Abstract
Time-dependent changes in nicotinic acetylcholine receptor (nAChR) function were studied in acutely isolated medial habenula neurons during whole-cell perfusion.
The peak amplitude of inward currents induced by 1 s pulses of nicotinic agonists, applied at 30 s intervals, gradually increased over the first several minutes of whole-cell recording. The ratio of response amplitudes at 1 and 15 min (t15/t1) was 1.9. Run-up of responses occurred independently of channel activation and was specific to nAChRs.
The channel blocker chlorisondamine (30 μM), co-applied with nicotine, was used to irreversibly block the majority (91 %) of the nAChRs that opened in the first 2 min of recording. Run-up in the remaining 9 % unblocked channels assessed at 15 min (t15/t2= 3.4) was similar to that in control cells not exposed to nicotine and chlorisondamine simultaneously, implying that run-up is not due to the incorporation of new receptors.
A marked alteration in the sensitivity of nAChRs to extracellular Ca2+ was also observed during whole-cell perfusion. The ratio of current amplitudes obtained in 0.2 and 4.0 mM Ca2+ changed from 0.54 (t= 5 min) to 0.82 (t= 30 min).
Inward rectification of nicotine-induced responses was reduced during internal dialysis. Voltages for half-maximal conductance were -23.0 and -13.8 mV at 2 and 15 min, respectively. Inclusion of either free Mg2+ (∼2 mM) or spermine (100 μM) in the internal solution counteracted the change in rectification, but did not prevent run-up.
The period of run-up was followed by a use-dependent run-down phase. Little run-down in peak current amplitude was induced provided that agonist was applied infrequently (5 min intervals), whereas applications at 30 s intervals produced a loss of channel function after ∼15 min whole-cell perfusion. The time at which run-down began (∼5-30 min) was correlated with the initial rate of nAChR desensitization (∼200-4000 ms); slowly desensitizing nicotinic currents demonstrated delayed run-down.
We suggest that run-up of nAChR-mediated responses does not require receptor activation and may result from a change in channel open probability. We also hypothesize that channel run-down reflects accumulation of nAChRs in long-lived desensitized/inactivated states.
Neuronal nicotinic acetylcholine receptors (nAChRs) are a molecularly diverse group of ion channels localized to pre- and postsynaptic sites in the brain (Sargent, 1993; McGehee & Role, 1995; Lindstrom et al. 1996; Role & Berg, 1996; Colquhoun & Patrick, 1997; Wonnacott, 1997; Jones et al. 1999). In addition to conventional roles in synaptic transmission, nAChRs are implicated in certain forms of plasticity by virtue of their relatively high Ca2+ permeability (Mulle et al. 1992a; Vernino et al. 1992; Lena & Changeux, 1997; Radcliffe & Dani, 1998). nAChRs are themselves amenable to both short- and long-term cellular regulation, although the extent of such modulation is at present unclear (Swope et al. 1992; Role & Berg, 1996). For a complete understanding of the influence of nAChRs at synapses, knowledge of how the function of these channels is controlled will be essential.
The extracellular environment, in particular the local concentration of Ca2+, has marked short-term effects on nAChR properties. Elevation of Ca2+ levels can increase the channel open probability (Popen) severalfold (Mulle et al. 1992b; Vernino et al. 1992) and can change the contribution of synaptically induced nicotinic currents to signalling in sympathetic neurons (Amador & Dani, 1995). The peptide transmitters, substance P and vasoactive intestinal peptide (VIP), affect the desensitization properties and increase peak currents through ganglionic nAChRs, respectively, via activation of various second messengers (Role, 1984; Downing & Role, 1987; Gurantz et al. 1994; Cuevas & Adams, 1996). The latter result may involve changes in the number of functional channels on the cell surface (Margiotta et al. 1987), and therefore provides a mechanism for long-term alteration of neuronal excitability. In the CNS, these types of receptor modulation may be relevant in cognitive processing (Levin & Simon, 1998) and could contribute to deficits in nAChR number and function that occur during nicotine addiction (Marks et al. 1983) and Alzheimer’s disease (Norberg, 1994).
Extensive use of the whole-cell patch-clamp recording technique has led to a greater understanding of intracellular control of ion channel function (Horn & Korn, 1992). By allowing exchange of the contents of the patch pipette and the intracellular milieu, and by permitting the selective introduction of various molecules into the cytoplasm, there have been remarkable insights into the internal regulation of voltage-gated ion channels, e.g. Ca2+ channels (Chad & Eckert, 1986), and ligand-gated ion channels, e.g. N-methyl-D-aspartate (NMDA) receptors (MacDonald et al. 1989; Rosenmund & Westbrook, 1993) and neuronal nAChRs (Liu & Berg, 1999). The present study has examined the modulation of nAChRs during whole-cell and perforated-patch recordings from medial habenula (MHb) neurons (McCormick & Prince, 1987; Mulle & Changeux, 1990; Lester & Dani, 1995a). In the present study, we show that the intracellular environment is important for regulation of the function of nAChRs including their ability to sense changes to extracellular Ca2+. Preliminary reports of this work have appeared in abstract form (Lester & Dani, 1995b; Hicks & Lester, 1999).
METHODS
Acute isolation of medial habenula neurons
Neurons were isolated from the habenula nuclei of 10- to 25-day-old rats using a method described previously (Lester & Dani, 1995a). In accordance with national guidelines, a single rat was anaesthetized using methoxyfluorane or halothane and decapitated. Following removal of the brain, both habenula nuclei (with as little underlying tissue as possible) were dissected in ice-cold Pipes buffer containing (mM): NaCl, 120; KCl, 5; MgCl2, 5; Pipes, 25; D-glucose, 25; pH 7.4. They were then immediately placed into 10 ml Pipes buffer containing papain (40-100 U; Worthington Biochemical Corporation, Freehold, NJ, USA and Sigma Chemical Co., St Louis, MO, USA), bovine serum albumin (10 mg) and cysteine (2 mg) and incubated at 37°C for 25-40 min. During incubation the tissue was constantly agitated using a gentle rocking motion. The tissue was washed 3 times with fresh Pipes buffer and transferred into Hepes-buffered, low-glucose Dulbecco’s modified Eagle’s medium (Gibco BRL, Grand Island, NY, USA) containing ∼1 mg ml−1 each of bovine serum albumin and trypsin inhibitor. Neurons were dissociated by gentle trituration with a fire-polished Pasteur pipette and plated onto sterile glass coverslips coated with 2-4 μg ml−1 poly-D-lysine (Collaborative Research, Bedford, MA, USA). Neurons were maintained at 37°C for at least 1 h and used up to 10 h following isolation.
Whole-cell patch-clamp recordings and analysis
Cells were transferred to a recording chamber mounted on the stage of an inverted microscope and continuously superfused with a control medium (mM): NaCl, 150 or sodium methanesulphonate, 150; KCl, 0 or 3; CaCl2, 2; Hepes, 10; pH 7.4. Recordings were restricted to small neurons (10-15 μm cell body diameter) with morphological characteristics similar to those of identified MHb neurons in brain slices (Quick et al. 1999). This may have biased the sampled cells to a subpopulation of MHb neurons but ensured a high success rate of finding cells that responded robustly to nicotine. Whole-cell recordings were obtained using fire-polished, Sylgard-coated glass pipettes (i.d., 1.15 mm; o.d., 1.65 mm; no. 7052; Garner Glass, Clairmont, CA, USA) filled in the majority of experiments with an internal solution containing (mM): sodium methanesulphonate, 140 or caesium methanesulphonate, 120; NaCl, 10; Hepes, 10; EGTA, 10 (control intracellular solution). In some experiments either MgCl2 (5 mM; estimated free Mg2+∼2 mM) or CaCl2 (1.1 or 6.3 mM; estimated free Ca2+∼30 and 100 nM, respectively) was added to these solutions to alter the level of divalent ions. In order to buffer internal Ca2+ more effectively some solutions contained (mM): caesium methanesulphonate, 90; CsCl, 50; Cs-BAPTA, 10; Hepes, 10. Intracellular solutions were at pH 7.3-7.4 and had osmolalities ∼270-290 mosmol kg−1. Pipettes had initial resistances of 2-5 MΩ.
Perforated patch-clamp experiments were performed following the methods of Rae et al. (1991). In brief, 3 mg amphotericin B was dissolved in 50 μl DMSO; 4 μl of this solution was added to 1 ml intracellular solution giving a final amphotericin B concentration of 240 μg ml−1. This solution was triturated using a Pasteur pipette until frothy (2-3 min). The tip of a recording pipette was dipped for ∼1 s into normal intracellular solution and then back-filled with amphotericin B-containing solution. Following gigaseal formation, whole-cell access was estimated from the decrease in the estimated series resistance (Rs; see below). Within 5-10 min Rs values were generally in the range ∼20-25 MΩ. Only after Rs had stabilized below 30 MΩ (see Fig. 6A) were cells exposed to nicotinic agonists. Cells were constantly superfused with control solution whilst the whole-cell access was improving.
Figure 6. Run-up does not occur in perforated-patch recordings.
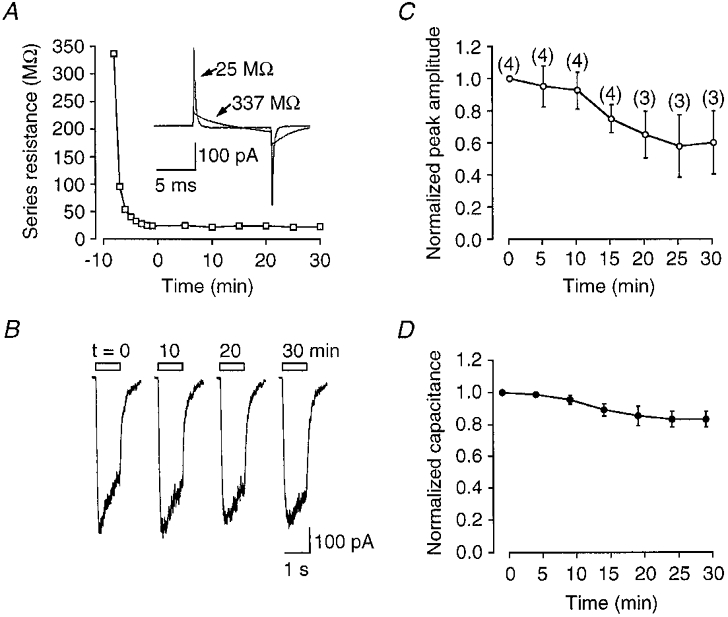
A, example of a typical decrease in Rs following formation of a cell-attached gigaohm seal with a patch pipette containing amphotericin B. Examples of capacitance currents resulting from 10 mV steps are shown in the inset. B, examples of currents evoked by nicotine (30 μM) at various times during the course of the experiment. C, peak nicotinic currents plotted with respect to time after Rs < 30 MΩ. The number of observations at each time point are shown in parentheses. D, plot of the change in whole-cell capacitance for the same group of cells over the same period of time. Data were corrected for Rs error using the off-line algorithm.
Nicotine-induced currents were recorded using either a Warner PC-501A (Warner Instruments, New Haven, CT, USA) or an Axopatch 1-D patch-clamp amplifier (Axon Instruments, Foster City, CA, USA), low-pass filtered at 200-500 Hz, digitized at 1 kHz and captured on an 80486-based computer using Axobasic software (Axon Instruments). For measurements of whole-cell capacitance and Rs, currents were low-pass filtered at 5-10 kHz and digitally sampled at 100 kHz. In order to assess changes in desensitization, a single exponential was fitted to the desensitizing phase of the agonist-induced current using a least-squares routine:
| (1) |
where It is the amplitude of the response at time, t, after the peak, Ip, and Iss is the steady-state (residual) current. The time constant of the relaxation is given by τ. If the fit produced a negative steady-state current, the data were rejected. In 129/130 cases (n = 19 cells) good exponential fits were obtained. In order to monitor changes in whole-cell capacitance and series resistance during the experiment, Rs was uncompensated. Whole-cell capacitance (C) was estimated on-line from the integral of the capacitive current (Ic): the total charge movement (Q) that resulted from a 10 mV voltage-step (V) using the expression C= Q/V. Series resistance, Rs, could be estimated from the time constant, τc, resulting from a single exponential fit to the decay of Ic using the equation τc=CRs. In 35 cells voltage errors due to Rs were reduced either by ∼80 % on-line Rs compensation or by using an off-line algorithm if Rs was uncompensated during the experiment (Traynelis, 1998; Quick et al. 1999). Briefly, currents induced by nicotine were corrected for the voltage error, Ve, resulting from an uncompensated Rs, using:
| (2) |
where Vh is the applied holding potential, Im is the measured current and Erev is the measured reversal potential for the nicotinic current. Except where Erev was determined in individual cells, Erev was set to +10 mV (see Fig. 6) and to +2 mV (n = 4) for cells recorded in NaCl- and sodium methanesulphonate-containing extracellular media, respectively. The differences in Erev values probably arose as a result of uncorrected junction potentials.
To assess changes in channel rectification, voltage ramps (300 mV s−1) over the range -90 to +60 mV were performed in the presence and absence of nicotine. In order to reduce any adverse effect of nAChR desensitization during the ramp, a low concentration of nicotine was used (10 μM) and the mean current was calculated from voltage ramps applied in both positive and negative directions. The conductance was calculated as:
| (3) |
The resulting conductance-voltage curve was fitted with a Boltzmann relation:
| (4) |
where Gmax is the maximum conductance (measured at -80 mV), q is the effective gating charge and V0.5 is the voltage at which the conductance is half-maximal.
Rapid application of agonists
Control and agonist-containing solutions were gravity fed into a linear array of glass barrels (i.d., 380 μm; o.d., 500 μm; Garner Glass) that were positioned close (∼100 μm) to the neuron. The barrels were attached to one of two types of rapid movement systems: (1) mounted on the horizontal axis of a 3-D manipulator that could be repositioned by a motorized driver (Newport, Irvine, CA, USA) under computer control; or (2) attached to a piezo-electric bimorph (Piezo Systems, Cambridge, MA, USA) driven to bend by a 50-100 V voltage supplied by a DC power supply also under computer control. The solution-exchange time of these systems has been previously estimated as ∼50 ms (Lester & Dani, 1995a). The rapid perfusion medium contained (mM): either NaCl, 150-155; Hepes, 10; glucose, 0 or 10; KCl, 0 or 3; or sodium methanesulphonate, 150; NaCl, 10; Hepes, 10; pH = 7.4 and osmolality ∼310-320 mosmol kg−1. There were no marked differences in the behaviour of nicotinic responses recorded using either the high or low Cl− extracellular media (but see Connolly et al. 1995), and results using these two solutions were pooled. CaCl2 or BaCl2 was added at a concentration of 0.2, 2 or 4 mM. In cases in which a single cell was exposed to agonist in different concentrations of Ca2+, a preincubation period of >10 s in control media containing the test concentration of Ca2+ was used. Acetylcholine chloride (ACh) and nicotine tartrate (nicotine) were diluted into this medium from frozen stock solutions (0.1-1 M). The pH was not readjusted after addition of nicotine, resulting in a slight decrease in pH by 0.15 units at the highest concentration (300 μM) of nicotine used. Unless otherwise stated, drugs and chemicals were obtained from Sigma Chemical Co. Chlorisondamine was a gift from Novartis Pharmaceuticals Corporation (Summit, NJ, USA).
Experimental design
In all experiments, agonists were applied for 0.5-2 s at specified intervals (generally 30 s or 5 min). All times are relative to the time that whole-cell access was achieved. For cell-to-cell comparison, data points were generally normalized to the peak nicotinic response at the 1 min time point. Cells were only included in the analysis if they had an initial amplitude > 50 pA and remained stable for >15 min. Stability was gauged as a steady holding current <-120 pA at -40 mV, and where measured, Rs did not exceed 30 MΩ and Ve was < 30 mV. The mean initial Rs was 9.2 ± 0.7 MΩ (n = 53) before compensation/correction. The data shown in Fig. 3 for 30 μM acetylcholine and 300 μM nicotine were obtained from cells from which data of a different nature have been published (Lester & Dani, 1995a). Data are expressed as means ±s.e.m. In order to assess significance across multiple data sets over time one- and two-way ANOVA with Tukey’s post hoc HSD test was performed. In addition Student’s two-tailed unpaired and paired t tests were performed on both raw and normalized data. Data sets were considered to be significantly different from each other if P < 0.05 (*) or P < 0.01 (**), as denoted in the figures and text.
Figure 3. Run-up is independent of the concentration or type of nicotinic agonist.

A, currents induced by 1 s applications of 300 μM nicotine (upper traces) or 30 μM acetylcholine (ACh; lower traces) at 1 min (left traces) and 5 min (right traces) after the start of whole-cell recording. B, bar chart comparing the extent of run-up during whole-cell perfusion on responses induced by 30 or 300 μM nicotine and 30 μM ACh. The data for 30 μM nicotine have been replotted from Fig. 1.
RESULTS
Receptor-specific run-up of nAChR responses
Data were analysed from 90 cells acutely isolated from the MHb region of rat brain. At negative holding potentials, ∼-40 mV, cells responded with inward currents to a brief application of nicotine (30 μM; 1 s). The peak amplitude of consecutive responses increased over the first several minutes of whole-cell recording during perfusion with control intracellular solutions (Fig. 1). The mean ratio of the peak currents measured at 1 and 15 min, I15/I1, was 1.9 ± 0.2 (n = 19). Because of uncompensated Rs the extent of this run-up is underestimated (see Methods). Although in this initial series of experiments Rs was not measured for each cell, an estimate of the error was made using the total mean Rs of 9.2 MΩ (n = 53 cells). Following application of eqn (2) to the peak nicotinic currents from each cell, the mean corrected increase in response amplitude at 15 min was estimated as 2.0-fold. Thus, run-up is underestimated by only ∼10 % if Rs is not taken into account. (A more detailed evaluation of the effects of uncompensated Rs is performed below in the section on altered extracellular Ca2+ sensitivity of nAChRs, where correction of Ve is much more critical.) Two other time-dependent changes in nicotine-induced response are immediately obvious, run-down of function and altered desensitization (Fig. 1). Both of these changes are discussed below.
Figure 1. Run-up of nicotinic currents during whole-cell perfusion of medial habenula neurons.

A, whole-cell currents induced by the rapid application of nicotine (30 μM; 1 s) at times indicated following initiation of the whole-cell recording configuration. B, plot of the peak current amplitudes with respect to time for the cell in A. In this cell responses were evoked every 30 s. C, time course of the mean changes in peak amplitude during whole-cell perfusion. Data were normalized to the response at 1 min. Error bars in this and all other figures show the s.e.m. and for clarity error bars are only shown at 5 min intervals. The number of observations at each time point are indicated in parentheses.
It is possible that run-up is due to non-specific changes (e.g. generalized to all membrane receptors) that occur in these neurons as a consequence of whole-cell recording. In a separate series of experiments, cells were exposed to pulses of nicotine (30 μM, 1 s) at 30 s intervals, and in addition to the glutamate receptor agonist kainate (50 μM, 1 s) at 5, 10, 15 and 20 min after commencement of whole-cell perfusion. Unlike nAChR-induced currents, responses to kainate did not run up (Fig. 2), but rather tended to run down (see Wang et al. 1994). In the same group of cells, there was no change in the measured series resistance (Rs). The uncompensated Rs values were 7.0 ± 1.5 and 7.3 ± 2.0 MΩ at 1 and 20 min, respectively. The cell capacitance decreased from 16.7 ± 2.2 to 13.4 ± 2.0 pF (n = 5; P < 0.05) over the same period, possibly implying some loss of cell membrane (also see below). While the effects of changes in cell volume were not systematically explored in this study, and therefore may have contributed to run-up, the decrease in cell capacitance (considered together with the relative osmolality of the intracellular solution) argues against a swelling-induced augmentation of nicotinic responses, as observed for other ligand-gated channels (see e.g. Paoletti & Ascher, 1994). On the other hand, both the decrease in kainate response and the membrane capacitance were of the same magnitude, ∼20 %, consistent with the idea that the run-down of kainate responses could be due to internalization of membrane and any associated glutamate receptors.
Figure 2. Run-up is specific to nicotinic receptors.

A, responses of a neuron to 1 s applications of 30 μM nicotine (upper traces) and 50 μM kainate (lower traces) at the times indicated after the start of whole-cell recording. B, the mean change in the peak amplitude of the nicotinic and kainate-induced currents at 1 min (open bars) and 20 min (hatched bars) recording time (n = 5 cells).
Run-up was also observed for responses induced by the neurotransmitter acetylcholine (ACh), implying that the time-dependent changes in currents induced by nicotine result from changes in the nAChR rather than being due to a peculiarity of nicotine (Fig. 3). In addition, because run-up was observed at near-saturating (Mulle & Changeux, 1990; Lester & Dani, 1995a; but see Covernton & Connolly, 2000) concentrations of agonist (Fig. 3), it seems unlikely that it results solely from a shift in the affinity of the nAChR for agonist (Colquhoun, 1998). Thus, run-up is likely to result either from a change in the functional properties of the receptors (e.g. increased channel Popen and/or single channel conductance), or from the incorporation of a new population of channels. Similar conclusions were reached from consideration of the time-dependent changes in nAChR channel function in PC12 cells (Ifune & Steinbach, 1993). In the present study, we have attempted to distinguish between these two possibilities.
Mechanism of nAChR run-up
There are two possible extreme experimental outcomes following irreversible inhibition of all functional nAChR channels present at the start of whole-cell recording. If run-up is due to new receptors then the nicotinic responses should recover to about 100 % of control levels by 10-15 min (i.e. because run-up reflects an approximate doubling of the response amplitude under normal conditions; see Fig. 1). Alternatively, if run-up is due to a change in the existing channel properties, then after complete receptor inhibition, no recovery of nicotine-induced currents should be observed. To irreversibly inactivate nAChRs we used the open channel blocker chlorisondamine (Neely & Lingle, 1986; Amador & Dani, 1995). It was not possible to inhibit completely or instantaneously all the nAChRs. At t= 2 min, a 91 ± 1 % (n = 4) block of the nicotinic current was achieved by exposure of cells to five co-applications of 30 μM nicotine and 30 μM chlorisondamine (Fig. 4A, filled circles, and B). For each of the two potential mechanisms, the time course and extent of run-up were then predicted from a series of control cells not exposed to chlorisondamine (Fig. 4A, squares). If run-up depends on a new channel population then the time course of recovery after chlorisondamine can be estimated as the fraction of unblocked channels plus the fractional increase in current observed in control cells after t= 2 min (Fig. 4A, dashed line). If run-up is due to a change in channel properties then recovery after chlorisondamine will be described by the fraction of unblocked channels multiplied by the relative increase in response (with respect to the response at t= 2 min) in control cells (Fig. 4A, continuous line). The data show that recovery after chlorisondamine closely follows the prediction for a change in the properties of existing channels (Fig. 4A). The slightly greater recovery observed experimentally could be due to partial relief of block (Neely & Lingle, 1986).
Figure 4. Run-up does not require the incorporation of new plasma membrane channels.

A, comparison of the changes in peak amplitude of nicotinic currents after five 1 s applications of either 30 μM nicotine alone (▪) or 30 μM nicotine plus 30 μM chlorisondamine (•) presented within the first 2 min of whole-cell recording. In both cases the subsequent changes in response amplitude are indicated by the open symbols. The lines are the predicted time courses of response amplitude recovery (see text) depending on whether run-up involves new channels (dashed line) or a change in channel properties (continuous line). B and C, examples of currents before (30 s), during and after (2 and 15 min) the exposure of cells to nicotine plus chlorisondamine (B) or chlorisondamine alone (C). Nicotine and chlorisondamine applications are indicated by the open and filled bars, respectively. All amplitude measurements were peak currents. Data were corrected for Rs error using the off-line algorithm.
It is possible, however, that chlorisondamine alone is capable of preventing the appearance of a new channel population, e.g. by interfering with nAChR assembly and/or trafficking of receptors to the cell surface. To discount this possibility and to confirm that chlorisondamine is acting as an open channel blocker of nAChRs on MHb cells, we performed an additional control. In these experiments cells were exposed to a sequence of five applications of chlorisondamine in the absence of nicotine (Fig. 4C). Chlorisondamine alone did not block nAChRs or prevent run-up. Responses induced by nicotine immediately after chlorisondamine exposure were 0.98 (1.13, 0.84) of control, and the fractional run-up between 2 and 15 min was 2.5-fold (1.9, 3.0; n = 2). Thus, in order to ‘prevent’ run-up, chlorisondamine requires the co-application of nicotine and presumably activation of intact surface nAChRs. Therefore the incorporation of new functional channels into the plasma membrane is an unlikely explanation for the time-dependent enhancement of response amplitude.
Changes in the sensitivity of nAChRs to external calcium
The results obtained with chlorisondamine are consistent with the suggestion that a change in receptor Popen and/or conductance underlies nAChR run-up. Indeed, because the Popen of nAChRs is proportional to the extracellular concentration of Ca2+ (Mulle et al. 1992b; Vernino et al. 1992; Amador & Dani, 1995; Galzi et al. 1996), it is possible that run-up results in part from an altered external Ca2+ sensitivity. Thus, we have examined the sensitivity of nAChRs to Ca2+ in MHb neurons during whole-cell perfusion (Fig. 5). Cells were stimulated with nicotine (30 μM; 1 s) at 30 s intervals in 4.0 mM extracellular Ca2+. At time points of 5, 10, 15, 20, 25 and 30 min the sensitivity of nAChRs to Ca2+ was tested by applying nicotine (30 μM) in a background of 0.2 mM Ca2+. As previously demonstrated (Mulle et al. 1992b), a reduction in extracellular Ca2+ caused a rapid and reversible decrease in the amplitude of responses induced by nicotine, at least at early time points, t= 5 min (Fig. 5B, left traces). However, over the duration of the experiment, there was a marked change in the sensitivity of nAChRs to Ca2+, apparent in recordings as a failure of receptors to discriminate between the two concentrations of Ca2+ (Fig. 5B). These results are not due to errors in Rs as the response undergoes run-up (larger responses would be more markedly attenuated by Rs error), because the amplitude of the responses in 4 mM Ca2+ was approximately the same at the 5 and 20 min time points (Fig. 5A and B). For comparative purposes the Ca2+ sensitivity was calculated as the ratio of Rs-corrected peak currents (see Fig. 5A) in response to the application of nicotine in 0.2 mM Ca2+ and the response immediately prior in 4 mM Ca2+ (I0.2Ca/I4.0Ca). After correction for Rs errors in individual cells, I0.2Ca/I4.0Ca was ∼0.5 at 5 min, but had increased significantly to >0.8 by 25-30 min (Fig. 5C). These results are consistent with the suggestion that internal factors may regulate the ability of nAChRs to respond to changes in extracellular [Ca2+]. Together with the results from the chlorisondamine experiments, these results are consistent with the idea that run-up could be due to an increase in Popen of nAChR channels mediated in part through an altered sensitivity of receptors to extracellular Ca2+.
Figure 5. Time-dependent changes in the sensitivity of nicotinic receptors to the concentration of external calcium.

A, peak nicotinic current plotted with respect to time. Cells were continuously bathed in a control solution containing 4 mM Ca2+ and responses were elicited by nicotine (30 μM; 1 s) in 4 mM extracellular Ca2+ (open symbols). At the 5, 10, 15, 20, 25 and 30 min time points, nicotinic currents were evoked in 0.2 mM external Ca2+ (filled symbols), with a pre-incubation period of at least 10 s in a control solution containing 0.2 mM Ca2+. The plot shows both uncorrected measured data (squares) and data corrected for series resistance error (circles). The series resistance (Rs) in this neuron was at the high end of the range, 24 MΩ, and illustrates the importance of the Rs correction. B, examples of the currents evoked in high (4 mM; open bars) and low (0.2 mM; filled bars) Ca2+-containing solutions at 5 min (left traces) and 20 min (right traces) after starting the whole-cell recording. C, plot of the mean fractional Ca2+ sensitivity calculated as the peak nicotinic current amplitude in 0.2 mM Ca2+/peak nicotinic current amplitude in 4 mM Ca2+ (I0.2Ca/I4.0Ca) versus recording time. The Ca2+ sensitivity was obtained from Rs-corrected data. The number of cells at each time point is indicated in parentheses. Estimation of significance was by comparison to the Ca2+ sensitivity at 5 min.
Perforated-patch recording prevents time-dependent run-up in nAChR function
We next addressed whether some of the time-dependent changes observed during whole-cell recording from MHb neurons arose as a result of internal perfusion. If true then elimination of whole-cell dialysis using the perforated patch-clamp technique (Rae et al. 1991; Horn & Korn, 1992) should prevent the alterations in nAChR behaviour. With amphotericin B in the patch pipette, cell-attached recordings were made from neurons, and whole-cell Rs was monitored on-line with a 10 mV voltage step. Figure 6A shows a plot of the change in RS (‘access resistance’) during a typical perforated patch-clamp recording. The RS decreased rapidly over the first 10 min and was <30 MΩ before nicotine was first applied. Run-up was never observed during perforated patch-clamp recording (Fig. 6B and C), although there was a non-significant run-down in channel activity over 30 min (Fig. 6C; P > 0.05). The tendency for channel activity to run down in these experiments may in part be related to a significant 20 % loss in cell capacitance, and presumably cell membrane that occurred over the same time period (Fig. 6D; P < 0.05). These data imply that run-up in nAChR function is dependent on whole-cell perfusion/access and may involve exchange of contents between the cell and patch pipette.
Run-up is not mediated by perfusion-induced changes in channel rectification
Neuronal nAChRs show strong inward rectification that is likely to be produced intracellularly through the interaction of a positively charged species, e.g. Mg2+ or spermine, with the open channel (Mathie et al. 1990; Ifune & Steinbach, 1991; Sands & Barish, 1992; Haghighi & Cooper, 1998). Although this block is responsible for an almost complete loss of outward currents at positive membrane potentials, channel rectification is clearly present at moderately negative voltages; the voltage for half-maximal conductance (V0.5) for a variety of expressed and native nAChRs is ∼-40 mV (Haghighi & Cooper, 1998). Because in the present studies run-up was monitored at ∼-40 mV, a complete removal of channel rectification, caused by the ‘washout’ of either Mg2+ or spermine, would result in a ∼2-fold increase in response amplitude. We have performed two sets of experiments to test the idea that altered rectification may contribute to run-up.
First, we ascertained whether the rectification of nAChR channels in MHb cells is affected by internal perfusion. Voltage ramps from -90 to +60 mV, performed in the presence and absence of 10 μM nicotine (Fig. 7A), were used to create current-voltage (i–V) plots at different times during the recording (Fig. 7B). These data demonstrate that run-up cannot be explained by an increase in the driving force for inward nicotinic currents. From i–V curves (Fig. 7B, inset), Erev was 10.0 ± 2.1 and 10.5 ± 1.4 mV (n = 3) at 2 and 15 min, respectively. In addition, although there was a general increase in current at all potentials, the development of a small outward current at positive potentials indicated a possible alteration in the voltage sensitivity of the channels (Fig. 7A, arrowhead, and B, inset). This phenomenon could be seen more clearly as a positive voltage shift in the normalized conductance- voltage plots (Fig. 7C). Based on the Boltzmann relation (eqn (4)), V0.5 shifted from -23.0 ± 0.9 to -13.8 ± 3.3 mV, and the effective gating charge (q) decreased from -1.71 ± 0.09 to -1.30 ± 0.07 (n = 3). Although activation of Ca2+-dependent Cl− channels can influence nAChR channel rectification in MHb cells (Connolly et al. 1995), we consistently observed strong rectification even in high extracellular Cl− media that would be expected to facilitate such Ca2+-dependent outward currents (Fig. 7B). The apparent hysteresis in the ramp-induced currents (see difference in the outward currents in positive and negative arms of the ramp; Fig. 7A, arrowhead) suggests a slight contamination from Ca2+-dependent Cl− channels (Connolly et al. 1995). However, it is unlikely that altered channel rectification is due to the time-dependent activation of a Cl− conductance, since such changes persist in low extracellular Cl− (Ifune & Steinbach, 1993). As a result of altered rectification, the fractional conductance increase at -40 mV was calculated to be only 1.06 ± 0.05 (Fig. 7C, arrowheads), and thus insufficient to account for a 2-fold run-up of nAChR function. The fact that prolonged internal whole-cell perfusion does not completely alleviate inward rectification of nAChR channels (see also Ifune & Steinbach, 1993) may be ascribed to a highly buffered reserve of spermine (Bowie & Mayer, 1995; Haghighi & Cooper, 1998).
Figure 7. Altered channel rectification during whole-cell perfusion.

A, current traces in response to voltage ramps applied in the presence (continuous lines) and absence (dashed lines) of nicotine (10 μM) after 2 (left traces) and 25 min (right traces) internal dialysis. The voltage ramp (-90 to +60 to -90 mV) was applied 200 ms into the nicotine application (lower left). The arrowhead indicates the time-dependent development of outward current at positive membrane potentials. B, transposition of the data in A into current-voltage (i–V) relationships. Inset, enlargement of the i–V curves around the reversal potential to indicate the development of outward current and the lack of change in reversal potential. C, mean conductance-voltage plots for 3 cells indicating the shift in rectification between 2 (open symbols) and 15 min (filled symbols) recording. In order to generate this plot conductances at both time points were normalized to -80 mV. Points around the reversal potential have been blanked for clarity. The arrowheads indicate the change in fractional conductance at -40 mV. Cells were compensated on-line for Rs.
If a loss of intracellular components responsible for inward rectification does not significantly contribute to run-up then the presence of these species should not prevent the time-dependent increase in current amplitude. Inclusion of ∼2 mM free Mg2+ in the patch pipette did not prevent run-up of nicotinic currents recorded at -40 mV, although the onset and time course of run-up were slightly slowed (Fig. 8A). In addition, intracellular Mg2+ caused a negative voltage shift in V0.5 compared to control cells (Fig. 8B and C). After the inclusion of Mg2+ in the internal solution, V0.5= -33.3 mV and q= -1.39 at 15 min (n = 2). The maximum effect of Mg2+ on channel rectification was very rapid, occurring in <2 min (Fig. 8C), compared to a much slower effect on run-up (Fig. 8A). Thus, the Mg2+-induced decrease in relative conductance at -40 mV (Fig. 8B, arrowheads) is unlikely to account for the altered time course of run-up. The effects of Mg2+ on run-up may be related to the more pronounced channel run-down observed in Mg2+ (Fig. 8A; see below). On further inspection, it was also noted that V0.5, even in the continued presence of Mg2+, underwent a slower time-dependent shift to more positive potentials (Fig. 8C), implying that factors other than Mg2+ regulate nAChR channel rectification. Recent experiments have implicated the polyamine spermine in the inward rectification of nAChRs (Haghighi & Cooper, 1998). We demonstrate here that spermine (100 μM) is extremely effective in preventing the development of outward current at +40 mV (Fig. 9A). The time-dependent increase in the ratio of response amplitudes at positive compared to that at negative potentials (rectification index; I+40mV/I-40 mV) in control cells is a good measure of altered channel rectification (Fig. 9B; Ifune & Steinbach, 1993). We find here that the change in the rectification index was completely prevented by the inclusion of spermine in the internal solution. The suppression of outward current by spermine did not, however, influence the run-up of nicotinic responses at negative potentials (Fig. 9A and C). Together these experiments clearly dissociate the mechanism of run-up from that of changes in channel rectification (see also Ifune & Steinbach, 1993).
Figure 8. Intracellular Mg2+ slows run-up and enhances channel rectification.

A, time course of the changes in peak amplitude during intracellular perfusion with a Mg2+-containing solution (•) is shown (left). The effects of perfusion with a control Mg2+-free solution (data from Fig. 1) are shown for comparison (continuous line). Examples of nicotinic currents in Mg2+-containing solutions are shown (right) at the times after establishment of whole-cell recording configuration. Cells were not corrected for Rs. B, the mean conductance-voltage plots after 15 min recording in the presence (filled symbols) or absence (open symbols) of free intracellular Mg2+. The arrowheads indicate the decreased fractional conductance at -40 mV in the presence of free intracellular Mg2+. Control data are from Fig. 7. Points around the reversal potential have been blanked for clarity. C, plot of the time dependence of the changes in voltage for half-maximal conductance (V0.5) induced by intracellular Mg2+ (•). Control data are shown for comparison (○). The curves are significantly different (P < 0.01). Cells were compensated on-line for Rs.
Figure 9. Intracellular spermine prevents the development of outward current during whole-cell perfusion.
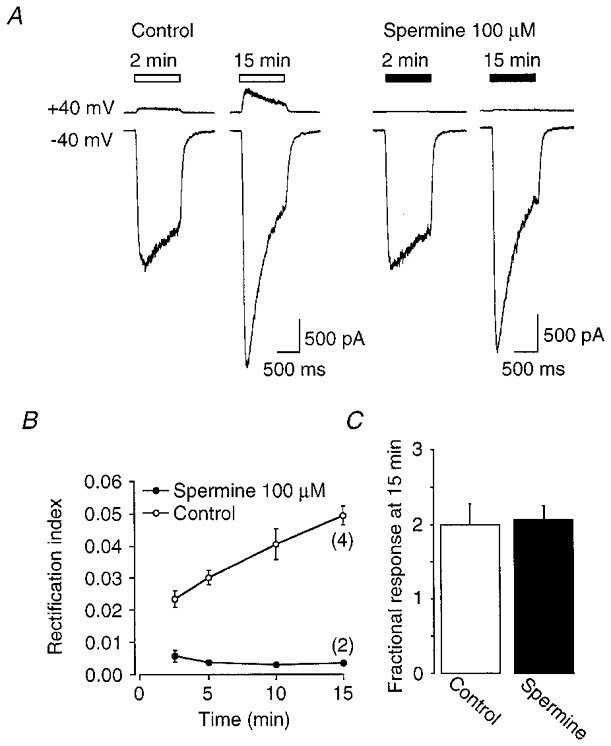
A, comparison of inward and outward currents induced by nicotine (30 μM) in control cells (left traces) and cells perfused with a solution containing 100 μM spermine (right traces) dialysed at the times indicated. B, plot of the relative changes in currents at +40 mV compared to -40 mV (rectification index) for control (○) and spermine (•). The curves are significantly different (P < 0.01). C, bar chart indicating the fractional run-up at 15 min in control and spermine perfused cells. Data were corrected for Rs error using the off-line algorithm.
Intracellular calcium and run-up
The regulation of nAChR channels by intracellular Ca2+ (Khiroug et al. 1997; Fenster et al. 1999a) raises the possibility that compromised Ca2+ buffering during whole-cell perfusion produces run-up. We first tested the hypothesis that a reduction in intracellular Ca2+ due to the introduction of the Ca2+ chelator EGTA contributed to run-up. CaCl2 was added to an EGTA-containing internal solution to produce free Ca2+ concentrations of approximately 30 nM (n = 3) and 100 nM (n = 2), close to estimated resting levels of free Ca2+ reported in MHb cells (Mulle et al. 1992a). Run-up was still observed under these conditions (data not shown). At 30 nM free intracellular Ca2+, the fractional run-up at the 15 min time point was 1.9 ± 0.03 (P > 0.05; compared with control cells in Fig. 1). Thus, a reduction in intracellular [Ca2+] is not responsible for run-up.
Alternatively, run-up may have been induced by an increase in intracellular [Ca2+] that may have occurred on gaining whole-cell access (see Tong & Jahr, 1994), possibly because of inefficient chelation by EGTA. To try to address this hypothesis, we assessed the time-dependent changes in response amplitude and Ca2+ sensitivity during whole-cell perfusion with the faster Ca2+ buffer BAPTA. Under these conditions both run-up (Fig. 10A and C) and an alteration of extracellular Ca2+ sensitivity (Fig. 10B) were still observed. On further examination of run-up in individual cells (see Fig. 10D), however, it was noted that there was a tendency for the magnitude of run-up to be less in BAPTA-perfused cells compared to EGTA-perfused cells: 4/5 cells exhibited less than mean run-up in BAPTA compared with only 3/6 cells in EGTA (Fig. 10C, right).
Figure 10. Effects of differential Ca2+ chelation on run-up and changes in Ca2+ sensitivity.

Time-dependent changes in the amplitude and sensitivity of nAChRs to extracellular Ca2+ during dialysis with BAPTA-containing internal solution. A, time course of changes in the peak amplitude of responses induced by nicotine (30 μM; 1 s) in a background of 4 mM (□) or 0.2 mM extracellular Ca2+ (▪) during whole-cell perfusion. Arrowheads indicate the slow recovery of responses after a brief period in low Ca2+. B, plot of the mean fractional Ca2+ sensitivity calculated as the peak nicotinic current amplitude in 0.2 mM Ca2+/peak nicotinic current amplitude in 4 mM Ca2+ (I0.2Ca/I4.0Ca) versus recording time. C, comparison of run-up of nAChRs in cells dialysed with EGTA-containing or BAPTA-containing internal solutions. The mean data during the first 15 min (left plot) and the fractional run-up for individual cells at 15 min (right plot) are shown. Filled circles labelled ‘cell A’ and ‘cell B’ represent the cells shown in A and D, respectively. All data are corrected off-line for Rs error. The number of cells at each time point is indicated in parentheses. The EGTA cells are the same as those shown in Fig. 5. D, time course of changes in the peak amplitude of responses induced by nicotine in another BAPTA-filled neuron.
Time-dependent regulation of receptor desensitization and channel run-down
In addition to the time-dependent run-up, altered Ca2+ sensitivity, and reduced channel rectification, nAChRs in MHb cells undergo changes in desensitization and an ultimate run-down (or loss) of function. Because receptor desensitization is a key mechanism through which chronic nicotine regulates nAChR number (Marks et al. 1983; Fenster et al. 1999b) and may result in permanent receptor inactivation (Lukas, 1991; Peng et al. 1994), we have investigated the relationship between receptor inactivation and desensitization in MHb neurons. As reported previously, alterations in nAChR desensitization occur during whole-cell perfusion of both PC12 cells (Ifune & Steinbach, 1993) and chromaffin cells (Inoue & Kuriyama, 1991) and also as a consequence of excised outside-out patch formation in MHb neurons (Lester & Dani, 1994). In the present study, a gradual time-dependent increase in the onset of desensitization of nicotinic currents occurred as a result of whole-cell perfusion. The change in desensitization was quantified by comparison of the time constants obtained from single exponential fits to the desensitization phase of nicotinic currents (Fig. 11A and B). In EGTA-perfused cells there was a progressive, albeit non-significant, decrease in the time constant from 1136 ± 269 ms at 1 min to 367 ± 83 ms at 30 min (P > 0.05). The lack of significance probably results from the large cell-to-cell variability in the fitted exponential time constants (see Fig. 11C) and prompted us to analyse the desensitization phase by another method. The change in the time course of desensitization results in a greater fraction of desensitized channels at the end of a 1 s nicotine application, as estimated from the steady-state/peak ratio. This ratio decreased from 0.59 ± 0.05 to 0.24 ± 0.05 from 1 to 30 min for the 10 cells that lasted ≥30 min (P < 0.01).
Figure 11. Time-dependent changes in nicotinic receptor desensitization.

A, examples of currents elicited by nicotine (30 μM; 1 s) at the recording times indicated. Continuous lines are single exponential fits. For clarity, traces have been normalized to their peak amplitudes. B, plot of the time constants resulting from single exponential fits to the desensitizing component of nicotinic currents with respect to recording time (n = 19; cells are the same as those shown in Fig. 1). For significance, all data were compared to the 1 min time point. C, histogram showing the distribution of initial desensitization time constants (at t= 1 min). Bin size = 250 ms.
The proportion of receptors in the desensitized state(s) will regulate response amplitude by controlling the number of channels available for activation by agonist (Katz & Thesleff, 1957). It is possible then that the perfusion-induced increase in the onset of desensitization may contribute to run-down by driving more receptors into desensitized conformations. In the present study, the large variability in both the time at which run-down started (∼5 to >30 min) and the initial rates of desensitization suggested to us that run-down and desensitization may be related. In order to assess any relationship, the cells (see Fig. 1) were divided into two groups based on time constants smaller or larger than the median value of 621 ms and designated as ‘fast’ (n = 9) and ‘slow’ (n = 10) desensitizing sets, respectively. The changes in peak amplitudes of the responses for these two groups were replotted and show that cells with initially faster desensitization also demonstrate faster run-down of their peak nicotine-induced responses (Fig. 12A and B; P < 0.01). For cells that exhibited run-down during the recording period, there was also a positive correlation between the rate of desensitization (measured at the 5 min time point) and the time at which run-down began (Fig. 12C). The onset of run-down was defined as the time at which the peak response started to decline in an irreversible manner, and where the decline was >10 % of the maximal response (Fig. 12A, arrowheads). In addition, because the mean initial amplitudes (at 1 min) of the two groups were not significantly different, 412 ± 90 pA (‘fast’) and 360 ± 52 pA (‘slow’), the potential for run-up was not a limitation in the ‘fast’ group. These data are consistent with the suggestion that the kinetics of desensitization and run-down may be related.
Figure 12. Run-down of nicotinic channels is related to the initial extent of receptor desensitization.

A, example responses from ‘slow’ and ‘fast’ desensitizing cells, at the time points indicated (open bars). The dotted line defines the complete time course of changes in peak amplitude for each cell. The arrowheads indicate the times at which channel run-down was gauged to begin (see text). B, mean peak responses for the ‘fast’ and ‘slow’ groups with respect to recording time. The curves are significantly different (P < 0.01). C, relationship between the rate of desensitization and the start of run-down in nAChR function. Plot of the desensitization time constant against the time to run-down for each cell (n = 13) in the peak response. The line is a linear regression fit to the data; correlation coefficient, r= 0.76. Six cells did not show run-down during the course of the recording and were not included in the plot (the mean desensitization time constant for these cells was τ= 945 ± 311 ms at 5 min and their mean recording duration was 21 ± 2 min).
Use-dependent nature of changes in nAChR response amplitude
If run-down purely reflects the build-up of receptors in the desensitized state(s), increasing the rate of onset of desensitization per se will not produce run-down, unless receptors become trapped. Such trapping could occur if the rate of recovery from desensitization was reduced during the recording (see Khiroug et al. 1997). According to this hypothesis run-down should be use dependent because it will depend on activity-driven desensitization. Infrequent applications of nicotine (5 min intervals) produced the same fractional run-up of peak responses as with higher frequency applications (30 s intervals). The mean ratio of the peak currents at 1 and 15 min (I15/I1) was 1.9 ± 0.2 (n = 19; from Fig. 1) and 2.2 ± 0.6 (n = 7) for high and low frequency stimulation, respectively (Fig. 13A). Moreover, during low frequency stimulation there was no apparent run-down of response amplitude (Fig. 13A) up to 30 min (data not shown). These results imply that while run-up of nicotine-induced responses is not dependent on the frequency of agonist exposure, run-down is highly use dependent. The use-dependent nature of run-down was confirmed by reducing the frequency of stimulation after run-down had begun (Fig. 13C and D). In eight experiments, run-up was allowed to develop over the first ∼15 min of recording, after which cells were exposed alternately to pulses of nicotine applied at 30 s intervals for 5 min followed by a 5 min rest period with no stimulation. Run-down was largely absent during the no-stimulation period, but returned when stimulation was resumed at 30 s intervals. In some cases, a slight reversal of run-down could be observed after the 5 min rest (Fig. 13C and D), although even after 15 min without agonist applications little further recovery was observed (data not shown). Run-down in nAChR activity was not accompanied by a significant loss in membrane surface area, estimated from whole-cell capacitance (Fig. 13C), implying that the decreased function was not caused by a non-specific internalization of membrane and associated channels.
Figure 13. Use dependence of nAChR channel run-up and run-down.

A, time courses of the change in peak amplitude of responses induced by nicotine (30 μM; 1 s) applied at 30 s (dashed line; data are replotted from Fig. 1) or 5 min intervals (•). B, plot of the fractional change in whole-cell capacitance for cells in the use-dependent experiments (n = 10). Significance was assessed with respect to the capacitance at t= 1 min. C, plot of the change in amplitude of nicotine-induced responses (□) and whole-cell capacitance (○) during alternating periods of agonist exposure at 30 s or 5 min interpulse intervals in an individual cell. Note that the plot starts 25 min into the recording. D, example traces from the cell in C at the times indicated (filled bars).
The role of divalent cations in nAChR desensitization and run-down
As shown above, run-down was more pronounced in cells perfused with millimolar concentrations of Mg2+ (Fig. 8). These results imply that divalent cations may be important intracellular modulators of nAChRs in MHb neurons. Because of the known importance of divalent ions on neuronal nAChR desensitization (Khiroug et al. 1997; Fenster et al. 1999a), we have further explored their involvement in the regulation of receptor function. Compared to control conditions (2 mM extracellular Ca2+- intracellular EGTA), the onset of desensitization was more pronounced when EGTA was replaced by BAPTA (Fig. 14A), when Ba2+ was substituted for extracellular Ca2+, and when Mg2+ was added to the internal solution (Fig. 14A, inset). In addition, stimulation of cells in the presence of Ba2+ only marginally affected run-up, whereas channel run-down was significantly more pronounced compared to control (Fig. 14B and C), similar to cells dialysed with free Mg2+ (see Fig. 8A). Interestingly, run-down was not augmented by inclusion of BAPTA in the intracellular solution (data not shown) although run-up was slightly less marked (see Fig. 10). Overall, these findings strongly implicate divalent cations in the regulation of both receptor desensitization and run-down.
Figure 14. Divalent cations, desensitization and run-down.

A, comparison of the change in desensitization time constant for cells perfused with either EGTA-containing (○) or BAPTA-containing (•) intracellular solutions. Data have been reanalysed from cells shown in Fig. 10. The curves are significantly different (P < 0.05). The mean time constants after 15 min whole-cell recording for cells recorded in the presence of 2 mM extracellular Ca2+ (from Fig. 1; n = 19), 2 mM extracellular Ba2+ (n = 4) and intracellular Mg2+ (from Fig. 8; n = 4) are shown (inset). B, comparison of the changes in peak amplitude of responses induced by nicotine in the presence of extracellular Ca2+ (line) and Ba2+ (•). The curves are significantly different (P < 0.01). C, example traces of responses recorded in Ba2+-containing media at the times indicated above the open bars.
DISCUSSION
We have examined the behaviour of nAChRs during whole-cell recording of MHb neurons. Internal perfusion results in several time-dependent changes in the properties of nAChR channels. These are: (1) a run-up in the peak current during the initial 0-15 min; (2) an alteration in receptor sensitivity to external Ca2+; (3) a reduction in inward channel rectification; (4) an increase in the rate of desensitization; and (5) a variable use-dependent run-down in the peak currents that occurs between 5 and >30 min. We will consider mechanistically how these changes could arise, how they are related to one another, their dependence on intracellular factors and their involvement in the physiological regulation of the amplitude and kinetics of nAChRs.
Subtype-specific time-dependent changes in nAChR function
In both hippocampal neurons and ciliary ganglion cells, the run-down of peak nicotinic currents during whole-cell perfusion has been thoroughly characterized and attributed to α7 subunit-containing nAChRs (Alkondon et al. 1994; Liu & Berg, 1999). Conversely, run-up of peak nAChR currents during whole-cell dialysis in superior cervical ganglion neurons (Covernton et al. 1994), chromaffin cells (Nooney & Feltz, 1995) and the peripherally derived PC12 cell line (Ifune & Steinbach, 1993) probably involves receptors formed by a combination of α3, β2, β4 and α5 subunits (Conroy & Berg, 1995). Unusual for CNS nuclei, the MHb expresses high levels of mRNA for β4 and α3 subunits (Duvoisin et al. 1989; Wada et al. 1989). These observations, together with functional data showing that nAChRs in MHb cells are extremely sensitive to both the β4 subunit-preferring agonist cytisine (Mulle & Changeux, 1990; Luetje & Patrick, 1991) and the α3β4 antagonist α-AuIB conotoxin (Luo et al. 1998; Quick et al. 1999) imply that nicotinic receptors in the MHb share many properties of the ‘ganglionic-type’ receptors, including the time-dependent changes presented here. Thus, the function of different subtypes of nAChRs may be under very different forms of cellular regulation.
Does run-up involve a change in the number of functional receptors or an altered channel open probability?
Run-up in the whole-cell nAChR current could occur through an increase in one or more of three independent variables: the number of functional channels, the single channel amplitude, and the channel Popen (e.g. Belardetti & Siegelbaum, 1988). It was reasoned here that irreversible inhibition of all channels open shortly after commencement of whole-cell recording should abolish run-up if it does not involve new channels. This is true if the agent used to achieve inhibition, chlorisondamine, blocks only channels opened by nicotine. In partial support of this assumption we find that chlorisondamine applied in the absence of agonist does not irreversibly block closed channels (also see Neely & Lingle, 1986; Amador & Dani, 1995). Moreover, chlorisondamine alone does not prevent run-up of nAChR responses, implying that any blocking action of chlorisondamine requires agonist, and therefore presumably intact channels. Thus, while this observation argues strongly against the formation of new channels during run-up, it does not eliminate the possibility that chlorisondamine and nicotine together block a functionally inactive population of channels that either are in slow equilibrium with the active population or represent a distinct pool of nAChRs. A silent pool of nAChRs is implied by the large excess of nicotine binding sites on the plasma membrane surface compared to functional channels (Fenster et al. 1999b). Moreover, such non-functional receptors may under some circumstances be slowly converted to functional channels (Margiotta et al. 1987). Finally, there is no independent evidence in support of modulation of single nAChR channel conductance. On the contrary, it has been noted that, despite run-down, the single channel amplitude of individual channels in outside-out patches excised from MHb neurons remains stable over several minutes (Mulle & Changeux, 1990; Lester & Dani, 1994; Connolly et al. 1995).
Thus, the most parsimonious explanation for run-up would appear to be a time-dependent increase in channel Popen, similar to that observed for NMDA receptors on membrane excision (Rosenmund et al. 1995). Furthermore, because the binding of Ca2+ to extracellular domains regulates nAChR function through a change in Popen (Mulle et al. 1992b; Vernino et al. 1992; Amador & Dani, 1995; Galzi et al. 1996), it is possible that the perfusion-induced run-up and altered Ca2+ sensitivity share a common mechanism. For example, the ability of Ca2+ to modulate nAChRs could become occluded if internal dialysis disrupted a separate but convergent regulation of Popen, such that channels could open efficaciously even in the absence of high extracellular Ca2+. In this respect, although Ca2+ modulation of nAChRs is present during the first few minutes after outside-out patch formation (Mulle et al. 1992b; Amador & Dani, 1995), the maximal Popen, albeit determined for nAChRs in membrane patches from different cell types, was estimated to be >0.9 in both the presence (Mathie et al. 1991) and absence of extracellular Ca2+ (Maconochie & Knight, 1992). Thus, under some circumstances, nAChRs may not discriminate well between Ca2+ concentrations in the low millimolar range. Although this could be viewed as a loss of sensitivity to Ca2+, i.e. channels fail to accurately translate a change in Ca2+ into a change in Popen, an equally plausible explanation is that nAChRs have become supersensitive to Ca2+. Alternatively, the time-dependent activation of a second population of normally non-functional channels that are differentially regulated by extracellular Ca2+ could account for both run-up and altered Ca2+ sensitivity. This latter idea is not unreasonable since MHb cells may express more than one pharmacologically and kinetically distinct nAChR channel (Connolly et al. 1995; Quick et al. 1999). Resolution of the exact mechanism of run-up and its relationship to external Ca2+ sensitivity will ultimately require precise estimates of Popen for different subtypes of nAChRs.
Can altered desensitization kinetics account for channel run-down?
The process of run-down of channel activity during whole-cell recording has led to a more complete understanding of intracellular factors that are necessary to maintain receptor function (Horn & Korn, 1992). Because nAChRs can exist in both functional and non-functional pools on the cell surface (Margiotta et al. 1987; Bencherif et al. 1995), run-down of nAChR channel activity could potentially reflect disruption of mechanisms involved in the interconvertion between these two populations. It is generally assumed that the functional pool of channels cycles between activatable and at least two desensitized states (Katz & Thesleff, 1957; Feltz & Trautmann, 1982; Boyd, 1989). If entry into desensitized channel conformations is a prerequisite for moving receptors from functional to inactive pools, then regulation of desensitization kinetics would be important. In this model run-down would be precipitated by increasing the rate of onset of desensitization or by slowing its recovery (see Lester & Dani, 1994). Both these requirements are met during whole-cell recording. As shown here, the fraction of receptors that are desensitized by the end of a 1 s application of 30 μM nicotine increases (i.e. decrease in the steady-state/peak current ratio) during whole-cell perfusion (see also Inoue & Kuriyama, 1991; Ifune & Steinbach, 1993). Moreover, for a given cell the development of run-down was related to the initial rate of desensitization during the brief agonist exposure (see Fig. 11). Together with the additional finding that recovery from desensitization is slowed during intracellular perfusion (Khiroug et al. 1997) these results predict that, as observed, run-down should occur in a highly use-dependent manner. However, the apparent correlation between desensitization and run-down does not necessarily imply a causal relationship, and run-down could occur via a desensitization-independent mechanism, similar to that suggested for GABA receptors (Frosch et al. 1992).
Intracellular factors involved in the regulation of nAChRs
For both NMDA receptors and α7-type nAChRs, run-down requires the use-dependent influx of extracellular Ca2+ and its subsequent downstream interaction with various effectors (Rosenmund & Westbrook, 1993; Liu & Berg, 1999). Unfortunately, run-down of nAChR channels is more difficult to explore in MHb neurons because of the added complexity arising from run-up. Assuming, however, that run-down of nAChRs in MHb neurons is largely use dependent (see above), the similarity in the time courses of run-up over the initial ∼10 min, monitored with agonist applications at either 30 s or 5 min interpulse intervals (see Fig. 12A), implies that run-down has a delayed onset. A delay is consistent with the need for either a sufficient loss/build-up of some factor that only leads to run-down after it or the process it is involved in reaches some critical level. Because, in addition, either the replacement of extracellular Ca2+ with Ba2+ or the inclusion of free intracellular Mg2+ facilitates run-down, we suggest that the run-down involves an intracellular factor with which Ca2+ normally interacts (see also Liu & Berg, 1999). When this interaction is disrupted channel function will be compromised, as observed even after a short period in low extracellular Ca2+ (see Fig. 10), and may ultimately lead to run-down. Furthermore, because Ca2+ and protein phosphorylation are important during recovery from desensitization of certain nAChRs (Khiroug et al. 1997, 1998; Paradiso & Brehm 1998; Fenster et al. 1999a), likely candidates for run-down factor(s) include protein kinases and/or proteins that associate them with the receptor (Liu & Berg, 1999). Thus, the influx of Ca2+ possibly directly through the nAChR channel (Mulle et al. 1992a; Vernino et al. 1992) may be part of a feedback regulation, via an intermediate factor, that maintains receptors in activatable states by facilitating recovery from desensitization. When this factor is perturbed or ‘washed-out’ Ca2+ is no longer effective. An intermediate factor need not be involved, however, and channel run-down (or altered desensitization) could result from the inability of Ca2+ to interact directly with a functionally important binding site at the intracellular face of the channel (Girod et al. 1999).
Ifune & Steinbach (1993) demonstrated that run-up could be attenuated to varying degrees by high concentrations of several intracellular polyphosphates. Based on their findings it was concluded that ATP prevented run-up independent of protein phosphorylation. In MHb neurons, we have obtained inconclusive results using either Mg-ATP or the non-hydrolysable ATP analogue adenyl-imidodiphosphate (Na-AMP-PNP). In some, but not all, experiments both these agents appeared to reduce run-up (data not shown). It is possible that ambiguous results could arise if ATP was involved in multiple forms of phosphorylation-dependent and -independent forms of nAChR channel up- and downregulation. In this respect, because nicotinic responses can be enhanced through ATP-dependent mechanisms, e.g. an increased number of surface receptors (Rothhut et al. 1996) or recruitment of receptors from inactive pools (Margiotta et al. 1987), the attenuation of run-up by ATP may be counteracted depending on the exact experimental conditions.
It has been argued previously that the mechanical stress of patch excision may have initiated certain time-dependent changes in NMDA receptor function in outside-out patches (Tong & Jahr, 1994; Rosenmund et al. 1995). In the present work, because run-up was not observed during perforated-patch recordings, we must consider whether the mere act of membrane disruption on gaining whole-cell access contributes to the time-dependent changes. The unmasking of latent channel mechanosensitivity by membrane perturbations/cytoskeleton disruption has been well-documented and can result in altered Popen (Hamill & McBride, 1997). In addition, we report that a significant decrease in whole-cell capacitance occurs during recording (see Fig. 13), and presumably reflects internalization of cell membrane. Although a general decrease in surface area could result in a non-specific loss of membrane-associated proteins such as ion channels, this is unlikely to account for run-down because the capacitance changes are largely complete within the first 20 min (see Fig. 13). On the contrary a loss of cell membrane could generate a change in membrane tension which in turn may influence ligand-gated ion channel gating (e.g. Paoletti & Ascher, 1994; Wang et al. 1999).
Acknowledgments
We thank Dr Michael W. Quick for his comments on the manuscript and Drs David S. Weiss, Stephen F. Traynelis and Derek Bowie for helpful discussions. This work was supported by PHS grants NS 31669 (R.A.J.L.) and NS 21229 (J.A.D.) and the W. M. Keck Foundation (grant 931360).
References
- Alkondon M, Reinhardt S, Lobron C, Hermsen B, Maelicke A, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. II. The rundown and inward rectification of agonist-elicited whole-cell currents and identification of receptor subunits by in situ hybridization. Journal of Pharmacology and Experimental Therapeutics. 1994;271:494–506. [PubMed] [Google Scholar]
- Amador M, Dani JA. Mechanism for modulation of nicotinic acetylcholine receptors that can influence synaptic transmission. Journal of Neuroscience. 1995;15:4525–4532. doi: 10.1523/JNEUROSCI.15-06-04525.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardetti F, Siegelbaum SA. Up- and down-modulation of single K+ channel function by distinct second messengers. Trends in Neurosciences. 1988;11:232–238. doi: 10.1016/0166-2236(88)90132-4. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Fowler K, Lukas RJ, Lippiello PM. Mechanisms of upregulation of neuronal nicotinic acetylcholine receptors in clonal cell lines and primary cultures of fetal rat brain. Journal of Pharmacology and Experimental Therapeutics. 1995;275:987–994. [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Boyd ND. Two distinct kinetic phases of desensitization of acetylcholine receptors of clonal rat PC12 cells. The Journal of Physiology. 1989;389:45–67. doi: 10.1113/jphysiol.1987.sp016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad JE, Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. The Journal of Physiology. 1986;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. British Journal of Pharmacology. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun LM, Patrick JW. Pharmacology of neuronal nicotinic acetylcholine receptor subtypes. Advances in Pharmacology. 1997;39:191–220. doi: 10.1016/s1054-3589(08)60072-1. [DOI] [PubMed] [Google Scholar]
- Connolly JG, Gibb AJ, Colquhoun D. Heterogeneity of neuronal nicotinic acetylcholine receptors in thin slices of rat medial habenula. The Journal of Physiology. 1995;484:87–105. doi: 10.1113/jphysiol.1995.sp020650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. Journal of Biological Chemistry. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- Covernton PJ, Connolly JG. Multiple components in the agonist concentration-response relationships of neuronal nicotinic acetylcholine receptors. Journal of Neuroscience Methods. 2000;96:63–70. doi: 10.1016/s0165-0270(99)00185-5. [DOI] [PubMed] [Google Scholar]
- Covernton PJ, Kojima H, Sivilotti LG, Gibb AJ, Colquhoun D. Comparison of neuronal nicotinic receptors in rat sympathetic neurones with subunit pairs expressed in Xenopus oocytes. The Journal of Physiology. 1994;481:27–34. doi: 10.1113/jphysiol.1994.sp020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas J, Adams DJ. Vasoactive intestinal polypeptide modulation of nicotinic ACh receptor channels in rat intracardiac neurones. The Journal of Physiology. 1996;493:503–515. doi: 10.1113/jphysiol.1996.sp021399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing JE, Role LW. Activators of protein kinase C enhance acetylcholine receptor desensitization in sympathetic ganglion neurons. Proceedings of the National Academy of Sciences of the USA. 1987;84:7739–7743. doi: 10.1073/pnas.84.21.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: β4. Neuron. 1989;3:487–96. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Feltz A, Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. The Journal of Physiology. 1982;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Beckman ML, Parker JC, Sheffield EB, Whitworth TL, Quick MW, Lester RAJ. Regulation of α4β2 nicotinic receptor desensitization by calcium and protein kinase C. Molecular Pharmacology. 1999a;55:432–443. [PubMed] [Google Scholar]
- Fenster CP, Whitworth T, Quick MW, Lester RAJ. Upregulation of surface α4β2 nicotinic receptors is initiated by receptor desensitization following chronic exposure to nicotine. Journal of Neuroscience. 1999b;19:4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch MP, Lipton SA, Dichter MA. Desensitization of GABA-activated currents and channels in cultured cortical neurons. Journal of Neuroscience. 1992;12:3042–3053. doi: 10.1523/JNEUROSCI.12-08-03042.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzi JL, Bertrand S, Corringer PJ, Changeux JP, Bertrand D. Identification of calcium binding sites that regulate potentiation of a neuronal nicotinic acetylcholine receptor. EMBO Journal. 1996;15:5824–5832. [PMC free article] [PubMed] [Google Scholar]
- Girod R, Crabtree G, Ernstrom G, RamirezLatorre J, McGehee D, Turner J, Role L. Heteromeric complexes of α5 and/or α7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Annals of the New York Academy of Sciences. 1999;868:578–590. doi: 10.1111/j.1749-6632.1999.tb11331.x. [DOI] [PubMed] [Google Scholar]
- Gurantz D, Harootunian AT, Tsien RY, Dionne VE, Margiotta JF. VIP modulates neuronal nicotinic acetylcholine receptor function by a cyclic AMP-dependent mechanism. Journal of Neuroscience. 1994;14:3540–3547. doi: 10.1523/JNEUROSCI.14-06-03540.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi AP, Cooper E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. Journal of Neuroscience. 1998;18:4050–4062. doi: 10.1523/JNEUROSCI.18-11-04050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr Induced membrane hypo/hyper-mechanosensitivity: a limitation of patch-clamp recording. Annual Review of Physiology. 1997;59:621–631. doi: 10.1146/annurev.physiol.59.1.621. [DOI] [PubMed] [Google Scholar]
- Hicks JH, Lester RAJ. Long-lasting increase in the open probability of nicotinic channels induced by intracellular calcium. Society for Neuroscience Abstracts. 1999;29:684.8. [Google Scholar]
- Horn R, Korn SJ. Prevention of rundown in electrophysiological recordings. Methods in Enzymology. 1992;207:149–155. doi: 10.1016/0076-6879(92)07010-l. [DOI] [PubMed] [Google Scholar]
- Ifune CK, Steinbach JH. Inward rectification of acetylcholine-elicited currents in rat phaeochromocytoma cells. The Journal of Physiology. 1991;457:143–165. doi: 10.1113/jphysiol.1992.sp019369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifune CK, Steinbach JH. Modulation of acetylcholine-elicited currents in clonal rat phaeochromocytoma (PC12) cells by internal polyphosphates. The Journal of Physiology. 1993;463:431–447. doi: 10.1113/jphysiol.1993.sp019603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Kuriyama H. Properties of the nicotinic-receptor-activated current in adrenal chromaffin cells of the guinea-pig. Pflügers Archiv. 1991;419:13–20. doi: 10.1007/BF00373741. [DOI] [PubMed] [Google Scholar]
- Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends in Neurosciences. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- Katz B, Thesleff S. A study of the desensitization produced by acetylcholine at the motor end-plate. The Journal of Physiology. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Sokolova E, Talantova M, Nistri A. Imaging of intracellular calcium during desensitization of nicotinic acetylcholine receptors of rat chromaffin cells. British Journal of Pharmacology. 1997;122:1323–1332. doi: 10.1038/sj.bjp.0701518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug L, Sokolova E, Giniatullin R, Afzalov R, Nistri A. Recovery from desensitization of neuronal nicotinic acetylcholine receptors of rat chromaffin cells is modulated by intracellular calcium through distinct second messengers. Journal of Neuroscience. 1998;18:2458–2466. doi: 10.1523/JNEUROSCI.18-07-02458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena C, Changeux JP. Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. Journal of Neuroscience. 1997;17:576–585. doi: 10.1523/JNEUROSCI.17-02-00576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RAJ, Dani JA. Time-dependent changes in central nicotinic acetylcholine channel kinetics in excised patches. Neuropharmacology. 1994;33:27–34. doi: 10.1016/0028-3908(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Lester RAJ, Dani JA. Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. Journal of Neurophysiology. 1995a;74:195–206. doi: 10.1152/jn.1995.74.1.195. [DOI] [PubMed] [Google Scholar]
- Lester RAJ, Dani JA. Changes in central nicotinic receptor properties during whole-cell perfusion. Society for Neuroscience Abstracts. 1995b;21:142.6P. [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Progress in Brain Research. 1996;109:125–137. doi: 10.1016/s0079-6123(08)62094-4. [DOI] [PubMed] [Google Scholar]
- Liu QS, Berg DK. Actin filaments and the opposing actions of CaM kinase II and calcineurin in regulating α7-containing nicotinic receptors on chick ciliary ganglion neurons. Journal of Neuroscience. 1999;19:10280–10288. doi: 10.1523/JNEUROSCI.19-23-10280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both α- and β-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. Journal of Neuroscience. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ. Effects of chronic nicotinic ligand exposure on functional activity of nicotinic acetylcholine receptors expressed by cells of the PC12 rat pheochromocytoma or the TE671/RD human clonal line. Journal of Neurochemistry. 1991;56:1134–1145. doi: 10.1111/j.1471-4159.1991.tb11403.x. [DOI] [PubMed] [Google Scholar]
- Luo S, Kulak JM, Cartier GE, Jacobsen RB, Yoshikami D, Olivera BM, McIntosh JM. α-Conotoxin AuIB selectively blocks α3β4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. Journal of Neuroscience. 1998;18:8571–8579. doi: 10.1523/JNEUROSCI.18-21-08571.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Acetylcholine causes rapid nicotinic excitation in the medial habenular nucleus of guinea pig, in vitro. Journal of Neuroscience. 1987;7:742–752. doi: 10.1523/JNEUROSCI.07-03-00742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JF, Mody I, Salter MW. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. The Journal of Physiology. 1989;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- Maconochie DJ, Knight DE. A study of the bovine adrenal chromaffin nicotinic receptor using patch clamp and concentration-jump techniques. The Journal of Physiology. 1992;454:129–153. doi: 10.1113/jphysiol.1992.sp019257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta JF, Berg DK, Dionne VE. Cyclic AMP regulates the proportion of functional acetylcholine receptors on chicken ciliary ganglion neurons. Proceedings of the National Academy of Sciences of the USA. 1987;84:8155–8159. doi: 10.1073/pnas.84.22.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics. 1983;226:817–825. [PubMed] [Google Scholar]
- Mathie A, Colquhoun D, Cull-Candy SG. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. The Journal of Physiology. 1990;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, CullCandy SG, Colquhoun D. Conductance and kinetic properties of single nicotinic acetylcholine receptor channels in rat sympathetic neurones. The Journal of Physiology. 1991;439:717–750. doi: 10.1113/jphysiol.1991.sp018690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Changeux J-P. A novel type of nicotinic receptor in the rat central nervous system characterized by patch-clamp techniques. Journal of Neuroscience. 1990;10:169–175. doi: 10.1523/JNEUROSCI.10-01-00169.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Choquet D, Korn H, Changeux J-P. Calcium influx through nicotinic receptor in rat central neurons: its relevance to cellular regulation. Neuron. 1992a;8:135–143. doi: 10.1016/0896-6273(92)90115-t. [DOI] [PubMed] [Google Scholar]
- Mulle C, Lena C, Changeux J-P. Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron. 1992b;8:937–945. doi: 10.1016/0896-6273(92)90208-u. [DOI] [PubMed] [Google Scholar]
- Neely A, Lingle CJ. Trapping of an open-channel blocker at the frog neuromuscular acetylcholine channel. Biophysical Journal. 1986;50:981–986. doi: 10.1016/S0006-3495(86)83538-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooney JM, Feltz A. Inhibition by cyclothiazide of neuronal nicotinic responses in bovine chromaffin cells. British Journal of Pharmacology. 1995;114:648–655. doi: 10.1111/j.1476-5381.1995.tb17188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg A. Human nicotinic receptors – their role in aging and dementia. Neurochemistry International. 1994;25:93–97. doi: 10.1016/0197-0186(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P. Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron. 1994;13:645–655. doi: 10.1016/0896-6273(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Paradiso K, Brehm P. Long-term desensitization of nicotinic acetylcholine receptors is regulated via protein kinase A-mediated phosphorylation. Journal of Neuroscience. 1998;18:9227–9237. doi: 10.1523/JNEUROSCI.18-22-09227.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Molecular Pharmacology. 1994;46:523–530. [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh MJ, Lester RAJ. α3β4 subunit-containing nicotinic acetylcholine receptors dominate function in medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. Journal of Neuroscience. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. Journal of Neuroscience Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Role LW. Substance P modulation of acetylcholine-induced currents in embryonic chicken sympathetic and ciliary ganglion neurons. Proceedings of the National Academy of Sciences of the USA. 1984;81:2924–2928. doi: 10.1073/pnas.81.9.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Feltz A, Westbrook GL. Synaptic NMDA receptor channels have a low open probability. Journal of Neuroscience. 1995;15:2788–2795. doi: 10.1523/JNEUROSCI.15-04-02788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Rothhut B, Romano SJ, Vijayaraghavan S, Berg DK. Post-translational regulation of neuronal acetylcholine receptors stably expressed in a mouse fibroblast cell line. Journal of Neurobiology. 1996;29:115–125. doi: 10.1002/(SICI)1097-4695(199601)29:1<115::AID-NEU9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sands SB, Barish ME. Neuronal nicotinic acetylcholine receptor currents in phaeochromocytoma (PC12) cells: dual mechanisms of rectification. The Journal of Physiology. 1992;447:467–487. doi: 10.1113/jphysiol.1992.sp019012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annual Review of Neuroscience. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Blackstone CD, Huganir RL. Phosphorylation of ligand-gated ion channels: a possible mode of synaptic plasticity. FASEB Journal. 1992;6:2514–2523. [PubMed] [Google Scholar]
- Tong G, Jahr CE. Regulation of glycine-insensitive desensitization of the NMDA receptor in outside-out patches. Journal of Neurophysiology. 1994;72:754–761. doi: 10.1152/jn.1994.72.2.754. [DOI] [PubMed] [Google Scholar]
- Traynelis SF. Software-based correction of single compartment series resistance errors. Journal of Neuroscience Methods. 1998;86:25–34. doi: 10.1016/s0165-0270(98)00140-x. [DOI] [PubMed] [Google Scholar]
- Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of α2, α3, α4, and β2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. Journal of Comparative Neurology. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang HL, Milone M, Ohno K, Shen XM, Tsujino A, Batocchi AP, Tonali P, Brengman J, Engel AG, Sine SM. Acetylcholine receptor M3 domain: stereochemical and volume contributions to channel gating. Nature Neuroscience. 1999;2:226–233. doi: 10.1038/6326. [DOI] [PubMed] [Google Scholar]
- Wang L-Y, Dudek EM, Browning MD, MacDonald JF. Modulation of AMPA/kainate receptors in cultured murine hippocampal neurones by protein kinase C. The Journal of Physiology. 1994;475:431–437. doi: 10.1113/jphysiol.1994.sp020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends in Neurosciences. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]


