Abstract
Background
Parkinson's disease (PD) is a neurodegenerative pathology whose molecular etiopathogenesis is not known. Novel contributions have come from familial forms of PD caused by alterations in genes with apparently unrelated physiological functions. The gene coding for alpha-synuclein (α-syn) (PARK1) has been investigated as α-syn is located in Lewy bodies (LB), intraneuronal inclusions in the substantia nigra (SN) of PD patients. A-syn has neuroprotective chaperone-like and antioxidant functions and is involved in dopamine storage and release. DJ-1 (PARK7), another family-PD-linked gene causing an autosomal recessive form of the pathology, shows antioxidant and chaperone-like activities too.
Methodology/Principal Findings
The present study addressed the question whether α-syn and DJ-1 interact functionally, with a view to finding some mechanism linking DJ-1 inactivation and α-syn aggregation and toxicity. We developed an in vitro model of α-syn toxicity in the human neuroblastoma cell line SK-N-BE, influencing DJ-1 and α-syn intracellular concentrations by exogenous addition of the fusion proteins TAT-α-syn and TAT-DJ-1; DJ-1 was inactivated by the siRNA method. On a micromolar scale TAT-α-syn aggregated and triggered neurotoxicity, while on the nanomolar scale it was neuroprotective against oxidative stress (induced by H2O2 or 6-hydroxydopamine). TAT-DJ-1 increased the expression of HSP70, while DJ-1 silencing made SK-N-BE cells more susceptible to oxidative challenge, rendering TAT-α-syn neurotoxic at nanomolar scale, with the appearance of TAT-α-syn aggregates.
Conclusion/Significance
DJ-1 inactivation may thus promote α-syn aggregation and the related toxicity, and in this model HSP70 is involved in the antioxidant response and in the regulation of α-syn fibril formation.
Introduction
Parkinson's disease (PD) is a neurodegenerative, multifactorial movement disorder affecting about 3% of the population over 65 years [1], [2]. To date, six different casual genes linked to rare familiar forms of PD have been identified, with few evident functional interactions [3]–[5]. Consequently, a major question is the relationship between these proteins whose alteration leads ultimately to a similar pathology. Genetic modifications in the proteins coded by the family-PD-linked genes PARK-1 [alias α-synuclein (α-syn); GenBank: NM_000345; OMIM: #168600] and PARK-7 (alias DJ-1; GenBank: NM_007262; OMIM: #606324) cause respectively an autosomal dominant and an autosomal recessive form of PD [6]–[9].
A-syn physiologic function is unclear, but the protein is present in Lewy bodies (LB), a PD hallmark [10]. An α-syn-dependent etiopathologic hypothesis states that in PD α-syn is able to aggregate and gain a toxic function, even if the noxious aggregated form (protofibrillar or fibrillar) is debated [11], [12]. Transgenic models developed to support this hypothesis resulted in mild to severe phenotypes [13], [14]. A-syn gene multiplication has been associated with familial PD [15], [16]. Other data support an alternative scenario where α-syn aggregation prevents the protein performing its physiological neuroprotective functions that deal with chaperone-like activity, dopamine homeostasis and correct synaptic vesicular trafficking [17]–[19]. A-syn null mice did not show a severe phenotype, suggesting that loss of function alone is not sufficient to explain the pathogenesis [20].
DJ-1 mutations are single-nucleotide substitutions or deletions leading to a non-functional protein [21], [22]. DJ-1 alterations are more frequent than α-syn mutations; consequently, DJ-1 has been evaluated as a genetic predisposing factor for sporadic PD though with mainly negative results [23], [24]. Information on DJ-1 function in the nervous system deals with antioxidant activity [25], [26], chaperone-like properties [27], [28], involvement in mitochondrial physiology [29], [30] and dopamine homeostasis [31], [32].
The interaction between α-syn and DJ-1 has been explored in cellular models of α-syn overexpression, suggesting that DJ-1 is involved in preventing α-syn toxicity by induction of HSP70 (GenBank NM_005345) [33]. HSP70 was also shown to prevent α-syn toxicity in vivo and in vitro [34], [35] and it was suggested that α-syn neuroprotective activity depended on HSP70 induction [36].
To clarify the relationship among α-syn, DJ-1 and HSP70 we developed an in vitro model of oxidative stress in the human neuroblastoma cell line SK-N-BE, where we transiently altered the α-syn and DJ-1 concentrations by adding TAT-fused proteins and by the siRNA approach.
Materials and Methods
Human neuroblastoma SK-N-BE cells
Cells were cultured at 37°C, with 5% CO2 in D-MEM supplemented with 10% foetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/mL penicillin and 100 µg/mL streptomycin (Invitrogen, Carlsbad-CA, USA), in a humidified atmosphere.
Alpha-synuclein and DJ-1: cloning and TAT-fused proteins generation
Human α-syn full-length cDNA and human DJ-1 full-length cDNA were cloned starting from a human brain cDNA library (Clontech, Palo Alto-CA, USA) by RACE-PCR. The amplified cDNAs were then cloned into pRSET expression vectors (Invitrogen, Carlsbad-CA, USA) and fully sequenced. To generate the fusion proteins TAT-α-syn and TAT-DJ-1 the sequence coding for a peptide containing the translocation domain of the HIV-1 protein TAT (underlined) (MRGSHHHHHHGMARGYGRKKRRPASPGAS) was inserted in frame before the N-terminal of the corresponding cDNA. The fusion proteins were then expressed and purified adapting standard recombinant techniques [37].
Oxidative stress challenge and TAT-fused protein treatment
To challenge SK-N-BE cells with oxidative stress, 60×103 cells were seeded in a 96-well plate and incubated overnight. The day after, the medium was changed, a freshly prepared H2O2 dilution (or 6-hydroxydopamine [6-OHDA]) was added (final concentration H2O2 75 µM; 6-OHDA 50 µM), and the cells were incubated for 24 h. Viable cells were estimated by erythrosine-dye exclusion assay or lysed in the presence of a broad-range protease inhibitor cocktail to obtain a total protein extract. To perform erythrosine-dye exclusion assay, cells were harvested by trypsinization and resuspended in complete D-MEM medium. An aliquot (10 µL) was stained 1∶1 (vol/vol) with a 0.05% erythrosine B-dye solution in PBS and viable cells (uncoloured) were counted using a cell counting chamber. To verify the protective action of α-syn (or DJ-1) against oxidative injury, 2–4 h before H2O2 (or 6-OHDA) was added, the cells were incubated with TAT-α-syn 0.5 µM (or TAT-DJ-1 3 µM) in order to assure that the media contained the TAT-fused proteins during the oxidative challenge. To trigger TAT-α-syn toxicity, cells were incubated with TAT-α-syn 3 µM for 24 h, then viability was assessed by erythrosine-dye exclusion assay or cells were lysed as described above.
Thioflavin-T binding assay for detection of amyloid aggregates
SK-N-BE cells were cultured on plastic chamber slides (Nalge Nunc International-NY, USA), and treated as previously reported, with TAT-α-syn (0.5–3 µM) for 24–48 h. Then, they were fixed with 2% paraformaldehyde and incubated 8 min with 0.05% thioflavin-T solution in PBS. After washing twice with PBS, fluorescence emission was monitored by a fluorescence microscope (Olympus Corporation, Tokyo, Japan) coupled to a digital camera (Olympus Corporation, Tokyo, Japan).
Western blotting
About 25 µg of total protein extract was subjected to gradient SDS-PAGE electrophoresis (5–12%) and transferred to a nitrocellulose membrane (BioRad Laboratories, Hercules-CA, USA). The membrane was incubated overnight with a primary antibody, for 1 hour with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz-CA, USA) and ECL-detected (GE Healthcare, Piscataway-NJ, USA). The resulting impressed film was quantified using a digital image analyser (Syngene Corporation, Cambridge, UK).
Immunocytochemistry
30×103 SK-N-BE cells were cultured on plastic chamber slide, fixed with 4% paraformaldehyde and permeabilized using 0.5% Triton-X 100, 0.2% FCS in PBS. Then, they were incubated overnight at 4°C with a primary antibody diluted 1:100 in PBS+1% horse serum (HS), followed by a FITC-conjugated secondary antibody (Jackson Immuno Laboratories, West Grove-PA, USA) diluted 1∶200 in PBS+1% HS. Cells were then analyzed with a fluorescence microscope coupled to a digital camera (Olympus Corporation, Tokyo, Japan).
RNA interference
RNA interference was performed as follows: 80×103 SK-N-BE cells were seeded in a 24-well plate and grown overnight. The next day, the medium was replaced with 250 µL of fresh complete medium and two pre-designed double-strand small-interfering RNAs (siRNA) against DJ-1 mRNA sequence (siRNA-A sense: 5′-GGG ACCAUAUGAUGUGGUGtt-3′; antisense: 5′-CACCACAUCAUAUGGUCCCtc-3′. siRNA-B sense: 5′-GGAGCAGGAAAACCGGAAGtt-3′; antisense: 5′-CUUCCGGUUUUCCUGCUCCtt-3) (Ambion, Austin-TX, USA) were added singularly in 50 µL serum-free Optimem (Invitrogen, Carlsbad-CA) at 100 nM final concentration using as vehicle SilentFect liposome formulation (BioRad Laboratories, Hercules-CA) for 24–96 h, without any other medium change, to achieve DJ-1 downregulation. To prove specific DJ-1 silencing, also a negative control siRNA was used (CT-), comprised of a 19 bp scrambled sequence with 3′ dT overhangs that has no significant homology to any known human gene sequence (Ambion, Austin-TX, USA). Cell viability was then assessed by erythrosine-dye exclusion assay or cells were lysed in the presence of a broad-range protease inhibitor to obtain a total protein extract.
Semiquantitative RT-PCR
Semiquantitative RT-PCR was performed starting from 100 ng of DNA-free total RNA extracted from SK-N-BE cells with a commercial kit (Qiagen, Valencia-CA, USA) using the following primers: HSP70 forward: 5′-TTT GAC AAC AGG CTG GTG AAC C-3′; HSP70 reverse: 5′-GTG AAG ATC TGC GTC TGG TTG G-3′; aldolase-A forward: 5′-CGC AGA AGG GGT CCT GGT GA-3′; aldolase-A reverse: 5′-CAG CTC CTT CTT CTG CTC CGG GGT-3′. The resulting PCR products were loaded and quantified on a capillary electrophoresis unit (Agilent Technologies, Palo Alto-CA, USA) in comparison to a standard internal marker.
Antibodies
The following primary antibodies were used to run immunoblotting or immunocytochemistry: anti α-syn monoclonal antibody (Transduction Laboratories, Lexington-KY, USA); anti DJ-1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz-CA. USA); anti α-tubulin monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz-CA, USA) and anti HSP70 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz-CA, USA).
Chemicals and reagents
All chemicals were of analytical grade. Where not stated otherwise, reagents were from Sigma (Sigma Aldrich, St Louis-MO, USA).
Statistical analysis
All experiments were run at least three times. Statistics was analyzed using the program StatView ver 5.0. One-way ANOVA (or two-way ANOVA) was used, followed by Dunnett's or Tukey's post-hoc test. The significance limit was set at p = 0.05.
Results
TAT-α-syn and TAT-DJ-1 prevent oxidative stress in SK-N-BE cells
SK-N-BE cells were exposed to H2O2 or 6-OHDA for 24 h and neurotoxicity was assessed. To establish the optimal experimental dosage, we first plotted a dose-response curve [Figure 1A–B]. We decided to run the ensuing experiments using 75 µM H2O2 or 50 µM 6-OHDA, that reduced cell viability by about 50%.
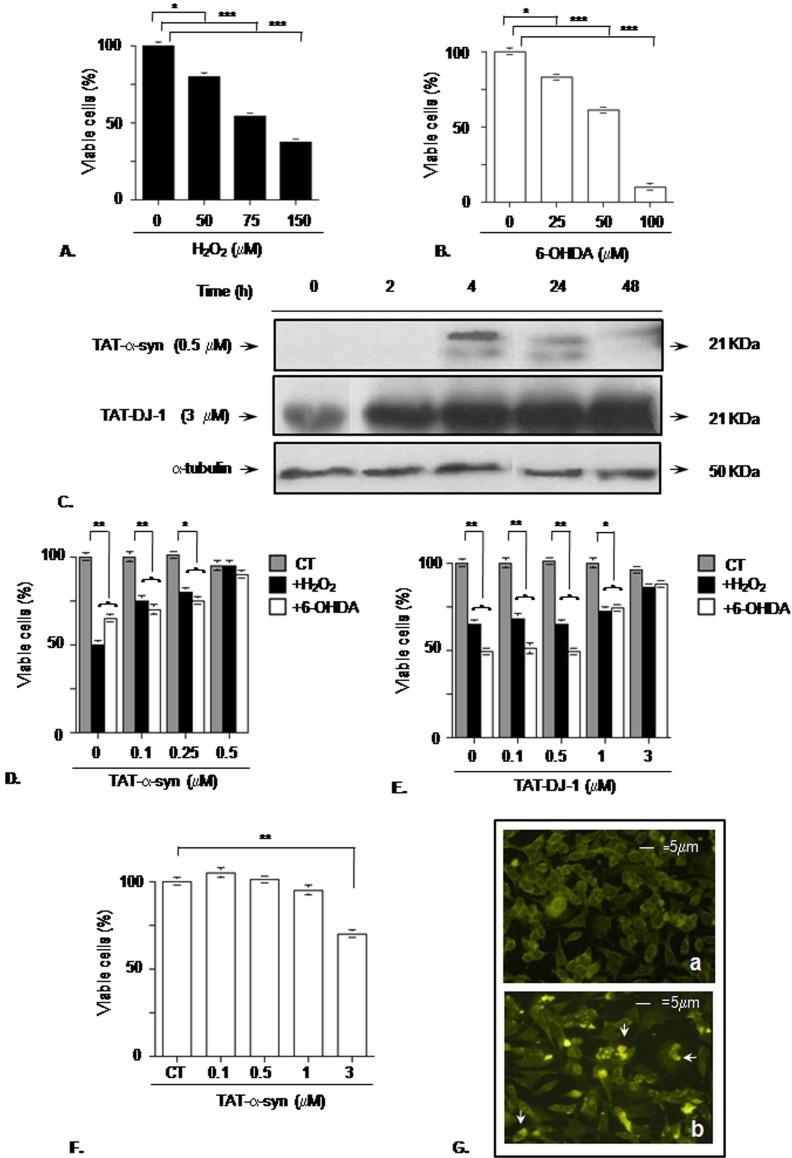
Figure 1. TAT-α-syn and TAT-DJ-1 prevent oxidative stress in SK-N-BE cells.
(A) Dose-response pattern of the toxic effect of hydrogen peroxide (H2O2) in SK-N-BE cells. (B) Dose-response curve of 6-hydroxydopamine (6-OHDA) toxicity in SK-N-BE cells. Cells were plated and challenged by oxidative stress for 24 h and cell viability was assessed by erythrosine-dye exclusion assay; *p<0.05; ***p<0.001 vs control group (0 µM H2O2 or 6-OHDA), Dunnett's post-hoc test. (C) Western blot assessing TAT-α-syn 0.5 µM and TAT-DJ-1 3 µM availability inside SK-N-BE cells. Cells were plated and incubated in presence of TAT-α-syn or TAT-DJ-1 for the reported time intervals. To demonstrate equal gel loading, α-tubulin immunoreactivity is also presented. (D) Protective effect of TAT-delivered α-syn and (E) TAT-delivered DJ-1 against oxidative stress. Cells were incubated with increasing amounts of TAT-α-syn or TAT-DJ-1 2–4 h before the toxic treatment and 24 h later cell viability was assessed by erythrosine-dye exclusion assay; *p<0.05; **p<0.01 vs control group at the same TAT-α-syn or TAT-DJ-1 concentration (CT, corresponding also to 0 µM H2O2 or 6-OHDA), Tukey's post-hoc test. (F) Toxicity of micromolar amounts of TAT-α-syn. Cells were incubated with increasing concentrations of TAT-α-syn for 24 h and cell viability was assessed by erythrosine-dye exclusion assay. **p<0.01 vs control group (CT, corresponding to 0 µM TAT-α-syn), Dunnett's post-hoc test. (G) Amyloid aggregation of TAT-α-syn 3 µM was detected by thioflavin-T staining after 24 h incubation; a) SK-N-BE control cells; b) SK-N-BE cells incubated with TAT-α-syn 3 µM. The arrows indicate intracellular thioflavin-T-positive inclusions (magnification 20X). Owing to concerns about similarities with previously published content in [36], the α-tubulin panel of Figure 1C is excluded from this PLOS ONE article’s CC-BY license; FASEB granted permissions to retain the image in this retracted article. See the accompanying retraction notice for more information.
To finely tune the α-syn or DJ-1 intracellular concentration, we incubated cells with the fusion proteins TAT-α-syn and TAT-DJ-1. First we used Western blotting to plot the kinetics of TAT-α-syn and TAT-DJ-1 availability inside cells. The basal level of endogenous α-syn in SK-N-BE cell was negligible (0h lane), while TAT-α-syn 0.5 µM fusion protein was detectable from 4 h after addition to the medium, migrated as a doublet around 21KDa and even if its total amount decreased over time it was still present 48h after treatment. At difference from α-syn, SK-N-BE expressed DJ-1 at basal level (0h lane) but after TAT-DJ-1 3 µM addition a stronger immunoreactive band was detectable starting from 2h and its level kept almost unchanged till 48h [Figure 1C].
Afterwards, we verified the protective effect of TAT-α-syn or TAT-DJ-1 against oxidative challenge, performing a dose-response curve. As shown in Figure 1D, TAT-α-syn protection started from 0.1 µM in comparison to untreated cells, but the optimal protective concentration was set at 0.5 µM, where we observed an almost complete prevention of oxidative stress-induced toxicity. TAT-DJ-1 too was neuroprotective against both H2O2 and 6-OHDA neurotoxicity [Figure 1E]. Viability increased starting from 1 µM for 6-OHDA treated cells, and the optimal protective dose against both H2O2 and 6-OHDA was set at 3 µM, where we found a survival rate that was not statistically different from control. Increasing further the TAT-DJ-1 concentration did not improve survival rate, even if no toxicity was detectable up to 5 µM (data not shown). We have also tried to further increase TAT-α-syn concentration but at doses higher than 1 µM it reduced cell viability and caused a thioflavin-T positive reaction, suggesting the appearance of intracellular aggregated forms of the fusion protein [Figure 1 F–G].
DJ-1 downregulation by siRNA increased SK-N-BE susceptibility to oxidative challenge
In order to verify how much DJ-1 protected SK-N-BE cells from oxidative damage, we downregulated endogenous DJ-1 expression by small-interfering RNA (siRNA) technology. Figure 2A shows a preliminary DJ-1 silencing experiment assessed by Western blotting, using two independent DJ-1-targeted siRNAs (siRNA-A and siRNA-B), 20 or 100 nM final concentration each, for 72 h. Both siRNAs achieved DJ-1 downregulation, but siRNA-B 100nM was the most effective, as confirmed by blot quantification [DJ-1/α-tubulin optical density (OD) ratio in control (mean±SD, n = 3): 1.1±0.1; DJ-1/α-tubulin OD ratio (mean±SD, n = 3) for siRNA-A 100 nM: 0.5±0.1; DJ-1/α-tubulin OD ratio for siRNA-B 100 nM (mean±SD, n = 3): 0.4±0.1, p<0.001 vs control, Tukey's test]. Consequently, for the ensuing silencing experiments we decided to use siRNA-B at 100 nM.
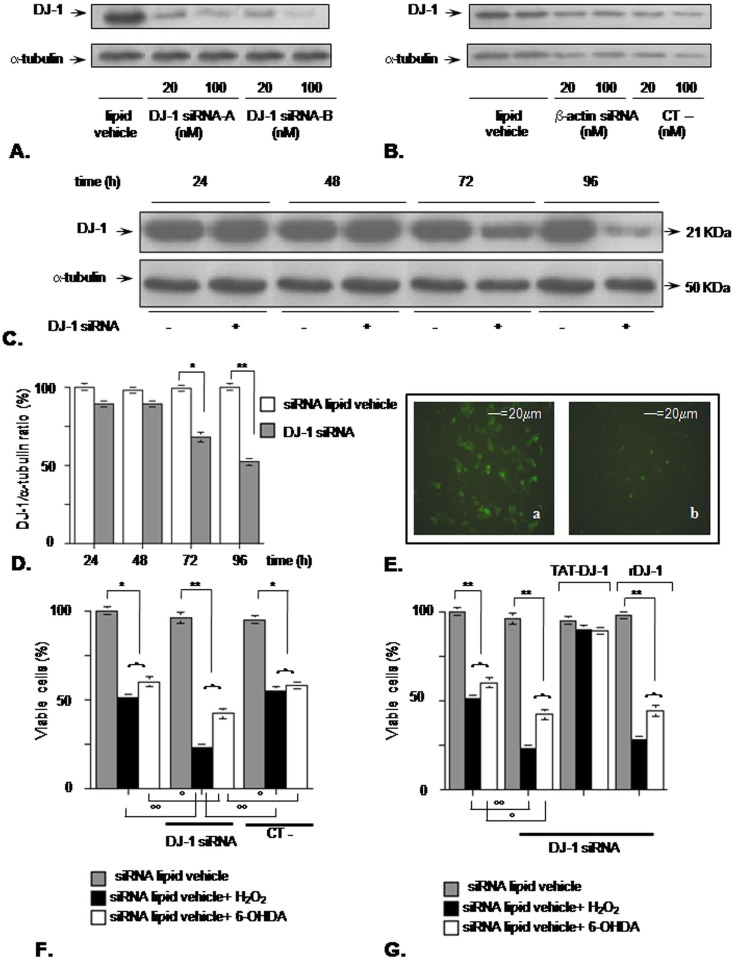
Figure 2. DJ-1 downregulation by siRNA increases SK-N-BE susceptibility to oxidative challenge.
(A) Preliminary Western blotting experiment aimed at modulating DJ-1 expression by siRNA. Two different siRNAs were tested by exposing SK-N-BE cells to 20 and 100 nM siRNA for 72 h. Each blot lane is representative of a triplicate assay in the same experiment (B) Western blotting assessing the specificity of the proposed silencing method. SK-N-BE cells were exposed for 72 h to β-actin targeted pre-designed siRNA and to a siRNA negative control (CT-) with no sequence similarity to DJ-1 mRNA. Each blot lane is representative of a triplicate assay in the same experiment. (C) Silencing DJ-1 expression by siRNA. Cells were incubated with 100 nM pre-annealed siRNA targeted to DJ-1. Starting 24 h after transfection, DJ-1 protein level was assessed by Western blotting and quantified by a digital image analyzer. The quantification (D) is representative of one of three independent experiments (n = 3 for each point); *p<0.05; **p<0.01 vs control group (siRNA lipid vehicle) at the same time, Tukey's post-hoc test. (E) DJ-1 silencing was also independently assessed by immunocytochemisty: a) SK-N-BE control cells exposed for 72 h to siRNA lipid vehicle alone (magnification 10X); b) SK-N-BE cells incubated for 72 h with 100nM DJ-1 siRNA (magnification 10X). (F) DJ-1 downregulation increases cell susceptibility to oxidative stress. Cells were silenced for DJ-1 expression for 72 h, then the oxidative stimuli were added for a further 24 h. Cell viability was assessed by erythrosine-dye exclusion assay. CT-: siRNA negative control; *p<0.05; **p<0.01 vs control group (siRNA lipid vehicle alone); °p<0.05; °°p<0.01 vs siRNA lipid vehicle+H2O2 or 6-OHDA alone; Tukey' post-hoc test. (G) Specific effect of DJ-1 on oxidative stress response. Cells were silenced for DJ-1 expression for 72 h, then TAT-DJ-1 3 µM or a recombinant DJ-1 at the same concentration (rDJ-1, a purified form of DJ-1 without the TAT sequence) was added 2 h before the oxidative challenge. After 24 h, cell viability was assessed by erythrosine-dye exclusion assay. DJ-1 silencing in the same experiment was confirmed by Western blotting (not shown); **p<0.01 vs control group (siRNA lipid vehicle alone), °p<0.05; °°p<0.01 vs siRNA lipid vehicle+H2O2 or 6-OHDA alone; Tukey's post-hoc test.
We also performed a specificity control experiment using a β-actin targeted-siRNA that was unable to affect DJ-1 expression, so did a siRNA negative control (CT-) with no sequence homology to DJ-1 mRNA [Western blot quantification: DJ-1/α-tubulin OD ratio for control (mean±SD, n = 3): 1.0±0.1; DJ-1/α-tubulin OD ratio (mean±SD, n = 3) for β-actin siRNA 100 nM: 1.0±0.1; DJ-1/α-tubulin OD ratio (mean±SD, n = 3) for CT- siRNA 100 nM: 0.9±0.1] [Figure 2B]. The silencing treatment using the lipid vehicle was not toxic to cells, with no statistically significant reduction in cellular viability in comparison to untreated cells (data not shown).
To fully characterize DJ-1 silencing by siRNA-B 100 nM, we performed a time-course experiment, shown in [Figure 2C]. DJ-1 protein level significantly decreased starting from 72 h and this reduction was even more evident at 96h, as reported in Figure 2D. DJ-1 downregulation at 72 h after siRNA treatment was also independently confirmed by immunocytochemistry, where a decrease of DJ-1 immunostaining was found in the DJ-1 siRNA treated-cells [Figure 2E].
Then, we challenged cells with the oxidative agent 72 h after DJ-1 silencing, in order to be sure to achieve DJ-1 downregulation in a detectable way. In this condition, SK-N-BE cells were more susceptible to oxidative damage induced by H2O2 or 6-OHDA, with a strong reduction in cellular viability that dropped under 25% in comparison to control after H2O2 and under 50% after 6-OHDA. This effect was specifically due to DJ-1 silencing, as the treatment with a siRNA negative control (CT-) did not influence the cell response to oxidative stress [Figure 2F].
To further check the specificity of this DJ-1-silencing effect, we tried to reverse the DJ-1 silencing by adding TAT-DJ-1 3 µM 2h before the oxidative treatment. We were able to reproduce the deleterious effect of DJ-1 silencing (as cell viability of the silenced groups in presence of oxidative stress was decreased in comparison to DJ-1 not silenced groups), and when we added TAT-DJ-1 this completely counteracted the negative effect of H2O2 or 6-OHDA. The extracellular add of 3 µM recombinant DJ-1 (a purified form of DJ-1 without the TAT sequence) had no such effect, confirming that only TAT-DJ-1 entered the plasma membrane and protected the cells [Figure 2G]. We found the same situation when we treated cells with a TAT-GFP fusion protein that entered inside cells but was unable to counteract the deleterious effect of DJ-1 silencing (Figure S1A).
DJ-1 silencing prevents TAT-α-syn protective action and increases TAT-α-syn dose-dependent toxicity
To explore a possible functional relationship between DJ-1 and α-syn we silenced DJ-1 expression by siRNA and tested TAT-α-syn mediated protection against H2O2 or 6-OHDA. As shown in Figure 3A, TAT-α-syn at nanomolar scale completely antagonized H2O2 or 6-OHDA toxicity, but this effect was no longer detectable in the presence of DJ-1 downregulation by siRNA. In this situation, cell viability dropped to under 50%, whit a more pronounced toxicity for H2O2 treatment. This effect was specifically dependent on DJ-1 inactivation, as TAT-α-syn maintained almost unchanged its protective effect against oxidative stress in the presence of a siRNA negative control (CT-) and when the DJ-1 protein level was restored by exogenous addition of TAT-DJ-1 2h before the oxidative challenge [Figure 3B].
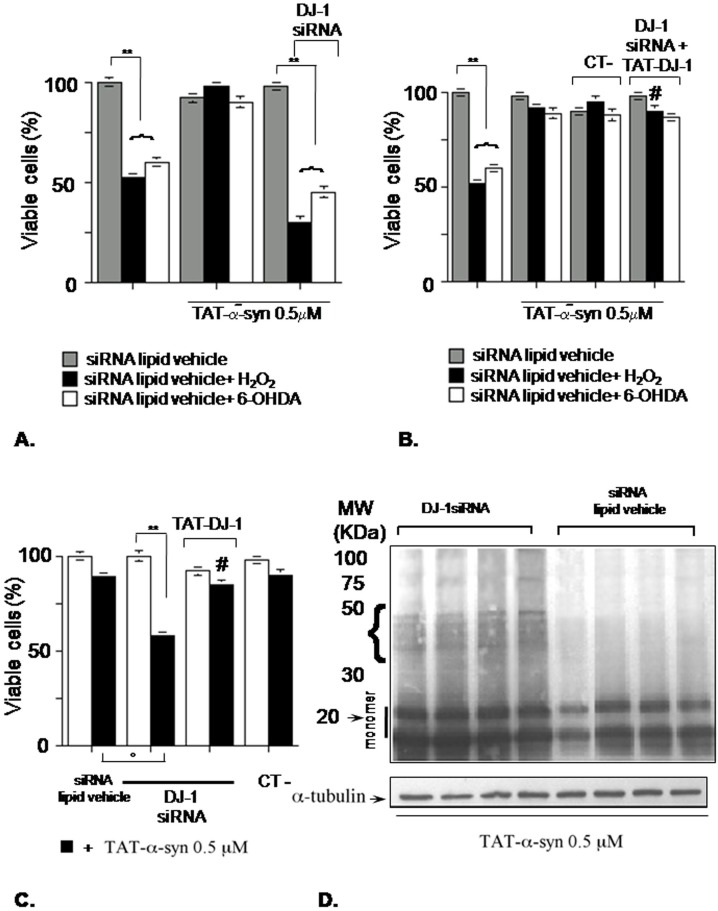
Figure 3. DJ-1 silencing prevents TAT-α-syn protective action and increases its dose-dependent toxicity.
(A) Cells were pre-incubated with TAT-α-syn 0.5 µM for 2 h. Then, the oxidative stimuli were added for a further 24 h. In the DJ-1 silenced group, before TAT-α-syn treatment, DJ-1 expression was silenced for 72 h. Cell viability was assessed by erythrosine-dye exclusion assay; **p<0.01 vs control group (siRNA lipid vehicle alone), Tukey's post-hoc test. (B) Cells were incubated with siRNA negative control (CT-) or with DJ-1 siRNA for 72 h. Then, in the DJ-1 siRNA cells, TAT-DJ-1 3 µM was added to culture medium and after 2 h TAT-α-syn was added to the indicated groups. After 2 h, the oxidative challenge was then carried out for 24 h. Cell viability was assessed by erythrosine-dye exclusion assay. **p<0.01 vs control group (siRNA lipid vehicle alone), Tukey's post-hoc test; #p<0.01, two-way ANOVA for TAT-α-syn (x) TAT-DJ-1. (C) Cells were silenced for DJ-1 expression for 72 h, then TAT-α-syn 0.5 µM was added for 24 h. In the TAT-DJ-1 group, TAT-DJ-1 was added to the culture medium 2 h before TAT-α-syn. CT-: siRNA negative control; **p<0.01 vs control group (no TAT-α-syn added); °p<0.05 vs TAT-α-syn alone; Tukey's post-hoc test; #p<0.01, two-way ANOVA for TAT-α-syn (x) TAT-DJ-1. (D) Western blot showing TAT-α-syn higher-molecular-weight immunoreactive bands after DJ-1 silencing. Cells were silenced for DJ-1 expression for 72 h, after that TAT-α-syn 0.5 µM was added for 24 h. Cell lysates were subjected to gradient SDS-PAGE (5–12%). The arrow indicates the monomeric form of TAT-α-syn around 20 KDa, while the bracket the presence of higher-molecular-weight immunoreactivity. Also α-tubulin immunoreactivity is shown to demonstrate equal gel loading.
We noticed another relevant effect of DJ-1 silencing on TAT-α-syn. In fact, after DJ-1 silencing by siRNA, if TAT-α-syn was added to cells at 0.5 µM for 24 h, it not only lost its protective property against oxidative stress but also became toxic in absence of a deleterious stimulus, triggering by itself cell death in a significant manner [Figure 3C]. To confirm that this effect was specifically due to DJ-1 silencing we tried a reversion by addition of TAT-DJ-1. In this context, TAT-α-syn was no more toxic, a situation that was replicated when instead of DJ-1 siRNA we used a no-match siRNA negative control (CT-).
The following step was to assess whether DJ-1 silencing caused TAT-α-syn 0.5 µM toxicity by modulation of its aggregation state and if DJ-1 silencing affected total TAT-α-syn availability inside cells. To this purpose, we used Western blotting to detect TAT-α-syn aggregates and to quantify intracellular available monomeric TAT-α-syn [Figure 3D]. Only when cells were treated with DJ-1 siRNA we noticed the appearance of higher-molecular-weight α-syn immunoreactive bands (around 40–50 KDa), suggesting of TAT-α-syn aggregation. On the contrary, in presence of siRNA lipid vehicle alone only the monomeric α-syn band around 20 KDa was detectable. Remarkably, Western blotting quantification data supported a statistically significant increase of about 25% of intracellular total monomeric TAT-α-syn in presence of DJ-1 siRNA [monomeric TAT-α-syn/α-tubulin OD ratio for siRNA lipid vehicle (mean±SD, n = 4): 2.4±0.2 ; monomeric TAT-α-syn/α-tubulin OD ratio for DJ-1 siRNA (mean±SD, n = 4): 3.0±0.2, p<0.02, Student's t-test].
We also tried to independently confirm TAT-α-syn 0.5 µM amyloid aggregation in presence of DJ-1 siRNA by thioflavin-T staining. However, we did not find any positive signal (data not shown), even if this method was able to clearly detect TAT-α-syn aggregates [see Figure 1G].
We have also verified an alternative scenario where we tried to trigger TAT-α-syn 0.5 µM neurotoxicity in SK-N-BE cells by increasing H2O2 amount (raised to 100 µM) and 6-OHDA amount (raised to 75 µM) for 24h in order to mimic an increase of oxidative stress due to DJ-1 depletion. No evident additive toxic effect of TAT-α-syn was assessed in presence of TAT-α-syn 0.5 µM and H2O2 or 6-OHDA. On the contrary, in 6-OHDA treated cells a weak but statistically significant neuroprotective action of TAT-α-syn was still present (Figure S1B).
TAT-DJ-1 affects HSP70 mRNA expression
Previous data suggested that the protective action of TAT-α-syn against oxidative stress involved an up-regulation of HSP70 [36]. In order to find out whether HSP70 was also involved in the DJ-1-mediated antioxidant response, we assessed the effect on HSP70 expression of TAT-DJ-1 and DJ-1 silencing by semiquantitative RT-PCR, using aldolase A (GenBank NM_184041) as internal standard [Figure 4A]. In the presence of TAT-DJ-1, HSP70 mRNA was about 2.5 times control, and this increase was statistically significant as reported in the gel quantification graph [Figure 4B]. Instead, the DJ-1 siRNA treatment or the siRNA negative control (CT-) treatment had no such effect on HSP70 expression that was similar to the basal level.
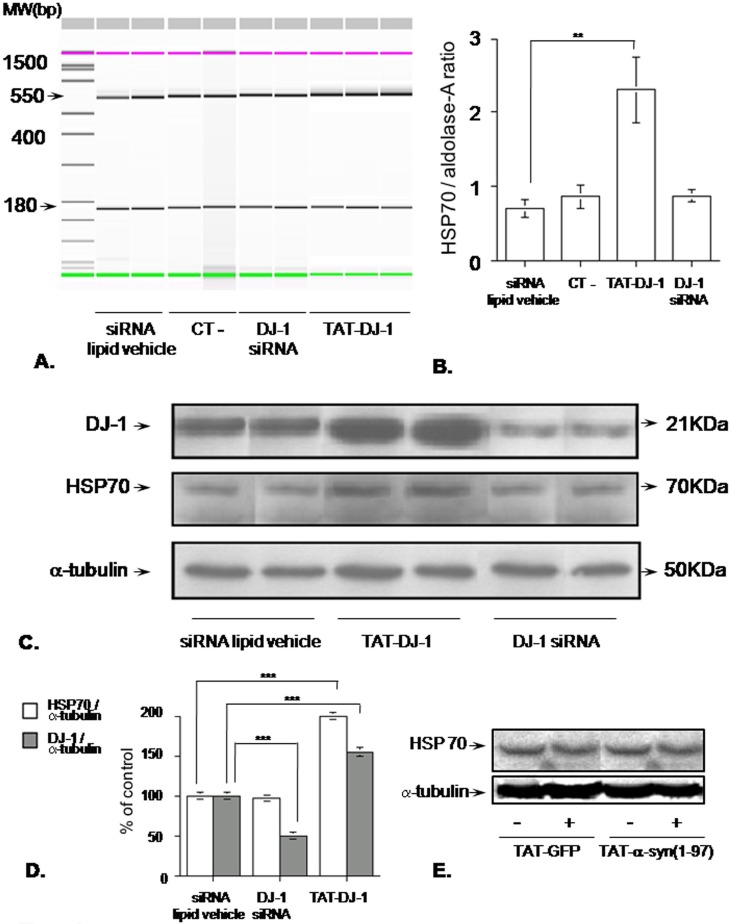
Figure 4. TAT-DJ-1 affects HSP70 mRNA expression.
A) Digital image of a capillary electrophoresis assessing the effect of DJ-1 on HSP70 mRNA expression. Cells were either incubated with TAT-DJ-1 for 24 h or silenced for DJ-1 expression for 72 h. Then total mRNA was extracted, reverse-transcribed and amplified to detect HSP70 (550 bp band) and aldolase-A (180 bp band), used as internal control. The bar graph (B) shows the RT-PCR assay values and is representative of one of three independent experiments (n = 4 for each group). CT-: siRNA negative control; **p<0.01 vs control (siRNA lipid vehicle alone), Tukey's post-hoc test. (C) Western blot showing HSP70 protein expression. Cells were incubated with either TAT-DJ-1 for 24h or DJ-1 siRNA for 72 h, then harvested for Western blotting. The bar graph (D) shows the calculated values normalized to α-tubulin as internal control and is representative of one of three independent experiments (n = 3 for each group); ***p<0.001 vs control group (siRNA lipid vehicle alone), Tukey's post-hoc test. (E) Western blotting assessing HSP70 expression level in SK-N-BE cells incubated with negative control TAT-fused proteins. Cells were incubated with TAT-GFP or TAT-α-syn(1-97) for 24 h and then harvested to perform HSP70 immunodetection. The western blot panels in Figure 4E report material from [36], reproduced with permission of FASEB. The images in Figure 4E are excluded from this PLOS ONE article’s CC-BY license. See the accompanying retraction notice for more information.
Then, we decided to confirm by Western blotting if HSP70 mRNA increase was also detectable at protein level. As shown in [Figure 4C], in the presence of TAT-DJ-1 the HSP70 immunoreactive band increased about 2 times the control level, and this increase was statistically significant as assessed by the blot quantification reported [Figure 4D]. However, HSP70 protein level kept unchanged after DJ-1 siRNA treatment, whose effect on its target was clearly detectable as the DJ-1 immunoreactive band was about half the control, with a statistically significant reduction of about 50%. The increase of DJ-1 protein level due to TAT-DJ-1 addition was also evident, with a statistically significant increase of DJ-1 immunoreactive band of about 1.5 times the basal level.
These TAT-DJ-1 mediated effect on HSP70 expression was specific, as when we used an unrelated TAT-GFP fusion protein no HSP70 increase at protein level was detectable. A similar pattern was obtained using another TAT-fused control protein represented by TAT-α-syn(1-97), a C-terminal truncated α-syn form that we previously showed to enter plasma membrane but was unable to induce HSP70 in PC12 cell line [36][Figure 4E].
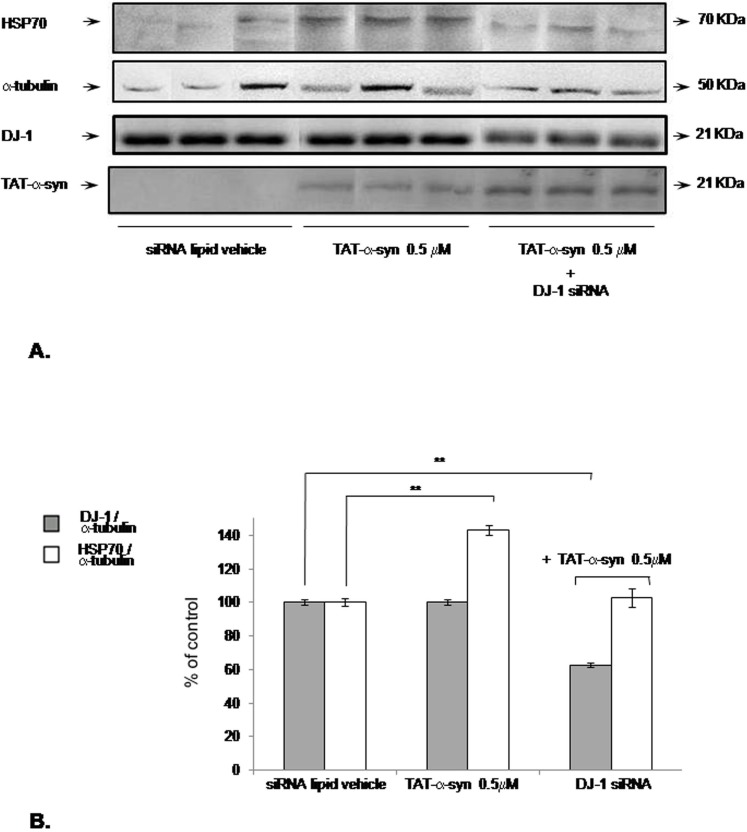
Finally, to verify whether DJ-1-dependent HSP70 upregulation was relevant for TAT-α-syn protective action, we performed an experiment where DJ-1 was downregulated by siRNA approach and cells where incubated with TAT-α-syn 0.5 µM, a neuroprotective condition involving HSP70 upregulation [36]. As assessed by Western blotting in Figure 5A, when we treated cells with TAT-α-syn 0.5 µM alone for 24h we confirmed an increase of HSP70 expression of about 40% the basal level. This effect was statistically significant as reported in the blot quantification graph [Figure 5B]. On the contrary, when TAT-α-syn 0.5 µM was added to cells for 24 h after a 72 h DJ-1 silencing, HSP70 increase was no more detectable and an increase of total intracellular monomeric TAT-α-syn was confirmed in analogy to Figure 3D (DJ-1 silencing was effective, as confirmed by blot quantification in Figure 5B). We were also able to restore HSP70 upregulation by adding TAT-DJ-1 3 µM before TAT-α-syn 0.5 µM incubation in SK-N-BE cells silenced for DJ-1 expression (data not shown).
Figure 5. DJ-1 silencing prevents TAT-α-syn-mediated HSP70 upregulation.
(A) In the DJ-1 silenced group, SK-N-BE cells were silenced for DJ-1 expression for 72 h, TAT-α-syn 0.5 µM was then added for 24 h and finally cells were harvested for Western blotting to assess HSP70, DJ-1 and TAT-α-syn immunoreactivity. The graph (B) shows blot quantification for HSP70 and DJ-1 expression using α-tubulin as internal standard (n = 3 for each group); **p<0.01, vs control group (siRNA lipid vehicle alone), Tukey's post-hoc test.
Finally, we verified whether TAT-α-syn 0.5 µM was able to induce DJ-1 upregulation at protein level, an effect that could in turn promote HSP70 expression. We were unable to find out a DJ-1 increase at protein level when we incubated cells with TAT-α-syn for 24 h (Figure S1C).
Discussion
We analyzed a possible functional relationship between DJ-1 and α-syn in a cellular model of oxidative stress, a detrimental condition that is believed to play a major role in neurodegenerative disorders. Both proteins have been linked to familial early-onset forms of PD, but it is still not clear why pathogenic mutations in these two apparently unrelated molecules lead ultimately to the same clinical features.
DJ-1 role was investigated after increasing its intracellular expression level by exogenous addition of a TAT-fused recombinant protein, while its expression was downregulated by siRNA. These experiments outlined a major role of DJ-1 in the antioxidant response, as its overexpression correlated positively with a survival outcome. This protective function was not limited to H2O2 challenge but was also clear against 6-OHDA, a neurotoxin whose noxious effect reproduces what happens in the dopamine-selective environment of PD neurodegeneration. Moreover, the antioxidant effect was specific, as neither a recombinant DJ-1 without the TAT fused-sequence (that was consequently unable to enter the cell) nor a siRNA negative control (that did not affect the level of DJ-1 mRNA) prevented the oxidative damage. Starting from these data, it is reasonable to conclude that one effect of DJ-1 inactivation by genetic alterations is to impair its prevention of oxidative stress, thus exposing dopaminergic neurons to exogenous or endogenous deleterious stimuli.
Zhou et al. suggested that DJ-1-mediated protective action against α-syn (A53T) overexpression ant toxicity involved HSP70 up-regulation, but this mechanism was not reported under oxidative stress conditions where a DJ-1-dependent increase of glutathione cellular levels was highlighted [33]. We found an increase of HSP70 both at mRNA and protein level after DJ-1 overexpression obtained by adding TAT-DJ-1. This effect missed using TAT-GFP or TAT-α-syn(1-97) fusion proteins, suggesting that HSP70 increase was not solely due to a generic cell response to molecular crowding after TAT-mediated protein delivery. HSP70 upregulation might mediate also TAT-DJ-1 antioxidant action, even if in our experiments under oxidative stress condition we have not directly demonstrate this aspect. Moreover, our data do not rule out the possible involvement of other DJ-1-mediated antioxidant mechanisms, like glutathione increase.
We have already demonstrated an antioxidant function of TAT-administered α-syn in rat PC12 cells, where it prevented H2O2 oxidative damage by raising the HSP70 protein level [36]. Here, we have extended this protective property to 6-OHDA, providing evidence that α-syn is an important neuroprotective element whose deficiency due to aggregation may concur in triggering PD.
An important issue addressed by our experiments was the possible functional relationship between DJ-1 and α-syn, in order to verify whether they do indeed belong to the same antioxidant pathway. When we downregulated DJ-1 expression by siRNA, TAT-α-syn alone was no longer able to protect cells from the oxidative damage, suggesting that DJ-1 presence is needed for an efficient antioxidant response, probably as a consequence of its positive action on HSP70 expression. So, when DJ-1 is inactivated this condition is deleterious not only directly but also indirectly as it negatively affects a second cell-protective mechanism based on α-syn neuroprotective function.
A second, unexpected but relevant consequence of DJ-1 downregulation on TAT-α-syn was that in this situation TAT-α-syn was not only unable to counteract the oxidative challenge but it became toxic by itself even in the absence of oxidative stress and starting from nanomolar intracellular concentration that was usually protective. As α-syn toxicity is generally considered a consequence of its aggregation, we verified that when TAT-α-syn had deleterious properties an aggregation pattern was detectable. In fact, only in the presence of DJ-1 siRNA we were able to identify TAT-α-syn oligomeric aggregates by Western blotting. This DJ-1 effect on TAT-α-syn nanomolar toxicity was specific as it could not be reproduced by recombinant DJ-1 (a purified form of DJ-1 without the TAT sequence) or siRNA negative control and was reversed by addition of TAT-DJ-1 after DJ-1 silencing. Moreover, when DJ-1 was silenced, TAT-α-syn at nanomolar scale was unable to induce HSP70 upregulation, an aspect that might contribute to TAT-α-syn aggregation and toxicity. A clear molecular mechanism leading to TAT-α-syn aggregation in presence of DJ-1 silencing by siRNA is not evident by our experiments. We can rule out that TAT-α-syn toxicity was just a consequence of increased oxidative stress after DJ-1 downregulation as an increased oxidative insult was unable to trigger TAT-α-syn 0.5 µM toxicity, thus suggesting that DJ-1 directly modulated α-syn aggregation properties. However, considering our data reporting an increased monomeric TAT-α-syn availability inside cells in presence of DJ-1 siRNA, we can suggest that DJ-1 downregulation might negatively affect TAT-α-syn degradation pathway, with a consequent TAT-α-syn accumulation that can promote its self-aggregation. So, in the presence of a reduced amount of DJ-1 TAT-α-syn aggregation can start at a nanomolar scale that is normally protective and too low to trigger aggregation by itself.
The fact that in the same condition we were unable to detect any amyloid-like aggregates by thioflavin-T staining might depend on the extent of α-syn aggregation. In fact, the formation of α-syn immunoreactive bands was limited to molecular weights 40-50 KDa, with no higher-molecular-weight oligomers, suggesting that larger amyloid fibrils probably require a more concentrated TAT-α-syn starting substrate. In accordance with this interpretation, Zhou et al. [33] did find higher-molecular-weight α-syn [A53T] aggregates but after strong α-syn overexpression due to adenoviral infection. However, even without large α-syn amyloid aggregates cell toxicity was evident, in agreement with the hypothesis that the early step of α-syn fibril formation is responsible for its toxicity.
In relation to PD pathogenesis, an inactivating DJ-1 genetic alteration might first of all prevent its direct antioxidant action but it might be also important in promoting α-syn aggregation and the related toxicity, that is accelerated by a pro-oxidant environment [38].
In summary, as schematically depicted in Figure 6, DJ-1 and α-syn are both involved in the antioxidant response against H2O2 and 6-OHDA and HSP70 is a common downstream mediator of the antioxidant properties of these two proteins. A direct mechanistic relationship between α-syn, DJ-1 and HSP70 is not supported by our data. In fact, TAT-α-syn was able to increase HSP70 level in presence of DJ-1 but TAT-α-syn was unable to increase DJ-1 expression that could in turn promote HSP70 upregulation. A possible interpretation could be that DJ-1 and α-syn promote HSP70 upregulation but at different levels. In fact, TAT-DJ-1 addition increased HSP70 at mRNA and protein expression level, at difference from TAT-α-syn that was unable to modulate HSP70 mRNA, as we have previously demonstrated [36]. So, it might happen that TAT-α-syn increases HSP70 protein level by a chaperone-like stabilization mechanism or by an action that reduces HSP70 degradation over time. This might also explain why in absence of DJ-1 TAT-α-syn was unable to increase HSP70 at protein level. In fact, when DJ-1 was downregulated TAT-α-syn formed insoluble aggregates and in this aggregated form it is likely that it was unable to interact with HSP70 and stabilize it. More importantly, we showed that DJ-1 expression is relevant not only for the effect on HSP70 but also in controlling α-syn function which, when there are only small amounts of DJ-1, is shifted from a soluble, neuroprotective condition to an aggregated, neurotoxic context.
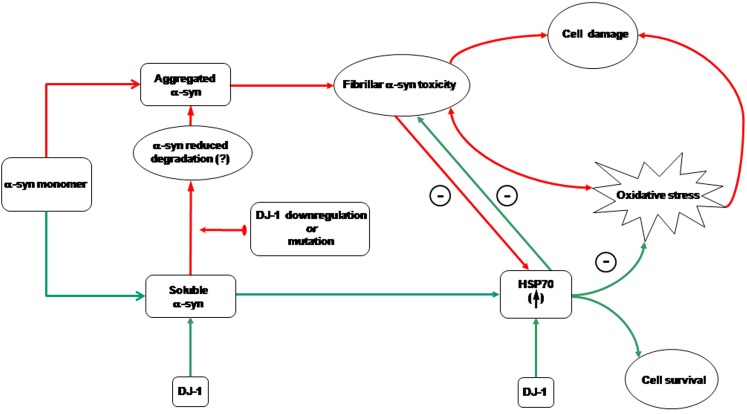
Figure 6. Schematic representation of the neuroprotective network against oxidative stress mediated by alpha-synuclein (α-syn) and DJ-1.
Red arrows identify noxious molecular events, while green arrows trace pro-survival cellular mechanisms. The symbol ⊙ stands for “antagonizes”. The free, soluble form of α-syn can be committed to aggregation and the related toxicity by DJ-1 downregulation (upper pathway), maybe through α-syn impaired degradation. In this situation, cell response to oxidative stress is impaired by DJ-1 deficiency, but also by α-syn aggregation-dependent indirect toxicity that prevents the protein neuroprotective role and stops α-syn-mediated HSP70 upregulation at protein level. In addition, α-syn aggregation has a direct toxic effect, probably pro-oxidant, that increases cell damage and triggers a positive feedback loop that promotes further α-syn aggregation. In non-aggregating conditions (lower pathway), in the presence of oxidative stress α-syn promotes cell survival and HSP70 up-regulation (Albani et al., 2004 [36]). In this situation, wild type DJ-1 contributes to keep α-syn in the soluble form. HSP70 is also a downstream molecular target of DJ-1, that increases HSP70 expression preventing α-syn aggregation [33]. DJ-1 pathogenic mutations might impair this function, leading to cell damage through the upper pathway.
In conclusion, DJ-1 and HSP70 might be therapeutic targets in PD for their role in counteracting oxidative stress and their involvement in α-syn solubility-related cell function.
Supporting Information
(9.17 MB TIF)
Acknowledgments
We are grateful to J. D. Baggott for English editing.
Funding Statement
This work was supported by the public grant N.530/F-A13 from Italian Istituto Superiore di Sanità. L.P. is recipient of a Cenci-Gallingani Foundation post-graduate fellowship.
References
- 1.Di Monte DA. The environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- 2.Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Cookson MR, Singleton A. Genes and parkinsonism. Lancet Neurol. 2003;2:221–228. doi: 10.1016/s1474-4422(03)00350-8. [DOI] [PubMed] [Google Scholar]
- 4.Gosal D, Ross OA, Toft M. Parkinson's disease: the genetics of a heterogeneous disorder. Eur J Neurol. 2006;13:616–627. doi: 10.1111/j.1468-1331.2006.01336.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein C, Schlossmacher MG. The genetics of Parkinson disease: Implications for neurological care. Nat Clin Pract Neurol. 2006;2:136–146. doi: 10.1038/ncpneuro0126. [DOI] [PubMed] [Google Scholar]
- 6.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 7.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, et al. Ala30-to-pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 8.Abeliovich A, Flint Beal M. Parkinsonism genes: culprits and clues. J Neurochem. 2006;99:1062–1072. doi: 10.1111/j.1471-4159.2006.04102.x. [DOI] [PubMed] [Google Scholar]
- 9.Tang B, Xiong H, Sun P, Zhang Y, Wang D, et al. Association of PINK1 and DJ-1 confers digenic inheritance of early-onset Parkinson's disease. Hum Mol Genet. 2006;15:1816–1825. doi: 10.1093/hmg/ddl104. [DOI] [PubMed] [Google Scholar]
- 10.Jellinger KA. The morphological basis of mental dysfunction in Parkinson's disease. J Neurol Sci [Epub ahead of print] 2006 doi: 10.1016/j.jns.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Volles MJ, Lansbury PT., Jr Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson's disease. Biochemistry. 2003;42:7871–7878. doi: 10.1021/bi030086j. [DOI] [PubMed] [Google Scholar]
- 12.Klucken J, Outeiro TF, Nguyen P, McLean PJ, Hyman BT. Detection of novel intracellular alpha-synuclein oligomeric species by fluorescence lifetime imaging. FASEB J. 2006;20:2050–2057. doi: 10.1096/fj.05-5422com. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto M, Rockenstein E, Masliah E. Transgenic models of alpha-synuclein pathology: past, present, and future. Ann N Y Acad Sci. 2003;991:171–188. [PubMed] [Google Scholar]
- 14.Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 16.Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, et al. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson's disease. Ann Neurol. 2006;59:298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- 17.Lykkebo S, Jensen PH. Alpha-synuclein and presynaptic function: implications for Parkinson's disease. Neuromolecular Med. 2002;2:115–129. doi: 10.1385/NMM:2:2:115. [DOI] [PubMed] [Google Scholar]
- 18.Wersinger C, Prou D, Vernier P, Niznik HB, Sidhu A. Mutations in the lipid-binding domain of alpha-synuclein confer overlapping, yet distinct, functional properties in the regulation of dopamine transporter activity. Mol Cell Neurosci. 2003;24:91–105. doi: 10.1016/s1044-7431(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 19.Ahn M, Kim S, Kang M, Ryu Y, Kim TD. Chaperone-like activities of alpha-synuclein: alpha-synuclein assists enzyme activities of esterases. Biochem Biophys Res Commun. 2006;346:1142–1149. doi: 10.1016/j.bbrc.2006.05.213. [DOI] [PubMed] [Google Scholar]
- 20.Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, et al. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonifati V, Oostra BA, Heutink P. Linking DJ-1 to neurodegeneration offers novel insights for understanding the pathogenesis of Parkinson's disease. J Mol Med. 2004;82:163–174. doi: 10.1007/s00109-003-0512-1. [DOI] [PubMed] [Google Scholar]
- 22.Hedrich K, Djarmati A, Schafer N, Hering R, Wellenbrock C, et al. DJ-1 (PARK7) mutations are less frequent than Parkin (PARK2) mutations in early-onset Parkinson disease. Neurology. 2004;62:389–394. doi: 10.1212/01.wnl.0000113022.51739.88. [DOI] [PubMed] [Google Scholar]
- 23.Eerola J, Hernandez D, Launes J, Hellstrom O, Hague S, et al. Assessment of a DJ-1 (PARK7) polymorphism in Finnish PD. Neurology. 2003;61:1000–1002. doi: 10.1212/01.wnl.0000083992.28066.7e. [DOI] [PubMed] [Google Scholar]
- 24.Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, et al. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menzies FM, Yenisetti SC, Min KT. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15:1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci U S A. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J Mol Biol. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Ved R, Saha S, Westlund B, Perier C, Burnam L, et al. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem. 2005;280:42655–42668. doi: 10.1074/jbc.M505910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, et al. Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Zhong N, Kim CY, Rizzu P, Geula C, Porter DR, et al. DJ-1 transcriptionally up-regulates the human tyrosine hydroxylase by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor. J Biol Chem. 2006;281:20940–20948. doi: 10.1074/jbc.M601935200. [DOI] [PubMed] [Google Scholar]
- 33.Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 34.Huang C, Cheng H, Hao S, Zhou H, Zhang X, et al. Heat Shock Protein 70 Inhibits alpha-Synuclein Fibril Formation via Interactions with Diverse Intermediates. J Mol Biol [Epub ahead of print] 2006 doi: 10.1016/j.jmb.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 35.Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 Reduces alpha-Synuclein Aggregation and Toxicity. J Biol Chem. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- 36.Albani D, Peverelli E, Rametta R, Batelli S, Veschini L, et al. Protective effect of TAT-delivered alpha-synuclein: relevance of the C-terminal domain and involvement of HSP70. FASEB J. 2004;18:1713–1715. doi: 10.1096/fj.04-1621fje. [DOI] [PubMed] [Google Scholar]
- 37.Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Zhou W, Freed CR. Tyrosine-to-cysteine modification of human alpha-synuclein enhances protein aggregation and cellular toxicity. J Biol Chem. 2004;279:10128–10135. doi: 10.1074/jbc.M307563200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(9.17 MB TIF)