Abstract
Memory function is largely mediated by medial temporal lobe (MTL), and its compromise has been observed in alcohol dependence and chronic cigarette smoking. The effects of heavy alcohol consumption and chronic smoking on hippocampal volumes and MTL metabolites and their recovery during abstinence from alcohol have not been assessed. Male alcoholics in treatment (ALC) [13 smokers (sALC) and 11 non-smokers (nsALC)] underwent quantitative magnetic resonance imaging and short-echo proton magnetic resonance spectroscopic imaging at one week and one month of sobriety. Outcome measures were compared to 14 age-matched, non-smoking light-drinkers and were related to visuospatial learning and memory. Over one month of abstinence, N-acetylaspartate, a neuronal marker, and membrane-associated choline-containing metabolites normalized in the MTL of nsALC, but remained low in the MTL of sALC. Metabolite concentration changes in both groups were associated with improvements in visuospatial memory. Hippocampal volumes increased in both groups during abstinence, but increasing volumes correlated with visuospatial memory improvements only in nsALC. In summary, chronic cigarette smoking in alcohol-dependent men appears to have adverse effects on MTL metabolite recovery during short-term sobriety. These data may also have implications for other conditions with established MTL involvement and significant smoking co-morbidity, such as schizophrenia-spectrum and mood disorders.
Keywords: hippocampus, magnetic resonance imaging, magnetic resonance spectroscopy, abstinence from alcohol, learning and memory, recovery
1. Introduction
The hippocampus and medial temporal lobe (MTL) play important role in encoding and consolidation of new declarative memories as well as encoding and retrieval of the spatial and temporal context of personal life events (Jarrard, 1995). Alcohol use disorders may result in neurocognitive deficits consistent with hippocampal and/or MTL dysfunction (Sullivan et al., 2000; Crews et al., 2005). Previous magnetic resonance studies in one-month-abstinent alcohol dependent individuals demonstrated volume reductions in the bilateral hippocampi (Beresford et al., 2006; Bleich et al., 2003; Sullivan et al., 1995; Agartz et al., 1999; Laakso et al., 2000), with some suggesting differential effects for sex and/or hemisphere (Agartz et al., 1999; Laakso et al., 2000). Small neuropathological studies found unchanged numbers of hippocampal neurons in alcoholics (Korbo, 1999; Harding et al., 1997), but profound loss in glial cell populations (Korbo, 1999) and hippocampal white matter (WM) volume (Harding et al., 1997). MRI volumetric studies, however, cannot distinguish between injury to neuronal or glial cells, and they are not sensitive to tissue density changes reported in recovering alcoholics (Trabert et al., 1995). Proton magnetic resonance spectroscopy (1H MRS) may distinguish between neuronal and glial injury and may be sensitive to changes in brain tissue density (Martin et al., 1995). We used 1H-MRS at short echo time to measure four major metabolites (Ross and Bluml, 2001): N-acetyl-aspartate (NAA), an accepted marker of neuronal viability, is observed only in mature neurons and their processes; decreased NAA may reflect neuronal loss, atrophied dendrites and axons, and/or derangements of neurometabolism; choline-containing compounds (Cho) are believed to be involved in cell membrane breakdown and synthesis; creatine-containing metabolites (Cr) consist of creatine and phosphocreatine, which are involved in cell bioenergetics, and myo-inositol (m-Ino) is a putative marker of astrocytes and may also function as an osmolyte (Schweinsburg et al., 2000). MRS studies in recently detoxified alcoholics generally reported lower NAA and Cho in the cerebellum and frontal lobe that tend to increase with sustained abstinence from alcohol (Durazzo et al., 2004; Durazzo et al., 2006; Ende et al., 2005; Jagannathan et al., 1996; Parks et al., 2002; Schweinsburg et al., 2001; Seitz et al., 1999)but see (Bendszus et al., 2001). Myo-inositol was elevated in multiple brain regions of one-month abstinent alcoholics (Schweinsburg et al., 2000). To date, however, there are no published MRS studies on MTL metabolites in chronic alcoholics.
Chronic cigarette smoking is a common comorbid condition in alcohol dependence (Romberger and Grant, 2004; Hurt et al., 1994; Pomerleau et al., 1997), illicit substance abuse, schizophrenia-spectrum and mood disorders (Patkar et al., 2006; Dani and Harris, 2005; Esterberg and Compton, 2005; Fergusson et al., 2003). While nicotine may acutely facilitate learning and memory (Levin and Simon, 1998; Newhouse et al., 2004; Sacco et al., 2004), a growing body of evidence suggests chronic cigarette smoking in non-alcoholic individuals adversely affects multiple domains of neurocognition (e.g., Deary et al., 2003; Razani et al., 2004), including learning and memory (Heffernan et al., 2005; Hill et al., 2003; Richards et al., 2003; Schinka et al., 2003). Chronic cigarette smoking, in addition to alcohol, results in additional oxidative stress to brain cells (Moriarty et al., 2003), and cigarette smoke contains many toxic compounds (Fowles et al., 2000) that may directly or indirectly compromise central nervous system tissue (Durazzo et al., 2006), possibly leading to volumetric and/or metabolic abnormalities. Cigarette smoking in the general population was associated with increased late-life brain atrophy (e.g, Akiyama et al., 1997), regionally specific gray matter reductions in adults (Brody et al., 2004), reduced MTL NAA (Gallinat et al., 2007), and global cerebral blood flow abnormalities (e.g., Rourke et al., 1997), possibly associated with compromised brain metabolic activity. Along this line, our studies in alcohol dependent individuals demonstrated detrimental effects of chronic cigarette smoking on lobar gray matter (GM) volumes and GM perfusion (Gazdzinski et al., 2005; Gazdzinski et al., 2006), as well as on NAA and Cho concentrations in the frontal lobes and subcortical structures (Durazzo et al., 2004). Additionally, smoking alcoholics demonstrated generally less recovery of regional NAA and Cho levels compared to non-smokers over one month of abstinence from alcohol (Durazzo et al., 2006).
In this study, we employed MRI and 1H magnetic resonance spectroscopic imaging (1H MRSI) to measure hippocampal volumes and mean MTL metabolite concentrations in a group of alcohol dependent individuals (ALC) at one week and one month of abstinence from alcohol. The alcoholics were retrospectively classified into smokers (sALC) and non-smokers (nsALC) and compared to an age-matched group of non-smoking, light drinking controls (nsLD). We hypothesized that 1) at one week of abstinence, sALC have smaller hippocampal volumes and lower MTL NAA and Cho concentrations than both nsLD and nsALC, whereas nsALC have smaller hippocampal volumes and lower MTL NAA and Cho concentrations than nsLD; 2) sALC show less volumetric and metabolite recovery over one month of abstinence than nsALC; 3) at one month of abstinence, both sALC and nsALC demonstrate smaller hippocampal volumes and lower MTL concentrations of NAA and Cho than nsLD; and 4) hippocampal volumes and MTL metabolite concentrations and their changes with abstinence are related to measures of drinking and smoking severity as well as neurocognition.
2. Methods
2.1. Participants
Twenty-four male alcohol-dependent individuals between the ages of 28 and 66, recruited from the San Francisco VA Medical Center Substance Abuse Day Hospital (SADH) and the San Francisco Kaiser Permanente Chemical Dependence Recovery Program, were retrospectively divided into smokers (sALC, n=13) and non-smokers (nsALC, n=11). All were scanned twice, 6±3 days after their last alcoholic drink at assessment point 1 (AP1) and 32±9 days after their last drink at assessment point 2 (AP2). The time between scans did not differ between groups (see Table 1). Fourteen healthy non-smoking, male light drinkers (nsLD) were recruited from the San Francisco Bay Area community and scanned only once. All participants of this study were examined as part of the corresponding spectroscopic, structural, and perfusion MRI studies (Durazzo et al., 2004; Durazzo et al., 2006; Gazdzinski et al., 2005; Gazdzinski et al., 2006).
Table 1.
Demographics and participant characteristics (mean ± standard deviation)
| Parameter | nsLD | nsALC | sALC |
|---|---|---|---|
| N = 14 | N = 11 | N = 13 | |
| Age [years] | 47.3 ± 8.2 | 50.2 ± 9.1 | 50.7 ± 9.0 |
| Education [years] | 16.4 ± 2.6 | 14.0 ± 2.6 | 13.9 ± 1.3 |
| AMNART | 121 ± 8 | 109 ± 11 | 114 ± 7 |
| 1-yr average alcohol consumption [drinks/month] | 10 ± 11 | 387 ± 178 | 425 ± 187 |
| 3-yr average alcohol consumption [dri/month] | 10 ± 11 | 385 ± 179 | 366 ± 140 |
| Lifetime average alcohol consumption [dri/month] | 17 ± 16 | 193 ± 129 | 271 ± 100 |
| Duration of drinking [years] | 26.0 ± 8.1 | 33.5 ± 9.3 | 33.4 ± 9.9 |
| Total lifetime alcohol consumption [kg] | 74 ± 68 | 1051 ± 818 | 1445 ± 594 |
| Onset of heavy drinking [years] | - | 26.6 ± 9.9 | 21.8 ± 3.7 |
| Months of heavy drinking | - | 225 ± 106 | 308 ± 95 |
| Time after last alcoholic drink [days] at AP1 | - | 5.4 ± 2.7 | 6.4 ± 3.3 |
| Time after last alcoholic drink [days] at AP2 | - | 34.3 ± 8.1 | 30.8 ± 9.2 |
| Fagerstrom score | - | - | 6.1 ± 1.9 |
| Cigarettes per day | - | - | 23 ± 10 |
| Duration of smoking [years] | - | - | 25 ± 12 |
| Pack-years | - | - | 31 ± 21 |
| Ethnicity | |||
| Caucasian | 10 | 8 | 9 |
| African-American | 1 | 0 | 3 |
| Latino | 0 | 2 | 0 |
| Native American | 0 | 1 | 1 |
| Asian | 2 | 0 | 0 |
| Pacific Islander/Polynesian | 1 | 0 | 0 |
AMNART = American National Adult Reading Test; 1-yr average alcohol consumption = number of drinks per month over 1 year prior to study; 3-yr average alcohol consumption = number of drinks per month over 3 years prior to study; Lifetime average alcohol consumption = number of drinks per month over lifetime; Duration of alcohol drinking = number of years of regular alcohol consumption (defined as consuming at least one standard drink/month. Total lifetime alcohol consumption = total amount of pure EtOH (kg) consumed over lifetime. Onset of heavy drinking = age, when alcohol consumption exceeded 100 drinks per month. Pack years = (number of cigarettes per day/20) × (duration of smoking at current level in years).
The inclusion and exclusion criteria are fully described in (Durazzo et al., 2004). In summary, all ALC met DSM-IV criteria for alcohol dependence with physiological dependence and consumed more than 150 standard alcoholic drinks per month for at least 8 years prior to enrollment into the study. A standard drink contains 13.6 grams of pure ethanol, equivalent of 12 oz. beer, 5 oz. wine, or 1.5 oz. liquor. All participants were free of general medical, neurologic, and psychiatric conditions, except unipolar mood disorders, hypertension, and hepatitis C in ALC. Unipolar mood disorders were not exclusionary in ALC due to their high reported incidence among alcohol-dependent individuals (e.g., Gilman and Abraham, 2001) and chronic cigarette smokers (e.g., Fergusson et al., 2003).
Participants completed structured clinical interviews as previously described (Durazzo et al., 2004). Two sALC and two nsALC met DSM-IV criteria for substance-induced (alcohol) mood disorder with depressive features and one nsALC was diagnosed with recurrent major depression with mood congruent psychotic symptoms. Exclusion of these participants from analyses did not affect the results. One nsALC met criteria for past methamphetamine dependence, whereas one sALC met criteria for past opioid dependence with physiologic dependence. However, both individuals were in sustained full remission, with last use five or more years before enrollment. One participant in each ALC group had (medication controlled) hypertension. Standard clinical labs and a brief neurocognitive battery were completed within one day of the MR studies. They assessed hepatocellular injury, red blood cell status, and visuospatial learning and memory (Brief Visual Memory Test-Revised; BVMT-R; (Benedict, 1997). Participants were allowed to smoke ad libitum prior to and during cognitive evaluation.
Alcohol consumption and smoking behavior over lifetime were assessed via the Lifetime Drinking History (LDH; Skinner and Sheu, 1982; Sobell et al., 1988; Sobell and Sobell, 1992) and the Fagerstrom Tolerance Test for Nicotine Dependence (Fagerstrom et al., 1991), respectively (see Table 1). All sALC continued to smoke at their baseline levels during the assessment interval, except for one individual, who stopped smoking and used a nicotine patch; all his MR measures improved after AP1 but were within the range of sALC group data. Six nsALC never smoked and five quit smoking more than five years prior to enrollment. These never-smokers and past smokers did not differ significantly on any of the neurocognitive or MR-derived measures at either AP. Twenty-one of 24 ALC participated in continued outpatient substance abuse treatment programs at the San Francisco VA Medical Center for the study duration and were randomly breathalized and given weekly drug screens to assure abstinence. The Institutional Review Boards of the University of California San Francisco and the San Francisco VA Medical Center approved all procedures, and informed consent was obtained from all participants prior to study.
2.2. Data Acquisition and Processing
Data were acquired on a standard 1.5T MR system (Siemens Vision, Iselin, NJ). For AP1, hippocampi were outlined using a semi-automated high dimensional brain warping algorithm (Medtronic Surgical Navigation Technologies, Louisville, CO; (Hsu et al., 2002) on T1-w magnetization-prepared rapid gradient echo images acquired with TR/TE/TI=10/7/300 ms, 15° flip angle, 1×1mm2 in-plane resolution, and 1.5-mm-thick coronal partitions oriented orthogonal to the long axes of hippocampi. AP2-images were coregistered to AP1-images to assure use of the same landmarks for hippocampal delineation (see Hsu et al., 2002) at both APs. This approach was validated using 20 elderly participants scanned about one year apart, and it yielded results similar to those obtained by independent hippocampal delineation for baseline and follow-up scans. Probability maps of GM, WM, and CSF within major lobes, subcortical nuclei, brainstem, and cerebellum (but not hippocampi) were obtained from T1-w images by combining 1) three-tissue probabilistic segmentation and 2) masks of major lobes, subcortical nuclei, brainstem, and cerebellum. The latter structures were outlined on individual scans utilizing a deformable registration method that used an MRI atlas from a single 36-year old control that had been manually divided into the aforementioned structures (Cardenas et al., 2005). To correct for inter-participant variability in head size, absolute hippocampal volumes were scaled to total intracranial volume (defined as sum of GM, WM, and CSF determined by segmentation).
A 1H MRSI dataset was acquired with TR/TE=1800/25 ms with PRESS pre-selection of a 100×60×15 mm3 volume of interest (VOI; Schuff et al., 1999). The VOI was placed parallel to the long axes of the hippocampi and positioned on the axial plane to cover both hippocampi (see Figure 1). The MRSI field of view was 210×210 mm2 and was sampled using a circular k-space scheme equivalent to a maximum of 24×24 phase encoding steps, resulting in a nominal and actual voxel resolution of 1.1 ml and 1.6 ml, respectively (Schuff et al., 2001b). Processing details were described in (Meyerhoff et al., 2004; Schuff et al., 2001a). In short, integrals of the resonances corresponding to NAA, Cho, Cr, and m-Ino were estimated including baseline correction (see Figure 2), adjusted for inter-participant differences in coil loading, transmitter voltage, the known SI point spread function, RF pulse profiles, and extrapolated to 100% tissue using fractional tissue contributions to individual voxels that were obtained from aligned GM, WM, and CSF probability maps. The final outcome measures were tissue-specific absolute metabolite concentrations expressed in institutional units; they were not reported in molar units to avoid possibly inaccurate assumptions about relaxation times. For statistical analyses, we used only those voxels that passed our spectral quality assurance test as described in (Meyerhoff et al., 2004) and contained less than 25% CSF. MTL voxels contained at least 20% of hippocampal tissue and generally covered the body of the hippocampus. The average volume contributions to MTL SI voxels were 31±3% from hippocampi, 32±5% from temporal WM, and 29±5% from temporal GM, without significant differences between groups.
Figure 1.
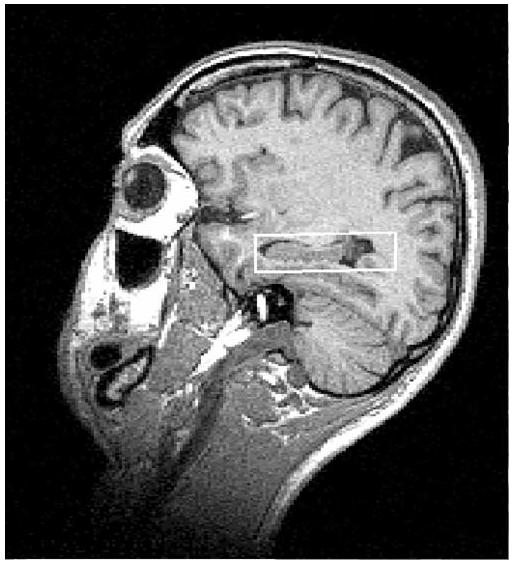
Position of spectroscopic volume of interest overlaid on sagittal brain scout image.
Figure 2.
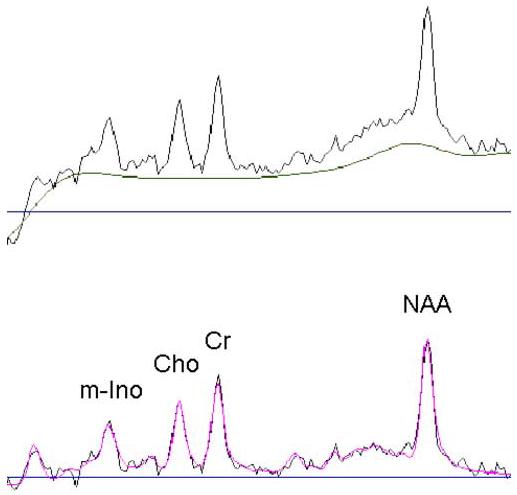
Exemplary spectrum: One experimental spectrum with fitted baseline (upper part) and fitted spectrum overlaid on experimental spectrum after subtraction of the fitted baseline (lower part).
2.3. Study design and statistical analyses
The nsLD group was scanned only once and was used in cross-sectional comparisons of hippocampal volumes and MTL metabolite concentrations with sALC and nsALC at one week and one month of abstinence. These cross-sectional assessments utilized analyses of covariance (ANCOVA), with age as the covariate, and followed up with pair-wise contrasts. The longitudinal analyses evaluated hippocampal volume and metabolite concentrations only in sALC and nsALC with repeated measures ANOVAs. The within-subject factor (AP) examined longitudinal changes in hippocampal volumes and absolute metabolite concentrations across APs in the combined ALC (i.e., nsALC+sALC). The AP-by-smoking status (between-subjects factor) interaction evaluated potential differences in recovery of hippocampal volumes and absolute metabolite concentrations between sALC and nsALC.
Additionally, we examined rates of change for hippocampal volumes and MTL metabolite concentrations for sALC and nsALC using independent t-tests. Change rates were defined as:
Hippocampal volumes and MTL metabolites were separately correlated with seven drinking and four smoking variables, three measures of depressive, withdrawal and anxiety symptomatology, nine laboratory variables, and two neurocognitive measures. To account for multiple comparisons, the alpha levels for the corresponding families were set to 0.007 (=0.05/7), 0.012, 0.017, 0.006, and 0.025, respectively. Relationships were assessed with Spearman correlations. All statistical tests were conducted with SPSS-12.0 for Windows (SPSS; Chicago, IL).
3. Results
3.1. Participant characterization
Groups were matched on age [(F(2,35)=0.62, P=ns], but nsLD had greater education than sALC and nsALC [F(2,35)=5.22, P=0.01]. sALC and nsALC had similar average number of drinks per month consumed over 1 and 3 years prior to enrollment (P=0.6). However, sALC tended to begin drinking at levels higher than 100 drinks/month at younger age (P=0.06) and tended to have more drinks per month over lifetime (P=0.10) than nsALC. Detailed demographics and participant characteristics for all groups are given in Table 1. sALC did not differ from nsALC at any AP on self-report measures of depressive, anxiety, and withdrawal symptomatology, and most laboratory variables. However, gamma-glutamyltransferase and aspartate aminotransferase levels in nsALC at AP1 were elevated but normalized by AP2 (see Table 2). The measures of withdrawal symptomatology for sALC and nsALC at AP1 were not clinically elevated. The sALC Fagerstrom score was 6.1 ± 1.9 (min=2, max=10), indicating a medium to high level of nicotine dependence.
Table 2.
Depressive, anxiety, and withdrawal symptomatology and laboratory variables by group and assessment point (AP) (mean ± standard deviation)
| Parameter | nsLD | nsALC | sALC | ||
|---|---|---|---|---|---|
| AP1 | AP2 | AP1 | AP2 | ||
| BDI | 3.9 ± 4.0 | 15.5 ± 9.7 | 5.7 ± 5.9 | 15.6 ± 10.3 | 11.8 ± 10.6 |
| STAI Y-2 | 33 ± 8 | 48 ± 11 | 43 ± 10 | 50 ± 13 | 44 ± 13 |
| CIWA-Ar | - | 3.5 ± 3.7 | 0.1 ± 0.3 | 2.5 ± 2.8 | 0.5 ± 1.3 |
| GGT [i.u.] | 28 ± 18 | 129 ± 164 | 59 ± 76 | 61 ± 35 | 48 ± 32 |
| AST [i.u.] | 27 ± 8 | 45 ± 47 | 24.8 ± 7.5 | 33 ± 9 | 31.0 ± 12.0 |
| ALT [i.u.] | 30 ± 13 | 59 ± 55 | - | 28 ± 7 | - |
| Albumin [g/dl] | 4.1 ± 0.3 | 3.9 ± 0.3 | 4.1 ± 0.4 | 4.0 ± 0.2 | 4.7 ± 2.3 |
| Prealbumin [mg/dl] | 31.4 ± 6.3 | 27.9 ± 7.1 | 24.7 ± 7.0 | 26.7 ± 4.1 | 27.9 ± 4.1 |
| WBC | 6.3 ± 1.5 | 7.2 ± 1.3 | 7.2 ± 1.9 | 6.6 ± 2.0 | 8.0 ± 1.9 |
| RBC | 4.90 ± 0.37 | 4.60 ± 0.48 | 4.76 ± 0.35 | 4.27 ± 0.32 | 4.55 ± 0.42 |
| Hemoglobin [g/dl] | 15.1 ± 1.1 | 14.7 ± 1.6 | 15.0 ± 1.4 | 13.8 ± 0.9 | 14.4 ± 1.0 |
| Hematocrit [%] | 43.9 ± 3.2 | 42.6 ± 3.7 | 43.1 ± 4.2 | 40.6 ± 2.4 | 42.3 ± 2.9 |
| MCV | 89.5 ± 2.9 | 91.1 ± 6.7 | 90.4 ± 5.8 | 95.1 ± 3.7 | 93.1 ± 3.7 |
| Hep-C [number of participants] | 0 | 2 | 2 | 2 | 2 |
BDI = Beck Depression Inventory; STAI Y-2 = State -trait Anxiety Inventory - State; CIWA-Ar = Addiction Research Foundation Clinical Institute of Withdrawal Assessment for Alcohol; GGT = gamma-glutamyltransferase; local normal range = 7-64 institutional units; AST = aspartate aminotransferase; local normal range = 5-35 institutional units; ALT = alanine aminotransferase; local normal range = 7-56 institutional units; Albumin local normal range = 3.3 - 5.2 g/dl; Prealbumin local normal range = 18-45 mg/dl; WBC = white blood cell count; local normal range = 4.8 - 10.8 K/cmm; RBC = red blood cell count; local normal range = 4.7 - 6.1 M/cmm; Hemoglobin local normal range = 14-18 g/dl; Hematocrit local normal range = 42-52 %.
3.2. Right-Left Hemisphere Comparisons
There were no significant group differences in hippocampal volumes and metabolite concentrations between right and left hemispheres in this cohort. Thus, bilateral hippocampal volumes and metabolite concentrations averaged over both hemispheres are reported.
3.3. Cross-sectional group comparisons at assessment point 1
Hippocampal volumes
ANCOVA comparing hippocampal volume between nsLD, nsALC and sALC was not significant [F(2,35)=1.85, P=0.17]. However, among the hypothesized contrasts, sALC volumes were 7.8% smaller than nsALC volumes (P=0.05) and tended to be 6.9% smaller than in nsLD (P=0.08; see Figure 3). No significant volume differences were observed between nsALC and nsLD.
Figure 3.
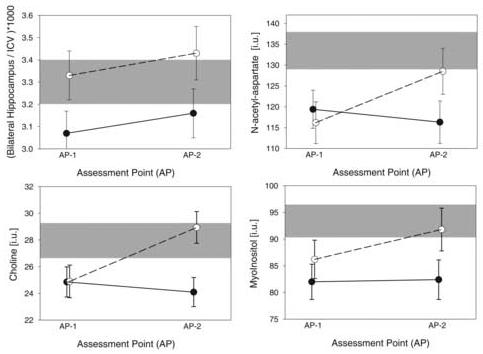
Baseline values and recovery of hippocampal volumes, scaled to intracranial volume (upper left), MTL - NAA (upper right), MTL - Cho (lower left), and MTL - m-Ino (lower right). Non-smoking and smoking alcohol dependent groups are depicted with open and closed symbols, respectively. Range of measures in nsLD is depicted in gray (mean ± standard error).
MTL metabolite concentrations
For NAA, groups were significantly different [F(2,34)=3.77, P=0.03], with 10.4% lower concentration in sALC (P=0.02) and 12.8% lower concentration in nsALC (P=0.008) compared to nsLD (see Figure 3). For Cho, there was a trend for group differences [F(2,34)=3.05, P=0.06]. Among the hypothesized contrasts, both nsALC and sALC demonstrated 12% lower Cho concentrations than nsLD (P=0.03 and P=0.02, respectively; see Figure 3). NAA and Cho concentrations were not significantly different between sALC and nsALC. A trend for group differences was apparent for m-Ino [F(2, 34)=2.98, P=0.06] (see Figure 3), but no significant group differences were found for MTL Cr [F(2, 34)=0.44, P=0.69. Upon co-variation for inter-participant differences in hippocampal tissue contributions to MTL voxels, the reported results remained virtually unchanged.
3.4. Longitudinal volumetric and metabolite changes for nsALC and sALC
3.4.1. Hippocampal volumes
There was a significant main effect for AP (i.e., time) [F(1,22)=7.51, P=0.01], indicating significant increases of hippocampal volume in the ALC group (i.e., sALC + nsALC) over approximately one month of abstinence from alcohol (see Figure 3). The interaction between smoking status and AP was not significant [F(1,22)=0.02, P=0.96], consistent with similar rates of change of hippocampal volume observed for sALC and nsALC (3.6 ± 5.8% vs. 3.4 ± 6.4%, P=0.48).
3.4.2. MTL metabolite concentrations
There were significant interactions between smoking status and AP for NAA [F(1,22)=4.25, P=0.05] and Cho [F(1,22)=6.29, P=0.02] (Figure 3). Compared to AP1, nsALC had higher Cho at AP2 (P=0.01) and tended to have higher NAA at AP2 (P=0.06). In sALC, there were no longitudinal changes in NAA and Cho. Correspondingly, sALC compared to nsALC had significantly smaller rates of change in NAA (-3 ± 26% vs. 12 ± 15%, P = 0.05) and Cho (-1 ± 31% vs. 19 ± 19%, P=0.04). The interactions and differences in rates of change for Cr and m-Ino were not significant. Also, no main effects for AP were observed for any metabolite [F(1,22)<2.57, P>0.12].
3.5. Cross-sectional group comparisons at assessment point 2
3.5.1. Hippocampal volumes
No significant group differences were observed for hippocampal volume at AP2 [F(2, 34)=1.42, P=0.26]. Among hypothesized contrasts, sALC had 7.7% smaller hippocampi than nsALC (P=0.05).
3.5.2. MTL metabolite concentrations
At AP2, significant group differences were seen for Cho [F(2, 34)=5.41, P=0.009] with a trend for in NAA [F(2, 34)=2.80, P=0.075]. Cho in sALC was 14.3% lower than in nsLD (P=0.007) and 16.7% lower than in nsALC (P=0.003). NAA was 12.3% lower in sALC compared to nsLD (P=0.02) and tended to be 10.5% lower in sALC than nsALC (P=0.06). Correspondingly, NAA and Cho were similar in nsALC and nsLD (P>0.40). Neither Cr nor m-Ino differed significantly between groups (P>0.59). Covarying for inter-participant differences in hippocampal tissue contributions to MTL voxels did not change the reported significances appreciably.
3.6. Correlations among outcome measures
3.6.1. Hippocampal volumes
Younger age of onset of heavy drinking in combined ALC (i.e., nsALC + sALC) correlated with larger hippocampal volumes at both AP1 and AP2 (rho<-0.51, P<0.008). This correlation was more pronounced in nsALC and remained significant after controlling for age and after eliminating from analyses all participants with depressive disorders. Higher average number of drinks per month over lifetime was associated with larger hippocampal volumes at both APs in nsALC (rho>0.89, P<0.001, N=11), but not in sALC (|rho|<0.22, P>0.44). No correlations between hippocampal volumes and measures of smoking severity reached the level of significance. In nsALC over one month of abstinence, improving visuospatial memory tended to correlate with increasing hippocampal volumes (rho=0.60, P=0.03); this relationship was not apparent in sALC.
3.6.2. MTL metabolite concentrations
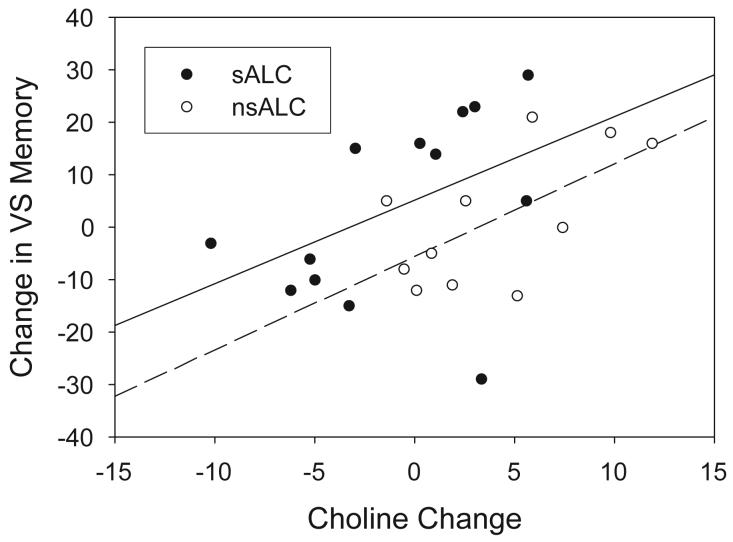
Between AP1 and AP2, in the combined ALC group, improving visuospatial memory correlated with increases of MTL Cho, Cr, and m-Ino (rho>0.40, P<0.025). These correlations were similar in sALC and nsALC (see Figure 4). In nsALC, but not in sALC, a larger m-Ino increase tended to correlate with greater average number of drinks per month over lifetime and earlier onset of heavy drinking (|rho|>0.63, P<0.04), whereas a larger Cr increase tended to be associated with greater average number of drinks per month over lifetime (rho=0.62, P=0.04). At AP2, but not at AP1, better visuospatial memory correlated with higher MTL NAA in the combined ALC group (rho=0.43, P=0.02). These relationships were similar in sALC and nsALC. Metabolite concentrations and their changes were not related to measures of drinking or smoking severities after correction for multiple comparisons. Furthermore, MTL metabolite concentrations and their changes did not correlate with hippocampal volumes and their changes.
Figure 4.
Significant correlation between change in concentration of choline-containing metabolites and visuospatial memory over the first month of abstinence from alcohol (rho>0.40, P<0.025).
4. Discussion
These preliminary MR studies in abstinent alcohol-dependent men describe hippocampal volumes and medial temporal lobe metabolite concentrations at one week of abstinence from alcohol and their changes over approximately one month of sobriety. At one week of abstinence, we observed smaller hippocampal volumes in sALC compared to nsALC, but no volume reductions in nsALC relative to nsLD; however, both sALC and nsALC demonstrated significantly lower MTL NAA and Cho compared to nsLD. Over one month of abstinence from alcohol, both sALC and nsALC demonstrated similar magnitudes of hippocampal volume increases, but sALC continued to exhibit smaller volumes than nsALC at one month of sobriety (i.e., AP2). Over the same period, NAA and Cho concentrations did not significantly change in sALC and, at one month of abstinence, sALC continued to demonstrate lower NAA and Cho than in both nsALC and nsLD. In contrast, MTL NAA and Cho concentrations normalized in nsALC. Improvements in visuospatial memory were associated with increases of hippocampal volumes in nsALC only and with changes of Cho, Cr, and m-Ino in both sALC and nsALC. At one month of sobriety, better visuospatial memory in nsALC correlated with higher NAA concentrations. In as much as these correlations were essentially not observed in other brain regions of a similar cohort (Durazzo et al., 2006), these associations suggest that the changes in hippocampal volumetric and MTL spectroscopic measures are functionally significant and reflect the integrity of MTL tissue, including pathways subserving memory and other related cognitive abilities.
4.1. Hippocampal Volumes
Hippocampal volume reductions were observed in recently detoxified alcoholics (Bleich et al., 2003). However, in our studies, only sALC at one week of abstinence showed smaller hippocampi than both nsALC and nsLD, whereas nsALC did not significantly differ from nsLD on this measure. This is consistent with our earlier report of smaller lobar GM volumes in sALC compared to both nsALC and nsLD (Gazdzinski et al., 2005). The significant hippocampal volume increase over the first month of abstinence is consistent with volumetric recovery observed in other brain regions during sobriety (e.g., Pfefferbaum et al., 1995; Gazdzinski et al., 2004). It suggests that previous cross-sectional studies that measured hippocampal volumes at about one month of abstinence (Agartz et al., 1999; Laakso et al., 2000; Sullivan et al., 1995) did not capture the full extent of (i.e., underestimated) chronic alcohol-associated hippocampal volume deficits. The mechanisms of hippocampal volume reductions at one-week of abstinence and subsequent volume increase in both sALC and nsALC may involve dearborization and rearborization of hippocampal dendrites (King et al., 1988; Lescaudron et al., 1989; Cadete-Leite et al., 1988; Durand et al., 1989; McMullen et al., 1984) or generalized changes in the neuropil of this structure. Although small human neuropathological studies did not show frank neuronal loss in hippocampi of alcoholics, they did not account for smoking status of the participants; thus, hippocampal neuron loss in smokers cannot be excluded. Cigarette smoke contains many toxic compounds in the gas and particulate phases [e.g., carbon monoxide, free radicals, nitrosamines, polynuclear aromatic compounds (Fowles et al., 2000)] that may directly or indirectly compromise central nervous system tissue and vascular endothelial function (for review see Durazzo et al., 2006). The chronic exposure of sALC to these potentially noxious compounds in cigarette smoke may contribute to the smaller volumes observed in sALC relative to both nsALC and nsLD at AP1 (∼8%), as well as the continued volume differences observed between sALC and nsALC at AP2 (∼8%).
The unexpected positive relationships between larger hippocampal volumes and earlier onset of heavy drinking in both sALC and nsALC, as well as associations between larger hippocampal volumes and more drinks per month over lifetime in nsALC were not apparent in our previous investigations of other brain regions in a similar patient cohort (Gazdzinski et al., 2005). We do not have a clear-cut explanation for these associations. They may reflect the influence of factors other than alcohol on hippocampal size such as psychosocial stress (Smith, 1996; Winter and Irle, 2004), mood disorders (Campbell and Macqueen, 2004; Videbech and Ravnkilde, 2004), personality disorders (Laakso et al., 2000), withdrawal induced neurotoxicity (Crews et al., 2005; Prendergast et al., 2000), genetic susceptibility (Hill et al., 2006), or the modest size of our cohort. .
4.2. MTL metabolite concentrations
Decreased NAA and Cho concentrations in MTL at one week of abstinence in both sALC and nsALC are consistent with metabolite abnormalities reported in other brain regions of alcohol dependent individuals during early abstinence (Durazzo et al., 2004; Ende et al., 2005; Parks et al., 2002; Seitz et al., 1999). In our corresponding 1H MRSI study (Durazzo et al., 2004), which assessed metabolite concentrations in lobar, subcortical, and cerebellar brain regions (but not in hippocampus) in many one-week-abstinent alcoholics also studied for this report, we found that chronic smoking was associated with lower NAA and Cho levels in frontal lobe and several subcortical structures. However, sALC and nsALC did not differ from each other or nsLD on temporal lobe WM or GM NAA and Cho levels.
No significant longitudinal changes in MTL NAA, Cho, and m-Ino levels were observed in sALC, whereas all MTL metabolite concentrations were normalized in nsALC after one month of sobriety. These different patterns of recovery between sALC and nsALC are consistent with our longitudinal 1H MRSI findings in recovering alcoholics (Durazzo et al., 2006). The absence of longitudinal NAA and Cho increases in sALC, again, may reflect the direct and indirect adverse effects of continued exposure to cigarette smoke on brain tissue integrity and vascular endothelial function (discussed in more detail in (Durazzo et al., 2006). Additionally, nicotine administration in rats inhibits dentate gyrus neurogenesis (Abrous et al., 2002; Shingo and Kito, 2005), which otherwise is increased during withdrawal from alcohol (Nixon and Crews, 2004). Since the dentate gyrus constitutes only about 3% of human hippocampal volume (Harding et al., 1998), it is not likely neurogenesis contributes significantly to hippocampal volume changes with abstinence from alcohol. However, the dentate gyrus is part of the essentially tri-synaptic network of the hippocampus and addition of relatively small numbers of new neurons in this structure may lead to relatively large differences in signal processing (Kempermann et al., 2004), potentially leading to larger synaptic and metabolic activity in hippocampus/MTL, and thus to increased NAA levels (Meyer-Lindenberg et al., 2005). This process may also be associated with cell membrane changes reflected in the observed Cho increases.
Although the sALC group did demonstrate hippocampal volume increases over one month of abstinence, the lack of significant MTL metabolite recoveries may suggest incomplete recovery of hippocampal and associated tissue integrity in sALC. Conversely, the longitudinal NAA increases observed in nsALC suggest greater recovery of neuronal structural elements or metabolism, while Cho increases may indicate normalization of parenchymal cell membrane synthesis/turnover or recovery of para-hippocampal cortex and myelin (Harding et al., 1997; Martin et al., 1995). Previous studies in abstinent alcoholics did not evaluate smoking effects, although the samples presumably included a significant number of smokers; these studies generally demonstrated little or no NAA recovery in major lobes and cerebellum over the first month of abstinence (Ende et al., 2005; Parks et al., 2002), but see (Bendszus et al., 2001). Finally, lower MTL NAA in sALC at one month of abstinence compared to nsALC and nsLD is consistent with smoking related hippocampal NAA reductions in non-alcoholic populations (Gallinat et al., 2007).
4.3. Functional significance
Over the first month of abstinence, MTL metabolite concentration increases in nsALC and sALC were related to improvements of visuospatial learning. These neurobiological changes may reflect rearborization, axonal regrowth, and/or neurogenesis and may have functional significance. For example, studies in rats demonstrated that the number of new cells generated in the dentate gyrus was associated with better performance on hippocampal dependent learning (Eisch, 2002). The fact that Cho, Cr, and m-Ino increases correlate with improvements of visuospatial memory in alcoholics over the first month of abstinence suggest that, MTL cell membranes and myelin integrity play important roles in facilitating performance on hippocampus-mediated tasks.
Our participants were allowed to smoke ad libitum prior to and during neurocognitive assessment. However, we do not believe nicotine-related cognitive enhancement or withdrawal significantly confounded our cognitive test results. In healthy non-smokers and in individuals with attention deficit hyperactivity disorder and schizophrenia-spectrum disorders acute nicotine administration transiently improves some areas of neurocognition, most appreciably sustained attention (see Rezvani and Levin, 2001; Sacco et al., 2004). Whether these acute effects on neurocognition are manifested also in non-smoking alcoholics and other substance abusers is unclear (see Ceballos et al., 2005; Ceballos et al., 2006). The adverse effects of nicotine withdrawal on neurocognition are not typically apparent until 8-12 hours after last nicotine dose, at least in non-alcoholic chronic smokers (see Sacco et al., 2004; Mendrek et al., 2006; Xu et al., 2005). This is likely attributable to the maintenance of relatively high levels of plasma nicotine due to repeated smoking during waking hours (Hukkanen et al., 2005).
4.4. Limitations
MTL 1H MRSI voxels contain contributions from hippocampi and surrounding WM and GM, making it impossible to determine the affected tissue type. Exploratory analyses not reported here, however, showed that metabolite concentrations in temporal WM did not change significantly over time in either sALC or nsALC. Therefore, the observed MTL metabolite changes likely originate in GM of the hippocampi and/or temporal cortex. The group sizes in this study were modest, as we included only those participants who had complete datasets at both AP1 and AP2. All cross-sectional results, however, remained unchanged when we analyzed about 40% larger groups at one week of abstinence (including those who did not have AP2) and at one month of abstinence (including those, who were enrolled between AP1 and AP2). Including only male participants in the analyses precluded assessment of sex effects. Finally, potential unrecorded group differences in nutrition, stress, exercise, overall physical health, and genetic predispositions may contribute to the results described in this study.
4.5. Conclusions
Hippocampal volume and recovery of MTL metabolites that reflect neuronal viability and membrane synthesis/turnover in abstinent alcohol-dependent men appear to be adversely affected by comorbid chronic cigarette smoking. MTL spectroscopic measures and their changes may be functionally significant as they correlate with performance and improvement of hippocampus mediated cognitive tasks. Our previous MR studies assessing regional lobar brain metabolites, tissue volumes, and perfusion in recovering alcoholics suggest that chronic cigarette smoking compounds aspects of alcohol-induced brain injury and modulates neurobiological recovery during short-term abstinence. If replicated, this research collectively will give support to the growing clinical movement of encouraging chronic smokers entering treatment for alcohol use disorders to consider concurrent participation in a smoking cessation program. On a more general note, our neuroimaging results may also be relevant to other conditions with high smoking co-morbidity and established hippocampal/MTL involvement, such as polysubstance abuse, schizophrenia and mood disorders. The long-term effects of cigarette smoking likely oppose the acute and positive effects of nicotine on attention, learning, and memory, by imparting biological injury on brain tissue and ultimately resulting in functional compromise and possibly poorer long-term outcome.
Acknowledgements
NIH AA10788 (DJM) supported this project. We thank Mary Rebecca Young, Bill Clift and Dr. Donald Tusel of the San Francisco VA Substance Abuse Day Hospital, Dr. David Pating, Karen Moise and their colleagues at the San Francisco Kaiser Permanente Chemical Dependency Recovery Program for their valuable assistance in recruiting research participants. We also thank Diana Truran and Derek Flenniken for assistance with data analyses and Erin Clevenger and Daniel Rosenbaum for hippocampal volume analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le Moal M, Piazza PV. Nicotine self-administration impairs hippocampal plasticity. Journal of Neuroscience. 2002;22:3656–62. doi: 10.1523/JNEUROSCI.22-09-03656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Archives of General Psychiatry. 1999;56:356–63. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Meyer JS, Mortel KF, Terayama Y, Thornby J, Konno S. Normal human aging: factors contributing to cerebral atrophy. Journal of Neurological Sciences. 1997;152:39–49. doi: 10.1016/s0022-510x(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, Boning J, Solymosi L. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. Ajnr.American Journal of Neuroradiology. 2001;22:1926–32. [PMC free article] [PubMed] [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test - Revised. Psychological Assessment Resources, Inc; Odessa, FL: 1997. [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, Liu D, Shen D, Davatzikos C. Hippocampus volume loss due to chronic heavy drinking. Alcohol Clinical Experimental Research. 2006;30:1866–70. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Bleich S, Sperling W, Degner D, Graesel E, Bleich K, Wilhelm J, Havemann-Reinecke U, Javaheripour K, Kornhuber J. Lack of association between hippocampal volume reduction and first-onset alcohol withdrawal seizure. A volumetric MRI study. Alcohol and Alcoholism. 2003;38:40–4. doi: 10.1093/alcalc/agg017. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biological Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Cadete-Leite A, Tavares MA, Uylings HB, Paula-Barbosa M. Granule cell loss and dendritic regrowth in the hippocampal dentate gyrus of the rat after chronic alcohol consumption. Brain Research. 1988;473:1–14. doi: 10.1016/0006-8993(88)90309-5. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. Journal of Psychiatry and Neuroscience. 2004;29:417–26. [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Research: Neuroimaging. 2005;138:115–30. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Tivis R, Lawton-Craddock A, Nixon SJ. Visual-spatial attention in alcoholics and illicit stimulant abusers: effects of nicotine replacement. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:97–107. doi: 10.1016/j.pnpbp.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Tivis R, Lawton-Craddock A, Nixond SJ. Nicotine and cognitive efficiency in alcoholics and illicit stimulant abusers: implications of smoking cessation for substance users in treatment. Subst Use Misuse. 2006;41:265–81. doi: 10.1080/10826080500409076. [DOI] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, He J, Innes D, Loh el W, Pfefferbaum A, Zou J, Sullivan EV. Alcoholic neurobiology: changes in dependence and recovery. Alcohol Clinical Experimental Research. 2005;29:1504–13. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nature Neuroscience. 2005;8:1465–70. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Pattie A, Taylor MD, Whiteman MC, Starr JM, Whalley LJ. Smoking and cognitive change from age 11 to age 80. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:1003–1007. doi: 10.1136/jnnp.74.7.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Saint-Cyr JA, Gurevich N, Carlen PL. Ethanol-induced dendritic alterations in hippocampal granule cells. Brain Research. 1989;477:373–377. doi: 10.1016/0006-8993(89)91430-3. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clinical Experimental Research. 2004;28:1849–60. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clinical Experimental Research. 2006;30:539–51. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Eisch AJ. Adult neurogenesis: implications for psychiatry. Progress in Brain Research. 2002;138:315–42. doi: 10.1016/S0079-6123(02)38085-3. [DOI] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, Heinz A, Mann K. Monitoring the Effects of Chronic Alcohol Consumption and Abstinence on Brain Metabolism: A Longitudinal Proton Magnetic Resonance Spectroscopy Study. Biological Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Esterberg ML, Compton MT. Smoking behavior in persons with a schizophrenia-spectrum disorder: a qualitative investigation of the transtheoretical model. Social Sciences and Medicine. 2005;61:293–303. doi: 10.1016/j.socscimed.2004.11.057. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1991;69:763–5. [PubMed] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychological Medicine. 2003;33:1357–67. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Fowles J, Bates M, Noiton D, Epidemiology and Toxicology Group . The Chemical Constituents in Cigarettes and Cigarette Smoke: Priorities for Harm Reduction. New Zealand: 2000. pp. 1–65. [Google Scholar]
- Gallinat J, Lang UE, Jacobsen LK, Bajbouj M, Kalus P, von Haebler D, Seifert F, Schubert F. Abnormal hippocampal neurochenistry in smokers: evidence from proton magnetic resonance spectroscopy at 3T. Journal of Clinical Psychopharmacology. 2007;27:80–84. doi: 10.1097/JCP.0b013e31802dffde. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Jahng G-H, Ezekiel F, Banys P, Meyerhoff DJ. Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion: A preliminary magnetic resonance study. Alcoholism: Clinical and Experimental Research. 2006;30:1–12. doi: 10.1111/j.1530-0277.2006.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff D. Brain Recovery During Abstinence from Alcohol: MRI, MR Spectroscopic Imaging, and Neurocognitive Studies; American Academy of Neurology 56th Annual Meeting; American Academy of Neurology, Moscone Convention Center, San Francisco, CA USA. 2004.p. A542. [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clinical Experimental Research. 2005;29:1484–95. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Abraham HD. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alcohol Depend. 2001;63:277–86. doi: 10.1016/s0376-8716(00)00216-7. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM, Kril JJ. Variation in hippocampal neuron number with age and brain volume. Cerebral Cortex. 1998;8:710–8. doi: 10.1093/cercor/8.8.710. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M, Kril JJ, Halliday GM. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus. 1997;7:78–87. doi: 10.1002/(SICI)1098-1063(1997)7:1<78::AID-HIPO8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Heffernan TM, Ling J, Parrott AC, Buchanan T, Scholey AB, Rodgers J. Self-rated everyday and prospective memory abilities of cigarette smokers and non-smokers: a web-based study. Drug Alcohol Dependence. 2005;78:235–41. doi: 10.1016/j.drugalcdep.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Hill RD, Nilsson LG, Nyberg L, Backman L. Cigarette smoking and cognitive performance in healthy Swedish adults. Age Ageing. 2003;32:548–50. doi: 10.1093/ageing/afg067. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar Volume in Offspring From Multiplex Alcohol Dependence Families. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, Weiner MW. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. Journal of Magnetic Resonance Imaging. 2002;16:305–10. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Peyton J, Benowitz NL. Metabolism and Disposition Kinetics of Nicotine. Pharmacological Reviews. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr., Morse RM, Palmen MA, Bruce BK. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol Clinical Experimental Research. 1994;18:867–72. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Jagannathan NR, Desai NG, Raghunathan P. Brain metabolite changes in alcoholism: An in vivo proton magnetic resonance spectroscopy (MRS) study. Magnetic Resonance Imaging. 1996;14:553–557. doi: 10.1016/0730-725x(96)00048-3. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. What does the hippocampus really do? Behavioural Brain Research. 1995;71:1–10. doi: 10.1016/0166-4328(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Current Opinion in Neurobiology. 2004;14:186–91. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- King MA, Hunter BE, Walker DW. Alterations and recovery of dendritic spine density in rat hippocampus following long-term ethanol ingestion. Brain Research. 1988;459:381–5. doi: 10.1016/0006-8993(88)90656-7. [DOI] [PubMed] [Google Scholar]
- Korbo L. Glial cell loss in the hippocampus of alcoholics. Alcohol Clinical Experimental Research. 1999;23:164–8. [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, Tiihonen J. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behavioural Brain Research. 2000;109:177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Lescaudron L, Jaffard R, Verna A. Modifications in number and morphology of dendritic spines resulting from chronic ethanol consumption and withdrawal: a Golgi study in the mouse anterior and posterior hippocampus. Experimental Neurology. 1989;106:156–63. doi: 10.1016/0014-4886(89)90089-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 1998;138:217–30. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Martin PR, Gibbs SJ, Nimmerrichter AA, Riddle WR, Welch LW, Willcott MR. Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcohol Clinical Experimental Research. 1995;19:1078–1082. doi: 10.1111/j.1530-0277.1995.tb00992.x. [DOI] [PubMed] [Google Scholar]
- McMullen PA, Saint-Cyr JA, Carlen PL. Morphological alterations in rat CA1 hippocampal pyramidal cell dendrites resulting from chronic ethanol consumption and withdrawal. Journal of Comperative Neurology. 1984;225:111–8. doi: 10.1002/cne.902250112. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addictive Behaviors. 2006;31:833–44. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff D, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner H. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clinical Experimental Research. 2004;28:650–61. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Sarpal D, Koch P, Steele S, Kohn P, Marenco S, Morris CA, Das S, Kippenhan S, Mattay VS, Weinberger DR, Berman KF. Functional, structural, and metabolic abnormalities of the hippocampal formation in Williams syndrome. Journal of Clinical Investigation. 2005;115:1888–95. doi: 10.1172/JCI24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radical Biology and Medicine. 2003;35:1582–8. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Current Opinions in Pharmacology. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. Journal of Neuroscience. 2004;24:9714–22. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, Price RR, Martin PR. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol Clinical Experimental Research. 2002;26:1368–80. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Mannelli P, Peindl K, Murray HW, Meier B, Leone FT. Changes in tobacco smoking following treatment for cocaine dependence. American Journal of Drug Alcohol Abuse. 2006;32:135–48. doi: 10.1080/00952990500479209. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clinical Experimental Research. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Aubin HJ, Pomerleau OF. Self-reported alcohol use patterns in a sample of male and female heavy smokers. Journal of Addictive Disorders. 1997;16:19–24. doi: 10.1300/J069v16n03_02. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mayer S, Littleton JM. Chronic, but not acute, nicotine exposure attenuates ethanol withdrawal-induced hippocampal damage in vitro. Alcohol Clinical Experimental Research. 2000;24:1583–92. [PubMed] [Google Scholar]
- Razani J, Boone K, Lesser I, Weiss D. Effects of cigarette smoking history on cognitive functioning in healthy older adults. American Journal of Geriatric Psychiatry. 2004;12:404–11. doi: 10.1176/appi.ajgp.12.4.404. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biological Psychiatry. 2001;49:258–67. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. American Journal of Public Health. 2003;93:994–8. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomedicine and Pharmacotherapy. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anatomical Record. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Dupont RM, Grant I, Lehr PP, Lamoureux G, Halpern S, Yeung DW, San Diego HIV Neurobehavioral Research Center Reduction in cortical IMP-SPET tracer uptake with recent cigarette consumption in a young group of healthy males. European Journal of Nuclear Medicine. 1997;24:422–7. doi: 10.1007/BF00881815. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. Journal of Psychopharmacology. 2004;18:457–74. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA, Belanger H, Mortimer JA, Graves AB. Effects of the use of alcohol and cigarettes on cognition in elderly African American adults. Journal of the International Neuropsychological Society. 2003;9:690–7. doi: 10.1017/S1355617703950028. [DOI] [PubMed] [Google Scholar]
- Schuff N, Amend D, Knowlton R, Tanabe J, Norman D, Fein G, Weiner MW. Age-related metabolite changes and volume loss in hippocampus by proton MR spectroscopic imaging and MRI neurobiology of aging. Neurobiology of Ageing. 1999;20:279–285. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst A, Amend D, Capizzano A, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using 1H magnetic resonance spectroscopic imaging. Magnetic Resonance in Medicine. 2001a;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biological Psychiatry. 2001b;50:952–9. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol Clinical Experimental Research. 2001;25:924–34. [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Videen JS, Alhassoon OM, Patterson TL, Grant I. Elevated myo-inositol in gray matter of recently detoxified but not long-term alcoholics: A preliminary MR spectroscopy study. Alcohol Clinical Experimental Research. 2000;24:699–770. [PubMed] [Google Scholar]
- Seitz D, Widmann U, Seeger U, Nagele T, Klose U, Mann K, Grodd W. Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifing alcoholics. Alcohol Clinical Experimental Research. 1999;23:158–163. [PubMed] [Google Scholar]
- Shingo AS, Kito S. Effects of nicotine on neurogenesis and plasticity of hippocampal neurons. Journal of Neural Transmission. 2005;112:1475–8. doi: 10.1007/s00702-005-0370-2. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J.Stud.Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith MA. Hippocampal vulnerability to stress and aging: possible role of neurotrophic factors. Behavioral Brain Research. 1996;78:25–36. doi: 10.1016/0166-4328(95)00220-0. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. The Humana Press Inc; 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. Journal of Studies on Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [published erratum appears in J Stud Alcohol 1989 Jan;50(1):92] [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clinical Experimental Research. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clinical Experimental Research. 2000;24:611–21. [PubMed] [Google Scholar]
- Trabert W, Betz T, Niewald M, Huber G. Significant reversibility of alcoholic brain shrinkage within 3 weeks of abstinence. Acta Psychiatrica Scandinavica. 1995;92:87–90. doi: 10.1111/j.1600-0447.1995.tb09548.x. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Winter H, Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. American Journal of Psychiatry. 2004;161:2194–200. doi: 10.1176/appi.ajp.161.12.2194. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biological Psychiatry. 2005;58:143–50. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]