Abstract
Changes in the concentration of interstitial K+ surrounding skeletal muscle fibres ([K+]I) probably play some role in the regulation of cardiovascular adjustments to muscular activity, as well as in the aetiology of muscle pain and fatigue during high-intensity exercise. However, there is very little information on the response of [K+]I to exercise in human skeletal muscle.
Five young healthy subjects performed plantar flexion exercise for four 5 min periods at increasing power outputs (∼1–6 W) with 10 min intervening recovery periods, as well as for two 5 min periods with ischaemia at ∼1 and ∼3 W. Microdialysis probes were inserted into the gastrocnemius medialis muscle of the right leg to measure [K+]I, and K+ release from the plantar flexors during and after incremental exercise was calculated from plasma flow and arterial–venous differences for K+. Calf muscle pain was assessed using a visual analogue scale.
On average, [K+]I was 4.4 mmol l−1 at rest and increased during minutes 3–5 of incremental exercise by ∼1–7 mmol l−1 as a positive function of power output. K+ release also increased as a function of exercise intensity, although there was a progressive increase by ∼1–6 mmol l−1 in the [K+] gradient between the interstitium and arterial–venous plasma.
[K+]I was lower during ischaemic exercise than control exercise. In contrast to this effect of ischaemia on [K+]I, muscle pain was relatively higher during ischaemic exercise, which demonstrates that factors other than changes in [K+]I are responsible for ischaemic muscle pain.
In conclusion, this study has demonstrated that during 5 min of dynamic exercise, [K+]I increases during the later period of exercise as a positive function of exercise intensity, ischaemia reduces [K+]I during rest and exercise, and the increase in [K+]I is not responsible for muscle pain during ischaemic exercise.
Changes in K+ levels in the extracellular, or interstitial, fluid surrounding skeletal muscle fibres ([K+]I) might contribute to several important physiological responses during muscular exercise. These responses include the excitation of the muscle chemoreflex which contributes to adjustments in cardiac output and pulmonary ventilation during exercise (Rybicki et al. 1984, 1985). Potassium is also a vasodilator which, when it accumulates in the muscle interstitial fluid during exercise, might contribute to vascular tone and the regulation of muscle blood flow (Saltin et al. 1998). In addition, increases in [K+]I during short-term, high-intensity exercise might contribute to muscle fatigue (Sjögaard, 1996) and muscle pain (Mense, 1993). Although direct measurement of [K+]I has enabled the investigation of its role in some of these responses in animal preparations, technical difficulties associated with direct, microelectrode recordings of [K+]I have impeded the study of these exercise responses in humans (Vyskocil et al. 1983; Green et al. 1999).
We recently proposed a method of assessing [K+]I in active, human skeletal muscle that used microdialysis and thallium (201Tl) as an internal reference (Green et al. 1999). [K+]I in the human gastrocnemius medialis muscle was found to range from ∼4 mmol l−1 at rest to 7-8 mmol l−1 during intermittent, static calf exercise (Green et al. 1999). These values were comparable to those recorded in active skeletal muscle using microelectrodes (Hnik et al. 1976; Vyskocil et al. 1983). In the same study (Green et al. 1999), [K+]I increased by 0.5 mmol l−1 as the force output increased from a low to moderate intensity, but there was no further increase in [K+]I as the force was increased from a moderate to a higher intensity. These data were consistent with the response of femoral venous [K+] to continuous static knee extensor exercise performed across similar intensities (Saltin et al. 1981), but differed from the response of femoral venous [K+] to dynamic exercise (i.e. cycling) which increased progressively as a function of intensity (Hallen et al. 1994; Völlestad et al. 1994). Although reasons for these different responses of [K+] to exercise are not known, they might relate to differences in the temporal resolution of measurements, the type of muscle action (i.e static vs. dynamic) and/or the site of measurement (i.e. venous vs. interstitial).
Consequently, the aims of the present study were (1) to improve the temporal resolution of microdialysis measurements of [K+]I to a level that would enable its role in the rapid physiological adjustments to exercise to be better studied, (2) to do so during dynamic exercise of different intensities, and (3) to contrast interstitial and arterial–venous [K+] during exercise. In addition, since it has been suggested that increases in [K+]I might cause ischaemic muscle pain (Harpuder & Stein, 1943; Mense, 1993), we tested the hypothesis that the effect of ischaemia on muscle pain during exercise is associated with greater increases in [K+]I.
METHODS
Experimental overview
Five healthy subjects (one female, four males; mean age = 22 ± 2 years) volunteered to participate in two experiments and provided written, informed consent. All procedures were conducted in accordance with the statutes of the Declaration of Helsinki (1989) and were approved by the ethics committee of the Copenhagen and Frederiksberg communities (project no. KF 01-257/98). An outline of the two experiments is shown in Fig. 1. Experiment 1 focused on the response of [K+]I to submaximal exercise performed at four different intensities, whereas Expt 2 focused on the response of [K+]I and muscle pain to ischaemic exercise. On one day the microdialysis measurements of [K+]I were made for Expts 1 and 2. The order of these two experiments was counterbalanced and a 25 min intervening rest period was used. On another day the incremental protocol (i.e. Expt 1) was repeated with additional exercise bouts to determine peak power output, and both plasma flow and arterial–venous differences for [K+] were measured.
Figure 1.
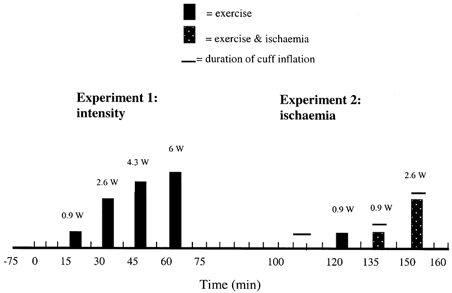
An illustration of the experimental protocol.
Experiment 1: effect of exercise intensity on muscle interstitial [K+] and K+ release
As illustrated in Fig. 1, the rest and incremental exercise protocol was characterised by a 15 min rest period and then four bouts of exercise (5 min long) of increasing intensity interspersed with 10 min rest periods. On a separate day when plasma flow and arterial–venous differences were measured, this protocol was repeated and, 10 min following the fourth exercise bout, subjects exercised at progressively higher intensities for 1 min each to determine a peak power output. Subjects were seated upright in a comfortable chair with the foot placed firmly onto a metal plate. The foot was positioned ∼10 cm above the ischial tuberosity and the knee angle maintained at ∼170 deg. Exercise consisted of repeated plantar flexion movements at a frequency of 0.67 Hz for 5 min. This frequency was well accepted by the subjects; whereas a problem in sustaining a constant rhythm at a higher, more common frequency (i.e. 1 Hz) was encountered with the present apparatus. The power output generated during each of the four bouts (Fig. 1) corresponded, on average, to 13, 37, 61 and 85% of peak power output. The different power outputs were generated by the displacement of four different weights through a vertical distance of 59 mm each time the foot was rotated about the ankle (i.e. plantar flexion) through a 10 deg range from a neutral position. Subjects were instructed to relax the calf muscle during the eccentric phase and allow the weight to fall quickly. Measurements of [K+]I and K+ release were made during this experiment as described in sections that follow.
Experiment 2: Effect of ischaemia on muscle interstitial [K+] during exercise
As shown in Fig. 1, the five subjects also completed a protocol which consisted of a 5 min period of leg ischaemia at rest, one control exercise bout (i.e. 0.9 W) and two ischaemic exercise bouts (i.e. 0.9 and 2.6 W). Exercise at 0.9 and 2.6 W was performed as described for Expt 1. Leg ischaemia was created by inflation of a cuff wrapped around the thigh to 260 Torr within 1 s. During the first minute immediately after ischaemic exercise the cuff remained inflated at 260 Torr and was then released so that cuff pressure returned to zero within 1 s. Pilot experiments using strain-gauge plethysmography showed that inflating the thigh cuff to 260 Torr increased leg volume through displacement of fluid by ∼1% as the cuff was being inflated, but that leg volume remained stable for 5 min while the cuff remained inflated. In addition, these pilot experiments also showed that there was no increase in leg volume in response to exercise at 0.9 and 2.6 W while the thigh cuff remained inflated at 260 Torr. Therefore, during both rest and exercise blood flow to the leg was eliminated.
Microdialysis measurement of [K+]I
At least 75 min prior to Expts 1 or 2, three or four microdialysis probes were inserted into the gastrocnemius medialis muscle of each subject's right leg. Prior to insertion of each probe, the skin, subcutaneous tissue and fascia close to both the insertion and exit points were anaesthetised with lidocaine (lignocaine, 20 mg ml−1). A sterile needle (18 G, 80 mm length) was then inserted into the muscle ∼5 mm below the fascia, pushed in a direction approximately parallel with the muscle fibres to then exit the skin ∼60 mm from the insertion site. The microdialysis probe was fed through the needle and then the needle was withdrawn to leave the probe in the muscle. Due to high resistance to perfusion flow and sample volumes 10% less than expected, only one or two probes per subject were used and the muscle data reported are based on responses measured by a total of nine probes.
Each probe consisted of 12-15 cm of microdialysis tubing (0.20 mm i.d., 0.22 mm o.d., molecular cut-off at 5-6 kDa; GFS 16-GFE 18; Gambro, Lund, Sweden). A length (∼40 cm) of 6/0 gauge OD suture thread (Vicryl, Ethicon, Denmark) was glued to the microdialysis tubing at both ends to provide stability when used in vivo. The microdialysis tubing and suture thread were then inserted and glued inside two hollow nylon tubes (i.d. = 0.5 mm, o.d. = 0.6 mm: Portex, Hythe, Kent, UK) and adjusted so that the exposed length of the microdialysis tubing was ∼4 cm. The inlet and outlet lengths of tubing were 16 and 8.1 cm, respectively. Both ends of the outlet tubing were glued in a manner that did not block dialysate flow through the microdialysis tubing and which restricted dialysate flow to within the microdialysis tubing. This was done so that the delay in the dialysate's transit from the exposed dialysate end of the microdialysis fibre to the end of the outlet tubing was ∼30 s and which minimised the possibility of significant mixing of dialysate on its transit to the collecting vial.
The microdialysis probes were perfused with Ringer acetate solution ([K+] = 4.1 mmol l−1) using a microdialysis pump (CMA 102; CMA Microdialysis, Sweden) at a rate of 5 μl min−1. To each perfusate was added a small amount of Thallium (201Tl; standard activity = 74 MBq; Amersham) to yield a radioactivity of ∼100000 d.p.m. in 2.5 μl of perfusate. The dialysate was collected in 250 μl vials, sealed and then weighed to the nearest 0.01 mg. 201Tl activity in the perfusate and dialysate was measured using an autogamma counter (Packard 5650). Three samples of perfusate preceded and followed each series of dialysate 201Tl measurements so that any drift, independent of the natural decay of 201Tl, could be checked. In no case was drift encountered. Potassium in the perfusate and dialysate was measured using a flame photometer (model FLM3, Radiometer, Copenhagen) after it had been calibrated using 0, 5 and 10 mm K+ standards (Precinorm BM No. 125130). The relative loss of 201Tl was calculated as the difference between the perfusate and dialysate 201Tl activities divided by the perfusate 201Tl activity, and then used to represent the relative recovery of K+ (RR) as described previously (Green et al. 1999). Interstitial [K+] was calculated using the following equation:
Dialysate was collected during periods of 30, 60 or 120 s. To see if these periods exerted any effect on [K+]I, the following sequence of dialysate collections was followed during the 15 min rest period of Expt 1: 30, 60, 120, 60, 30, 30, 60, 120, 60, 30 s. During the rest-exercise protocols of Expts 1 and 2, the shortest period (30 s) was used across rest-exercise transitions, the intermediate period (60 s) was used during minutes 2-4 of exercise and minutes 2-3 of rest, and the longest period (i.e. 120 s) was used between minutes 4 and 9 of rest. As a result of these different periods, the dialysate volume ranged between 2.5 and 10 μl. To minimise any effect of evaporation of dialysate within the collecting vial on [K+]I, 250 μl of lithium buffer (Radiometer S3376) was added soon after the dialysate was weighed.
Measurement of arterial and venous [K+] and K+ release
Arterial and venous [K+] and K+ release to the circulation were measured during Expt 1 only. Prior to exercise and following the application of a local anaesthetic (2% lidocaine), a catheter was inserted into a radial artery and another was inserted into the popliteal vein of the exercising leg using the Seldinger technique and ultrasound colour Doppler imaging of the vessel. The catheter tip was placed distal to the junction with the small saphenous vein and just proximal to the anterior-posterior tibial venous bifurcation. This means that the popliteal venous effluent drained both anterior and posterior regions of the leg, as well as some regions of the foot, but would be less affected by venous effluent from superficial regions such as the skin because these regions are drained primarily by the small and large saphenous veins. We are unaware of the extent to which venous [K+] during exercise would be contaminated by venous effluent draining regions other than the plantar flexors; however, the similarity between exercise venous [K+] in the present study (see Table 1 in Results) and other studies of the response to knee extensor exercise (Saltin et al. 1981; Hallen et al. 1994; Völlestad et al. 1994) suggests that, relative to this latter type of exercise, the extent of contamination is similar. Blood was sampled anaerobically from the artery and vein 2 min prior to each exercise bout and at the third and fifth minute of each exercise bout, except at the maximum power output during which only one measurement was made near the end of exercise. Haemoglobin concentration ([Hb]) (Oxylite, Radiometer) and haematocrit were determined, and the fractional difference in plasma volume between arterial and venous blood (ΔPV) was calculated (Harrison, 1985). Haematocrit was corrected for trapped plasma (Hct × 0.96) and peripheral sampling (Hct × 0.91) (Gillen et al. 1991). Plasma [K+] was determined using an automated K+ analyser (Radiometer KNA2) and the plasma arterial–venous difference ([K+]a-v) was corrected for differences in plasma volume between arterial (a) and venous (v) blood using the equation:
Table 1.
Leg blood flow (LBF), arterial–venous haematocrit (Hct), arterial–venous haemoglobin (Hb), percentage change in plasma volume from arterial to venous blood (%ΔPV), arterial–venous K+ and K+ release during rest and dynamic plantar flexion exercise (Expt 1)
| LBF (ml min−1) | Arterial Hct (%) | Venous Het (%) | Arterial Hb (mmol l−1) | Venous Hb (mmol l−1) | %δPV | Arterial K+ (mmol l−1) | Venous K+ release(μmol min−1) | K+ release (μmol min−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Rest | 58 ± 12 | 43.8 ± 1.7 | 43.5 ± 1.6 | 7.9 ± 0.5 | 8.5 ± 0.2 | −7.1 ± 4.6 | 4.1 ± 0.1 | 4.3 ± 0.1 | −1 ± 10 | |
| 0.9 W | Min 3 | 136 ± 25 | 43.1 ± 1.4 | 43.5 ± 1.7 | 8.3 ± 0.4 | 8.6 ± 0.5 | −4.1 ± 3.2 | 4.1 ± 0.1 | 4.5 ± 0.2 | 13 ± 10 |
| Min 5 | 114 ± 11 | 43.1 ± 1.2 | 43.4 ± 1.8 | 8.5 ± 0.6 | 8.6 ± 0.3 | −2.8 ± 3.6 | 4.1 ± 0.1 | 4.4 ± 0.1 | 12 ± 9 | |
| 2.6 W | Min 3 | 265 ± 29 | 43.3 ± 1.5 | 44.5 ± 1.3 | 8.9 ± 0.4 | 8.7 ± 0.4 | −0.1 ± 2.7 | 4.2 ± 0.1 | 4.6 ± 0.16 | 69 ± 11 |
| Min 5 | 280 ± 37 | 43.3 ± 1.5 | 44.5 ± 1.1 | 8.5 ± 0.4 | 8.7 ± 0.5 | −5.0 ± 2.2 | 4.2 ± 0.1 | 4.5 ± 0.2 | 5 ± 13 | |
| 4.3 W | Min 3 | 672 ± 100 | 43.8 ± 1.7 | 44.5 ± 1.7 | 8.7 ± 0.5 | 8.9 ± 0.3 | −4.6 ± 2.6 | 4.3 ± 0.1 | 5.0 ± 0.1 | 180 ± 49 |
| Min 5 | 697 ± 110 | 43.7 ± 1.6 | 44.5 ± 1.9 | 8.8 ± 0.3 | 9.0 ± 0.3 | −4.1 ± 1.6 | 4.4 ± 0.1 | 5.0 ± 0.2 | 128 ± 77 | |
| 6.0 W | Min 3 | 817 ± 153 | 44.8 ± 1.5 | 45.8 ± 1.1 | 8.4 ± 0.1 | 9.0 ± 0.4 | −7.9 ± 4.8 | 4.5 ± 0.1 | 5.5 ± 0.4 | 258 ± 109 |
| Min 5 | 973 ± 82 | 44.7 ± 1.3 | 45.9 ± 1.3 | 8.9 ± 0.6 | 9.1 ± 0.1 | −4.6 ± 5.6 | 4.6 ± 0.1 | 5.3 ± 0.5 | 210 ± 139 | |
| Max | 1103 ± 210 | 45.2 ± 1.4 | 45.6 ± 1.4 | 8.4 ± 0.4 | 9.2 ± 0.4 | −9.1 ± 3.5 | 4.7 ± 0.1 | 5.7 ± 0.4 | 246 ± 103 |
as described elsewhere (McKenna et al. 1997). Blood flow was measured immediately after blood sampling (i.e. minutes 3 and 5) using venous occlusion plethysmography and a single strand mercury-in-Silastic strain gauge. The strain gauge was calibrated prior to each exercise test and then placed around the largest circumference of the calf muscle. A large cuff was placed above the knee and venous occlusion was induced by inflating the cuff to 40 Torr for no more than 3 s during exercise and 10 s during rest. Exercise blood flows were made during a 5 s pause when the subjects were asked to relax the calf muscles and keep the foot in the rested, plantarflexed position. Since blood flow was measured at ∼2-3 s after the cessation of exercise, the extent to which our values underestimate blood flow during exercise will be small and consistent across the four power outputs. Blood flows (ml (100 ml)−1 min−1) were transformed to absolute values (l min−1) based on an anthropometric estimation of leg volume that excludes the foot (Jones & Pearson, 1969). Potassium release (μmol min−1) from the calf to the venous effluent was calculated as the product of plasma [K+]a-v and plasma flow, where plasma flow was calculated as the product of blood flow and the reciprocal of the corrected haematocrit obtained at the same time for a given individual.
Muscle pain
The visual analogue scale was a 10 cm line used to subjectively assess muscle pain. Subjects were asked to rate the intensity of calf muscle pain by marking the 10 cm line with a pen at some point that ranged from zero on the left end (no pain) to ten on the right end (maximum pain). A visual analogue (VA) score was recorded during rest, three times during each exercise bout, and several times during recovery as illustrated in Fig. 4 (Expt 1) and 6 (Expt 2).
Figure 4. Perception of pain during rest and exercise.
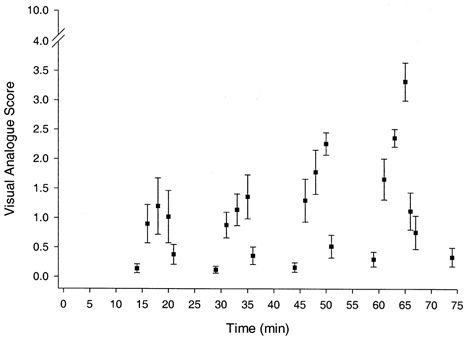
The subjective assessment of calf muscle pain, as represented by the visual analogue score, during rest and dynamic plantar flexion exercise of increasing intensity (Expt 1).
Statistics
Repeated measures ANOVA was used to test for differences in independent variables across time and between exercise responses at the same power output under different conditions (i.e. ischaemia vs. normal). Tukey's Honestly Significant Difference test was used to locate any differences found. The level of significance was set at P < 0.05.
RESULTS
Experiment 1
The response of [K+]I (mean ± s.e.m.) during the pre-exercise rest period, and the four exercise and four recovery periods is shown in Fig. 2. [K+]I during the pre-exercise rest period ranged between 4.0 and 4.6 mmol l−1, and the measurements obtained from the three sampling intervals (i.e. 30, 60 and 120 s) were not different from each other. When considering all time points during exercise (i.e. mean ± s.e.m.) there was a significant increase in [K+]I compared with the pre-exercise rest period. [K+]I was significantly different between three of the four exercise intensities, with the exception being the comparison between 2.6 and 4.2 W. During the initial 1-2 min of each exercise bout, the increase in [K+]I was rapid and non-linear and, assuming it to be monoexponential as an approximation of the temporal response, the half-times (derived from the time constants) were 208, 33, 30 and 17 s for the four increasing power outputs. To describe the [K+]I response due to exercise only and during a relatively more stable period (i.e. minutes 3-5), the average of the [K+]I values during the last 2 min of the preceding rest (for 0.9 W) or recovery periods (for 2.6, 4.3 and 6 W) were subtracted from the average of the [K+]I values measured during minutes 3-5 of exercise for each individual. These difference scores (i.e. Δ[K+]I) at the four power outputs for each subject are shown in Fig. 3. For all five subjects, Δ[K+]I was positively related to power output (R2 = 0.65-0.96), and there was up to a 3-fold difference in the slope of this relationship between subjects.
Figure 2.

Interstitial K+ response (mean ± s.e.m.) in the human gastrocnemius medialis muscle (n = 5) during rest and dynamic plantar flexion exercise of increasing intensity (Expt 1).
Figure 3.
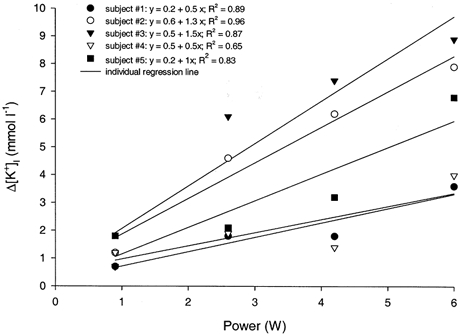
The relationship between Δ[K+]I and power output during dynamic plantar flexion exercise for five subjects. See Results for explanation of Δ[K+]I.
During the initial 1-2 min of recovery, [K+]I rapidly decreased but was still significantly higher than resting values during the pre-exercise period. [K+]I returned to resting levels within 2-3 min after exercise at the three lowest power outputs, and took 5 min to return to these levels at the highest power output. Assuming the recovery responses were monoexponential, the half-times were 60, 45, 64 and 60 s for the four increasing power outputs.
Exercise significantly increased the relative loss of 201Tl beyond that observed at rest (mean = 0.53), although there were no significant differences between exercise intensities (mean = 0.59-0.64). Relative 201Tl loss values during the initial 5 min of recovery were always lower than the exercise responses preceding them, but were not relatively lower during the final 5 min of recovery. This lack of difference during the last 5 min of recovery is probably due to the change in leg position (from horizontal to vertical) during minutes 5-8 of recovery which was done to eliminate numbness experienced in the subjects’ feet.
Leg blood flow, arterial–venous [K+], haematocrit and haemoglobin concentration, as well percentage ΔPV and K+ release are shown in Table 1. Leg blood flow during exercise was always significantly higher than at rest, and was always significantly different between power outputs. By the eighth minute of recovery after each of the four exercise bouts (Expt 1), blood flow had returned to resting levels (data not shown). Arterial K+ was not increased above rest at 0.9 and 2.6 W, but was significantly higher at 4.3 and 6 W, as well as at the maximum power output. By the eighth minute of recovery arterial [K+] had returned to resting levels at the four submaximal power outputs (data not shown). Venous [K+] during exercise was always higher (P < 0.05) than at rest and was always greater than arterial [K+] at corresponding times. Venous [K+] was significantly different between all power outputs and progressively increased as a function of power output. By the eighth minute of recovery after each exercise bout, venous [K+] was slightly, but significantly, lower than the value observed during the pre-exercise period (4.3 ± 0.1 mmol l−1) for 0.9 W (3.8 ± 0.3 mmol l−1), 2.6 W (4.1 ± 0.1 mmol l−1), 4.3 W (4.0 ± 0.2 mmol l−1) and 6 W (3.8 ± 0.1 mmol l−1). K+ release at rest was not different from zero, except for that response during recovery after 0.9 W where there was a small, but significant K+ uptake, rather than release. With the exception of the fifth minute at 2.6 W, exercise at all intensities significantly increased K+ release above resting values and progressively up to 6 W. The insignificant increase in K+ release at 2.6 W (minute 5) appears to be related to the relatively large correction for %ΔPV (compared with minute 3) between arterial and venous blood (see Table 2). Except for 0.9 W, K+ release was always greater at minute 3 than minute 5 of exercise.
Table 2.
[K+]I and the differences between [K+]I and arterial [K+] (i.e. [K+]I–[K+]a) ad venous [K+] (i.e. [K+]I–[K+]v) before and during dynamic plantar flexionn exercise (n = 5)
| [K+]I (mmol I−1) | [K+]I-[K+]a (mmol I−1) | [K+]I-[K+]v (mmol I−1) | ||
|---|---|---|---|---|
| Rest | 4.5 ± 0.4 | 0.4 ± 0.1 | 0.2 ± 0.1 | |
| 0.9 W | Min 3 | 5.3 ± 0.2 | 1.2 ± 0.1 | 0.8 ± 0.1 |
| Min 5 | 5.7 ± 0.2 | 1.6 ± 0.1 | 1.3 ± 0.1 | |
| 2.6 W | Min 3 | 8.2 ± 0.8 | 4.0 ± 0.4 | 3.6 ± 0.4 |
| Min 5 | 8.7 ± 0.8 | 4.5 ± 0.4 | 4.2 ± 0.3 | |
| 4.3 W | Min 3 | 8.3 ± 0.9 | 4.0 ± 0.3 | 3.3 ± 0.3 |
| Min 5 | 9.1 ± 1.1 | 4.7 ± 0.5 | 4.4 ± 0.4 | |
| 6.0 W | Min 3 | 10.9 ± 1.2 | 6.4 ± 0.6 | 5.4 ± 0.6 |
| Min 5 | 11.1 ± 1.4 | 6.5 ± 0.8 | 5.8 ± 0.7 |
At all times during exercise, [K+]I was greater than arterial or venous [K+]. The gradients between interstitial, arterial and venous [K+] at the same time during pre-exercise rest and exercise are shown in Table 2. The interstitial-arterial and interstitial-venous [K+] gradients during exercise at all power outputs were significantly higher than at rest and the eighth minute of the four recovery periods (data not shown). With the exception of the comparison between [K+] gradients at 2.6 and 4.3 W (minute 3 only), the interstitial-arterial or interstitial-venous [K+] gradients progressively increased (P < 0.05) as a function of power output at the corresponding time (i.e. minutes 3 or 5). By minute 8 of recovery, the interstitial-arterial [K+] gradients had decreased to levels not different from the pre-exercise value (0.4 ± 0.2 mmol l−1) after 0.9 W (0.0 ± 0.3 mmol l−1), 2.6 W (0.6 ± 0.2 mmol l−1) and 4.3 W (0.5 ± 0.2 mmol l−1); whereas after 6 W the gradient was still significantly higher (1.2 ± 0.5 mmol l−1) than that observed during pre-exercise rest. Similar results were found for the interstitial-venous [K+] gradients, with only the value during recovery after 6 W (1.5 ± 0.6 mmol l−1) being significantly higher than that observed during pre-exercise rest (0.2 ± 0.2 mmol l−1).
The subjective assessment of muscle pain, represented by the visual analogue (VA) score, is shown in Fig. 4. Exercise at all power outputs significantly increased the VA score relative to pre-exercise values. The VA score was higher (P < 0.05) during exercise at 4.3 W than either 2.6 or 0.9 W, and higher at 6 W than at the other power outputs; in contrast, the VA score during exercise at 2.6 W was not different from that recorded during exercise at 0.9 W. The mean VA score increased with increasing power output, being 1.0, 1.1, 1.8 and 2.5 at 2.6, 4.3 6 and 9 W, respectively. During recovery after each exercise bout, the VA score declined and at 9 min it was not different from zero.
Experiment 2
The [K+]I response to Expt 2 is shown in Fig. 5. The resting values during the initial 5 min period (4.5 ± 0.3 mmol l−1) were not different from those observed during the initial 15 min period of Expt 1. Exercise at 0.9 W increased [K+]I to values (5.4 ± 0.5 mmol l−1) that were similar to the corresponding response at 0.9 W during Expt 1 (5.5 ± 0.4 mmol l−1).
Figure 5. Effect of ischaemia on [K+]I during rest and exercise.
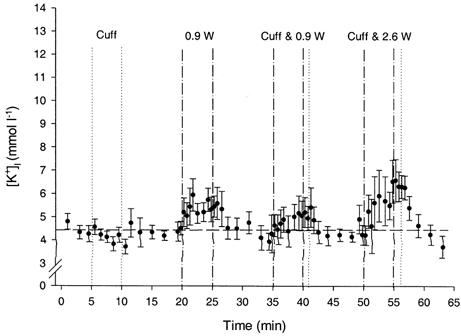
Interstitial K+ response (mean ± s.e.m.) in the human gastrocnemius medialis muscle (n = 5) during rest and dynamic plantar flexion exercise with and without ischaemia (Expt 2). The dashed, horizontal line is set at the mean of the pre-exercise values obtained during the initial 15 min period of Expt 1 for comparison. The dashed vertical lines define the periods of exercise, whereas the dotted vertical lines define the period of cuff inflation (i.e. ischaemia) which, in the case of the periods labelled ‘cuff & 0.9 W’ and ‘cuff & 2.6 W’, begins at the start of exercise (i.e. minutes 35 and 50).
[K+]I during ischaemia (i.e. cuff inflation without exercise) was not different from those values observed during the initial 5 min rest period of Expt 2. However, [K+]I immediately following this period of ischaemia (3.8 ± 0.3 mmol l−1) was significantly lower than those values observed during the initial 5 min rest period. All subsequent resting measures, taken after either exercise or ischaemic exercise, were not different from these initial resting values in Expt 2.
[K+]I was significantly increased by exercise with and without ischaemia. When considering all exercise values, at 0.9 W [K+]I tended to be lower (P = 0.08) with than without ischaemia (4.8 ± 0.9 vs. 5.4 ± 0.5 mmol l−1). At 2.6 W, [K+]I was significantly lower with than without ischaemia (5.4 ± 1.3 vs. 7.8 ± 0.9 mmol l−1). During the minute immediately following ischaemic exercise, the cuff remained inflated and [K+]I was maintained at levels that were not different from the peak exercise responses observed at the end of exercise. After the cuff pressure was released, [K+]I returned to resting levels within 1 and 2 min at 0.9 and 2.6 W, respectively.
There were no significant differences in relative 201Tl loss across the resting and exercise conditions, despite the fact that exercise increased relative 201Tl loss (mean = 0.59-0.61) compared with preceding and following rest periods (mean = 0.50-0.55). Such effects appeared to be too small relative to the within-subject variation across time.
The VA score values during Expt 2 are shown in Fig. 6. Ischaemia at rest caused a small, non-significant increase in VA score from 0.2 ± 0.1 to 0.5 ± 0.1. The VA scores during exercise at 0.9 W were not different with (1.0 ± 0.3) and without ischaemia (1.3 ± 0.3), and were comparable to those VA scores recorded at 0.9 W in Expt 1. In contrast, VA scores were significantly higher during ischaemic exercise at 2.6 W (3.0 ± 0.5) than the control condition (1.1 ± 0.4; Expt 1). The VA score remained elevated during the period of cuff inflation after exercise, and for 1 or 2 min after cuff release it remained greater than resting values for 0.9 and 2.6 W, respectively. By the eighth minute of recovery these VA scores were not different from resting levels.
Figure 6. Effect of ischaemia on perception of pain during rest and exercise.
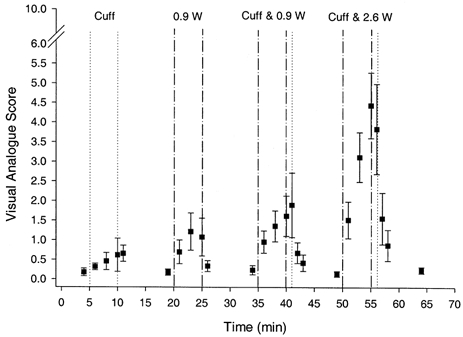
The subjective assessment of calf muscle pain, as represented by the visual analogue score, during rest and dynamic plantar flexion exercise with and without ischaemia (Expt 2). The dashed vertical lines define the periods of exercise, whereas the dotted vertical lines define the period of cuff inflation (i.e. ischaemia) which, in the case of the periods labelled ‘cuff & 0.9 W’ and ‘cuff & 2.6 W’, begins at the start of exercise (i.e. minutes 35 and 50).
Experiments 1 and 2: muscle [K+]I and pain
As illustrated in Fig. 7, there was no correlation between [K+]I and VA scores recorded during exercise at 0.9 and 2.6 W with and without ischaemia. This is in contrast to a significant relationship found between [K+]I and the VA score (r = 0.82, P < 0.05) during Expt 1 only.
Figure 7. Pain-[K+]I relationship during ischaemic nad non-ischaemic exercise.
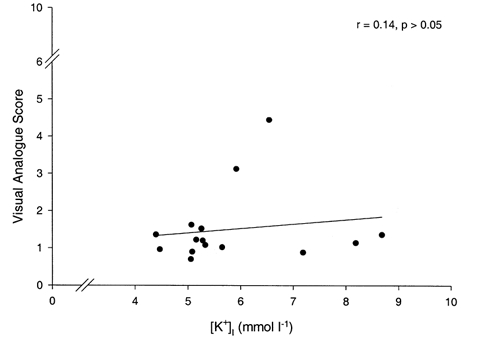
The relationship between mean values of calf muscle pain (i.e. visual analogue score) and muscle [K+]I during dynamic plantar flexion exercise at 0.9 and 2.6 W with and without ischaemia (i.e. Expts 1 and 2).
DISCUSSION
The main findings of the present study were that, for dynamic exercise, (1) there was a positive effect of exercise intensity on [K+]I when the later period of exercise (i.e. minutes 3-5) was considered, (2) a K+ gradient existed between interstitial and arterial or venous blood during exercise and progressively widened from ∼1 to 6 mmol l−1 as the exercise intensity increased, (3) ischaemia reduced the exercise [K+]I response relative to normal conditions, and (4) changes in [K+]I were not responsible for the increased muscle pain experienced during ischaemic exercise.
Experiment 1: effect of exercise intensity on muscle [K+]I and arterial–venous [K+]
An aim of the present study was to improve the temporal resolution of microdialysis measurements to enable a more detailed and accurate description of the [K+]I response to exercise. We recently reported the first measurements of [K+]I during exercise using microdialysis and 201Tl as an internal reference, and stated that a limitation of the method was its relatively poor temporal resolution (Green et al. 1999). In the present study the sampling intervals varied between 30, 60 and 120 s and this in itself did not affect [K+]I levels determined during the pre-exercise period. The resting [K+]I was, on average, 4.4 mmol l−1, which was similar to the resting values observed in our previous study when longer sampling intervals were used (Green et al. 1999). Therefore, the accuracy of measuring [K+]I using microdialysis does not appear to be compromised when reducing the sampling interval to 30 s, and doing so enables a more detailed description of relatively rapid changes in [K+]I during exercise and recovery.
The [K+]I values at rest and during exercise (Fig. 2) are comparable to other data for active, human skeletal muscle recorded using the microelectrode in animal preparations (Hnik et al. 1976; Hirche et al. 1980) and human muscle (Vyskocil et al. 1983), and 1-4 mmol l−1 higher than our previous data for intermittent, static exercise (Green et al. 1999). In response to the four power outputs the mean [K+]I increased from 5.3 to 10.5 mmol l−1, and the peak responses increased from 5.6 to 11.5 mmol l−1. When only the latter, more stable [K+]I responses during minutes 3-5 of exercise were considered, there was a positive effect of power output on the change in [K+]I (relative to rest) across the entire range of intensities for all subjects (Fig. 3). This contrasts with our previous data for intermittent, static exercise where the increase in [K+]I across force outputs (i.e. 15-45% maximum voluntary contraction) comparable to those used in the present study was considerably less (i.e. < 1 mmol l−1) (Green et al. 1999). There might be several reasons for this difference. First, it might be attributed to the muscle action (i.e. static vs. dynamic), although there is little evidence to support this. Second, if the temporal response of [K+]I is similar to that seen for venous [K+] during dynamic exercise where it declines some time after reaching a peak value (Völlestad et al. 1994), then the less frequent sampling used during a longer exercise period (15 min) in our earlier study (Green et al. 1999) might have failed to capture an initial and relatively large increase in [K+]I and to distinguish differences in [K+]I that might have been present during the earlier period of exercise. In addition, unlike the present study, we previously used a longer and continuous exercise protocol, without recovery periods, during which processes that would reduce [K+]I and which are time dependent (e.g. K+ reuptake, K+ release and water fluxes) might have exerted a greater influence on [K+]I. Consequently, the type of muscle action, muscle activation pattern, frequency of sampling, and/or duration of exercise might all contribute to whether or not an intensity effect on [K+]I is observed.
As illustrated in Fig. 3, the positive effect of exercise intensity on [K+]I was observed for all subjects, although there were considerable differences in the slope of this relationship when it was described as a linear function. Visual inspection of the individual items of data suggests that in some subjects the positive effect of exercise intensity on [K+]I was, in fact, non-linear. Given the role of K+ as a vasodilator and the current interest in studying human skeletal muscle blood flow during exercise (Saltin et al. 1998), it could be of value to study the significance of this variability in the [K+]I response in relation to the heterogeneity of flow through active muscle. However, since the individual [K+]I values were taken from only one or two probes in each muscle, caution should be applied to interpreting the variability of these responses between subjects until a more thorough study of the sources of such variability has been done.
The overall temporal pattern of the [K+]I responses during and following each of the four exercise bouts (Fig. 2) is similar to the [K+]I response observed during and after static contractions of the human forearm (Vyskocil et al. 1983) and femoral venous K+ during and after cycling (Hallen et al. 1994; Völlestad et al. 1994). The temporal response of [K+]I will be influenced by the rates of change in K+ efflux to the interstitium, K+ release to the circulation, K+ reuptake by muscle fibres and water fluxes into and out of the interstitial space (Hallen & Sejersted, 1993). Given that K+ efflux and reuptake are probably the most important determinants of the [K+]I response during exercise (Hallen et al. 1994), it seems that one or both of these processes will best explain the effect of exercise intensity on [K+]I. A simple, but untested explanation is that exercise intensity exerts a relatively greater effect on K+ efflux than K+ reuptake during the initial period of exercise until these two rates, as well as the rates of K+ release out of, and water fluxes into, the interstitium become balanced during the later, more stable period of the exercise [K+]I response.
It has been suggested that during the dynamic exercise of a small muscle group, the relatively high muscle blood flow will serve to maximise K+ release and keep [K+]I low so that it is ‘mirrored’ by, and perhaps just slightly higher than, venous [K+] (Saltin et al. 1981; Hallen & Sejersted, 1993; Sjögaard, 1996). On this basis changes in venous [K+] have frequently been used to represent changes in [K+]I (Hallen et al. 1994; Vollestad et al. 1994; Gullestad et al. 1995). While this might be valid during the initial period of exercise (Hirche et al. 1980), the present findings suggest that it is not valid by the third minute of exercise despite the fact that the magnitude of K+ release and its positive response to increasing power output (Table 1) were similar to those reported in these other studies (Hallen et al. 1994; Vollestad et al. 1994). The [K+] gradient between the interstitium and the venous plasma, measured during the last 2 min of exercise, increased from ∼1 to 6 mmol l−1 as exercise intensity and plasma flow increased (Table 2). This is consistent with the effect that increasing blood flow has on the ratio of equilibration of 42K between the circulation and the interstitial fluid of isolated active muscle, which decreased from 0.7-0.9 to 0.2 as flows increased from resting levels to higher values, consistent with exercise values observed in the present study (Renkin, 1959; Sheehan & Renkin, 1972). These data suggest that as blood flow rises during exercise, the increase in K+ release is insufficient to resist the decline in the equilibration of K+ between the interstitial fluid and circulation and reduce the widening of the difference between interstitial and venous [K+]. And the present data demonstrate that venous [K+] underestimates the corresponding changes in [K+]I across different power outputs, at least after the rapid increase in [K+]I and plasma flow is complete.
K+ release to the circulation from the active plantar flexors increased as a positive function of power output, an effect that was calculated by determining the increase in blood or plasma flow and the widening of the arterial–venous K+ gradient (Table 1). An exception to this effect of exercise intensity on K+ release occurred at 2.6 W (minute 5), where no significant release occurred despite a widening of the arterial–venous K+ gradient (Table 2) and increased plasma flow (Table 1). Since the calculation of K+ release in the present study included correction factors for water fluxes into or out of the plantar flexors, errors in measuring haematocrit and/or haemoglobin (see Methods) might have contributed to this apparently anomalous result. Despite this potential error, we, like others (McKenna et al. 1997), felt that this correction for water fluxes was important to include in an attempt to estimate K+ fluxes more accurately and reduce any contaminating effect of venous drainage from inactive regions of the active limb.
Experiment 2: effect of ischaemia on muscle [K+]I
Prior to this study, no data have been reported on the effect of ischaemia on skeletal muscle [K+]I during muscular activity. In the heart, ischaemia progressively increases [K+]I and this has been thought to be a function of increased K+ efflux and suppression of K+ reuptake (Fiolet et al. 1984). Intense and sustained activation of skeletal muscle, which causes it to fail, also increases K+ efflux (Castle & Haylett, 1987). Eliminating the blood flow to active muscle would also be expected to minimise K+ release to the circulation. On the basis of this evidence, ischaemia in contracting, skeletal muscle was expected to increase [K+]I. However, during exercise at 0.9 and 2.6 W the [K+]I response was reduced by ischaemia.
The [K+]I response during ischaemia at rest provides some insight into why this occurred, because it progressively decreased by 0.5 mmol l−1 throughout and just beyond the ischaemic period (Fig. 5). In pilot experiments we observed (authors’ unpublished observations) that the leg volume at rest increased by 1% or ∼24 ml during the initial seconds of ischaemia. This was probably due to a displacement of some portion of the blood to the leg caused by compression of the proximal limb. This same maneouvre has increased the interstitial fluid volume in the gastrocnemius muscle, and it is thought to occur mainly by an ultrafiltration of water from the vascular space due to elevated capillary hydrostatic pressure (Binzoni et al. 1998). Such an effect will cause [K+]I to decline and helps to explain the decline in [K+]I during ischaemic rest. It has also been suggested that cellular swelling could stimulate the Na+-K+ pump (Semb & Sejersted, 1996), and thus an increased Na+-K+ activity and K+ reuptake during and soon after ischaemia might also contribute to the decline in [K+]I.
During muscle activity, the accumulation of osmotically active substances in the interstitium (e.g. inorganic phosphate, lactate) would be expected to occur to a relatively greater extent under ischaemia due to enhanced phosphocreatine hydrolysis and lactate production, and further increase water fluxes into the interstitium (Watson et al. 1993; Lindinger et al. 1994). At the second minute of exercise at 2.6 W, [K+]I was, on average, 5.9 and 8.2 mmol l−1 for the ischaemic and control condition, respectively. A relative increase in water content of ∼40% is required to explain the relatively lower ischaemic [K+]I value. Assuming that the interstitial water volume for gastrocnemius is 320 ml (kg muscle dry weight)−1 (Sjögaard & Saltin, 1982) or ∼80 ml (kg muscle)−1, and that the mass of the gastrocnemius medialis is ∼350 g (Clarys & Marfell-Jones, 1986), then the total interstitial water volume of the muscle is ∼28 ml. The interstitial water volume in the vastus lateralis muscle increased by ∼40% during combined submaximal and maximal knee extension exercise lasting 16-26 min (Sjögaard et al. 1985), and from this it would perhaps be more reasonable to apply an increase of ∼10% to the present data. Consequently, the interstitial water volume might have increased to 31 ml (from 28 ml) during exercise at 2.6 W without ischaemia, and thus would need to increase by a further 12 ml (i.e. 40%) to ∼43 ml during ischaemia to explain the relatively lower [K+]I under this condition. This extent of fluid shift is not unreasonable given that ∼24 ml of extra fluid, primarily of vascular origin, was probably trapped within the limb by the inflated cuff. Although this is speculative, increased water fluxes into the interstitium during exercise could, at least in part, explain the relatively lower [K+]I response during ischaemia.
The reduction in [K+]I during exercise with ischaemia might also be influenced by an increase in Na+-K+ pump activity, perhaps secondary to a greater cellular swelling as stated previously. For the reasons given below, however, this effect might only be manifest during an initial period of exercise before factors such as a decrease in muscle pH and the free energy change (ΔG) of ATP hydrolysis begin to inhibit Na+-K+ pump activity (Fiolet et al. 1984).
After exercise, ischaemia was maintained and [K+]I remained elevated (Fig. 5), an effect that has been observed for venous [K+] (Fallentin et al. 1992; Boushel et al. 1998). When the cuff was released [K+]I did not decline significantly during the first minute and thereafter declined with a longer half-time (∼100 s) than was observed at any intensity without flow restriction (i.e. ∼60 s: Expt 1). This suggests that nearing the end, and soon after, ischaemic exercise, K+ reuptake and K+ efflux were similar, resulting in no significant change in [K+]I. Given that K+ efflux is mainly a function of muscle stimulation (Nielsen & Overgaard, 1996), then it should return rapidly to zero after exercise. The rate at which this occurs, however, might be reduced by the acidosis created during ischaemic exercise, which remains elevated during ischaemic recovery (Blei et al. 1993; Boushel et al. 1998), since a decrease in pH could increase K+ ‘leakage’ into the interstitial fluid through an increase in opening of ATP-dependent K+ (KATP) channels (Davies, 1990). Na+-K+ pump activity and K+ reuptake might be slowed by the reduced increase in [K+]I (and thus intracellular Na+) (Clausen & Everts, 1988; Nielsen & Overgaard, 1996) and lower ΔG of ATP hydrolysis associated with ischaemia (Fiolet et al. 1984). Consequently, K+ efflux might have been relatively high and K+ reuptake relatively low after ischaemic exercise, which would explain how [K+]I remained unchanged during and soon after the post-exercise ischaemic period.
Ischaemia, muscle [K+]I and pain
In studying physiological responses to ischaemic exercise, many investigators have inflated a cuff around a proximal limb to eliminate blood flow. This type of ischaemic exercise is often associated with considerable muscle pain of a type that has been suggested to be no different from intermittent claudication (Lewis et al. 1931). Considerable evidence has emerged that links [K+]I with ischaemic muscle pain (Fock & Mense, 1976; Mense & Stahnke, 1983; Kaufman et al. 1984; Rybicki et al. 1985; Adreani & Kaufman, 1998). In this study, muscle pain was significantly higher during exercise at 0.9 and 2.6 W with than without ischaemia (Fig. 4vs.Fig. 6). When the cuff remained inflated for 1 min after exercise, muscle pain also remained elevated, as did [K+]I (Fig. 5). However, when the cuff was released pain rapidly decreased in the first minute, whereas [K+]I still remained relatively high. Moreover, there were opposing effects of ischaemia on the exercise-induced pain and [K+]I responses: ischaemia increased exercise-induced pain, but decreased [K+]I. There was also no correlation between [K+]I and calf muscle pain during exercise under ischaemic and control conditions (Fig. 7). Therefore, when a cuff is used to induce ischaemia, the behaviour of [K+]Iper se does not explain the increased muscle pain during ischaemic exercise.
Therefore, other substances such as bradykinin, prostaglandins, histamine and 5-hydroxytryptamine might be involved in the muscle pain response (Fock & Mense, 1976; Mense, 1981). However, the role of [K+]I in the ischaemic muscle pain that is experienced during normal, ambulatory conditions cannot be dismissed, since the muscle swelling that occurs during cuff inflation is probably far more pronounced than would occur in a muscle poorly perfused as a result of vessel narrowing caused by compression, obstruction or ligation. The progressive increase in [K+]I in the contracting heart during ischaemia created by arterial clamping (Fiolet et al. 1984), which differs from the present findings, suggests that the method used to induce ischaemia might exert different effects on [K+]I.
It has been suggested that during high-intensity exercise the increase in [K+]I probably contributes to muscle fatigue by causing both membrane depolarisation and excitation transmission block at the sarcolemma or t-tubule (Sjögaard, 1996). Although muscle fatigue was not assessed in the present study, the [K+]I measurements provide some insight into the potential influence of [K+]I on muscle fatigue. Increases in muscle [K+]I up to 10-12.5 mmol l−1 can reduce maximum twitch or tetanic force by 40-100% (Juel, 1988; Clausen et al. 1993; Bouclin et al. 1995), whereas increases to lower values (7-8 mmol l−1) exerted only minor effects on tetanic force production (Bouclin et al. 1995). This effect of [K+]I on muscle force production is accentuated in the presence of decreases in extracellular [Na+] which occurs during high-frequency fatigue (Bouclin et al. 1995). During the later stages of high-intensity exercise in the present study (i.e. 6 W), [K+]I increased to values beyond 11 mmol l−1 which suggests that it could contribute to fatigue at and perhaps beyond this intensity, even though oxygen uptake was submaximal (data not shown). At the end of ischaemic exercise (Expt 2), the increase in muscle pain was associated with the subjects’ comments of impending failure to complete the exercise, although the increase in [K+]I was far less than that observed during exercise without ischaemia at a higher intensity. This suggests that muscle fatigue during this sort of ischaemic exercise at a relatively low intensity is less likely to be caused by the increase in [K+]I.
In conclusion, this study has demonstrated that during 5 min of dynamic exercise, (1) the elevation in [K+]I observed 3-5 min into exercise increased as a positive function of exercise intensity, (2) venous [K+] is not an accurate marker of [K+]I and its later response to exercise, (3) ischaemia reduced [K+]I during rest and exercise, and (4) the increase in [K+]I is not responsible for muscle pain during ischaemic exercise when blood flow is eliminated by the inflation of a cuff around a proximal limb.
Acknowledgments
The authors would like to thank Annie Høj for her technical assistance, and the Danish National Research Council (504-14) and the Danish Medical Research Council (9802636) for funding this research.
References
- Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. Journal of Applied Physiology. 1998;84:1827–1833. doi: 10.1152/jappl.1998.84.6.1827. [DOI] [PubMed] [Google Scholar]
- Binzoni T, Quaresima V, Barattelli G, Hiltbrand E, Gurke I, Terrier F, Cerretelli P, Ferrari M. Energy metabolism and interstitial fluid displacement in human gastrocnemius during short ischemic cycles. Journal of Applied Physiology. 1998;85:1244–1251. doi: 10.1152/jappl.1998.85.4.1244. [DOI] [PubMed] [Google Scholar]
- Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. Journal of Physiology. 1993;465:203–222. doi: 10.1113/jphysiol.1993.sp019673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouclin R, Charbonneau E, Renaud JM. Na+ and K+ effect on contractility of frog sartorius muscle: implications for the mechanism of fatigue. American Journal of Physiology. 1995;268:C1528–1536. doi: 10.1152/ajpcell.1995.268.6.C1528. [DOI] [PubMed] [Google Scholar]
- Boushel R, Madsen P, Nielsen HB, Quistorff B, Secher NH. Contribution of pH, diprotonated phosphate and potassium for the reflex increase in blood pressure during handgrip. Acta Physiologica Scandinavica. 1998;164:269–275. doi: 10.1046/j.1365-201X.1998.00429.x. [DOI] [PubMed] [Google Scholar]
- Castle NA, Haylett DG. Effect of channel blockers on potassium efflux from metabolically exhausted frog skeletal muscle. Journal of Physiology. 1987;383:31–43. doi: 10.1113/jphysiol.1987.sp016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarys JP, Marfell-Jones MJ. Anthropometric prediction of component tissue masses in the minor limb segments of the human body. Human Biology. 1986;58:761–769. [PubMed] [Google Scholar]
- Clausen T, Andersen SLV, Flatman JA. Na+-K+ pump stimulation elicits recovery of contractility in K+-paralysed rat muscle. Journal of Physiology. 1993;472:521–536. doi: 10.1113/jphysiol.1993.sp019960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Everts ME. Is the Na,K-pump capacity in skeletal muscle inadequate during sustained work? Progress in Clinical and Biological Research. 1988;268B:239–244. [PubMed] [Google Scholar]
- Davies NW. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature. 1990;343:375–377. doi: 10.1038/343375a0. [DOI] [PubMed] [Google Scholar]
- Fallentin N, Jensen BR, Bystrom S, Sjogaard G. Role of potassium in the reflex regulation of blood pressure during static exercise in man. Journal of Applied Physiology. 1992;85:1583–1592. doi: 10.1113/jphysiol.1992.sp019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiolet JWT, Baartscheer A, Schumacher CA, Coronel R, ter Welle HF. The change of the free energy of ATP hydrolysis during global ischemia and anoxia in the rat heart. Its possible role in the regulation of transsarcolemmal sodium and potassium gradients. Journal of Molecular and Cellular Cardiology. 1984;16:1023–1036. doi: 10.1016/s0022-2828(84)80015-2. [DOI] [PubMed] [Google Scholar]
- Fock S, Mense S. Excitatory effects of 5-hydroxytryptamine, histamine and potassium ions on muscular group IV afferent units: a comparison with bradykinin. Brain Research. 1976;105:459–469. doi: 10.1016/0006-8993(76)90593-x. [DOI] [PubMed] [Google Scholar]
- Gillen CM, Lee R, Mack GW, Tomaselli CM, Nishiyasu T, Nadel ER. Plasma volume expansion in humans after a single intense exercise protocol. Journal of Applied Physiology. 1991;71:1914–1920. doi: 10.1152/jappl.1991.71.5.1914. [DOI] [PubMed] [Google Scholar]
- Green S, Bulow J, Saltin B. Microdialysis and the measurement of muscle interstitial K+ during rest and exercise in humans. Journal of Applied Physiology. 1999;87:460–464. doi: 10.1152/jappl.1999.87.1.460. [DOI] [PubMed] [Google Scholar]
- Gullestad L, Hallen J, Sejersted OM. K+ balance of the quadriceps muscle during dynamic exercise with and without β-adrenoceptor blockade. Journal of Applied Physiology. 1995;78:513–523. doi: 10.1152/jappl.1995.78.2.513. [DOI] [PubMed] [Google Scholar]
- Hallen J, Gullestad L, Sejersted OM. K+ shifts of skeletal muscle during stepwise bicycle exercise with and without β-adrenoreceptor blockade. Journal of Physiology. 1994;477:149–159. doi: 10.1113/jphysiol.1994.sp020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen J, Sejersted OM. Intravasal use of pliable K+-selective electrodes in the femoral vein of humans during exercise. Journal of Applied Physiology. 1993;75:2118–2325. doi: 10.1152/jappl.1993.75.5.2318. [DOI] [PubMed] [Google Scholar]
- Harpuder K, Stein ID. Studies on the nature of pain arising from an ischemic limb. American Heart Journal. 1943;25:438–447. [Google Scholar]
- Harrison MH. Effects of thermal stress and exercise on blood volume in humans. Physiological Reviews. 1985;65:149–209. doi: 10.1152/physrev.1985.65.1.149. [DOI] [PubMed] [Google Scholar]
- Hirche H, Schumacher E, Hagemann H. Extracellular K+ concentration and K+ balance of the gastrocnemius muscle of the dog during exercise. Pflügers Archiv. 1980;387:231–237. doi: 10.1007/BF00580975. [DOI] [PubMed] [Google Scholar]
- Hnik P, Holas M, Krekule I, Kriz N, Mejsnar J, Smiesko V, Ujec E, Vyskocil F. Work-induced potassium changes in skeletal muscle and effluent venous blood assessed by liquid ion-exchanger microelectrodes. Pflügers Archiv. 1976;362:85–94. doi: 10.1007/BF00588685. [DOI] [PubMed] [Google Scholar]
- Jones PRM, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. Journal of Physiology. 1969;204:63–66. P. [PubMed] [Google Scholar]
- Juel C. The effect of β2-adrenoceptor activation on ion-shifts and fatigue in mouse soleus muscles stimulated in vitro. Acta Physiologica Scandinavica. 1988;134:209–216. doi: 10.1111/j.1748-1716.1988.tb08481.x. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrup TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. Journal of Applied Physiology. 1984;57:640–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- Lewis T, Pickering GW, Rothschild P. Observations upon muscular pain in intermittent claudication. Heart. 1931;15:359–383. [Google Scholar]
- Lindinger MI, Spriet LL, Hultman E, Putman T, McKelvie RS, Lands LC, Jones NL, Heigenhauser GJF. Plasma volume and ion regulation during exercise after low- and high-carbohydrate diets. American Journal of Physiology. 1994;266:R1896–1906. doi: 10.1152/ajpregu.1994.266.6.R1896. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Heigenhauser GJF, McKelvie RS, MacDougall JD, Jones NL. Sprint training enhances ionic regulation during intense exercise in men. Journal of Physiology. 1997;501:687–702. doi: 10.1111/j.1469-7793.1997.687bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Sensitization of group IV muscle receptors to bradykinin by 5-hydroxytryptamine and prostaglandin E2. Brain Research. 1981;225:95–105. doi: 10.1016/0006-8993(81)90320-6. [DOI] [PubMed] [Google Scholar]
- Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241–289. doi: 10.1016/0304-3959(93)90027-M. [DOI] [PubMed] [Google Scholar]
- Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. Journal of Physiology. 1983;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, Overgaard K. Ion gradients and contractility in skeletal muscle: the role of active Na+, K+ transport. Acta Physiologica Scandinavica. 1996;156:247–256. doi: 10.1046/j.1365-201X.1996.204000.x. [DOI] [PubMed] [Google Scholar]
- Renkin EM. Exchangeability of tissue potassium in skeletal muscle. American Journal of Physiology. 1959;197:1211–1215. doi: 10.1152/ajplegacy.1959.197.6.1211. [DOI] [PubMed] [Google Scholar]
- Rybicki KJ, Kaufman MP, Kenyon JL, Mitchell JH. Arterial pressure responses to increasing interstitial potassium in hindlimb muscle of dogs. American Journal of Physiology. 1984;247:R717–721. doi: 10.1152/ajpregu.1984.247.4.R717. [DOI] [PubMed] [Google Scholar]
- Rybicki KJ, Waldrop TG, Kaufman MP. Increasing gracilis muscle interstitial potassium concentrations stimulate group III and IV afferents. Journal of Applied Physiology. 1985;58:936–941. doi: 10.1152/jappl.1985.58.3.936. [DOI] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiologica Scandinavica. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Saltin B, Sjøgaard G, Gaffney FA, Rowell LB. Potassium, lactate, and water fluxes in human quadriceps muscle during static contractions. Circulation Research. 1981;48:I-18–I-24. [PubMed] [Google Scholar]
- Semb SO, Sejersted OM. Fuzzy space and control of Na+, K+ pump rate in heart and skeletal muscle. Acta Physiologica Scandinavica. 1996;156:213–225. doi: 10.1046/j.1365-201X.1996.211000.x. [DOI] [PubMed] [Google Scholar]
- Sheehan RM, Renkin EM. Capillary, interstitial, and cell membrane barriers to blood-tissue transport of potassium and rubidium in mammalian skeletal muscle. Circulation Research. 1972;30:588–607. doi: 10.1161/01.res.30.5.588. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G. Potassium and fatigue: the pros and cons. Acta Physiologica Scandinavica. 1996;156:257–264. doi: 10.1046/j.1365-201X.1996.207000.x. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. American Journal of Physiology. 1985;248:R190–196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G, Saltin B. Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. American Journal of Physiology. 1982;243:R271–280. doi: 10.1152/ajpregu.1982.243.3.R271. [DOI] [PubMed] [Google Scholar]
- Vøllestad NK, Hallen J, Sejested OM. Effect of exercise intensity on potassium balance in muscle and blood of man. Journal of Physiology. 1994;475:359–368. doi: 10.1113/jphysiol.1994.sp020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F, Hnik P, Rehfeldt H, Vejsada R, Ujec E. The measurement of K+e concentration changes in human muscles during volitional contractions. Pflügers Archiv. 1983;399:235–237. doi: 10.1007/BF00656721. [DOI] [PubMed] [Google Scholar]
- Watson PD, Garner RP, Ward DS. Water uptake in stimulated cat skeletal muscle. American Journal of Physiology. 1993;264:R790–796. doi: 10.1152/ajpregu.1993.264.4.R790. [DOI] [PubMed] [Google Scholar]


