Abstract
The formation of estrogens from C19 steroids is catalyzed by aromatase cytochrome P450 (P450arom), the product of the cyp19 gene. The actions of estrogen include dimorphic anatomical, functional, and behavioral effects on the development of both males and females, considerations that prompted us to examine the consequences of deficiency of aromatase activity in mice. Mice lacking a functional aromatase enzyme (ArKO) were generated by targeted disruption of the cyp19 gene. Male and female ArKO mice were born with the expected Mendelian frequency from F1 parents and grew to adulthood. Female ArKO mice at 9 weeks of age displayed underdeveloped external genitalia and uteri. Ovaries contained numerous follicles with abundant granulosa cells and evidence of antrum formation that appeared arrested before ovulation. No corpora lutea were present. Additionally the stroma were hyperplastic with structures that appeared to be atretic follicles. Development of the mammary glands approximated that of a prepubertal female. Examination of male ArKO mice of the same age revealed essentially normal internal anatomy but with enlargement of the male accessory sex glands because of increased content of secreted material. The testes appeared normal. Male ArKO mice are capable of breeding and produce litters of approximately average size. Whereas serum estradiol levels were at the limit of detection, testosterone levels were elevated, as were the levels of follicle-stimulating hormone and luteinizing hormone. The phenotype of these animals differs markedly from that of the previously reported ERKO mice, in which the estrogen receptor α is deleted by targeted disruption.
The final step in the biosynthesis of estrogens from C19 steroids is catalyzed by aromatase cytochrome P450 (P450arom) the product of the cyp19 gene (1). Aromatase activity is present in many human tissues, and the tissue-specific expression of this gene is regulated by means of tissue-specific promoters using alternative splicing, but the protein translated from the message is the same in all tissues (2).
A number of cases of aromatase deficiency in humans caused by mutations in the cyp19 gene have been reported (3–9). In the case of the females this condition leads to an autosomal-recessive form of female pseudohermaphroditism and virilization of the mother during pregnancy, as a consequence of impaired or absent conversion of fetal and maternal androgens to estrogens by the placental syncytiotrophoblasts. Subsequently, the consequences of aromatase deficiency at puberty in affected females include pubertal failure, hypergonadotropic hypogonadism, virilization, cystic ovaries, delayed bone age, and the potential for tall stature.
In the case of the males, childhood development appears unremarkable; however, there is continued linear bone growth throughout puberty, resulting in tall stature, delayed bone age, and osteopenia with failure of epiphysial closure in the affected young adult males. One of these individuals was placed on estrogen replacement with resulting marked improvement in his bone mineralization indices (7). Additionally, this individual had subnormal testicular volume and oligospermia, whereas the other individual had apparently normal testicular function (6).
Recently a mouse was generated in which the estrogen receptor gene (now recognized to be the α isoform) was inactivated by insertional disruption (the ERKO mouse) (10). Ovaries from homozygous mutant females contained cystic hemorrhagic follicles with few, if any, granulosa cells. A few primary follicles were present but no corpora lutea. Males homozygous for the condition were also infertile with testicular atrophy and seminiferous tubule dysmorphogenesis, resulting in decreased spermatogenesis and inactive sperm. Interpretation of these findings is complicated by the recent description (11) of a second estrogen receptor (the β isoform), which is still active in the ERKO mice (12). In an effort to resolve some of these discrepancies, as well as to investigate further the full range of phenotypic actions of estrogens in both males and females, we generated mice in which the aromatase gene was inactivated by targeted disruption. In this way we sought to remove the source of estrogen rather than neutralize its action. Our preliminary findings are presented here.
MATERIALS AND METHODS
Genomic Clone Isolation.
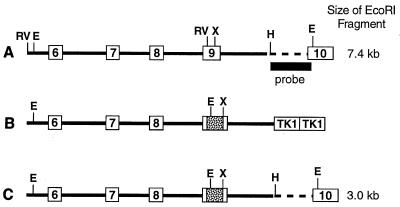
A 14.3-kilobase (kb) genomic clone containing exons V through IX of the mouse cyp19 gene was isolated from a J129/sv λFIXII (Stratagene) library using a rat aromatase cDNA probe (nucleotides 673-1268) (13). A portion of the structure of the gene is shown in Fig. 1A. The exonic sequences and boundaries were confirmed by comparison with the mouse cDNA sequence (14).
Figure 1.
Diagram of part of the structure of the wild-type mouse cyp19 gene (A), the targeting vector (B), and the disrupted gene (C).
Targeting Vector Construction.
The targeting plasmid pPollshort-neoPA-HSVTK used in this study has been described elsewhere (15). Briefly, the plasmid contains the positive selection marker, neo, driven by the murine RNA polymerase II promoter and followed by the bovine growth hormone 3′ untranslated region. Two herpes simplex virus-thymidine kinase (HSV-TK) genes (type 1) are inserted in tandem at the unique XhoI site after the neo gene. Exon IX of the mouse cyp19 gene was selected for disruption. A 5.5-kb EcoRV fragment spanning intron 5 to the EcoRV site within exon IX was subcloned upstream of the neo gene in the targeting plasmid. The targeting plasmid contains a unique BamHI site upstream of the neo gene, which was filled in using Klenow enzyme to produce blunt ends suitable for ligation of the EcoRV fragment. After confirmation that the EcoRV fragment was ligated in the correct 5′-to-3′ orientation this incomplete targeting plasmid was digested with XhoI and a 1.2-kb XhoI–SalI fragment containing the 3′ portion of exon IX and the remaining portion of intron 9 isolated from the genomic clone was inserted. The structure of the completed targeting plasmid (Fig. 1B) was confirmed by restriction enzyme digestion and sequencing. The aromatase gene sequence, neo and HSV-TK genes are all in the same 5′-to-3′ orientation.
Exon IX of the mouse cyp19 gene was selected for disruption because the coding region sequence between the EcoRV (bp 1047) and XhoI (bp 1210) sites present in this exon is highly conserved among all aromatase cDNAs reported thus far (2). Computer alignment of the aromatase amino acid sequence with the amino acid sequence of several crystallized cytochrome P450 enzymes (16) indicated this sequence is involved in several important structural features of the enzyme, namely the K and K′ helices as well as several of the β-sheet regions. Insertion of the neo gene between these two restriction sites in exon IX deleted 163 bp of coding sequence (amino acid residues 349–403).
Embryonic Stem (ES) Cell Culture.
KG-1 (J129) ES cells were grown in DMEM with 15% fetal bovine serum/0.1 mM MEM nonessential amino acids/0.1 mM β-mercaptoethanol/2 mM glutamine on neomycin-resistant SNL 6–76 feeder layers to maintain an undifferentiated state. Ten million cells were electroporated (275 volts, 330 μF) with 50 μg of linearized targeting plasmid. Cells were replated onto SNL 6–76 feeder layers and neomycin selection (300 μg active compound/ml) began the next day and was continued for 9 days. On day 3, 0.2 μM 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl-5-iodouracil was added to the media for 2 successive days. Colonies surviving the drug selection procedure were split with one-half being frozen under oil at −80°C and the remaining half grown up for DNA extraction.
Screening of Targeted Clones.
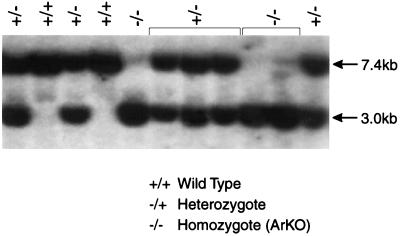
DNA was extracted from each individual clone and analyzed by restriction digestion and Southern blot analysis. Introduction of the neo gene also introduced an EcoRI restriction site used to detect the targeted allele (Fig. 1B). DNA from the ES cell clones was digested with EcoRI and subjected to electrophoresis on a 0.8% agarose in 0.5× TBE gel (90 mM Tris boric acid/2.5 mM EDTA, pH 8.3). After transfer to Hybond NEO+ (Amersham) the blots were UV-crosslinked, prehybridized in Church’s buffer (50 mM sodium phosphate/1 mM EDTA/1% bovine serum albumin, pH 7.2) with 1% SDS (17), and probed with a HindIII–EcoRI fragment (Fig. 1A). The probe hybridizes to the 3′ end of intron 9 and the 5′ portion of exon X up to the internal EcoRI site within exon X. This region is not included within the targeting construct (Fig. 1C). After hybridizing overnight at 65°C, the blots were washed in 2× standard saline citrate (SSC), 0.1% SDS twice at room temperature for 20 min and once at 60°C in 0.5× SSC, 1% SDS for 10 min. and then exposed to film (Hyperfilm MP, Amersham) for 1–3 days at −80°C. The wild-type allele results in a band of expected size, 7.4 kb, whereas the targeted allele gives a band of 3.0 kb (Fig. 2).
Figure 2.
Southern blot analysis of tail DNA of offspring from a heterozygous mating pair. For details, see text.
Mouse Breeding.
The targeted ES cells were injected into blastocysts from C57BL/6J females and implanted into pseudopregnant ICR mothers to proceed to term. Male chimeras were identified by coat color and bred to C57BL/6J females to determine germ-line transmission of the targeted allele. Tail DNA from agouti coat-colored F1 offspring were screened by Southern blot analysis as described above. Heterozygous F1 animals were interbred to produce F2 generations, which also were screened by Southern blotting.
Aromatase Activity.
Aromatase activity was measured in minced ovaries from wild-type and ArKO mice by the tritiated water release assay (18). Ovaries were dissected from 12-week-old animals, weighed, and placed in 2 ml of DMEM without serum in a 35-mm tissue culture dish. For controls a portion of the quadraceps muscle of an equivalent weight as the gonad was dissected from each animal and placed in a separate dish. Tissues were minced with two 23-gauge needles in a scissor-like fashion until the tissue was disrupted. Tissues then were incubated with 300 pmol of [1β-3H] androstenedione for 24 hr with or without the addition of 100 nM Arimidex (Zeneca, Wilmington, DE), a high-affinity high specificity inhibitor of aromatase. Results were calculated as fmol of [3H]water released per 24 hr per mg of tissue after subtraction of the cpm obtained for the quadraceps muscle samples.
Steroid and Gonadotropin Assays.
Steroid analyses were performed for estradiol, progesterone, testosterone, and androstenedione by RIA at the Oregon Regional Primate Center, Beaverton, OR by David L. Hess. Serum gonadotropins were determined by using the National Institute of Arthritis, Digestive Disease and Kidney (NIADDK)-anti rFSH-II and NIADDK-anti rLH-S-II antisera. The standards used were rat follicle-stimulating hormone (FSH) AFP 11454B and rat luteinizing hormone (LH) AFP 10250C. For the steroid assays, animals of 12–14 weeks were used. For the gonadotropin assays, all of the wild-type females were in estrus as gauged by labial swelling, but their ages varied. Three hundred microliters of serum per animal was collected for the steroid assays and 450 μl per animal for the gonadotropin assays.
RESULTS
Gene Targeting.
After electroporation of the targeting construct (Fig. 1B) into the ES cells followed by drug selection, 144 surviving colonies were screened by Southern blot analysis. Nine of the 144 clones gave the expected sized bands of 7.4 kb for the wild-type allele and 3.0 kb for the targeted allele (Fig. 1C), indicating that homologous recombination had occurred. Southern blot analysis of DNA from these clones with a neo coding region probe confirmed that the neo gene segregated with the 3.0-kb allele as expected and also indicated that the neo insertion was at a single site (data not shown).
Production of F2 Generations.
Five ES cell clones were expanded for injection into blastocysts. Clones 76 and 91 were pooled as well as clones 107 and 108 for the injections. Clone 100 was injected alone. Altogether these ES cell clones produced 19 male chimeras and one female chimera. Three male chimeras from clones 76 + 91, two male chimeras from clone 100, and two male chimeras from clones 107 + 108 were bred to C57BL/6J females for production of an F1 generation. The female chimera also was bred to a fertile male but proved to be sterile. The three chimeras from clones 76 + 91 and the two chimeras from clone 100 transmitted the targeted allele through their germ line and led to the production of two lines of transgenic animals. The chimeras from clones 107 + 108 did not transmit the targeted allele. All F1 offspring were screened by Southern blot analysis, and heterozygous male and female mice were paired for production of homozygous animals in the F2 generation. Fig. 2 shows a representative Southern blot for offspring from a heterozygous mating pair. Animals from both lines (clones 76 + 91 and clone 100) were examined, and the phenotypes were found to be identical. All data reported in this paper are from animals derived from clones 76 + 91.
Reproductive Performance of the F1 Generation.
Six pairs of F1 heterozygotes were allowed to produce 37 litters of offspring with an average litter size of nine pups. As shown in Table 1, the mutant allele was transmitted to the F2 generation with the expected Mendelian inheritance pattern. An approximately equal number of male and female offspring were produced.
Table 1.
Genotype frequency in the F2 generation
| +/+ | +/− | −/− | Total | |
|---|---|---|---|---|
| Males | 50 (14.3) | 92 (26.4) | 36 (10.3) | 178 (51) |
| Females | 38 (10.9) | 89 (25.5) | 44 (12.6) | 171 (49) |
| Total | 88 (25.2) | 181 (51.9) | 80 (22.9) | 349 (100) |
Offspring from a total of 37 heterozygous matings were genotyped and the numbers of male or female offspring of each genotype are reported: wild type (+/+), heterozygous (+/−), and homozygous mutant (−/−). Numbers in parentheses are the percentage of offspring in each group.
The ArKO Phenotype.
Mice homozygous for the disrupted aromatase gene (ArKO) are born phenotypically normal. At approximately 3 weeks of age the coats of both male and female ArKO animals are dull compared with those of littermates. As the animals age, the labial folds of the female ArKO mice do not develop. At sexual maturity, female ArKO mice begin to develop a male body habitus with excessive internal fat deposition (data not shown). Male ArKO mice appear to develop normally with the exception of hair coat dullness noted above.
Gross necropsy of young mature animals at approximately 12–14 weeks of age revealed underdeveloped uteri and ovaries lacking corpora lutea in the female ArKO mice. The clitoral glands also were enlarged approximately 3-fold over age-matched control females (Table 2). The internal anatomy of the male ArKO mice of the same approximate age appeared normal with the exception of increased organ weights for the seminal vesicles and combined urinary bladder/prostate weights (Table 2). The increased weights of the seminal vesicles appeared to be caused by increased volume of secretions as opposed to structural changes (Table 3). There was no difference in the testicular weights, nor in the weights of brain, heart, lungs, liver, kidney, or spleen for any group (data not shown). In the ArKO females the mammary and gonadal fat pads were increased in weight by 50–80%. In the males, the gonadal fat pads were increased in weight by 50% (data not shown).
Table 2.
Organ weights, g
| Wild type | ArKO | ||
|---|---|---|---|
| Females | |||
| Uterus | 0.15 ± 0.02 | 0.07 ± 0.01 | P < 0.001 |
| Clitoral gland | 0.01 ± 0.005 | 0.04 ± 0.006 | P = 0.008 |
| Males | |||
| Testes | 0.25 ± 0.005 | 0.24 ± 0.02 | NS |
| Seminal vesicles | 0.25 ± 0.02 | 0.39 ± 0.01 | P < 0.001 |
| Bladder/prostate | 0.08 ± 0.005 | 0.12 ± 0.01 | P = 0.006 |
Mice ranging in age from 12–14 weeks were sacrificed, and organs were removed, blotted, and weighed on an analytical balance. Values are reported as means ± SEM. Significance was determined by using the Student’s t test. NS, not significant.
Table 3.
Serum steroid hormone concentrations
| Females
|
Males
|
|||||||
|---|---|---|---|---|---|---|---|---|
| E2 | P4 | T | A4 | E2 | P4 | T | A4 | |
| Wild type | ||||||||
| 1. | 5 | 3 | 247 | 97 | 2 | 1 | 203 | 41 |
| 2. | 25 | 52 | 252 | 76 | 2 | 1 | 1,520 | 35 |
| 3. | 4 | 2 | 198 | 209 | 5 | 3 | 3,370 | 103 |
| 4. | 4 | 0 | 116 | 9 | 3 | 1 | 10,460 | 206 |
| 5. | 4 | 3 | 251 | 63 | 5 | 5 | 1,320 | 63 |
| 6. | 4 | 20 | 497 | 132 | 4 | 1 | 230 | 32 |
| ArKO | ||||||||
| 1. | — | 1 | 2,440 | 160 | 4 | 2 | 19,400 | 488 |
| 2. | — | 2 | 2,150 | 117 | 3 | 3 | 24,590 | 628 |
| 3. | 3 | 5 | 2,060 | 52 | 3 | 1 | 740 | 53 |
| 4. | 8 | 1 | 3,710 | 87 | 3 | 1 | 8,910 | 124 |
| 5. | 6 | 1 | 1,390 | 113 | ||||
Data are presented for serum from each animal analyzed.
Steroid hormone values are in pg/ml.
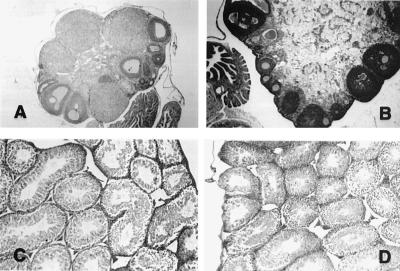
Histology of the ovaries of the homozygous females at 12–14 weeks showed the presence of many large follicles filled with granulosa cells and evidence of antrum formation, but no corpora lutea (Fig. 3). An occasional mitotic figure was evident. The stromal cells were hypertrophied, and there were a number of structures suggestive of atretic follicles. Although the uteri of these animals were markedly diminished in size (Table 2) they appeared normal on histological examination.
Figure 3.
Optical micrographs of ovaries (A and B) and testes (C and D) of wild-type (A and C) and ArKO (B and D) mice at 12–14 weeks of age. (Magnification: A, ×10; B–D, ×20.)
The male ArKO reproductive tract also appeared normal at the cellular level with the exceptions noted above, and the gross morphology of the testes at the light microscope level also appeared normal. Sperm were present in the testis and epididymis.
Steroid and Gonadotropin Assays.
With the exception of one wild-type female animal the serum estradiol concentrations for all animals were beneath the level of detection for the assay (Table 3; ≤6–8 pg/ml). In this one case the estradiol level was elevated, as was the progesterone. Progesterone levels were basal for all groups except for two wild-type females, including the one above, indicating that those two animals were in the diestrous/estrous phase of the cycle. Testosterone concentrations were markedly elevated in the female ArKO mice, to values approximately 10 times those of wild-type females. Testosterone levels varied among ArKO males; however, there was a definite trend toward elevated levels above those of the wild-type males. Androstenedione concentrations varied among animals within each group with the only notable elevation occurring in the ArKO males where several of the group had values at least twice as high as values reported for the control males.
FSH and LH assays revealed (Table 4) that LH levels were elevated 2- to 10-fold in the ArKO females as compared with wild type, and also in the ArKO males as compared with wild type. ArKO females also had elevated FSH values as compared with wild type (3- to 4-fold). However, insufficient serum was available from the male mice to allow determination of FSH levels.
Table 4.
Serum gonadotropin concentrations
| Females
|
Males
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mFSH, ng/ml | 95% | Limits | mLH, ng/ml | 95% | Limits | mFSH, ng/ml | mLH, ng/ml | 95% | Limits | |
| Wild type | 3.4 | 2.1 | 5.3 | 1.8 | 1.5 | 2.1 | ND | <0.1 | <0.1 | <0.1 |
| 1.8 | 1.0 | 3.1 | 1.7 | 1.4 | 2.0 | <0.1 | <0.1 | <0.1 | ||
| 5.8 | 3.7 | 8.8 | 2.3 | 2.0 | 2.7 | 0.3 | 0.2 | 0.3 | ||
| 2.7 | 1.7 | 4.2 | 0.3 | 0.3 | 0.4 | 0.1 | 0.1 | 0.2 | ||
| 3.3 | 2.0 | 5.0 | 0.2 | 0.2 | 0.3 | 0.1 | <0.1 | 0.2 | ||
| ArKO | 16.5 | 11.1 | 24.1 | 3.2 | 2.7 | 3.7 | ND | 0.9 | 0.8 | 1.1 |
| 10.9 | 7.3 | 16.0 | 2.7 | 2.4 | 3.2 | 0.3 | 0.2 | 0.3 | ||
| 9.9 | 6.5 | 14.7 | 3.9 | 3.4 | 4.6 | 1.2 | 1.0 | 1.4 | ||
| 8.7 | 5.7 | 12.8 | 3.6 | 3.1 | 4.2 | 0.4 | 0.3 | 0.5 | ||
| 9.8 | 6.5 | 14.4 | 3.5 | 3.0 | 4.1 | 1.8 | 1.5 | 2.1 | ||
Gonadotropins were measured in wild-type female mice that were in estrous as evidenced by labial swelling and reddening of vaginal membranes. ND, not determined.
Aromatase Assay.
As indicated in Table 5, there was no detectable aromatase activity in the ArKO female ovary whereas the activity of the wild-type female ovary was 136 ± 6.6 fmol/mg wet wt/24 hr. Treatment of the wild-type ovary with 100 nM Arimidex (Zeneca), a specific, high-affinity inhibitor of aromatase, resulted in an approximately 14-fold reduction in activity.
Table 5.
Aromatase activity of ArKO and wild-type ovaries
| Ovary | fmol [3H]water released/ mg wet wt/24 h |
|---|---|
| +/+ Wild-type ovary | 136 ± 6.6 |
| +/+ Wild-type ovary + Arimidex | 10 ± 3.5 |
| ArKO ovary | ND |
Ovaries from each of three animals per genotype were dissected, minced, and incubated with substrate +/− addition of Arimidex, a potent inhibitor of aromatase, as described in the text.
ND, not detectable.
Estrogen Response.
To determine whether the uteri of the ArKO mice were responsive to estrogen, 12-week-old wild-type and ArKO female mice were given 500 μg of Premarin (Wyeth-Ayerst Laboratories, Marietta, PA) conjugated equine estrogens i.p. for 6 days. Control groups of ArKO and wild-type females were injected with sterile vehicle for the same duration. The day after the last dose of test substance the animals were sacrificed, and the ovaries, uteri, cervix, and upper two-thirds of the vagina were carefully dissected and weighed. At that time the uteri of the ArKO females had become hyperemic. Uterine wet weights were approximately equivalent in the Premarin-treated ArKO and wild-type groups. The uterine wet weights of the vehicle-treated ArKO females were approximately 3-fold lower than those of the Premarin-treated groups (data not shown).
Fertility.
Male ArKO mice are capable of siring litters of average size (eight pups vs. nine pups for heterozygous pairs) when mated with heterozygous females. Four male ArKO mice (8–11 weeks of age) were placed with sexually mature heterozygous females, and each male sired a litter approximately 3 weeks later. Thereafter, only two of the males sired litters within another 3–4 weeks. A third male sired a litter within 6 weeks of the birth of the first litter, and the fourth male never sired another litter over the ensuing 11 weeks. Of the two males siring two litters within their first two consecutive breeding cycles one male sired two litters with two separate females approximately 6 weeks after the birth of his last litter and the second male consistently has sired litters approximately every 6 weeks up until the time of writing, when he was 6 months old.
DISCUSSION
The present work represents the beginning of a dissection of the phenotype of mice in which the activity of aromatase, the enzyme responsible for estrogen biosynthesis, is eliminated by targeted disruption of the cyp19 gene (ArKO mice). The efficacy of this treatment is indicated by the lack of aromatase activity in the ovaries of female mice homozygous for the mutation, as well as by the absence of detectable levels of estradiol in the plasma of these mice. On the other hand, testosterone levels are markedly elevated, especially in the females, as are the levels of the gonadotropins, LH, and FSH. This finding is indicative of the important role of estrogens in the negative feedback regulation of gonadotropins in both male and female. However, a number of wild-type females had undetectable estradiol levels. For technical reasons, gonadotropins were determined in a different group of animals from those in which steroid analyses were performed. Further determinations using larger groups of animals are required to clarify these points. The high testosterone levels in the females presumably reflect stimulation of the cells of the theca interna by LH, and the high testosterone levels in the male presumably reflect stimulation of the interstitial cells of the testes by the high circulating LH levels.
These findings are similar to those previously reported for both ArKO-deficient male and female humans (6, 9, 19, 20), as well as the estrogen receptor-deficient male (21), and for the ERKO mice in which the estrogen receptor is inactivated by targeted disruption (10), except that in the latter, serum estradiol levels are markedly elevated (12). However, there are several important differences between the phenotype of the ArKO mice and that of the ERKO mice. In the first place, the morphology of the ovaries is strikingly different. Whereas the ERKO mice had hyperemic cystic ovaries with few granulosa cells (10), the ArKO mice had ovaries in which the follicles contained numerous granulosa cells and displayed signs of antrum formation, as well as mitotic figures. However their development was arrested at a stage before ovulation and hence there were no corpora lutea and thus progesterone levels in the plasma were at the limit of detectability. Additionally the ovaries had hyperplastic stroma, indicative of overstimulation by LH, although there was no thickening of the theca interna layer. Additionally, there were a number of figures in the stroma, which may be indicative of atretic follicles.
Although the ERKO mice originally were considered to be lacking totally in estrogen action, it is now apparent that these mice lack the α form of the estrogen receptor, but continue to express the β isoform. Because the β isoform is abundantly expressed in ovarian granulosa cells and serum estradiol levels are markedly elevated in these animals (12), the difference in phenotype between the two mutant mice models may be a consequence of the continuing action of estrogen via the β form of the receptor in the ovaries of the ERKO mice, although why this should result in the observed differences is not entirely apparent at this time.
Another major difference between the two groups of mice lies in the male phenotype, in that the male ERKO mice display markedly diminished fertility and a grossly dysmorphic seminiferous epithelium (10). The ArKO mice displayed more or less normal fertility when young, although a decreased rate of litter siring became apparent with advancing age. Consistent with this finding, the gross morphology of the testes at the light microscopy level in the younger animals appeared normal. Hess et al. (22) recently presented evidence that estrogen regulates reabsorption of luminal fluid in the head of the epididymis, through the action of the estrogen receptor α. Inhibition of this reabsorption in the ERKO mice and resulting back pressure was suggested to be responsible for the testicular atrophy observed from day 20 onward. However if this was the only action of estrogen in the testes, then the ArKO mice should display a similar phenotype. The fact that this is not the case suggests that other effects of estrogen, perhaps mediated via the β isoform of the receptor, which is expressed in Sertoli cells, spermatogonia, and pachytene spermatocytes of the rat (23), also play a role. Detailed analysis of the morphology of the testes of the ArKO mice may help to resolve these issues. Nevertheless, these observations raise the possibility that estrogen might have a direct action on the testes to diminish testicular function, in addition to its action to suppress gonadotropin release at the level of the hypothalamic/pituitary axis.
It is also possible that undefined estrogenic substances are present in the circulation of these animals other than C18 steroids, which interact with the estrogen receptors and thus blunt the response of the ArKO mice to aromatase deficiency. To rule out the possibility that dietary factors could play a role, we currently are studying the effects of a phytoestrogen-free diet on the ArKO phenotype. Preliminary results would indicate that this diet has little effect (data not shown).
In terms of the parallel to humans with aromatase deficiency, the appearance of the ovaries is somewhat different because large cystic follicles in various stages of atresia were noticed in the women with aromatase deficiency (5, 6, 9). These were not apparent in the ovaries of the ArKO mice at 12–14 weeks, although the ovaries of older and younger animals have not yet been evaluated. Hemorrhagic cystic follicles were noted in the ovaries of the ERKO mice (10). The ovaries of the ArKO mice are also somewhat different from those of women with polycystic ovarian disease, which display numerous small cystic follicles in various stages of atresia.
The situation with respect to the phenotype of the ArKO males remains somewhat confused because one of the men with this mutation had apparently normal testicular development (6), whereas the other had low testicular weight and oligospermia (7); however, the latter individual has a sibling with complete azoospermia and no aromatase deficiency. So in this case the situation may be confused by a second genetic condition in this consanguinous family.
In conclusion, the development of this model of estrogen insufficiency will throw light on the role of estrogens in both male and female physiology and pathophysiology. Preliminary results indicate specific phenotypes of the adipose tissue, the bones, and the skin, in addition to the reproductive organs. Studies currently are underway to analyze these phenotypes as well as the biochemical basis underlying them. Additionally we are investigating potential neuroendocrinological and behavioral aspects of estrogen deficiency by using this model.
Acknowledgments
We thank Faye Coates for skilled editorial assistance and Professor J. K. Findlay for critically reviewing the manuscript. This work was supported, in part, by National Institutes of Health Grants R37AG08174 and R01HD13234, as well as the Victorian Breast Cancer Research Consortium, Melbourne, Australia.
ABBREVIATIONS
- ArKO
aromatase-deficient
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- kb
kilobase
- ES
embryonic stem
References
- 1.Nelson D R, Kamataki T, Waxman D J, Guengerich F P, Estabrook R W, Feyereisen R, Gonzalez F J, Coon M J, Gunsalus I C, Gotoh O, et al. DNA Cell Biol. 1993;12:1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- 2.Simpson E R, Zhao Y, Agarwal V R, Michael M D, Bulun S E, Hinshelwood M M, Graham-Lorence S, Sun T, Fisher C R, Qin K, Mendelson C R. Rec Prog Horm Res. 1997;52:185–214. [PubMed] [Google Scholar]
- 3.Shozu M, Akasofu K, Harade T, Kubota Y. J Clin Endocrinol Metab. 1991;72:560–566. doi: 10.1210/jcem-72-3-560. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Fisher C R, Conte F A, Grumbach M M, Simpson E R. Proc Natl Acad Sci USA. 1993;90:11673–11677. doi: 10.1073/pnas.90.24.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conte F A, Grumbach M M, Ito Y, Fisher C R, Simpson E R. J Clin Endocrinol Metab. 1994;78:1287–1292. doi: 10.1210/jcem.78.6.8200927. [DOI] [PubMed] [Google Scholar]
- 6.Morishima A, Grumbach M M, Simpson E R, Fisher C, Kenan Q. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 7.Carani C, Qin K, Simoni M, Fanstini-Fustini M, Serpente S, Boyd J, Korach K S, Simpson E R. N Engl Med J. 1997;337:91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 8.Portrat-Doyen S, Forest M G, Nicolino M, Morel Y, Chatelain P C. 10th Int. Cong. Endocrinol. San Francisco. 1996. p. 586. abstr. [Google Scholar]
- 9.Mullis P E, Yoshitawa N, Kuhlmann B, Lippuner K, Jaeger P, Harada N. J Clin Endocrinol Metab. 1997;82:1739–1745. doi: 10.1210/jcem.82.6.3994. [DOI] [PubMed] [Google Scholar]
- 10.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuiper G G, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J A. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couse J F, Lindsey J, Grandrian K, Gustafsson J A, Korach K S. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 13.Hickey G T, Krasnow J S, Beattie W G, Richards J S. Mol Endocrinol. 1990;4:3–12. doi: 10.1210/mend-4-1-3. [DOI] [PubMed] [Google Scholar]
- 14.Terashima M, Toda K, Kawamoto T, Kuribayashi I, Ogawa Y, Maeda T, Shizuta Y. Arch Biochem Biophys. 1991;285:231–237. doi: 10.1016/0003-9861(91)90354-l. [DOI] [PubMed] [Google Scholar]
- 15.Ishabashi S, Schwartz M, Frykman P K, Herz J, Russell D W. J Biol Chem. 1996;271:18017–18023. doi: 10.1074/jbc.271.30.18017. [DOI] [PubMed] [Google Scholar]
- 16.Graham-Lorence S, Amarneh B, White R E, Peterson J A, Simpson E R. Protein Sci. 1995;4:1065–1080. doi: 10.1002/pro.5560040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Church G M, Gilbert W. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackerman G E, Smith M E, Mendelson C R, MacDonald P C, Simpson E R. J Clin Endocrinol Metab. 1981;53:412–417. doi: 10.1210/jcem-53-2-412. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Fisher C R, Hall P F, Waterman M R. Proc Natl Acad Sci USA. 1993;90:11673–11677. doi: 10.1073/pnas.90.24.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conte F A, Grumbach M M, Ito Y, Fisher C R, Simpson E R. J Clin Endocrinol Metab. 1994;78:1287–1292. doi: 10.1210/jcem.78.6.8200927. [DOI] [PubMed] [Google Scholar]
- 21.Morishima A, Grumbach M M, Simpson E R, Fisher C, Kenan Q. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 22.Hess R A, Bunick D, Lee K-H, Bahr J, Taylor J A, Korach K S, Lubahn D B. Nature (London) 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders P T K, Fisher J S, Sharpe R M, Millar M R. J Endocrinol. 1998;156:R13–R17. doi: 10.1677/joe.0.156r013. [DOI] [PubMed] [Google Scholar]