with repeated measures and the Fisher's post hoc test to identify significant differences between mean values.
For each Northern blot both adrenal TH mRNA expression and PNMT mRNA expression were calculated as a ratio to 18 S rRNA expression. Expression data derived from from multiple Northern blots were analysed by normalising data within each blot to a lane which was present in all blots. The normalised data from the different blots, expressed as arbitrary units, were then able to be compared. Within each age group, the ratios of adrenal TH and PNMT mRNA to 18 S rRNA after normoxia or at different time points after hypoxia were compared using one-way ANOVA and Fisher's post hoc tests. Similarly, one-way ANOVA was also used to compare the effects of pretreatment with or without hexamethonium on adrenal TH:18 S rRNA and PNMT mRNA:18 S rRNA ratios at 3-5 h after either normoxia or hypoxia at > 125 days gestation.
Regression analysis was used to determine the relationship between the average change in fetal arterial PO2 measured during the normoxia and hypoxia experiments and adrenal TH:18 S rRNA or PNMT mRNA:18 S rRNA ratios. For the regression analyses, the post hypoxia time point at which a significant change in adrenal TH or PNMT mRNA expression first occurred, was used in each age group.
The proportional changes in plasma catecholamines from basal values (100%) in the normoxia and hypoxia experiments were compared using Student's t tests. In all analyses, a probability of less than 5% (i.e. P < 0.05) was taken to be significant.
RESULTS
Fetal body and adrenal gland weights
Body weight in the 96-105 days fetal sheep was not found to be significantly different between the control normoxia (1.20 ± 0.02 kg), 3-5 h post-hypoxia (0.92 ± 0.10 kg), 12 h post-hypoxia (1.08 ± 0.06 kg), and 20 h post-hypoxia groups (1.13 ± 0.09 kg). Similarly no significant differences in body weight were observed between experimental groups for the 129-144 days (control normoxia, 4.52 ± 0.22 kg; 3-5 h post-hypoxia, 3.48 ± 0.34 kg; 12 h post-hypoxia, 4.25 ± 0.21 kg; 20 h post-hypoxia, 4.13 ± 0.39 kg), and hexamethonium-treated (normoxia + hexamethonium, 3.51 ± 0.38 kg; hypoxia + hexamethonium, 4.02 ± 0.24 kg) fetal sheep.
The adrenal gland weights of the 96-105 days fetal sheep in the control normoxia (112 ± 8 mg), 3-5 h post-hypoxia (94 ± 7 mg), 12 h post-hypoxia (91 ± 4 mg) and 20 h post-hypoxia (104 ± 4 mg) groups were not significantly different from each other. Adrenal gland weights were also similar amongst the experimental groups for the 129-144 days (control normoxia, 231 ± 30 mg; 3-5 h post-hypoxia, 254 ± 26 mg; 12 h post-hypoxia, 250 ± 24 mg; 20 h post-hypoxia, 192 ± 9 mg) and hexamethonium-treated (normoxia + hexamethonium, 164 ± 18 mg; hypoxia + hexamethonium, 213 ± 16 mg) fetal sheep.
Fetal arterial blood gases and experimental hypoxia
There were no significant changes in fetal arterial PO2 during normoxia at either 96-105 days (Fig. 1A) or at 129-144 days (Fig. 1B). Fetal arterial PO2 decreased (P < 0.005) during hypoxia at both 96-105 days and 129-144 days and recovered to baseline values by 30 min after hypoxia in both age groups (Fig. 1A and B). There was no effect of hexamethonium administration at -30 min on basal arterial PO2 (Fig. 1B) or on the change in PO2 values which occurred during hypoxia (Fig. 1B).
Figure 1. Effects of experimental hypoxia and normoxia upon fetal arterial blood gas parameters.
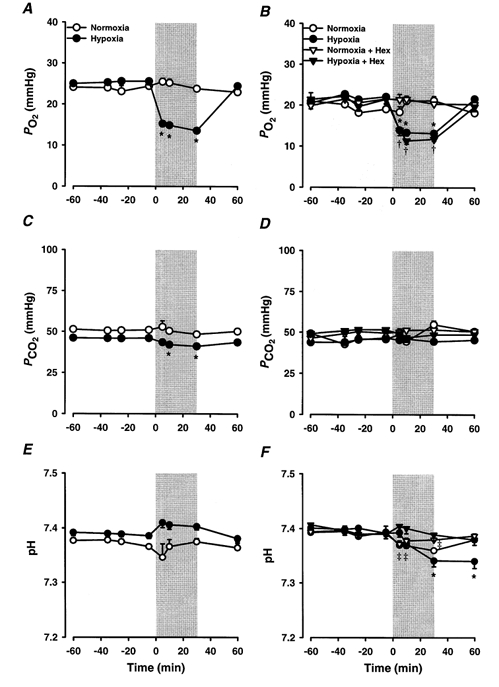
Average arterial PO2 (A, B), PCO2 (C, D), and pH (E, F) values for 96-105 days (left-hand panel; A, C, E) and 129-144 days (right-hand panel; B, D, F) fetal sheep exposed to a 30 min period of experimental hypoxia (•; 96-105 days, n = 14; 129-144 days, n = 15) or normoxia (○; 96-105 days, n = 5; 129-144 days, n = 4) as indicated by the shaded areas on the graphs. Values from 129-144 days fetal sheep treated with hexamethonium (Hex) and exposed to either normoxia (▿, n = 6) or hypoxia (▾, n = 6) are also shown. *P < 0.05, significant difference from basal values for hypoxia group; †P < 0.05, significant difference from basal values for hypoxia + Hex group; ‡P < 0.05, significant difference from basal values for normoxia + Hex group.
In the younger age group, there was no change in fetal arterial PCO2 during normoxia, although during hypoxia, fetal arterial PCO2 decreased from 46.0 ± 0.7 mmHg to 41.0 ± 1.0 mmHg at 30 min (Fig. 1C). In the older fetal sheep, fetal arterial PCO2 did not change during either normoxia or hypoxia in the presence or absence of hexamethonium (Fig. 1D).
At 96-105 days, there was no change in arterial pH during either hypoxia or normoxia (Fig. 1E). In the later age group, there was also no change in arterial pH during normoxia, although during hypoxia, arterial pH decreased (P < 0.0005) from 7.389 ± 0.006 to 7.369 ± 0.004 at 30 min (Fig. 1F). There was also a small reduction in arterial pH in the hexamethonium-treated fetuses during normoxia and no change in arterial pH in the hexamethonium-treated group during hypoxia (Fig. 1F).
Fetal plasma catecholamines
At 96-105 days, plasma adrenaline concentrations were frequently undetectable and basal NA and adrenaline concentrations (NA, 0.32 ± 0.09 nmol l−1, n = 14; adrenaline, 0.13 ± 0.04 nmol l−1, n = 6) were less (P < 0.05) than at 129-144 days (NA, 0.78 ± 0.11 nmol l−1; adrenaline, 0.36 ± 0.04 nmol l−1; n = 19). At 129-144 days, there was no significant effect of hexamethonium administration on basal catecholamine concentrations.
In the younger fetuses, the net change in plasma NA from basal values was greater (P < 0.05) during hypoxia than during control normoxia (normoxia, 0.09 ± 0.05 nmol l−1; hypoxia, 0.43 ± 0.11 nmol l−1). In this age group, changes in plasma adrenaline concentrations during hypoxia and normoxia could not be compared as plasma adrenaline was only detectable during one normoxia experiment. At 129-144 days gestation, the changes in plasma NA and adrenaline concentrations as a proportion of baseline were greater (P < 0.05) during hypoxia (NA, 223.7 ± 35.2%; adrenaline, 167 ± 16.0%) than during normoxia (NA, 100.5 ± 7.8%; adrenaline, 93.6 ± 15.1%). After hexamethonium pretreatment, there was no significant increase in plasma NA levels during hypoxia (hypoxia + hexamethonium, 182.3 ± 31.2%) when compared with during normoxia (normoxia + hexamethonium, 143.6 ± 27.3%), whilst there was a residual plasma adrenaline response to hypoxia (P < 0.05) after hexamethonium (hypoxia + hexamethonium, 143.7 ± 18.5%; normoxia + hexamethonium, 90.1 ± 11.8%).
Hypoxia and adrenal TH mRNA
At 96-105 days a decrease (P < 0.05) in the relative expression of adrenal TH mRNA at 20 h occurred after exposure to hypoxia (post-normoxia, 0.58 ± 0.12; 20 h post-hypoxia, 0.23 ± 0.06; Fig. 2A). At 129-144 days there was also a decrease (P < 0.005) in relative TH mRNA expression, which occurred at 3-5 h after hypoxia (post-normoxia, 0.42 ± 0.09; 3-5 h post-hypoxia, 0.13 ± 0.03) and which was maintained until 12 h (0.10 ± 0.01) before recovery to control levels by 20 h post-hypoxia (Fig. 2B and 3).
Figure 2. Time course of acute hypoxia induced changes in adrenal TH and PNMT mRNA expression in the fetal sheep.
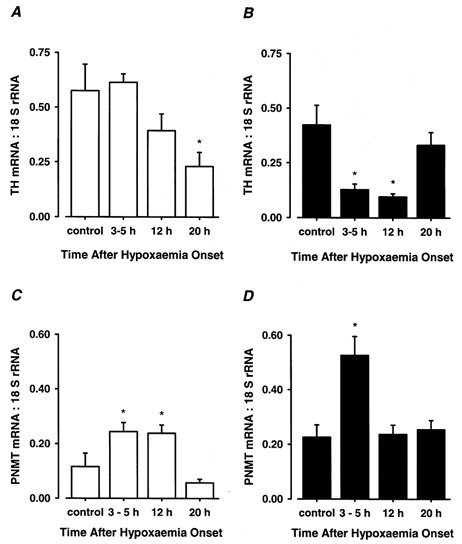
The ratios of adrenal TH mRNA:18 S rRNA (A, B) and PNMT mRNA:18 S rRNA (C, D) expression in 96-105 days (left-hand panel; A, C) and 129-144 days (right-hand panel; B, D) fetal sheep 3-5 h (96-105 days, n = 5; 129-144 days, n = 5), 12 h (96-105 days, n = 5; 129-144 days, n = 5), and 20 h (96-105 days, n = 4; 129-144 days, n = 5) after the onset of a 30 min period of experimental hypoxia or normoxia (control; 96-105 days, n = 5; 129-144 days, n = 5). *P < 0.05, significant difference from control value.
Figure 3. Autoradiographs of Northern blots of total adrenal RNA from fetal sheep exposed to acute hypoxia.
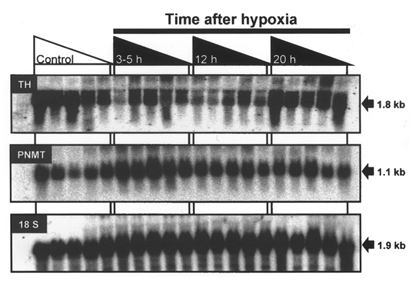
Representative autoradiographs of a Northern blot after hybridisation of radiolabelled TH cDNA, PNMT and 18 S antisense oligonucleotide probes with total RNA (20 μg lane−1) extracted from adrenal glands collected from fetal sheep at 129-144 days gestation after experimental normoxia (Control, indicated by area under open triangle, n = 5) or 3-5 h (n = 5), 12 h (n = 5), and 20 h (n = 5) after 30 min of experimental hypoxia (indicated by the areas under the filled triangles). The approximate sizes of the relevant transcripts are indicated on the right-hand side of the panels.
In the younger fetuses, adrenal TH mRNA expression after normoxia and at 20 h post-hypoxia was significantly related to the average change in arterial PO2 (ΔPO2) which occurred during the 30 min period of normoxia or hypoxia (TH mRNA = 3.1ΔPO2+ 54.1, r2= 0.55, P < 0.05) (Fig. 4A). At 129-144 days, the relationship between adrenal TH mRNA expression and ΔPO2 in the post-normoxia and 3-5 h post-hypoxia groups was more complex (TH mRNA = -0.5(ΔPO2)3– 4.1(ΔPO2)2+ 8.3(ΔPO2) + 117.9, r2= 0.82, P < 0.05; Fig. 4A).
Figure 4. Relationships of adrenal TH and PNMT mRNA expression with experimentally induced changes of arterial PO2 in fetal sheep.
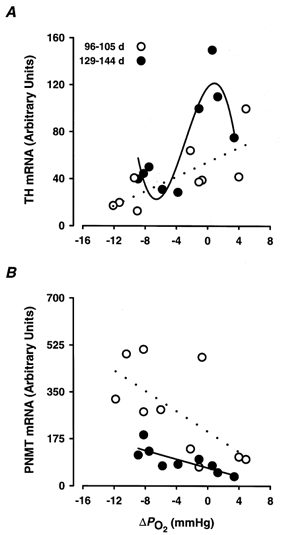
The expression of adrenal TH mRNA:18 S rRNA (A) and PNMT mRNA:18 S rRNA (B) plotted against the experimentally induced changes in arterial PO2 (ΔPO2) for 96-105 days (○; A, control and 20 h values; B, control and 3-5 h values) and 129-144 days fetal sheep (•; A, control and 3-5 h values; B, control and 3-5 h values). Dotted and continuous lines represent the lines of best fit derived by regression analysis for the 96-105 days (TH mRNA = 3.1ΔPO2+ 54.1, r2= 0.55, P < 0.05; PNMT mRNA = -19.0ΔPO2+ 202.9, r2= 0.42, P < 0.05) and the 129-144 days groups (TH mRNA = -0.5(ΔPO2)3– 4.1(ΔPO2)2+ 8.3(ΔPO2) + 117.9, r2= 0.82, P < 0.05; PNMT mRNA = -9.4 ΔPO2+ 75.6, r2= 0.69, P < 0.01), respectively.
Hypoxia and adrenal PNMT mRNA
In the younger age group, relative adrenal PNMT mRNA expression increased (P < 0.01) at 3-5 h post-hypoxia (post-normoxia, 0.12 ± 0.05; 3-5 h post-hypoxia, 0.24 ± 0.03), and this increase was maintained at 12 h (0.24 ± 0.03) before recovery to control levels by 20 h post-hypoxia (Fig. 2C). In the older fetuses, relative adrenal PNMT mRNA expression also increased (P < 0.005) at 3-5 h after hypoxia (post-normoxia, 0.23 ± 0.05; 3-5 h post-hypoxia, 0.53 ± 0.07) but returned to control values by 12 h post-hypoxia (Fig. 2D and 3).
In contrast to adrenal TH mRNA, adrenal PNMT mRNA expression at 96-105 days was inversely related to the average changes in fetal arterial PO2 during normoxia or hypoxia (PNMT mRNA = -19.0 ΔPO2+ 202.9, r2= 0.42, P < 0.05; Fig. 4B). There was also an inverse relationship between PNMT mRNA expression and ΔPO2 (PNMT mRNA = -9.4ΔPO2+ 75.6, r2= 0.69, P < 0.01) in the older age group (Fig. 4B).
Effects of hexamethonium pretreatment on TH mRNA and PNMT mRNA responses to hypoxia and normoxia
At 129-144 days, administration of hexamethonium before exposure to normoxia significantly reduced (P < 0.0005) TH mRNA expression after normoxia (Fig. 5A) and there was subsequently no difference between adrenal TH mRNA levels after either normoxia or 3-5 h post-hypoxia. There was no relationship between adrenal TH mRNA levels at 3-5 h post-hypoxia or normoxia and the mean changes in fetal arterial PO2 during hypoxia or normoxia after hexamethonium pretreatment (Fig. 5B).
Figure 5. The effect of ganglionic blockade upon the hypoxia-induced changes in adrenal TH and PNMT mRNA expression in 129-144 days fetal sheep.
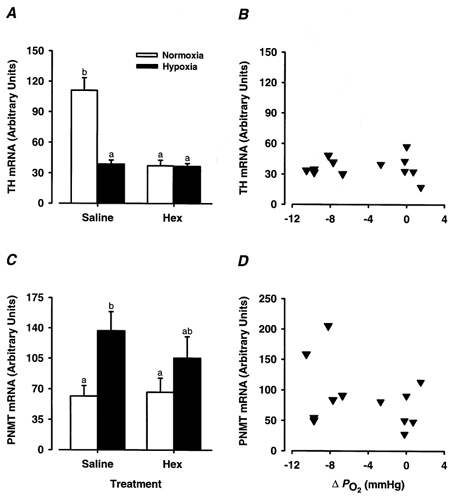
Adrenal TH (A) and PNMT mRNA (C) expression in 129-144 days fetal sheep following exposure to experimental hypoxia (filled columns, 3-5 h post hypoxia) or normoxia (open columns) following the administration of either hexamethonium (25 mg kg−1) or saline. Different superscript letters denote significant differences (P < 0.05) in the average adrenal expression of TH mRNA or PNMT mRNA between groups. The right-hand panel shows the expression of adrenal TH mRNA (B) and PNMT mRNA (D) plotted against the experimentally induced changes in arterial PO2 (ΔPO2) for hexamethonium-treated 129-144 days fetal sheep.
After normoxia, adrenal PNMT mRNA levels were the same in fetuses which were or were not pretreated with hexamethonium (Fig. 5C). There was no significant increase, however, in PNMT mRNA levels at 3-5 h after hypoxia in fetuses pretreated with hexamethonium. There was also no relationship between adrenal PNMT mRNA expression at 3-5 h post-normoxia or hypoxia and the experimentally induced changes in fetal arterial PO2 during normoxia or hypoxia in fetal sheep pretreated with hexamethonium (Fig. 5D).
DISCUSSION
We have demonstrated that adrenal TH mRNA but not PNMT mRNA expression is maintained by nicotinic stimulation in the late gestation sheep fetus. In late gestation (< 125 days) acute hypoxia suppresses adrenal TH mRNA and increases PNMT mRNA expression and these responses are not present after pretreatment with the nicotinic antagonist hexamethonium. Interestingly, acute hypoxia also suppresses TH mRNA and increases PNMT mRNA expression in the fetal adrenal gland before the development of adrenal innervation (< 105 days gestation). These changes in the level of expression of key catecholamine biosynthetic enzymes may have important physiological and clinical consequences for the fetus exposed to acute or repeated episodes of hypoxia in early or late gestation.
Basal TH mRNA expression and adrenal innervation
We have previously reported that there is an increase in TH mRNA expression in the fetal sheep adrenal gland at around the time of adrenal innervation, i.e. at 125 days gestation (Adams et al. 1998). Interestingly in the present study, pretreatment of fetuses after 125 days gestation with the nicotinic antagonist, hexamethonium, resulted in a marked reduction in adrenal TH mRNA levels in normoxic fetuses. As fetal arterial blood gases remained within the normal physiological range during normoxia, this suggests that TH mRNA expression in the fetal adrenal gland may be maintained by tonic nicotinic stimulation during late gestation. In the adult adrenal medulla, muscarinic or nicotinic stimulation results in an increase in intracellular Ca2+ (O'Sullivan et al. 1989), activation of a range of second messengers including cyclic AMP, protein kinase A (PKA) and protein kinase C (PKC) and an upregulation of catecholamine synthetic enzyme gene expression (Terbush et al. 1988; Anderson et al. 1992; Icard-Liepkalns et al. 1992; Morita et al. 1995; Hwang et al. 1997). Thus in the adult adrenal gland, there is a direct coupling of catecholamine secretion and synthesis. In PC12 and bovine adrenomedullary chromaffin cells, basal TH mRNA expression has also been shown to be dependent on a cyclic AMP/PKA-dependent pathway acting via a cyclic AMP response element (CRE) on the TH gene promoter (Kim et al. 1993; Hwang et al. 1997). It is possible therefore that in the fetal sheep in vivo tonic nicotinic stimulation acts through raised intracellular cAMP levels to stimulate TH gene expression in the adrenal medulla.
Adrenal TH mRNA expression after acute hypoxia and the impact of adrenal innervation
Several studies have previously shown that circulating catecholamines increase in response to hypoxia and/or asphyxia in either acutely exteriorised fetal sheep or in fetal sheep in utero (Comline & Silver, 1961; Jones et al. 1988; Cheung, 1990). Plasma noradrenaline and adrenaline responses to acute hypoxia are virtually abolished after adrenal demedullation in the sheep fetus and therefore the predominant source of circulating catecholamines during hypoxia is the fetal adrenal medulla (Jones et al. 1988). In the present study, plasma noradrenaline concentrations increased in response to acute hypoxia after 129 days gestation and this response was abolished by pretreatment with the nicotinic antagonist hexamethonium, confirming that the noradrenaline response is neurogenic in origin. We have also found that in these older fetuses, there was a suppression of adrenal TH mRNA levels from as early as 3 h and for up to 12 h post-hypoxia. Whilst it is possible that hypoxia may have stimulated a rise in TH mRNA expression at a time point earlier than 3-5 h, this is unlikely given that TH mRNA is relatively stable with a half-life of around 10 h (Czyzyk-Krzeska et al. 1994b; Czyzyk-Krzeska, 1997). As TH mRNA stability is also increased during hypoxia by the binding of hypoxia-induced protein to stability elements in the 3′ untranslated region of the TH mRNA (Czyzyk-Krzeska et al. 1994a, 1997), it is probable that any reduction in TH mRNA expression post-hypoxia is due to decreased TH gene transcription.
There was a complex relationship between the adrenal TH mRNA levels measured after normoxia or hypoxia and the average change which occurred in fetal arterial PO2 during normoxia or hypoxia, but there was a clear decrease in TH mRNA levels as arterial PO2 fell by up to 7 mmHg. Interestingly, pretreatment of the older fetuses with the nicotinic antagonist hexamethonium resulted in the loss of this relationship. This may, however, have been due to the effect of hexamethonium pretreatment on basal TH mRNA expression, i.e. basal TH mRNA levels may have been suppressed to a point where a further hypoxia-related decrease was not possible. Thus the suppressive effect of hypoxia on adrenal TH mRNA levels in the older animals might require the prior activation of TH gene expression by tonic neurogenic stimulation in vivo.
We have also demonstrated that hypoxia stimulated an increase in circulating noradrenaline concentrations and suppressed adrenal TH mRNA levels in younger fetuses prior to the development of adrenal innervation. Synapses between preganglionic nerve terminals and the chromaffin cells of the fetal sheep adrenal gland develop between 100 and 130 days gestation and adrenal innervation is not fully functional until around 125 days gestation (Comline & Silver, 1961; Boshier et al. 1989). In the younger fetuses, the suppression of adrenal TH mRNA levels occurred later than in the older group, i.e. at some 20 h after the onset of hypoxia, and the relationship between the average changes in arterial PO2 and TH mRNA was different in the two age groups. Nevertheless it is possible that there are common intracellular mechanisms which result in the suppression of TH mRNA levels by hypoxia before and after the development of adrenal innervation.
Hypoxic regulation of adrenal TH mRNA expression
Oxygen-sensitive potassium channels are present on fetal sheep and neonatal rat adrenomedullary chromaffin cells and on PC12 cells and these channels may mediate the non-neurogenic catecholamine secretory response to hypoxia (Zhu et al. 1996; Thompson et al. 1997; Rychkov et al. 1998). The closing of these oxygen-sensitive channels in response to hypoxia results in a depolarisation of the chromaffin cells and opening of voltage-sensitive calcium channels (Thompson et al. 1997). In the isolated perfused adrenal gland, hypoxia stimulates catecholamine secretion directly by a mechanism dependent on voltage-sensitive Ca2+ channels (Adams et al. 1996). Thus both non-neurogenic and neurogenic stimulation of the fetal adrenal gland could result in an influx of Ca2+ into the chromaffin cell and a potential activation of a number of second messenger systems including isoforms of PKC, the Ca2+-calmodulin-dependent protein kinases, and PKA via Ca2+-calmodulin-sensitive adenylate cyclase. Stimulation of cAMP synthesis in the adrenal gland and PC12 cells can induce the expression of an inducible cAMP early represser (ICER) which binds to the TH CRE to repress the activity of the TH gene promoter and inhibit induction by PKA (Molina et al. 1993; Tinti et al. 1996). Acute hypoxia may preferentially induce ICER expression in the fetal sheep adrenal gland and thus lead to a decrease in adrenal TH mRNA expression via either non-neurogenic or neurogenic pathways.
Hypoxia can stimulate TH gene transcription in PC12 cells by a calcium-dependent mechanism which involves the induction and binding of hypoxia inducible factor-1 and activator protein-1 (AP-1) transcription factors to regulatory elements on the TH gene (Icard-Liepkalns et al. 1992; Norris & Millhorn, 1995; Raymond & Millhorn, 1997). The temporal pattern of induction of the various AP-1 transcription factors, such as the Fos and Jun proteins, appears to determine whether there is ultimately a positive or negative effect on TH gene expression (Ginzang-Ginsberg & Ziff, 1994). Alternatively, Fos proteins in conjunction with c-jun, have been demonstrated to function as transrepressers of TH gene transcription through their interaction with the AP-1 site of the TH ‘fat’ specific element, which is an important regulator of basal activity (Ginzang-Ginsberg & Ziff, 1994).
In contrast to these studies in the sheep fetus, acute hypoxia in the rat does not alter adrenal TH gene expression in vivo either before the development of adrenal innervation, in the 1-day-old neonate, or after innervation, in the adult (Czyzyk-Krzeska et al. 1992; DeCristofaro & LaGamma, 1994). Arterial PO2 is of course higher in postnatal than in fetal life, and it may be that there is a PO2 response threshold for the hypoxia-induced changes in adrenal TH gene expression which is more likely to be reached in utero (Czyzyk-Krzeska et al. 1992; DeCristofaro & LaGamma, 1994).
We have also previously shown that adrenal TH mRNA levels are not inhibited or stimulated by chronic hypoxia in sheep in which the growth and development of the placenta was experimentally restricted throughout gestation (Adams et al. 1998). In contrast, Holgert and colleagues (1995) reported that adrenal TH mRNA expression was increased in fetal rats at E21 after 48 h of maternal hypoxia. Thus the effects of hypoxia on the expression of adrenal TH mRNA appears to be dependent on the length of the hypoxic episode, the degree of hypoxia and the timing of the insult in relation to the development of adrenal innervation. The hypoxia-induced suppression of adrenal TH expression may be clinically relevant, as it would lead to an impairment of a subsequent noradrenaline response to repeated episodes of intrauterine hypoxia such as that experienced by the fetus during labour and delivery.
Adrenal PNMT mRNA expression after acute hypoxia and the impact of cholinergic innervation
In contrast to TH, adrenal PNMT mRNA levels were not suppressed by hexamethonium pretreatment in the older fetuses exposed to normoxia. This suggests that basal PNMT mRNA levels are not maintained by tonic splanchnic stimulation acting via nicotinic mechanisms in the late gestation sheep fetus. Alternatively, there is strong evidence to suggest that muscarinic pathways may also stimulate PNMT gene expression in the chromaffin cells of a number of species (Evinger et al. 1994; Morita et al.1996). In the older fetuses, hypoxia stimulated PNMT mRNA expression in the fetal adrenal and there was a direct relationship between the PNMT mRNA levels and the fall in fetal arterial PO2 levels. Hexamethonium pretreatment completely abolished the PNMT mRNA response to hypoxia, which indicates that this response is dependent on nicotinic stimulation of the adrenaline synthesising cells of the fetal adrenal. In studies on the adult rat adrenal gland, the transcription factor Egr-1 has been implicated in the neural stimulation of adrenal PNMT gene expression. Splanchnic nerve stimulation induces Egr-1 expression and there is a functional Egr-1 binding site in the promoter region of the PNMT gene (Ebert & Wong, 1995; Morita et al. 1996). Whilst pretreatment with hexamethonium completely abolished the stimulatory effect of hypoxia on adrenal PNMT mRNA levels in the older fetuses, it did not completely abolish the plasma adrenaline response to hypoxia. One possibility is that the residual circulatory adrenaline response after hexamethonium pretreatment may be derived from sources other than the adrenal medulla, such as the C1 neurones which project from the ventrolateral medulla of the brainstem (Walker & Schuijers, 1989).
Interestingly, hypoxia also stimulated adrenal PNMT mRNA expression in the sheep fetus prior to the development of adrenal innervation. This finding suggests that PNMT gene expression may be directly regulated by oxygen availability. Whilst the regulation of TH gene expression by oxygen has been intensely studied due to its potential role in the hypoxic response of the carotid body type I glomus cells (Czyzyk-Krzeska et al. 1992; Czyzyk-Krzeska, 1997) little is known about the regulation of PNMT gene expression by oxygen. The increase in PNMT mRNA levels observed in the current study was sustained for up to 12 h post hypoxia and was quantifiably related to the fall in arterial PO2 during the hypoxic episode. It is unlikely that this increase in PNMT mRNA levels is related to an increase in fetal glucocorticoid concentrations, as the fetal sheep adrenal gland is relatively unresponsive to ACTH stimulation at 95-105 days gestation (Challis & Brooks, 1989). A second possibility is that hypoxia acts via the oxygen-sensitive potassium channels to depolarise the adrenaline-synthesising cells and activate second messenger systems, including PKC. Activation of PKC stimulates PNMT mRNA expression by a mechanism involving Egr-1 binding to the PNMT gene promoter region (Morita et al. 1995). PKC has also been implicated in the cellular adaptations of PC12 cells to hypoxia (Raymond & Millhorn, 1997). Stimulation of PNMT mRNA expression by hypoxia before and after the development of adrenal innervation may therefore utilise a final common pathway such as the stimulation of Egr-1 expression within the chromaffin cell.
It is intriguing that in the older fetal sheep, there is no residual non-neurogenic activation of PNMT mRNA expression by hypoxia after hexamethonium pretreatment. In the fetal sheep and neonatal rat, there is also a disappearance of the non-neurogenic stimulation of catecholamine secretion by hypoxia, after the development of functional splanchnic innervation in vivo (Comline & Silver, 1961; Seidler & Slotkin, 1986; Cheung, 1990). The lack of catecholamine synthetic and secretory responses to hypoxia after hexamethonium pretreatment in the late gestation sheep fetus suggests that non-neurogenic adrenal responses to hypoxia may be dependent on intracellular mechanisms which are inhibited by non-cholinergic components of adrenal innervation.
During labour, fetal plasma concentrations of catecholamines are often markedly increased (Padbury et al. 1982) in response to a number of events including contraction-induced reductions in uterine blood flow and umbilical cord and fetal compression (Giussani et al. 1994). Given the key role of adrenaline in lung maturation and in the metabolic response to neonatal hypoglycaemia (Padbury et al. 1984, 1987; Sperling et al. 1984), an increase in adrenal PNMT mRNA levels may be critically important in maintaining an adrenaline synthetic and secretory capacity in the transition from fetal to neonatal life.
Conclusions
Our results demonstrate that adrenal TH mRNA but not PNMT mRNA expression is maintained by nicotinic stimulation in the late gestation sheep fetus. Acute hypoxia in vivo decreases TH mRNA and increases PNMT mRNA expression in fetal sheep both before and after the development of adrenal innervation. We suggest therefore that there may be common intracellular mechanisms which underlie the neurogenic and non-neurogenic responses of fetal adrenomedullary chromaffin cells to hypoxia. Arguably the effects of acute hypoxia on adrenal catecholamine synthesis and secretion may be more important in fetal life than at any subsequent time given the prevailing low fetal arterial PO2 and the potential exposure of the fetus to further falls in oxygenation throughout gestation, labour and delivery. Further work is required to integrate our current understanding of the molecular basis of the responses of isolated adrenomedullary chromaffin cells to hypoxia and the physiological responses of the fetal adrenal medulla in vivo to intrauterine hypoxia during late gestation.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the Australian Research Council for the conduct of these studies.
References
- Adams MB, Phillips ID, Simonetta G, McMillen IC. Differential effects of increasing gestational age and placental restriction on tyrosine hydroxylase, phenylethanolamine N-methyltransferase, and proenkephalin A mRNA levels in the fetal sheep adrenal. Journal of Neurochemistry. 1998;71:394–401. doi: 10.1046/j.1471-4159.1998.71010394.x. [DOI] [PubMed] [Google Scholar]
- Adams MB, Simonetta G, McMillen IC. The non-neurogenic catecholamine response of the fetal adrenal to hypoxia is dependent on activation of voltage sensitive Ca2+ channels. Brain Research Developmental Brain Research. 1996;94:182–189. doi: 10.1016/0165-3806(96)00054-5. [DOI] [PubMed] [Google Scholar]
- Anderson K, Robinson PJ, Marley PD. Cholinoceptor regulation of cyclic AMP levels in bovine adrenal medullary cells. British Journal of Pharmacology. 1992;106:360–366. doi: 10.1111/j.1476-5381.1992.tb14341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshier DP, Gavin CB, Holloway H. Morphometric analyses of adrenal gland growth in fetal and neonatal sheep II. The adrenal medulla with some observations on its ultrastructure. Journal of Anatomy. 1989;167:15–30. [PMC free article] [PubMed] [Google Scholar]
- Brace RA, Brittingham DS. Fetal vascular pressure and heart rate responses to non-labour uterine contractions. American Journal of Physiology. 1986;251:R409–416. doi: 10.1152/ajpregu.1986.251.2.R409. [DOI] [PubMed] [Google Scholar]
- Bygdeman S, von Euler U. Resynthesis of catechol hormones in the cat's adrenal medulla. Acta Physiologica Scandanavica. 1958;44:375–383. doi: 10.1111/j.1748-1716.1958.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Brooks AN. Maturation and activation of hypothalamic-pituitary-adrenal function in fetal sheep. Endocrine Reviews. 1989;10:182–204. doi: 10.1210/edrv-10-2-182. [DOI] [PubMed] [Google Scholar]
- Chan Y-L, Gutell R, Noller HF, Wool IG. The nucleotide sequence of a rat 18 S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18 S ribosomal ribonucleic acid. Journal of Biological Chemistry. 1984;259:224–230. [PubMed] [Google Scholar]
- Cheung CY. Fetal adrenal medulla catecholamine response to hypoxia – direct and neural components. American Journal of Physiology. 1990;258:R1340–1346. doi: 10.1152/ajpregu.1990.258.6.R1340. [DOI] [PubMed] [Google Scholar]
- Comline RS, Silver M. The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. Journal of Physiology. 1961;156:424–444. doi: 10.1113/jphysiol.1961.sp006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF. Molecular aspects of oxygen sensing in physiological adaption to hypoxia. Respiration Physiology. 1997;110:99–111. doi: 10.1016/s0034-5687(97)00076-5. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Bayliss DA, Lawson EE, Millhorn DE. Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. Journal of Neurochemistry. 1992;58:1538–1546. doi: 10.1111/j.1471-4159.1992.tb11376.x. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Dominski Z, Kole R, Millhorn DE. Hypoxia stimulates binding of a cytoplasmic protein to a pyrimidine-rich sequence in the 3′-untranslated region of rat tyrosine hydroxylase mRNA. Journal of Biological Chemistry. 1994a;269:9940–9945. [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Furnari BA, Lawson EE, Millhorn DE. Hypoxia increases rate of transcription and stability of tyrosine hydroxylase mRNA in pheochromocytoma (PC12) cells. Journal of Biological Chemistry. 1994b;269:760–764. [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Paulding WR, Beresh JE, Kroll SL. Post-transcriptional regulation of tyrosine hydroxylase gene expression by oxygen in PC12 cells. Kidney International. 1997;51:585–590. doi: 10.1038/ki.1997.84. [DOI] [PubMed] [Google Scholar]
- Decristofaro JD, Lagamma EF. Neonatal stress: effects of hypoglycemia and hypoxia on adrenal tyrosine hydroxylase gene expression. Pediatric Research. 1994;36:719–723. doi: 10.1203/00006450-199412000-00006. [DOI] [PubMed] [Google Scholar]
- D'Mello SR, Weisberg EP, Stachowiak MK, Turzai LM, Gioio AM, Kaplan BB. Isolation and nucleotide sequence of a cdna clone encoding bovine adrenal tyrosine hydroxylase: comparative analysis of tyrosine hydroxylase gene products. Journal of Neuroscience Research. 1988;19:440–449. doi: 10.1002/jnr.490190408. [DOI] [PubMed] [Google Scholar]
- Ebert SN, Wong DL. Differential activation of the rat phenylethanolamine N-methyltransferase gene by Sp1 and Egr-1. Journal of Biological Chemistry. 1995;270:17299–17305. doi: 10.1074/jbc.270.29.17299. [DOI] [PubMed] [Google Scholar]
- Evinger MJ, Ernsberger P, Regunathan S, Joh TH, Reis DJ. A single transmitter regulates gene expression through two separate mechanisms: cholinergic regulation of phenylethanolamine N-methyltransferase mRNA via nicotinic and muscarinic pathways. Journal of Neuroscience. 1994;14:2106–2116. doi: 10.1523/JNEUROSCI.14-04-02106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluharty SJ, Snyder GL, Zigmond MJ, Stricker EM. Tyrosine hydroxylase activity and catecholamine biosynthesis in the adrenal medulla of rats during stress. Journal of Pharmacology and Experimental Therapeutics. 1985;233:32–38. [PubMed] [Google Scholar]
- Giussani DA, Spencer JA D, Hanson MA. Fetal cardiovascular reflex responses to hypoxaemia. Fetal and Maternal Medicine Reviews. 1994;6:17–37. [Google Scholar]
- Gizang-Ginsberg E, Ziff EB. Fos family members successively occupy the tyrosine hydroxylase gene AP-1 site after nerve growth factor or epidermal growth factor stimulation and can repress transcription. Molecular Endocrinology. 1994;8:249–262. doi: 10.1210/mend.8.2.7909583. [DOI] [PubMed] [Google Scholar]
- Holgert H, Pequignot J-M, Lagercrantz H, Hokfelt T. Birth-related up-regulation of mRNA encoding tyrosine hydroxylase, dopamine β-hydroxylase, neuropeptide tyrosine, and prepro-enkephalin in rat adrenal medulla is dependent on postnatal oxygenation. Pediatric Research. 1995;37:701–706. doi: 10.1203/00006450-199506000-00005. [DOI] [PubMed] [Google Scholar]
- Hwang O, Park SY, Kim KS. Protein kinase A coordinately regulates both basal expression and cyclic AMP-mediated induction of three catecholamine synthesizing enzyme genes. Journal of Neurochemistry. 1997;68:2241–2247. doi: 10.1046/j.1471-4159.1997.68062241.x. [DOI] [PubMed] [Google Scholar]
- Icard-Liepkalns C, Biguet NF, Vyas S, Robert JJ, Sassone-Corsi P, Mallet J. AP-1 complex and c-fos transcription are involved in TPA provoked and transynaptic inductions of the tyrosine hydroxylase gene: insights into long term regulatory mechanisms. Journal of Neuroscience Research. 1992;32:290–298. doi: 10.1002/jnr.490320219. [DOI] [PubMed] [Google Scholar]
- Jones CT, Robinson RO. Plasma catecholamines in foetal and adult sheep. Journal of Physiology. 1975;248:15–33. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CT, Roebuck MM, Walker DW, Johnston BM. The role of the adrenal medulla and peripheral sympathetic nerves in the physiological response to hypoxia. Journal of Developmental Physiology. 1988;10:17–36. [PubMed] [Google Scholar]
- Kim KS, Lee MK, Caroll J, Joh TH. Both basal and inducible transcription of the tyrosine hydroxylase gene are dependent upon a cAMP response element. Journal of Biological Chemistry. 1993;268:15689–15695. [PubMed] [Google Scholar]
- Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- Morita K, Bell RA, Siddall BJ, Wong DL. Neural stimulation of egr-1 messenger RNA expression in the rat adrenal gland: possible relation to phenyethanolamine N-methyltransferase gene regulation. Journal of Pharmacology and Experimental Therapeutics. 1996;279:379–385. [PubMed] [Google Scholar]
- Morita K, Ebert SN, Wong DL. Role of transcription factor egr-1 in phorbol ester-induced phenylethanolamine N-methyltransferase gene expression. Journal of Biological Chemistry. 1995;19:11161–11167. doi: 10.1074/jbc.270.19.11161. [DOI] [PubMed] [Google Scholar]
- Norris ML, Millhorn DE. Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. Journal of Biological Chemistry. 1995;270:23774–23779. doi: 10.1074/jbc.270.40.23774. [DOI] [PubMed] [Google Scholar]
- O'Sullivan AJ, Cheek TR, Moreton RB, Berridge MJ, Burgoyne RD. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO Journal. 1989;8:401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padbury J, Agata Y, Ludlow J, Ikegami M, Baylen B, Humme J. Effect of fetal adrenalectomy on catecholamine release and physiologic adaption at birth in sheep. Journal of Clinical Investigation. 1987;80:1096–1103. doi: 10.1172/JCI113166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padbury JF, Jacobs HC, Lam RW, Conaway D, Jobe AH, Fisher DA. Adrenal epinephrine and the regulation of pulmonary surfactant release in neonatal rabbits. Experimental Lung Research. 1984;7:177–186. doi: 10.3109/01902148409087911. [DOI] [PubMed] [Google Scholar]
- Padbury JF, Roberman B, Oddie TH, Hobel CJ, Fisher DA. Fetal catecholamine release in response to labor and delivery. Obstetrics and Gynecology. 1982;60:607–611. [PubMed] [Google Scholar]
- Raymond R, Millhorn D. Regulation of tyrosine hydroxylase gene expression during hypoxia: The role of Ca2+ and PKC. Kidney International. 1997;51:536–541. doi: 10.1038/ki.1997.74. [DOI] [PubMed] [Google Scholar]
- Robinson JS, Kingston EJ, Jones CT, Thorburn GD. Studies on experimental growth retardation in sheep. The effect of removal of endometrial caruncles on fetal size and metabolism. Journal of Developmental Physiology. 1979;1:379–398. [PubMed] [Google Scholar]
- Rychkov GY, Adams MB, McMillen IC, Roberts ML. Oxygen-sensing mechanisms are present in the chromaffin cells of the sheep adrenal medulla before birth. Journal of Physiology. 1998;509:887–893. doi: 10.1111/j.1469-7793.1998.887bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Ontogeny of adrenomedullary responses to hypoxia and hypoglycaemia: role of splanchnic innervation. Brain Research Bulletin. 1986;16:11–14. doi: 10.1016/0361-9230(86)90005-5. [DOI] [PubMed] [Google Scholar]
- Sperling MA, Ganguli S, Leslie N, Landt K. Fetal-perinatal catecholamine secretion: role in perinatal glucose homeostasis. American Journal of Physiology. 1984;247:E69–74. doi: 10.1152/ajpendo.1984.247.1.E69. [DOI] [PubMed] [Google Scholar]
- Terbush DR, Bittner MA, Holz RW. Ca2+ influx causes rapid translocation of protein kinase C to membranes. Studies of the effects of secretagogues in adrenal chromaffin cells. Journal of Biological Chemistry. 1988;263:18873–18879. [PubMed] [Google Scholar]
- Thompson RJ, Jackson A, Nurse CA. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. Journal of Physiology. 1997;498:503–510. doi: 10.1113/jphysiol.1997.sp021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinti C, Conti B, Cubells JF, Kim K-S, Baker H, Joh TH. Inducible cAMP early repressor can modulate tyrosine hydroxylase gene expression after stimulation of cAMP synthesis. Journal of Biological Chemistry. 1996;271:25375–25381. doi: 10.1074/jbc.271.41.25375. [DOI] [PubMed] [Google Scholar]
- Wakade AR, Wakade TD, Malhotra RK. Restoration of catecholamine content of previously depleted adrenal medulla in vitro: Importance of synthesis in maintaining the catecholamine stores. Journal of Neurochemistry. 1988;51:820–829. doi: 10.1111/j.1471-4159.1988.tb01817.x. [DOI] [PubMed] [Google Scholar]
- Walker DW, Schuijers JA. Effect of thyroidectomy on cardiovascular responses to hypoxia and tyramine infusion in fetal sheep. Journal of Developmental Physiology. 1989;12:337–345. [PubMed] [Google Scholar]
- Wan DC-C, Scanlon D, Choi C-L, Bunn SJ, Howe PRC, Livett BG. Co-localization of RNAs coding for phenylethanolamine N-methyltransferase and proenkephalin A in bovine and ovine adrenals. Journal of the Autonomic Nervous System. 1989;26:231–240. doi: 10.1016/0165-1838(89)90172-0. [DOI] [PubMed] [Google Scholar]
- Widmark C, Hokegard K-H, Lagercrantz H, Lilja H, Rosen KG. Electrocardiographic waveform changes and catecholamine responses during acute hypoxia in the immature and mature fetal lamb. American Journal of Obstetrics and Gynecology. 1989;160:1245–1250. doi: 10.1016/0002-9378(89)90204-4. [DOI] [PubMed] [Google Scholar]
- Wong DL, Bildstein CL, Siddall B, Lesage A, Yoo YS. Neural regulation of phenylethanolamine N-methyltransferase in vivo: transcriptional and translational changes. Brain Research Molecular Brain Research. 1993;18:107–114. doi: 10.1016/0169-328x(93)90178-r. [DOI] [PubMed] [Google Scholar]
- Zhu WH, Conforti L, Czyzyk-Krzeska MF, Millhorn DE. Membrane sensitive depolarization in PC-12 cells during hypoxia is regulated by an O2-sensitive K+ current. American Journal of Physiology. 1996;271:C658–665. doi: 10.1152/ajpcell.1996.271.2.C658. [DOI] [PubMed] [Google Scholar]


