Abstract
The heritability of chronic hepatitis in the Labrador Retriever is studied with the aim of identifying the related gene mutation. Identification of cases and controls is largely based on instrumental neutron activation analysis (INAA) Cu determination in liver biopsies. The burden for these companion animals may be reduced if nail clippings and hair (fur) could serve as a noninvasive indicator for the hepatic Cu concentrations. No correlation was found between hepatic Cu concentrations and Cu concentrations in hair and nail samples. However, hair and nail samples were also analyzed by X-ray tube excitation, taking advantage of the X-ray Compton, Rayleigh, and Raman scattering which reflects the organic components such as the type of melanin. Principal component analysis provided first indications that some differentiation between healthy and sick dogs could indeed be obtained from hair and nail analysis.
Figure.
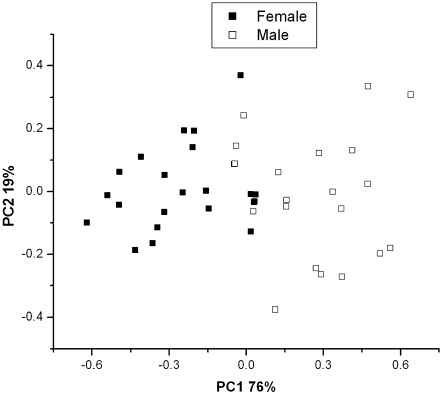
Principal component analysis of scattered region of x-ray fluorescence spectra of Labrador dog nails, demonstrating the differentiation towards dogs with high and low Cu liver levels (respectively positive and negative PC2 values) reflecting hepatitis, as well as gender (PC1: negative values for female and positive values for males)
Keywords: Neutron activation analysis, X-ray spectroscopy, Chemometrics, Trace elements, Biological samples
Introduction
Chronic hepatitis in dogs is a histological diagnosis, characterized by the presence of fibrosis, inflammation, and necrosis of the liver cells. Symptoms of chronic hepatitis are quite variable but the most common signs are lethargy, loss of appetite, and diarrhea. Pets may also drink and urinate more. As the disease progresses, many dogs develop yellowish gums, eyes, and skin (icterus/jaundice), and a swollen abdomen which is filled with fluid (ascites). In some cases toxins affect the nervous system and the dogs become blind and obtuse. This can progress to seizures, coma, and death. The disease is characterized, amongst others, by copper accumulation in the liver. Previous studies have shown elevated hepatic Cu concentrations in the order of 1,000–2,000 mg kg−1 compared with normal Cu levels of < 200–500 mg kg−1. Such hepatic copper accumulation can result from increased uptake of copper, a primary metabolic defect in hepatic copper metabolism, or from altered biliary excretion of copper. Several studies have already been conducted at Utrecht University on inherited copper toxicosis and hepatic copper storage in companion dogs such as Bedlington Terriers [1] and Dobermanns [2]. Currently, one of the programs is investigating the genetic basis of the disease by examination of family members from Labrador Retrievers with copper-associated chronic hepatitis.
The selected dogs undergo blood sampling, and retrieval of a liver biopsy (by use of the Menghini technique). The liver biopsy is examined histologically and stained for copper granules. As this project deals with companion animals from the Labrador population in The Netherlands and not with test animals, cooperation of the dog owners is crucial. Following the Delft group’s involvement and over 20 years’ experience investigating nail clippings as a bioindicator for the trace element status in man [3, 4], a feasibility study was undertaken to assess the usefulness of nail clippings and hair (fur) as a noninvasive indicator for the Cu liver levels with the aim of replacing the transcuteaneous full-needle biopsy.
Two approaches were tested. The most straightforward was to verify if Cu levels in nail clippings and hair would reflect the Cu liver levels. The other approach was initiated by the observation that a copper deficiency may result in hair/coat color changes. This is ascribed to the activity of cuproenzymes, which catalyze the biosynthesis of melanin from L-tyrosine. A copper deficiency causes, among others, pigmented hair on the head and face to lose its normal color and get a “washed out” appearance and become gray [5].
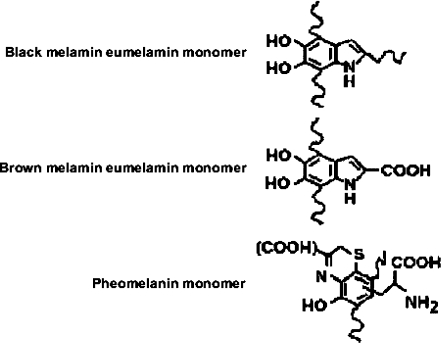
In 2005, Bueno et al. demonstrated that X-ray Rayleigh peaks and especially the peakless part in the X-ray Raman scattering continuum in X-ray spectra obtained by tube excitation carry information on the organic constituents of biological materials [6, 7]. A study of Poodles in which all spectral information was processed by dedicated principal component analysis (PCA) resulted in a clear differentiation of all dogs examined on the basis of their hair color and, moreover, the separation of healthy and sick dogs on the basis of the hair analysis. Hair pigmentation is due to two major types of melanin, viz., eumelanin and phaeomelanin (Fig. 1). The type of melanin manifesting in the hair can be attributed to the hair color genotype [8, 9], whereas the intensity and distribution of the granules may also be affected by diseases in which sulfur metabolism is involved.
Fig. 1.
Chemical structures of eumelanin and phaeomelanin
Both approaches were tested here, i.e., (i) Cu determination via instrumental neutron activation analysis (INAA) in liver biopsies, nail clippings, and hair samples; and (ii) X-ray spectrum acquisition after X-ray tube excitation, followed by full spectrum data (i.e. all channel contents) processing by PCA, including the often neglected peakless part of the X-ray scattering region resulting from Compton, Rayleigh and Raman effects.
Experimental
Liver biopsy samples, nail clippings, and hair were collected from approximately 100 dogs with chronic hepatitis as well as from healthy animals. Nail clippings were taken from phalanx I (the thumb nail), since this nail is not in contact with the ground, thus minimizing contamination problems. Nail clippings could not be collected from all animals as in some cases breeders had removed the nail permanently shortly after birth, since sometimes such a thumb nail hinders the dog in working trials. Hair was sampled from the median abdominal wall, just cranial to the belly button.
The liver biopsy samples were freeze-dried at the veterinary clinic. The nail clippings and hair samples were analyzed without cleaning to prevent changes in the nail and hair morphology that might affect the scattering information from the X-ray fluorescence (XRF) analysis. It should be noted that a Cu contamination of the nail clipping and hair is highly unlikely; the nail of the thumb nail is never in contact with, e.g., soil, and external contamination of the hair was also unlikely.
INAA
The needle biopsies were transferred into polyethylene capsules and lyophilized, resulting in sample masses varying typically between 1.5 and 50 mg dry weight. Nail clippings had masses of 5–50 mg, and the typical mass of the hair samples was approximately 100–200 mg.
The Cu levels were established by INAA via the determination of 66Cu. This is the radionuclide of choice although 64Cu is also produced; however, the associated 511-keV peak is insufficient for reliable Cu determination, whereas the 1,345-keV peak provides insufficient sensitivity. Quantification of all element mass fractions was based on the single comparator method [10]. Samples were irradiated one-by-one for 3 min in the Hoger Onderwijs Reactor in a thermal neutron fluence rate of 1.7 × 1017 m−2 s−1. A Zn flux monitor was included in each irradiation; the neutron fluence rate was determined by measurement of the induced 69mZn activity. The activated biopsy, nail, and hair samples were measured 30 s after irradiation for 3 min at a 1-cm distance from the end-cap of a side-looking 12% Ge detector. The samples were rotated during counting. The Zn monitors were measured in the same geometry for 60 s after a 4-min decay time. The samples were processed in batches of 14 samples, one internal quality control sample, and a blank. A sample of NBS 1577b Bovine Liver (certified Cu content 160 ± 8 mg kg−1) was used for internal quality control. Details of the irradiation facility, gamma-ray spectrometer, and associated quality assurance have been published elsewhere [11]. The spectrometer is equipped with ORTEC DSPEC-PLUS electronics, which allows for adequate dead-time correction [12]. It is obvious that, particularly in the nail and hair samples, several other elements such as Ca, Mg, and S were also quantified on the basis of their short halflife radionuclides, measured simultaneously with 66Cu.
X-ray tube excitation
The measuring procedure consisted of weighing approximately 100 mg of each hair sample and 10 mg of each nail sample directly into appropriate cells and submitting them to blank rhodium X-ray tube radiation, in triplicate. A common laboratory energy dispersive XRF instrument (EDX 700, Shimadzu) was used under the same irradiation conditions: 50-kV applied voltage in the tube, 25% dead time, 10-mm beam collimation, and 100-s irradiation time. It should be noted that the methodology of using the region in the X-ray spectrum resulting from scattering implies that only the spectrum’s shape is needed for the data processing by PCA. There is no need for quantification towards Cu mass fractions, and hence no reference materials were used for quality control as was the case in the hair, nail, and liver biopsy analysis by INAA.
The chemometric method [13] used for data analysis was PCA, processed via the software The Unscrambler, version 9.2, from Camo.
Results and discussion
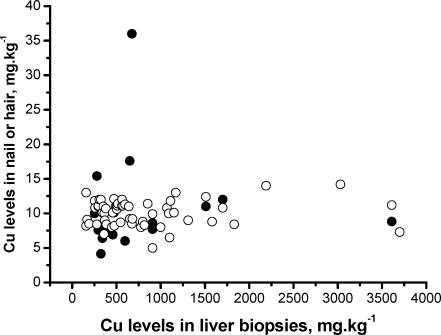
Cu could not be determined in all available nail samples because of the small sample masses and the detection limit of the analytical protocol selected. Detection limits on the basis of 66Cu were typically in the order of 25 ng, corresponding to a minimum detectable Cu mass fraction of 5 mg kg−1 in a 5-mg nail clipping sample. Cu levels in hair and nail are given in Fig. 2, together with the associated Cu liver levels. Uncertainties are not depicted for clarity reasons; typical uncertainties (combined standard uncertainty) were approximately 5–20% for the liver analyses, 15–30% for the nail, and 5–10% for the hair analyses. It is clear from Fig. 2 that the Cu levels in hair and nail do not reflect the Cu liver levels. This indicates that the gene(s) responsible for the Cu accumulation in the liver is(are) only active in the liver cells and not in the cells at the nail base or in the hair pocket.
Fig. 2.
Cu levels in nail clippings and hair of Labrador Retrievers as a function of the Cu levels in liver biopsies taken from the same dogs: ○ hair, ● nail; uncertainties are omitted for clarity (see text)
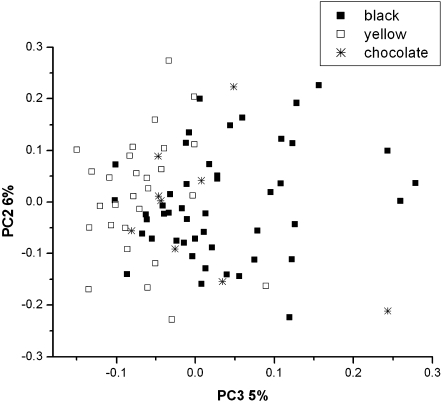
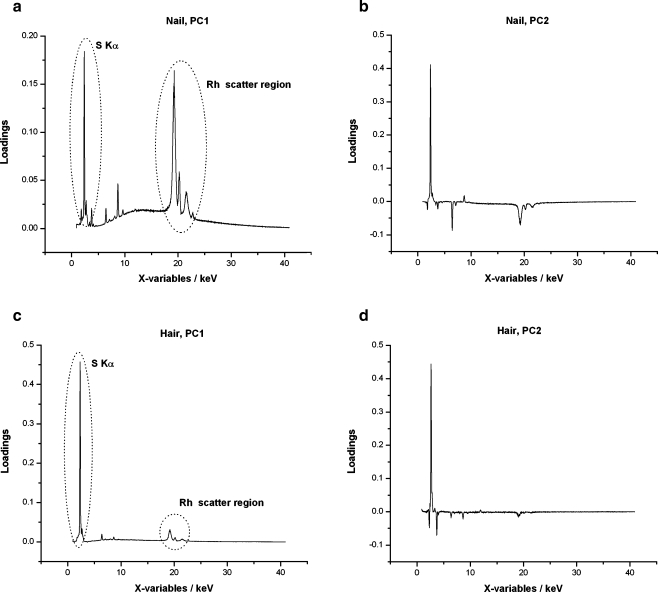
Principal component analysis (PCA) results of the processing of the channel contents of the X-ray spectra of all hair samples are given in Fig. 3, and the related factor loadings of PC1 and PC2 are shown in Fig. 4 (similar results were found for the processing of the channel contents of the X-ray spectra of the nail samples, although these are not shown here). The PC1 loading graph indicates the dependent variables that explain most of the data variance, and the PC2 loading graph indicates variables that explain the second data variance. PC1 and PC2 are orthogonals and therefore independent of each other.
Fig. 3.
PCA analysis of all hair samples, classified here by the hair color: □ yellow, * chocolate, ■ black
Fig. 4.
PC1 loadings for hair samples (top). PC2 loadings for hair samples (bottom)
The variables included in the PCA analysis were from the channel contents between 0 and 40 keV, i.e., covering the entire spectum recorded.The symbols in Fig. 3 reflect the hair color. The differentiation with respect to coat color results most likely from differences in type of melanin in Labradors with a black, liver (chocolate), or yellow-colored coat. Table 1 shows the genotypes of the hair color inheritance of Labrador Retrievers [8], in which each character represents a specific gene (denoted in pairs, of course). An uppercase character represents a dominant gene, a lowercase character represents a recessive gene; no character in a gene pair implies that it is not relevant for the genotype if the locus is occupied by a dominant or recessive gene. As produces black without any tan on the dog and there are other genes that can modify the black to liver (chocolate Labrador). If As is present, in most cases the dog will be able to produce only eumelanin pigment. Some shades of liver (chocolate), though a eumelanin pigment, overlap some shades of tan, a phaeomelanin pigment. The e, recessive red, overrides whatever gene is present at the A locus to produce a dog which shows only phaeomelanin pigment in the coat. The related melanin types are also included in Table 1 and the color differentiation by the principal component analysis is most probably based on the differences in melanin corresponding to the different genotypes. The wide spread of the groups might reflect homozygotes and heterozygotes for the genes involved.
Table 1.
Labrador genotypes [8] for hair color inheritance and corresponding types of melanin
| Color | Genotype | Melanin type |
|---|---|---|
| Black | As– B– E– | Eumelanin |
| Liver (chocolate) | As– bb E– | Eumelanin |
| Yellow, liver nose | – – B– ee | Phaeomelanin |
| Yellow, black nose | – – bb ee | Phaeomelanin |
The PCA also revealed a differentiation to gender, as is further exemplified in Fig. 5 for nails. This is in agreement with earlier work for Poodles in which a similar gender separation was observed [6].
Fig. 5.
PCA analyses of all nail samples, classified by gender
These results (Figs. 3, 4, 5) indicate that PC1 allows for a separation of dogs by gender and PC2 for separation by color, particularly when using hair samples. The loading graphs of the PCs (Fig. 4) show that sulfur and Rh scatter (Compton, Rayleigh, and Raman) explain the variance in PC1. This is most likely attributed to variations in (sulfur bondings in) keratin structures. This effect contributes to almost 100% of the data variance. The rest of it, PC2, is not very large (approximately 1–10%) and allows for separations due to differences in sulfur, and to some extent with melanin.
The physiological cause of the variation of the keratin structures in hair and nail by gender is not yet fully clear, but there are several indications that the structure of hair is different for males and females. It is well known that there is a relationship between testosterone and hair formation (andropogenic hair). Andropogenic hair has a different growth rate and a higher weight than other hair. It has also been observed that the type of sulfur is different for females and males: rhombic (alpha) sulfur predominates in female hairs, monoclinical (beta) sulfur in male hairs [14]. Less information is available about nails. Genetic variation in the proteins of the human nail has been observed [15], as well as a sex variation in lipid composition of human fingernail plates [16].
Another possible explanation for the separation of dogs in terms of genders in the hair analysis might be found in the results of Scott et al. [17] who concluded that the human melanocortin 1 receptor (MC1R) is regulated by, amongst others, specific endocrine sex hormones and by UV radiation. In confirmation of the latter study, Broekmans et al. [18] found that male subjects were more sensitive to UV irradiation than female subjects. It was already known that eumelanin is photoprotective, whereas phaeomelanin may contribute to UV-induced skin damage due to its potential to generate free radicals in response to UV radiation [19]. Skin melanin content, which was positively associated with hair color in men, was the main phenotypical determinant of sensitivity to UV irradiation.
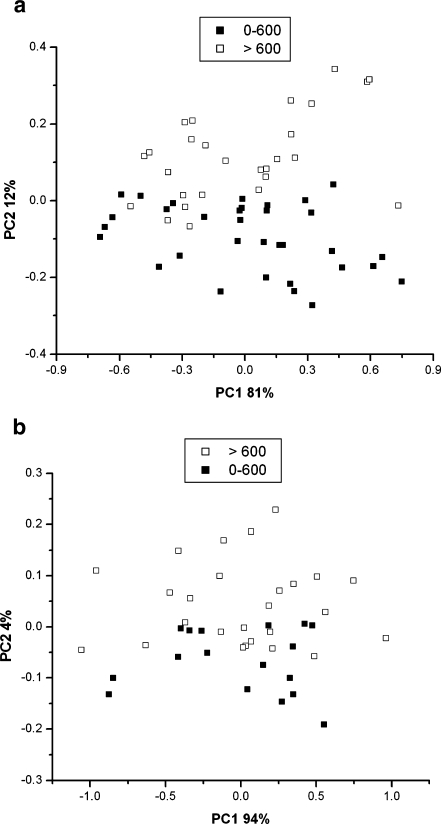
The PCA results could also be plotted as a function of the INAA Cu liver biopsy values. Results for hair and nail are given in Fig. 6 for a differentiation towards dogs with Cu liver levels of < 600 mg kg−1 or > 600 mg kg−1. A Cu biopsy level of > 600 mg kg−1 is considered to be positively indicative for liver Cu accumulation. In addition, plots were also made in which the hair and nail samples are identified by correlating with Cu biopsy values > and < 200 mg kg−1 as well as at > and < 400 mg kg−1. At these lower Cu levels no clear differentiation is obtained, as could be expected, which confirms that the PCA is not producing an artifact, accidentally coinciding at the 600 mg kg−1 Cu liver biopsy level.
Fig. 6.
PCA results, nail X-ray spectral data vs. INAA Cu liver biopsy values (top): ■ dogs with Cu liver levels 0–600 mg kg−1, □ dogs with Cu liver levels > 600 mg kg−1 , N = 60. PCA results, hair X-ray spectral data vs. INAA Cu liver biopsy values (bottom): ■ dogs with Cu liver levels 0–600 mg kg−1, □ dogs with Cu liver levels > 600 mg kg−1, N = 43
In all PCA, it could be justified to remove some outliers on the basis of high leverage values and high energy variance residuals in PCs. High values of leverage and residuals are caused, for instance, by imperfect quality and positioning of the sample during the X-ray irradiation. In addition, outliers may sometimes result from the presence of dogs with strong parental relationships [6].
The strength of using the information of the scattered radiation is emphasized by the role of sulfur in the separation towards gender and Cu accumulation. Total sulfur, as determined by INAA via 37S, hardly shows any correlation with INAA Cu liver values (correlation coefficients 0.03 and 0.32 for nail and hair, respectively). However, the INAA results provide only the total sulfur content, i.e., sulfur present in keratin, melanin, and any other compound, whereas the PCs of the channel contents of the X-ray spectra, especially due to the peakless scattered region, apply to sulfur directly related to keratin and/or melanin. This explains the much better correlation of “keratin/melanin” sulfur with Cu liver values compared with total S with Cu.
The results of our study show that a fairly sharp differentiation can be obtained between dogs with low and high Cu liver levels if PCA is performed on a dataset obtained from all channel contents of X-ray spectra obtained by X-ray tube excitation of nail or hair samples together with the spectral information of often neglected X-ray (Rayleigh, Raman) scattered radiation.
A practical application of this technique would start with construction of a dataset using samples of known origin, encompassing all varieties expected. New and unknown samples can then be projected on this model, and subsequently classified. This approach will be further validated with new samples to be collected from Labrador Retrievers still being observed and treated along medicinal and dietary approaches.
Conclusions
Hair and nail clippings are widely used as bioindicators for the trace element status of man and, to a much lesser extent, also for animals. The results of this study emphasize that it is quite risky to assume a priori that the trace element levels in hair and nail reflect differences between classes of individuals, e.g., “healthy” and “sick” people or animals. In this study related to chronic hepatitis, the Cu levels in liver biopsies are not reflected by Cu levels in hair and nail.
The X-ray Rayleigh scattered and X-ray Raman continuum contain valuable information about the organic, low-Z elemental matrix composition of an object. In this study, first indications were obtained that the Cu accumulation possibly may have an effect on the nail and hair melanin, thus offering a potentially favorable outlook for noninvasive monitoring of the Cu liver status. An additional advantage of the approach is that a measurement by X-ray tube excitation takes only about 2 min per sample so that, once a database has been built up, the technique might be valuable for large-scale screening purposes.
Acknowledgment
The authors wish to thank Ms. Anneke Koster-Ammerlaan and Ms. Delia van Rij for performing the neutron activation analyses of all samples.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Van de Sluis B, Rothuizen J, Pearson JH, Van Oost BA, Wijmenga C (2002) Hum Mol Genet 11:165–173 [DOI] [PubMed]
- 2.Mandigers PJJ (2005) Insights in the pathogenesis of Dobermann hepatitis. PhD Dissertation, Utrecht University ISBN 90–393–3872–2, p 244
- 3.Van Noord PA, Colette HJ, Maas MJ, De Waard F (1987) Int J Epidemiol 16:318–322 [DOI] [PubMed]
- 4.Favaro PC, Bode P, De Nadai Fernandes EA (2005) J Radioanal Nucl Chem 264:61–65 [DOI]
- 5.Roudebusch P, Sousa CA, Logas DE (2000) Skin and hair disorders. In: Hand MS, Thatcher CD, Remillard RL, Roudebusch P (eds) Small animal clinical nutrition, 4th edn. Mark Morris Institute, Topeka, pp 462–463
- 6.Maretti Silveira Bueno MI, Castro MTPO, Moreira De Souza A, Borges Santana De Oliveira E, Paixão Teixeira A (2005) Chemometr Intell Lab Syst 78:96–102 [DOI]
- 7.Bortoleto GG, Pataca LCM, Maretti Silveira Bueno MI (2005) Anal Chim Acta 539:283–287 [DOI]
- 8.Robinson R (1990) Genetics for dog breeders, 2nd edn. Pergamon, Oxford, ISBN 0-08-037492-1
- 9.Canine Color Genetics. http://bowlingsite.mcf.com/Genetics/ColorGen.html. Accessed 4 Oct 2007
- 10.Korthoven PJM, De Bruin M (1977) J Radioanal Chem 35:127–137 [DOI]
- 11.Bode P (2000) J Radioanal Nucl Chem 245:127–132 [DOI]
- 12.Blaauw M, Fleming RF, Keyser R (2001) J Radioanal Nucl Chem 248:309–313 [DOI]
- 13.Mathias O (1999) Chemometrics: statistics and computation in analytical chemistry. Wiley-VCH, Weinheim
- 14.Isusifli RM, Eiiubova NA, Buniatov MO (1995) Sud Med Ekspert 38:19–21 [PubMed]
- 15.Marshall RC (1980) J Invest Dermatol 75:264–269 [DOI] [PubMed]
- 16.Helmdach M, Thielitz A, Ropke EM, Gollnick H (2000) Skin Pharmacol Appl Skin Physiol 13:111–119 [DOI] [PubMed]
- 17.Scott MC, Suzuki I, Abdel-Malek ZA (2002) Pigment Cell Res 15:433–439 [DOI] [PubMed]
- 18.Broekmans WM, Vink AA, Boelsma E, Klopping-Ketelaars WA, Tijburg LB, Van’t Veer P, Van Poppel G, Kardinaal AF (2003) Eur J Clin Nutr 10:1222–1229 [DOI] [PubMed]
- 19.Harsanyi ZP, Post PW, Brinkmann JP, Chedekel MR, Deibel RM (1980) Experientia 36:291–292 [DOI] [PubMed]