Abstract
Prazosin is a readily available alpha-adrenergic antagonist that may be useful in the management of functional urethral obstruction in companion animals. This study used urethral pressure profilometry to evaluate the urethral effects of prazosin and phenoxybenzamine in healthy, non-sedated, male Beagle dogs. Heart rate, indirect systolic, diastolic and mean arterial blood pressures were measured, and saline perfusion urethral pressure profilometry was performed at 0, 10, 20, and 40 min following intravenous administration of prazosin (0.025 mg/kg), phenoxybenzamine (0.2 mg/kg), or placebo. Maximal urethral pressure, maximal urethral closure pressure, post peak nadir, and all blood pressure parameters decreased significantly at nearly all treatment intervals following administration of prazosin compared with placebo. Less consistently significant reductions were observed following phenoxybenzamine administration. Maximal decreases in urethral pressure parameters were observed 20 min following the injection of prazosin; maximal blood pressure decreases were evident by 10 min post- injection. In this non-sedated dog model, urethral pressure profilometry was a sensitive method of detecting urethral effects of alpha antagonists. Repeatable reductions in urethral pressure measurements were observed, with prazosin effecting more consistently significant changes than phenoxybenzamine. Significant decreases in systolic, diastolic, and mean arterial blood pressures were seen with prazosin, but not phenoxybenzamine or placebo. Further study of selective alpha-1 antagonists in dogs is needed to determine appropriate oral dosing protocols that will produce maximal urethral effects with minimal hemodynamic effects, and to demonstrate clinical efficacy in dogs with functional urethral obstruction.
Introduction
During the urine storage phase of micturition, stimulation of alpha-1 receptors in the bladder neck and proximal urethra narrow the bladder outlet maintaining continence. Inappropriate contraction of the bladder neck or muscular urethra during the voiding phase prevents normal urine outflow and is termed “functional outlet obstruction” or “functional urethral obstruction” (1,2). Traditionally, the drug of choice for the treatment of functional urethral obstruction in companion animals has been phenoxybenzamine (PBZ); a non-selective, non-competitive alpha-adrenergic antagonist. Phenoxybenzamine decreases urethral resistance in conscious and anaesthetized healthy male cats (3,4) and anaesthetized, healthy female dogs (5), as demonstrated by changes in urethral pressure profile (UPP) measurements. Phenoxybenzamine also blocks epinephrine-mediated urethral contraction (6), and urethral contraction induced by hypogastric nerve stimulation in dogs (7). Phenoxybenzamine clinically improves voiding in humans with functional urethral obstruction due to benign prostatic hyperplasia or spinal cord injury (8,9,10,11).
Phenoxybenzamine, often in combination with diazepam, has been empirically recommended to facilitate voiding in dogs with upper motor neuron bladders (detrusor-external sphincter dyssynergia) due to spinal trauma or disc disease, for other causes of increased urethral tone in dogs and in cats with post-obstructive voiding dysfunction (9,12,13,14,15,16). Clinical response to PBZ therapy has been variable, however. This variability may be related, at least in part, to the nature, severity, and chronicity of the obstructive disease (for example, partial cord lesion versus complete cord lesion; acute, reversible cord injury versus idiopathic functional obstruction) (1,3,4,8,9,15,17).
Phenoxybenzamine is considered obsolete for the treatment of functional urethral obstruction in humans, following the advent of selective alpha-1 antagonists and concerns regarding the potential carcinogenicity of PBZ (6,18,19). Peritoneal sarcomas, intestinal malignancies, and lung tumours have been reported in lab animals after repeated administration of PBZ. Mutagenicity has been demonstrated by the Ames test, a bacteria-based assay designed to screen for mutagenicity of chemical substances (20). These findings have rendered PBZ available in Canada only by emergency drug release, and it may well become unavailable in the United States in the future due to its limited use in human medicine and potential human carcinogenicity. Additionally, a recent change in manufacturer has resulted in a nearly 10-fold increase in the per-capsule cost of PBZ, limiting its practicality for use in companion animals (personal communication, Smith Kline French, 1999).
Selective alpha-1 antagonists; such as, prazosin (PRA), terazosin, doxazosin, and others, have become the cornerstone for functional urethral obstruction in humans. Prazosin and the other drugs in its class have high alpha-1:alpha-2 affinity ratios (for example, a ratio of 500–1000:1 for PRA) and their alpha-2 activity is not considered clinically significant (20). In general, the use of selective alpha-1 antagonists in humans produces a lower incidence and a lesser severity of adverse effects than the non-selective alpha antagonists (21). Initial studies examining the effect of selective alpha-1 antagonists on the lower urinary tract have produced promising results in humans, dogs, and cats. Multiple clinical trials in men with benign prostatic hyperplasia have indicated that prazosin and the other drugs in its class, safely and effectively improve both subjective voiding symptoms and measurable parameters associated with bladder outlet obstruction (for example, urine flow rates and volumes and UPP parameters) (6,22,23,24,25,26,27,28).
This study was performed to evaluate the potential of PRA, a readily available and economic alpha-1 antagonist, as an alternative to PBZ for the management of functional urethral obstruction in dogs. The specific study objectives were: 1) to evaluate the effects of PRA and PBZ on the UPP of normal, non-sedated male dogs; 2) to determine the hemodynamic effects of intravenous PRA and PBZ in non-sedated dogs; and 3) to determine if the UPP is a useful method of detecting the effect of alpha antagonists on the urethras of healthy, non-sedated male dogs.
Materials and methods
Animals
This study used 6 healthy, male research beagles (5 sexually intact, 1 castrated), group housed in the university animal research facilities and previously conditioned to UPP procedures as puppies. Physical examination, urinalysis, and urine cultures were performed on each dog prior to each study session. All study procedures were approved by the University of Prince Edward Island Animal Care Committee, and conformed to the guidelines set forth by the Canadian Council on Animal Care.
Drug preparation
Pharmacological agents were prepared within 24 h of each study session. Prazosin (Sigma Aldrich Canada, Oakville, Ontario) or PBZ (Wiler Fine Chemicals, London, Ontario) powder was dissolved into a carrier solution (10% ethanol, 40% propylene glycol, 50% saline) and then filtered through a 0.22 micron drug filter. The formulation of the carrier solution was empirically determined by the hospital pharmacist to be a base that both the prazosin and phenoxybenzamine powders would dissolve in, while minimizing the amount of ethanol used. Solution concentrations (PBZ at 0.5 mg/mL and PRA at 0.1 mg/mL) were formulated to provide each drug dose with approximately equivalent volumes of the carrier. The placebo (PL) solution consisted of the carrier alone.
Experimental design
Each dog received PRA, PBZ, and PL treatments. The order of treatment administration was randomized by the Latin square method. A 7- to 14-day washout period was allowed between each treatment. Food was withheld for 6 to 12 h preceding each study session, and dogs were allowed to void immediately before the UPP procedure was performed. At the beginning of each of the sessions, each dog was placed in left lateral recumbency. An 18 gauge, 3 inch indwelling catheter (Angiocath; Becton-Dickinson Medical Systems, Sandy, Utah, USA) was placed in the right jugular vein for drug administration and blood sampling. A blood pressure cuff was placed over the right dorsal pedal artery to provide an indirect oscillometric measurement of blood pressure (Dinamap Blood Pressure Monitor; Critikon Corporation, Tampa, Florida, USA). Baseline (0 min) heart rate, baseline UPP, and systolic (SYST); diastolic (DIAST); and mean arterial pressure (MAP) were performed and recorded.
After the baseline data were collected, PRA (0.025 mg/kg), PBZ (0.2 mg/kg), or PL (4.0 mL) was given intravenously over 2 min. Doses used for PRA and PBZ were extrapolated from previous studies on dogs and cats (3,5,7,24,25). Urethral pressure profile measurements were performed at 10, 20, and 40 min post-treatment. Heart rate and blood pressure were also measured at corresponding intervals. The dogs were in lateral recumbency only for actual performance of the UPP procedures (each procedure required approximately 2 to 3 min for positioning, catheter insertion, mechanical arm alignment, and catheter withdrawal). Between procedures dogs were free to sit, lie, or stand at will, and between the 20 and 40 min sessions they were permitted to roam in the procedure room. Attitude, mucous membrane colour, capillary refill time, and behaviour were recorded for each dog periodically throughout each study session. Dogs were continuously observed for 2 to 3 h following completion of the UPPs. One dose of ampicillin (22 mg/kg subcutaneously) was administered prophylactically to each dog at the end of each session to help prevent urinary tract infection.
Urethral pressure profiles
With the dog lightly restrained in lateral recumbency, the penis was extruded and cleaned with dilute chlorhexidine solution. A lubricated, 7 French, soft, dual lumen urethral catheter (Model DLC-7D; Life-Tech Incorporated, Houston, Texas, USA) was inserted through the urethral orifice into the bladder and the bladder was emptied of any residual urine. The urethral catheter port was connected to a pressure transducer by a fluid column allowing the continuous measurement and recording of urethral pressures by an integrated monitor (Urolab Janus System III; Life-Tech Incorporated). The urethral catheter was connected to a mechanical arm (Profilometer 1723; Life-Tech, Incorporated), which withdrew the catheter at a rate of 5 mm/s, while saline was infused at 5 mL/min by syringe pump (Model # 341B; Sage Instruments, Freedom, California, USA).
Data analysis
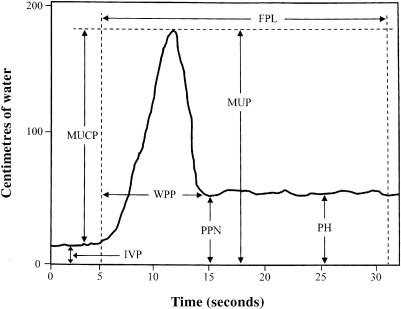
Computer generated measurements of the following variables were obtained from UPP tracings according to International Continence Society guidelines (Figure 1): intravesicular pressure (IVP) = the pressure recorded with the catheter tip within the urinary bladder; maximal urethral pressure (MUP) = the highest recorded pressure along the profile length; maximal urethral closure pressure (MUCP) = the difference between MUP and IVP; plateau height (PH) = pressure in the membranous (penile) urethra; and width of the prostatic peak (WPP) = a measure of the prostatic segment, measured from the beginning of the initial sharp pressure rise to the low point at the caudal edge of the prostate. In this study we also recorded post-peak nadir (PPN) defined as the lowest pressure recorded immediately after the prostatic peak. The functional profile length (FPL), defined as the length of the profile in which the recorded pressure exceeds the initial intravesicular pressure, was not measured due to the potential error introduced in resetting the withdrawal apparatus (necessary because of the length of these profiles). Though FPL was not specifically measured here, all UPPs were performed and assessed along the complete urethral length, from bladder to external urethral orifice, and the entire profile was visually reviewed in each instance.
Figure 1. Schematic male canine urethral pressure profile (UPP). FPL; functional profile length, MUCP; maximal urethral closure pressure, MUP; maximal urethral pressure, WPP; a measure of the prostatic segment, measured from the beginning of the initial sharp pressure rise to the low point at the caudal edge of the prostate, IVP; the pressure recorded with the catheter tip within the urinary bladder, PPN; post-peak nadir, and PH; pressure in the membranous (penile) urethra.
Statistical analysis
The data were analyzed using commercial statistical software (Release 8; Minitab, Incorporated, State College, Pennsylvania, USA). Differences in mean pre-treatment and post-treatment UPP measurements were determined within each treatment group by one-way analysis of variance (ANOVA). Differences in post-treatment UPP measurements among treatment groups were determined by Kruskal Wallis test for differences in the population medians and by one-way ANOVA for differences in the population means. Multiple comparisons to determine the specific differences between treatment groups were completed using Fisher's least significant difference method and Dunnett's procedure for comparing experimental treatments to a control (or placebo) group. The level of significance for all analyses was established at P < 0.05.
Results
Urethral pressure profilometry measurements
Comparison of mean pre- and post-treatment values within each group were performed for PL, PBZ, and PRA. This comparison showed a statistically significant difference in MUP, MUCP, and PPN between baseline and post-treatment intervals for PRA at all post-treatment intervals. No statistically significant differences were detected between baseline and post-treatment means for any parameter for PL or PBZ.
Significant differences in baseline UPP measurements were not observed among the 3 groups for any parameter. Statistically significant decreases between post-treatment MUP, MUCP, and PPN measurements were seen with PRA at all post-treatment intervals when compared with PL, with maximally diminished pressures observed at 20 min post-treatment. For MUP and MUCP, PRA values were not significantly different than PBZ values, though for PPN 10- and 20-minute values for PRA were significantly lower than for PBZ. For MUCP and MUP, PBZ was significantly lower than PL only at 20 min. For PH, PRA was lower than PL at 10 and 20 min, and lower than PBZ at 10 min; PBZ did not differ from PL. The 20 min WPP measurement showed differences between PRA and PBZ, but neither differed from PL and, thus, this finding was not considered important. No statistically significant differences among groups were observed in IVP at any post-treatment interval. These data are presented in Table I.
Table I.
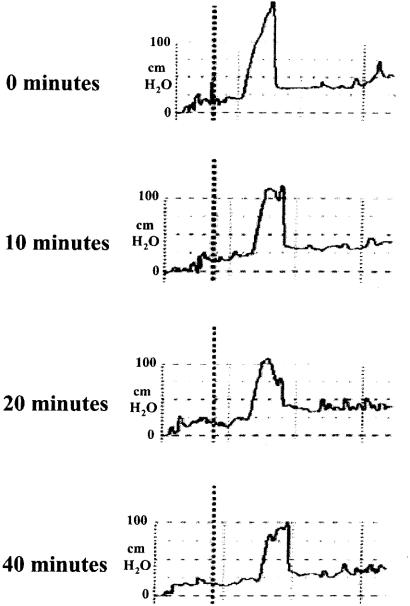
Examples of representative UPP traces from each of the 3 treatment groups are presented. The PL traces (Figure 2) are similar over the study period, and are also similar to the baseline traces for PBZ (Figure 3) and the 2 sets of PRA traces (Figure 4 and Figure 5). For PBZ (Figure 3), slight to moderate blunting of the maximal pressure peak following drug administration was a consistent observation. For PRA, 2 trends were observed. Some of the dogs demonstrated blunting of the maximal pressure peak which was similar, though more pronounced, than that seen with PBZ (Figure 4). While others demonstrated profound and sustained pressure decreases, almost obliterating the peak entirely (Figure 5).
Figure 2. Placebo — representative urethral pressure profile (UPP) traces.
Figure 3. Phenoxybenzamine — representative urethral pressure profile (UPP) traces.
Figure 4. Prazosin — representative urethral pressure profile (UPP) traces.
Figure 5. Prazosin — representative urethral pressure profile (UPP) traces.
Hemodynamic measurements
Baseline hemodynamic measurements and post-treatment heart rates were not different among the 3 groups for all hemodynamic parameters measured (Table II). No significant difference was found between the PL and PBZ groups for any post-treatment blood pressure parameter at any interval. Post-treatment SYS, DIAST, and MAP were significantly decreased after PRA administration compared with both PL and PBZ, with the exception of the 10 min MAP where PRA did not differ from PL. Maximal decreases for PRA were apparent by 10 min post-treatment and persisted up until the 40 min measurement.
Table II.
Adverse treatment effects
All treatments were administered intravenously over 2 min, with the dogs lightly restrained in lateral recumbency. Mild sedation (2 out of 6 dogs), lip-licking (2 out of 6 dogs), grimacing (1 out of 6 dogs), head-shaking (1 out of 6 dogs), and mild salivation (1 out of 6 dogs) were observed following PL administration. Mild to moderate sedation (1 out of 6 dogs), mild salivation (2 out of 6 dogs), panting (1 out of 6 dogs), lip-licking (2 out of 6 dogs), head-shaking (1 out of 6 dogs), injected mucous membranes (1 out of 6 dogs), and trembling (1 out of 6 dogs) were observed following PBZ administration. Salivation (3 out of 6 dogs), mild-moderate sedation (3 out of 6 dogs), moderate-marked sedation (2 out of 6 dogs), head-shaking (2 out of 6 dogs), injected mucous membranes (2 out of 6 dogs), grimacing (1 out of 6 dogs), and trembling (1 out of 6 dogs) were seen following PRA administration.
One dog developed a partial mechanical urethral obstruction, which rendered urethral catheterization difficult and uncomfortable, despite his continued ability to urinate normally. The obstruction became evident during this dog's 3rd set of procedures, which for him was the PRA session. This dog's UPP tracings at that point demonstrated pressure spikes in the plateau region of the profile, characteristic of focal mechanical obstruction, like that which might be produced by a partial urethral stricture. These spikes on the tracings corresponded to the site where difficulty with catheter passage was noted. This dog's prior UPPs were scrutinized; evidence of abnormal pressure spikes was not detected, nor was any difficulty or discomfort with urethral catheterization noted during prior sessions. Based on these observations, all data from this dog's first 2 sessions were included in the data analysis, but no data from his 3rd session was included. This dog was withdrawn from the study for humane reasons, without completing the 3rd session.
Discussion
In this study, intravenously administered PRA significantly and consistently lowered urethral pressures (MUP, MUCP, PPN), chiefly in the region of the prostatic urethra. The 2 described and illustrated responses to PRA (Figures 4 and 5) were qualitatively similar, though quantitatively different, and may well represent differing degrees of the same drug response. The decreases in UPP parameters observed in this study following PRA administration are generally consistent with previously reported male canine urethral effects of other alpha-1 antagonists, though serial, full UPPs in conjunction with alpha antagonist administration in non-sedated dogs have not previously been reported (6,22). Kenny et al (23) reported serial prostatic urethral pressure measurements following oral alpha-antagonist dosing, but the data gathered was limited to a single point in the prostatic urethra. The study reported here evaluated the response to alpha antagonists along the entire urethral length. Although FPL was not quantitated, UPPs were performed from the bladder to external urethral orifice at each study point and the entirety of each profile assessed visually. Maximal urethral pressure, MUCP, and WPP measurements quantitated the post-prostatic and distal urethral responses, respectively. Since the proximal urethra is the area of greatest urethral resistance in the dog, and is the portion of the canine urethra with the highest smooth muscle content (and should thus be most sensitive to the alpha-1 blockade), our investigation concentrated on this region (29,30).
Urethral pressure profilometry used to assess responses to drug administration, may be more clinically relevant when performed in non-sedated animals and the results may be more easily translated to patient populations. Such studies may also be interpreted without the potentially confounding influence of tranquilizers or anaesthetic agents on skeletal and smooth muscle. Additionally, drug administration to conscious, non-sedated animals allows for a more accurate assessment of incidental and potentially adverse effects; such as, mentation change, gastrointestinal signs, and clinical evidence of hypotension.
Significant decreases in SYST, DIAST, and MAP were seen with PRA administration, concomitant with decreases in urethral pressure parameters. Vascular smooth muscle is rich in alpha-1 receptors and hypotension is the most significant anticipated adverse effect of alpha-1 selective blockade used for bladder outlet obstruction. In humans, this most commonly clinically manifests as orthostatic (postural) hypotension when patients move quickly from sitting or recumbency to standing (usually as a “first dose” phenomenon) (19,21). Postural hypotension tends to be more frequent and severe in hypovolemic patients or patients on diuretics or other hypotensive agents. This effect is minimized by titrating up to therapeutic dosing over several days and by having patients take medication at bedtime (if once daily medication is used) (19,21). In this study, the dogs' mean DIAST remained greater than 60 mmHg and the MAP remained greater than 75 mmHg for all measured intervals. Unfortunately, blood pressures were only measured with the dogs in lateral recumbency, thus measurable postural differences in blood pressure were not evaluated. No clinical evidence of postural hypotension was detected, though the dogs moved several times from lateral recumbency to a standing position; even dogs who were somewhat sedated appeared to “snap out of it” when allowed to rise from a lateral position. All dogs were conscious, could right themselves, and could stand and walk without ataxia, incoordination, weakness, stumbling, or unsteadiness at all times during the sessions.
Sedation was the most frequent adverse effect noted in this study. Dogs from all 3 groups demonstrated some degree of tranquilization, indicating that the drug carrier may have been partially responsible for this effect. Dogs in placebo studies received 4.0 mL of carrier composed of 10% ethanol, 40% propylene glycol, and 50% saline, intravenously over 2 min. Though the dose of ethanol was small (approximately 0.025 mL/kg), the central depressant effects of even a small rapid intravenous bolus of ethanol can not be discounted. In addition, alpha-adrenergic antagonists may act within the central nervous system to suppress sympathetic outflow (20,31,32), and such a decrease in overall sympathetic tone could cause or compound sedative effects, as well as cause a degree of skeletal muscle relaxation. Several studies have shown that PRA suppresses central sympathetic and somatic nervous output, and does so more strongly than phentolamine, a non-selective antagonist similar to PBZ (31,32). The magnitude and frequency of sedation seen here appeared more dramatic for PRA than PBZ, and greater for PBZ than PL. These findings support a hypothesis that the combination of mild tranquilization from ethanol in the carrier plus central suppression of sympathetic outflow (which should be stronger with PRA than PBZ) was responsible for the varying degrees of sedation seen. Effects such as head-shaking, grimacing, lip-licking, and hypersalivation, tended to occur within 5 min of injection in all groups and were very transient, which may be supportive of a reaction to the carrier.
In the authors' experience (JRF and IFL), neither central effects nor clinical hypotension associated with oral prazosin administration (1 mg/15 kg PO q 12 h) is apparent in the normally hydrated canine or feline patient. This dosing protocol was extrapolated from standard canine doses of PRA used in the management of heart failure and hypertension; the low end of the dose range was used. Despite the noted lack of observed signs of hypotension in clinical patients, adherence to an incremental initial dosing regimen (1/2 of the calculated dose given for 48 h, then increased to the full calculated dose) and periodic monitoring of indirect blood pressure in the veterinary patient receiving alpha-1 antagonists seemed to be prudent surveillance measures. Particular attention should be paid to monitoring the patient that may already be predisposed to hypotension or hypovolemia, due to other disease processes or concurrent pharmacotherapy (for example, beta blockers, angiotensin converting enzyme inhibitors, or diuretics).
In conclusion, the UPP seems to be an appropriate and sensitive method of detecting the effects of alpha antagonists on the urethras of conscious, healthy male dogs. This model allows the relatively non-invasive completion of multiple studies over time without the risk, expense, or confounding effects of anaesthesia or sedation, and permits the collection of data in more clinically relevant circumstances. The authors' clinical experience with non-sedated UPP procedures in client-owned dogs corroborates their experience with the conditioned dogs used in this study; sedation is rarely needed for effective, repeated performance of this procedure in male dogs (12). Prazosin administration produced substantial reductions in MUP, MUCP, and PPN, as well as in measured blood pressure parameters, but did not produce clinically detectable hypotension. Adverse effects were minor, but were noted to be the most numerous and severe with PRA, PBZ administration resulted in fewer and less severe signs, and PL administration resulted in the fewest and mildest adverse effects. Prazosin appears to be an effective alternative alpha antagonist to PBZ in the male dog. Pharmacokinetic and dose-response studies are needed to determine the optimal oral dosing protocols, and clinical trials in dogs and cats with bladder outflow obstruction due to thoracolumbar spinal injury, dyssynergia, or urethrospasm will help confirm and delineate the clinical efficacy and value of this drug in veterinary medicine.
Footnotes
Acknowledgments
The authors thank the late Dr. Brian Hill and the Atlantic Veterinary College Department of Companion Animals for their generous support, Ms. Kendra Day for drug preparation, and Dr. Stephanie Rudich for procedural assistance.
Dr. Fischer's current address is Hemodialysis Center, University of California Veterinary Medical Center-San Diego, PO Box 9415, Rancho Santa Fe, California, USA 92067. Dr. Lane's current address is Department of Clinical Sciences, University of Tennessee, PO Box 1071, Knoxville, Tennessee, USA 37901-1071.
Address all correspondence and reprint requests to Dr. Julie R. Fischer; telephone: (858) 759-7235; fax: (858) 759-7234; e-mail: jrofischer@ucdavis.edu
Received February 4, 2002. Accepted June 27, 2002.
References
- 1.Chew DJ, DiBartola SP, Fenner WR. Pharmacologic manipulation of urination. In: Kirk RW, ed, Current Veterinary Therapy XI, Philadelphia, WB Saunders, 1986:1207–1212.
- 2.Fillippich LJ, Read RA, Riesz G. Functional urethral obstruction in a cat. Aust Vet Pract 1989;19:202–206.
- 3.Mawby DI, Meric SM, Crichlow EC, et al. Pharmacologic relaxation of the urethra in male cats: A study of the effects of phenoxybenzamine, diazepam, nifedipine, and xylazine. Can J Vet Res 1990;55:28–32. [PMC free article] [PubMed]
- 4.Marks SL, Straeter-Knowlen IM, Knowlen GG, et al. The effects of phenoxybenzamine and acepromazine maleate on urethral pressure profiles of anaesthetized healthy, sexually intact male cats. Am J Vet Res 1996;57:1497–500. [PubMed]
- 5.Khanna OP, Gonick P. Effects of phenoxybenzamine hydrochloride on canine lower urinary tract: Clinical implications. Urology 1975;6:323–330. [DOI] [PubMed]
- 6.Breslin D, Fields DW, Chou TC, et al. Medical management of benign prostatic hyperplasia: A canine model comparing the in vivo efficacy of alpha-1 adrenergic antagonists in the prostate. J Urol 1993;14:395–399. [DOI] [PubMed]
- 7.Poirier M, Riffaud JP, Lacolle JY, Dupont C. Effects of five alpha-blockers on the hypogastric nerve stimulation of the canine lower urinary tract. J Urol 1988;140:165–167. [DOI] [PubMed]
- 8.Caine M, Perlberg S, Meretyk S. A placebo-controlled double-blind study of the effects of phenoxybenzamine in benign prostatic obstruction. Br J Urol 1978;50:551–554. [DOI] [PubMed]
- 9.Abrams PH, Shah PJR, Stone R, Choa RG. Bladder outflow obstruction treated with phenoxybenzamine. Br J Urol 1982;54:527–530. [DOI] [PubMed]
- 10.Al-Ali M, Salman G, Rasheed A, et al. Phenoxybenzamine in the management of neuropathic bladder following spinal cord injury. Aust N Z J Surg 1999;69:660–663. [DOI] [PubMed]
- 11.Buczynski AZ. Urodynamic studies in evaluating detrusor sphincter dyssynergia and their effects on the treatment. Paraplegia 1984;22:168–172. [DOI] [PubMed]
- 12.Lane IF, Fischer JR, Miller E, Grauer GF, Lappin MR. Functional urethral obstruction in 3 dogs: Clinical and urodynamic findings. J Vet Intern Med 2000;14:43–49. [DOI] [PubMed]
- 13.Moreau PM. Neurogenic disorders of micturition in the dog and cat. Compend Contin Educ Pract Vet 1982;4:12–21.
- 14.Oliver JE. Dysuria caused by reflex dyssynergia. In Kirk RW, ed: Current Veterinary Therapy XIII. Philadelphia, WB Saunders, 1983;1088.
- 15.Barsanti JA, Coates JR, Bartges JW, Brown SA, Oliver JE, Finco DR. Detrusor-sphincter dyssynergia. Vet Clin North Am Small Anim Pract 1996;26:327–38. [DOI] [PubMed]
- 16.Diaz Espineira MM, Viehoff FW, Nickel RF. Idiopathic detrusor-urethral dyssynergia in dogs: A retrospective analysis of 22 cases. J Small Anim Pract 1998;39:264–270. [DOI] [PubMed]
- 17.Gookin JL, Bunch SE. Detrusor-striated sphincter dyssynergia in a dog. J Vet Intern Med 1997;10:339–344. [DOI] [PubMed]
- 18.Lepor H. Role of alpha adrenergic blockers in the treatment of benign prostatic hyperplasia. Prostate 1990;66:75–84. [DOI] [PubMed]
- 19.Carruthers SG. Adverse effects of α1-adrenergic blocking drugs. Drug Safety 1994;11:12–20. [DOI] [PubMed]
- 20.Hoffman BB, Lefkowitz RJ. Adrenergic receptor antagonists. In: Gilman AG, Rall TW, Nies AS, Taylor P, eds. Goodman and Gilman's The Pharmacologic Basis of Therapeutics. 8th ed. New York: Pergamon Press. 1980:221–243.
- 21.Jønler M, Riehmann M, Bruskewitz RC. Benign prostatic hyperplasia current pharmacologic treatment. Drugs 1994;47:66–81. [DOI] [PubMed]
- 22.Brune ME, Katwala SP, Milicic I, et al. Effects of selective and non-selective alpha-1-adrenoreceptors on intraurethral and arterial pressures in intact conscious dogs. Pharmacology 1996;53:356–368. [DOI] [PubMed]
- 23.Kenny BA, Miller AM, Williamson IJ, et al. Evaluation of the pharmacological selectivity profile of alpha 1 adrenoceptor antagonists at the prostatic alpha 1 adrenoceptors: binding, functional and in vivo studies. Br J Pharmacol 1996;118:871–878. [DOI] [PMC free article] [PubMed]
- 24.Frenier SL, Knowlen GG, Speth RC, Moore MP. Urethral pressure response to alpha-adrenergic agonist and antagonist drugs in anaesthetized male cats. Am J Vet Res 1992;53:1161–1165. [PubMed]
- 25.Straeter-Knowlen IM, Marks SL, Rishniw M, Speth RC, Wirth W, Knowlen GG. Urethral pressure response to smooth and skeletal muscle relaxants in anaesthetized, adult male cats with naturally acquired urethral obstruction. Am J Vet Res 1995;56:919–923. [PubMed]
- 26.Lepor H. The role of alpha blockade in the therapy of benign prostatic hyperplasia. In: Lepor H, Lawson RK, eds. Prostatic Diseases. WB Saunders Co. Philadelphia, 1993.
- 27.Lepor H. Medical therapy for benign prostatic hyperplasia. Urology 1993;42:483–501. [DOI] [PubMed]
- 28.Nakamura K, Kawashita E, Osumi Y. Effects of prazosin HCl on the urethral pressure profile in patients with benign prostatic hyperplasia. Urol-Int 1990;45:30–35. [DOI] [PubMed]
- 29.Awad SA, Downie JW. Relative contributions of smooth and striated muscles to the canine urethral pressure profile. Br J Urol 1976;48:347–354. [DOI] [PubMed]
- 30.Gookin JL, Stone EA, Sharp NJ. Urinary incontinence in dogs and cats Part I: urethral pressure profilometry. Compend Contin Educ Pract Vet 1996;18:407–418.
- 31.Gajewski J, Downie JW, Awad SA. Experimental evidence for a central nervous system site of action in the effect of alpha-adrenergic blockers on the external urinary sphincter. J Urol 1984;133:403–409. [DOI] [PubMed]
- 32.Danuser H, Thor K. Inhibition of central sympathetic and somatic outflow to the lower urinary tract of the cat by the alpha 1 adrenergic receptor antagonist prazosin. J Urol 1995;153:1308–1312. [PubMed]