Abstract
Models of ecological speciation predict that certain types of habitat should be more conducive to species diversification than others. In this study, I test this hypothesis in waders of the sub-order Charadrii using the number of morphological sub-species per species as an index of diversity. I classified all members of this clade as spending the non-breeding season either coastally or inland and argue that these represent fundamentally different environments. Coastal mudflats are characterised by high predictability and patchy worldwide distribution, whilst inland wetlands are widespread but unpredictable. The results show that migratory species that winter coastally are sub-divided into more sub-species than those that winter inland. This was not the case for non-migratory species. I argue that coastal environments select for more rigid migratory pathways, whilst inland wetlands favour more flexible movement patterns. Population sub-division could then result from the passive segregation of breeding sites or from the active selection for assortative mating of ecomorphs.
Keywords: Speciation, Waders, Non-breeding habitat, Sub-species
Introduction
Models of ecological speciation show that the propensity of a species to diverge is affected by the shape of the fitness landscape generated by its environment (Gavrilets 2004). This predicts that some environments should be more conducive to speciation than others. Recent comparative studies have supported this prediction (Funk et al. 2006; Phillimore et al. 2007). However, these studies do not discuss aspects of habitats that promote or inhibit speciation.
In this study, I test this prediction for wading birds of the sub-order Charadrii. This group was chosen because its species can be divided into those that are adapted to coastal habitats and those that feed mostly inland (Piersma 1997, 2003). On a year-to-year basis, coastal habitats offer a more predictable environment than inland wetlands (Roshier et al. 2001). Inter-tidal mudflats and ocean beaches will be located in the same place each year and offer a highly predictable pool of food resources. They are distributed patchily around the globe, and patches differ in their food resources and other conditions (Piersma et al. 1993a). On the other hand, the inland wetlands favoured by other waders are unpredictable and include waterbodies in arid regions that flood infrequently. Flooded areas are often large, but may be located in different areas between years and shift within a single non-breeding season, promoting extensive movements of birds that rely on these habitats (Roshier et al. 2002). The suitability of grasslands in semi-arid regions varies from year to year due to the differences in rainfall level. Little is known about the composition of the food resources used by waders in these inland environments, but it seems likely that these may be similar on broad spatial scales. I thus predict that coastal habitats should be more favourable to species diversification than inland habitats.
Many of the wader species that utilise coastal or inland habitats for the greater part of the year migrate to very different habitats, such Arctic tundras or boreal swamps, to breed. Whilst the reasons for this are beyond the scope of this study (but see Piersma 1997), it means that in these species reproduction is spatially separated from the feeding habitat to which they are adapted. Red knot Calidris canutus illustrate this point. For 10 months of the year, this species feeds on bivalves on mudflats around the world. The species has evolved a range of adaptations to feeding on bivalves, including a muscular stomach and sensitive bill tip (Piersma et al. 1993b, 1998). Nevertheless, it breeds exclusively on high Arctic tundras where feeding on abundant insect larvae and berries require few special adaptations. In such cases, local adaptation to, for example, temperate or tropical inter-tidal mudflats requires that birds from the same non-breeding area mate assortatively on the breeding grounds (Webster et al. 2002).
Investigating ecological speciation in this group of birds thus adds two new dimensions to the general pattern found by Funk et al. (2006) and Phillimore et al. (2007). First, as argued above, I have a priori reasons to expect that one type of habitat (coastal) should be more conducive to speciation than the other (inland). Second, many of the species investigated reproduce away from the feeding grounds to which they are adapted and speciation thus requires differential migration to achieve assortative mating. To test these ideas, I use the number of sub-species per species as a measure of diversification. Thus, I treat sub-species as ‘incipient species’. Morphological sub-species are considered useful in estimating the patterns of divergence among populations (Phillimore et al. 2007). Evidence that wader sub-species represent phylogenetically distinct groups is available for Dunlin Calidris alpina (Wenink et al. 1993), and to a lesser extent, for Red knot (Buehler and Baker 2005). Because older species could have differentiated into more sub-species than younger species, I investigated whether the number of sub-species was correlated to species age.
Materials and methods
Data collection
I collated data on the number of sub-species, migratoriness, breeding and non-breeding habitat from Del Hoyo et al. (1996) for all 215 species of the sub-order Charadrii (see Table 2 of S1). Crude estimates of species age were obtained from Thomas et al. (2004). Due to high levels of polytomy in parts of the phylogeny, some of these are likely to be over-estimates; however, no better estimates are available at present. I adopted the number of sub-species from one reputable source rather than search for the more recent updates because some of these revisions are still controversial. Migratoriness was scored as non-migrant, partial migrant or migrant (>80%, between 20% and 80% and <20% of non-breeding range overlaps with breeding range, respectively). Breeding habitat was scored as one of seven classes: tundra, boreal/temperate, mountains, steppe, (sub)tropical wetlands (either inland or coastal), (sub)tropical forest or oceanic. Non-breeding habitat was scored as coastal, inland or pelagic. For species that regularly use both coastal and inland habitats, I scored the habitat considered by Del Hoyo et al. (1996) to be used by the largest part of the population.
Table 2.
Data used in the analysis
| Genus | Species | Migratory | Winter | Summer | Sub-species | Species age |
|---|---|---|---|---|---|---|
| Actitis | Hypoleucos | Migrant | Inland | Boreal | 1 | 5.779 |
| Actitis | Macularia | Migrant | Inland | Boreal | 1 | 5.779 |
| Actophilornis | Africanus | Non-migrant | Inland | (Sub)tropical wetland | 1 | 8.547 |
| Actophilornis | Albinucha | Non-migrant | Inland | (Sub)tropical wetland | 1 | 8.547 |
| Anarhynchus | Frontalis | Migrant | Coastal | Boreal | 1 | 26.7 |
| Aphriza | Virgata | Migrant | Coastal | Mountain | 1 | 6.019 |
| Arenaria | Interpres | Migrant | Coastal | Tundra | 2 | 5.68 |
| Arenaria | Melanocephala | Migrant | Coastal | Tundra | 1 | 5.68 |
| Attagis | Gayi | Non-migrant | Inland | Mountain | 3 | 18.2 |
| Attagis | Malouinus | Non-migrant | Inland | Mountain | 1 | 18.2 |
| Bartramia | Longicauda | Migrant | Inland | Steppe | 1 | 20.299 |
| Burhinus | Bistriatus | Non-migrant | Inland | Steppe | 4 | 7.933 |
| Burhinus | Capensis | Non-migrant | Inland | Steppe | 4 | 3.967 |
| Burhinus | Grallarius | Non-migrant | Inland | Steppe | 1 | 6.287 |
| Burhinus | Oedicnemus | Partial migrant | Inland | Steppe | 6 | 3.967 |
| Burhinus | Senegalensis | Non-migrant | Inland | (Sub)tropical wetland | 1 | 3.967 |
| Burhinus | Superciliaris | Non-migrant | Inland | Steppe | 1 | 11.9 |
| Burhinus | Vermiculatus | Non-migrant | Inland | (Sub)tropical wetland | 2 | 19.8 |
| Calidris | Acuminata | Migrant | Inland | Tundra | 1 | 3.798 |
| Calidris | Alba | Migrant | Coastal | Tundra | 1 | 8.707 |
| Calidris | Alpina | Migrant | Coastal | Tundra | 9 | 7.836 |
| Calidris | Bairdii | Migrant | Inland | Tundra | 1 | 7.4 |
| Calidris | Canutus | Migrant | Coastal | Tundra | 5 | 3.798 |
| Calidris | Ferruginea | Migrant | Coastal | Tundra | 1 | 17.413 |
| Calidris | Fuscicollis | Migrant | Coastal | Tundra | 1 | 6.965 |
| Calidris | Maritima | Partial migrant | Coastal | Tundra | 1 | 2.588 |
| Calidris | Mauri | Migrant | Coastal | Tundra | 1 | 9.142 |
| Calidris | Melanotos | Migrant | Inland | Tundra | 1 | 4.136 |
| Calidris | Minuta | Migrant | Coastal | Tundra | 1 | 3.483 |
| Calidris | Minutilla | Migrant | Inland | Boreal | 1 | 6.965 |
| Calidris | Ptilocnemis | Partial migrant | Coastal | Tundra | 4 | 2.588 |
| Calidris | Pusilla | Migrant | Coastal | Tundra | 1 | 3.483 |
| Calidris | Ruficollis | Migrant | Coastal | Tundra | 1 | 2.609 |
| Calidris | Subminuta | Migrant | Inland | Boreal | 1 | 2.609 |
| Calidris | Temminckii | Migrant | Inland | Boreal | 1 | 10.013 |
| Calidris | Tenuirostris | Migrant | Coastal | Mountain | 1 | 3.798 |
| Catoptrophorus | Semipalmatus | Partial migrant | Coastal | Steppe | 2 | 19.701 |
| Charadrius | Alexandrinus | Partial migrant | Coastal | (Sub)tropical wetland | 5 | 22.992 |
| Charadrius | Alticola | Non-migrant | Inland | Mountain | 1 | 22.992 |
| Charadrius | Asiaticus | Migrant | Inland | Steppe | 1 | 7.224 |
| Charadrius | Bicinctus | Partial migrant | Coastal | Boreal | 2 | 7.224 |
| Charadrius | Collaris | Non-migrant | Inland | (Sub)tropical wetland | 1 | 22.992 |
| Charadrius | Dubius | Partial migrant | Inland | (Sub)tropical wetland | 3 | 12.882 |
| Charadrius | Falklandicus | Partial migrant | Coastal | Boreal | 1 | 16.317 |
| Charadrius | Forbesi | Partial migrant | Inland | Steppe | 1 | 22.992 |
| Charadrius | Hiaticula | Migrant | Coastal | Tundra | 2 | 8.793 |
| Charadrius | Javanicus | Non-migrant | Coastal | (Sub)tropical wetland | 1 | 22.992 |
| Charadrius | Leschenaultii | Migrant | Coastal | Steppe | 3 | 22.992 |
| Charadrius | Marginatus | Non-migrant | Coastal | (Sub)tropical wetland | 4 | 22.992 |
| Charadrius | Melodus | Migrant | Coastal | (Sub)tropical wetland | 1 | 22.992 |
| Charadrius | Modestus | Partial migrant | Inland | Boreal | 1 | 19.283 |
| Charadrius | Mongolus | Migrant | Coastal | Mountain | 5 | 7.224 |
| Charadrius | Montanus | Migrant | Inland | Steppe | 1 | 16.317 |
| Charadrius | Morinellus | Migrant | Inland | Tundra | 1 | 26.7 |
| Charadrius | Novaeseelandi | Non-migrant | Coastal | Oceanic island | 1 | 4.558 |
| Charadrius | Obscurus | Partial migrant | Coastal | Boreal | 2 | 22.992 |
| Charadrius | Pallidus | Non-migrant | Inland | (Sub)tropical wetland | 2 | 22.992 |
| Charadrius | Pecuarius | Non-migrant | Inland | (Sub)tropical wetland | 1 | 22.992 |
| Charadrius | Peronii | Non-migrant | Coastal | (Sub)tropical wetland | 1 | 22.992 |
| Charadrius | Placidus | Migrant | Inland | (Sub)tropical wetland | 1 | 22.992 |
| Charadrius | Rubricollis | Non-migrant | Coastal | (Sub)tropical wetland | 1 | 22.992 |
| Charadrius | Ruficapillus | Non-migrant | Coastal | (Sub)tropical wetland | 1 | 22.992 |
| Charadrius | Sanctaehelena | Non-migrant | Inland | Oceanic island | 1 | 22.992 |
| Charadrius | Semipalmatus | Migrant | Coastal | Tundra | 1 | 11.096 |
| Charadrius | Thoracicus | Non-migrant | Coastal | (Sub)tropical wetland | 1 | 22.992 |
| Charadrius | Tricollaris | Non-migrant | Inland | (Sub)tropical wetland | 2 | 5.548 |
| Charadrius | Veredus | Migrant | Inland | Steppe | 1 | 7.224 |
| Charadrius | Vociferus | Partial migrant | Inland | (Sub)tropical wetland | 3 | 5.548 |
| Charadrius | Wilsonia | Partial migrant | Coastal | (Sub)tropical wetland | 3 | 5.548 |
| Chionis | Alba | Partial migrant | Coastal | Oceanic island | 1 | 11.988 |
| Chionis | Minor | Non-migrant | Coastal | Oceanic island | 4 | 11.988 |
| Cladorhynchus | Leucocephalus | Partial migrant | Inland | (Sub)tropical wetland | 1 | 10.182 |
| Coenocorypha | Aucklandica | Non-migrant | Inland | Oceanic island | 4 | 4.91 |
| Coenocorypha | Pusilla | Non-migrant | Inland | Oceanic island | 1 | 4.91 |
| Cursorius | Coromandelicu | Non-migrant | Inland | Steppe | 1 | 9.9 |
| Cursorius | Cursor | Partial migrant | Inland | Steppe | 5 | 4.95 |
| Cursorius | Rufus | Non-migrant | Inland | Steppe | 1 | 4.95 |
| Cursorius | Temminckii | Partial migrant | Inland | Steppe | 1 | 9.9 |
| Elseyornis | Melanops | Non-migrant | Inland | (Sub)tropical wetland | 1 | 22.992 |
| Erythrogonys | Cinctus | Non-migrant | Inland | (Sub)tropical wetland | 1 | 23.256 |
| Esacus | Magnirostris | Non-migrant | Coastal | (Sub)tropical wetland | 1 | 3.967 |
| Esacus | Recurvirostris | Non-migrant | Inland | (Sub)tropical wetland | 1 | 9.21 |
| Eurynorhynchus | Pygmeus | Migrant | Coastal | Tundra | 1 | 17.413 |
| Gallinago | Gallinago | Migrant | Inland | Boreal | 3 | 19.576 |
| Gallinago | Hardwickii | Migrant | Inland | Boreal | 1 | 19.576 |
| Gallinago | Imperialis | Non-migrant | Inland | Mountain | 1 | 19.576 |
| Gallinago | Jamesoni | Non-migrant | Inland | Mountain | 1 | 19.576 |
| Gallinago | Macrodactyla | Non-migrant | Inland | (Sub)tropical wetland | 1 | 4.894 |
| Gallinago | Media | Migrant | Inland | Boreal | 1 | 4.894 |
| Gallinago | Megala | Migrant | Inland | Boreal | 1 | 4.894 |
| Gallinago | Nemoricola | Partial migrant | Inland | Mountain | 1 | 19.576 |
| Gallinago | Nigripennis | Non-migrant | Inland | Mountain | 3 | 4.894 |
| Gallinago | Nobilis | Non-migrant | Inland | Mountain | 1 | 19.576 |
| Gallinago | Paraguaiae | Non-migrant | Inland | (Sub)tropical wetland | 3 | 19.576 |
| Gallinago | Solitaria | Partial migrant | Inland | Mountain | 2 | 19.576 |
| Gallinago | Stenura | migrant | Inland | Boreal | 1 | 19.576 |
| Gallinago | Stricklandii | Non-migrant | Inland | Boreal | 1 | 19.576 |
| Gallinago | Undulata | Non-migrant | Inland | (Sub)tropical wetland | 2 | 19.576 |
| Glareola | Cinerea | Non-migrant | Inland | (Sub)tropical wetland | 1 | 13.896 |
| Glareola | Lactea | Partial migrant | Inland | (Sub)tropical wetland | 1 | 13.896 |
| Glareola | Maldivarum | Migrant | Inland | Steppe | 1 | 13.896 |
| Glareola | Nordmanni | Migrant | Inland | Steppe | 1 | 13.896 |
| Glareola | Nuchalis | Non-migrant | Inland | (Sub)tropical wetland | 2 | 13.896 |
| Glareola | Ocularis | Migrant | Inland | (Sub)tropical wetland | 1 | 13.896 |
| Glareola | Pratincola | Migrant | Inland | (Sub)tropical wetland | 3 | 13.896 |
| Haematopus | Ater | Non-migrant | Coastal | Boreal | 1 | 4.283 |
| Haematopus | Bachmani | Non-migrant | Coastal | Boreal | 1 | 4.283 |
| Haematopus | Chathamensis | Non-migrant | Coastal | Oceanic island | 1 | 6.789 |
| Haematopus | Fuliginosus | Non-migrant | Coastal | (Sub)tropical wetland | 2 | 12.849 |
| Haematopus | Leucopodus | Non-migrant | Coastal | Boreal | 1 | 14.817 |
| Haematopus | Longirostris | Non-migrant | Coastal | (Sub)tropical wetland | 1 | 14.817 |
| Haematopus | Meadewaldoi | Non-migrant | Coastal | Oceanic island | 1 | 14.817 |
| Haematopus | Moquini | Non-migrant | Coastal | Boreal | 1 | 11.072 |
| Haematopus | Ostralegus | Migrant | Coastal | Boreal | 4 | 4.283 |
| Haematopus | Palliatus | Non-migrant | Coastal | (Sub)tropical wetland | 2 | 12.024 |
| Haematopus | Unicolor | Non-migrant | Coastal | Boreal | 1 | 4.283 |
| Heteroscelus | Brevipes | Migrant | Coastal | Mountain | 1 | 5.324 |
| Heteroscelus | Incanus | Migrant | Coastal | Mountain | 1 | 5.324 |
| Himantopus | Himantopus | Partial migrant | Inland | (Sub)tropical wetland | 5 | 8.77 |
| Himantopus | Novaezelandia | Non-migrant | Inland | Boreal | 1 | 8.77 |
| Hydrophasianus | Chirurgus | Partial migrant | Inland | (Sub)tropical wetland | 1 | 17.118 |
| Ibidorhyncha | Struthersii | Non-migrant | Inland | Mountain | 1 | 16.5 |
| Irediparra | Gallinacea | Non-migrant | Inland | (Sub)tropical wetland | 3 | 8.547 |
| Jacana | Jacana | Non-migrant | Inland | (Sub)tropical wetland | 6 | 10.8 |
| Jacana | Spinosa | Non-migrant | Inland | (Sub)tropical wetland | 3 | 10.8 |
| Limicola | Falcinellus | Migrant | Coastal | Tundra | 2 | 3.798 |
| Limnodromus | Griseus | Migrant | Coastal | Boreal | 3 | 4.951 |
| Limnodromus | Scolopaceus | Migrant | Inland | Tundra | 1 | 4.951 |
| Limnodromus | Semipalmatus | Migrant | Coastal | Boreal | 1 | 7.847 |
| Limosa | Fedoa | Migrant | Coastal | Steppe | 2 | 11.739 |
| Limosa | Haemastica | Migrant | Coastal | Tundra | 1 | 9.303 |
| Limosa | Lapponica | Migrant | Coastal | Tundra | 3 | 5.869 |
| Limosa | Limosa | Migrant | Coastal | Steppe | 3 | 5.869 |
| Lymnocryptes | Minimus | Migrant | Inland | Boreal | 1 | 21.8 |
| Metopidius | Indicus | Non-migrant | Inland | (Sub)tropical wetland | 1 | 13.546 |
| Micropalama | Himantopus | Migrant | Inland | Tundra | 1 | 17.413 |
| Microparra | Capensis | Non-migrant | Inland | (Sub)tropical wetland | 1 | 8.547 |
| Numenius | Americanus | Migrant | Inland | Steppe | 2 | 6.404 |
| Numenius | Arquata | Migrant | Coastal | Boreal | 2 | 6.404 |
| Numenius | Borealis | Migrant | Inland | Tundra | 1 | 19.211 |
| Numenius | Madagascarien | Migrant | Coastal | Boreal | 1 | 6.404 |
| Numenius | Minutus | Migrant | Inland | Boreal | 1 | 19.211 |
| Numenius | Phaeopus | Migrant | Coastal | Boreal | 4 | 10.15 |
| Numenius | Tahitiensis | Migrant | Coastal | Tundra | 1 | 6.404 |
| Numenius | Tenuirostris | Migrant | Inland | Boreal | 1 | 19.211 |
| Oreopholus | Ruficollis | Partial migrant | Inland | Mountain | 2 | 30.733 |
| Pedionomus | Torquatus | Non-migrant | Inland | Steppe | 1 | 45.8 |
| Peltohyas | Australis | Non-migrant | Inland | Steppe | 1 | 4.558 |
| Phalaropus | Fulicaria | Migrant | Pelagic | Tundra | 1 | 2.158 |
| Phalaropus | Lobatus | Migrant | Pelagic | Tundra | 1 | 2.158 |
| Phegornis | Mitchellii | Non-migrant | Inland | Mountain | 1 | 26.7 |
| Philomachus | Pugnax | Migrant | Inland | Tundra | 1 | 6.019 |
| Pluvialis | Apricaria | Migrant | Inland | Tundra | 2 | 8.33 |
| Pluvialis | Dominica | Migrant | Inland | Tundra | 1 | 5.256 |
| Pluvialis | Fulva | Migrant | Coastal | Tundra | 1 | 5.256 |
| Pluvialis | Squatarola | Migrant | Coastal | Tundra | 1 | 10.511 |
| Pluvianellus | Socialis | Partial migrant | Coastal | Boreal | 1 | 19 |
| Pluvianus | Aegyptius | Non-migrant | Inland | (Sub)tropical wetland | 1 | 36.9 |
| Prosobonia | Cancellata | Non-migrant | Coastal | Oceanic island | 1 | 5.517 |
| Recurvirostra | Americana | Migrant | Inland | Boreal | 1 | 4.385 |
| Recurvirostra | Andina | Non-migrant | Inland | Mountain | 1 | 4.385 |
| Recurvirostra | Avosetta | Partial migrant | Inland | (Sub)tropical wetland | 1 | 8.77 |
| Recurvirostra | Novaehollandi | Partial migrant | Inland | (Sub)tropical wetland | 1 | 6.95 |
| Rhinoptilus | Africanus | Non-migrant | Inland | Steppe | 8 | 9.9 |
| Rhinoptilus | Bitorquatus | Non-migrant | Inland | Steppe | 1 | 7.846 |
| Rhinoptilus | Chalcopterus | Partial migrant | Inland | Steppe | 1 | 4.95 |
| Rhinoptilus | Cinctus | Non-migrant | Inland | Steppe | 3 | 4.95 |
| Rostratula | Benghalensis | Non-migrant | Inland | (Sub)tropical wetland | 2 | 27.5 |
| Rostratula | Semicollaris | Non-migrant | Inland | (Sub)tropical wetland | 1 | 27.5 |
| Scolopax | Celebensis | Non-migrant | Inland | (Sub)tropical forest | 1 | 12.057 |
| Scolopax | Minor | Partial migrant | Inland | (Sub)tropical forest | 1 | 12.057 |
| Scolopax | Mira | Non-migrant | Inland | (Sub)tropical forest | 1 | 4.664 |
| Scolopax | Rochussenii | Non-migrant | Inland | (Sub)tropical forest | 1 | 12.057 |
| Scolopax | Rusticola | Migrant | Inland | Boreal | 1 | 4.664 |
| Scolopax | Saturata | Non-migrant | Inland | (Sub)tropical forest | 2 | 12.057 |
| Steganopus | Tricolor | Migrant | Inland | Steppe | 1 | 3.42 |
| Stiltia | Isabella | Partial migrant | Inland | Steppe | 1 | 14.85 |
| Thinocorus | Orbignyianus | Non-migrant | Inland | Mountain | 2 | 19.352 |
| Thinocorus | Rumicivorus | Partial migrant | Inland | Mountain | 4 | 19.352 |
| Tringa | Erythropus | Migrant | Inland | Tundra | 1 | 5.324 |
| Tringa | Flavipes | Migrant | Inland | Boreal | 1 | 13.762 |
| Tringa | Glareola | Migrant | Inland | Boreal | 1 | 8.438 |
| Tringa | Guttifer | Migrant | Coastal | Boreal | 1 | 19.086 |
| Tringa | Melanoleuca | Migrant | Inland | Boreal | 1 | 5.324 |
| Tringa | Nebularia | Migrant | Inland | Boreal | 1 | 13.762 |
| Tringa | Ochropus | Migrant | Inland | Boreal | 1 | 19.086 |
| Tringa | Solitaria | Migrant | Inland | Boreal | 2 | 19.086 |
| Tringa | Stagnatilis | Migrant | Inland | Boreal | 1 | 13.762 |
| Tringa | Totanus | Migrant | Coastal | Boreal | 6 | 13.762 |
| Tryngites | Subruficollis | Migrant | Inland | Tundra | 1 | 10.448 |
| Vanellus | Albiceps | Non-migrant | Inland | (Sub)tropical wetland | 1 | 22.961 |
| Vanellus | Armatus | Non-migrant | Inland | (Sub)tropical wetland | 1 | 5.008 |
| Vanellus | Cayanus | Non-migrant | Inland | (Sub)tropical wetland | 1 | 22.961 |
| Vanellus | Chilensis | Non-migrant | Inland | (Sub)tropical wetland | 4 | 5.008 |
| Vanellus | Cinereus | Migrant | Inland | (Sub)tropical wetland | 1 | 22.961 |
| Vanellus | Coronatus | Non-migrant | Inland | Steppe | 2 | 22.961 |
| Vanellus | Crassirostris | Non-migrant | Inland | (Sub)tropical wetland | 2 | 5.008 |
| Vanellus | Duvaucelii | Non-migrant | Inland | (Sub)tropical wetland | 1 | 7.937 |
| Vanellus | Gregarius | Migrant | Inland | Steppe | 1 | 5.008 |
| Vanellus | Indicus | Non-migrant | Inland | (Sub)tropical wetland | 4 | 5.008 |
| Vanellus | Leucurus | Partial migrant | Inland | (Sub)tropical wetland | 1 | 22.961 |
| Vanellus | Lugubris | Non-migrant | Inland | Steppe | 1 | 5.008 |
| Vanellus | Macropterus | Non-migrant | Inland | (Sub)tropical wetland | 1 | 5.008 |
| Vanellus | Malabaricus | Non-migrant | Inland | Steppe | 1 | 22.961 |
| Vanellus | Melanocephalus | Non-migrant | Inland | Mountain | 1 | 22.961 |
| Vanellus | Melanopterus | Non-migrant | Inland | Steppe | 2 | 22.961 |
| Vanellus | Miles | Non-migrant | Inland | Steppe | 2 | 5.008 |
| Vanellus | Resplendens | Non-migrant | Inland | Mountain | 1 | 5.008 |
| Vanellus | Senegallus | Non-migrant | Inland | (Sub)tropical wetland | 3 | 22.961 |
| Vanellus | Spinosus | Non-migrant | Inland | (Sub)tropical wetland | 1 | 5.008 |
| Vanellus | Superciliosus | Migrant | Inland | Steppe | 1 | 22.961 |
| Vanellus | Tectus | Non-migrant | Inland | Steppe | 2 | 5.008 |
| Vanellus | Tricolor | Non-migrant | Inland | Steppe | 1 | 5.008 |
| Vanellus | Vanellus | Migrant | Inland | Steppe | 1 | 22.961 |
| Xenus | Cinereus | Migrant | Coastal | Boreal | 1 | 32.1 |
Statistical analysis
I first investigated whether the dependent variable (number of sub-species) showed phylogenetic auto-correlation (i.e. whether closely related species resembled each other more in their tendency to form sub-species than expected by chance) using the phylogenetic topology from Thomas et al. (2004). I used runs test as implemented in the programme Phylogenetic Independence (Abouheif 1999) to compare the observed pattern to that generated by randomly redistributing the data over the tips of the phylogeny 1,000 times. For this analysis, the number of sub-species per species was dichotomised as 1 (i.e. only the nominate form) or >1. Finding no evidence for phylogenetic autocorrelation (see the “Results” section), I treated species as independent data points in further analysis.
As the number of sub-species per species followed a Poisson distribution, I analysed the data using generalised linear models with a Poisson error distribution and a log link function. GLMStat (Beath 2001) was used to generate glms. Significance was tested by dropping each term from the fuller model and comparing the resulting change in deviance to a chi-square distribution.
Results
The results of runs test showed that there was no evidence for auto-correlation in the phylogenetic distribution of the number of sub-species (runs test P = 0.46). In fact, 461 of the 1,000 randomly generated runs averages were greater than that of the real data set, indicating a non-significant tendency towards over-dispersion.
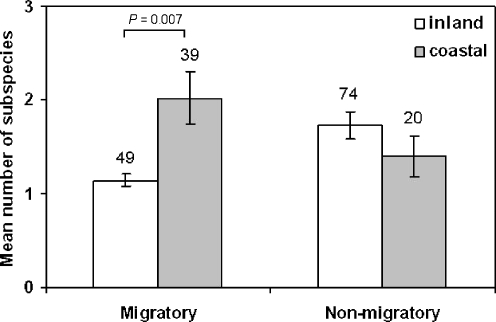
A glm containing migratoriness, non-breeding habitat, breeding habitat and species age and their interactions showed no significant effects of three- and four-way interactions. However, there was a significant two-way interaction between migratoriness and non-breeding habitat (Table 1), indicating that the pattern of covariance between non-breeding habitat and sub-species richness differed between migrants and non-migrants. Individual models revealed a significant effect of non-breeding habitat on the number of sub-species in migrants, but not in non-migrants or partial migrants (Table 1). These results are illustrated in Fig. 1: species wintering coastally are on average sub-divided into more sub-species than inland wintering species, but only in migrants. The results are not confounded by differences in species age (Table 1). Whilst there was a significant association between breeding and non-breeding habitat among migrants (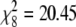 , P = 0.009), this did not result in an association between breeding habitat and the number of sub-species (Table 1).
, P = 0.009), this did not result in an association between breeding habitat and the number of sub-species (Table 1).
Table 1.
Results of the generalised linear model with the number of sub-species per species as independent variable
| Estimate ± SE | Deviance (df = 1) | P value | |
|---|---|---|---|
| Minimal adequate model | |||
| Migratoriness | −0.10 ± 0.08 | 0.23 | |
| Non-breeding habitat | −0.16 ± 0.19 | 0.39 | |
| Breeding habitat | 0.06 ± 0.04 | 0.17 | |
| Species age | −0.01 ± 0.007 | 0.11 | |
| Migratoriness × non-breeding habitat | 0.27 ± 0.12 | 4.92 | 0.03 |
| Separate models | |||
| Non-breeding habitat | |||
| Migrants | 0.42 ± 0.15 | 7.39 | 0.007 |
| Partial migrants | 0.13 ± 0.10 | 0.07 | 0.79 |
| Non-migrants | −0.21 ± 0.21 | 1.07 | 0.30 |
Non-significant interaction terms were removed. P values for the main effects were obtained by using the parameter estimate divided by its standard error as the test statistic. Also shown are the results of separate models for each category of migratoriness.
Fig. 1.
Mean number of sub-species per species for migratory and non-migratory waders. Pelagic wintering species (N = 2) and partial migrants (N = 31) not shown. Error bars represent the Poisson standard errors
Discussion
The prediction that coastal habitats should be more conducive to diversification in waders than freshwater habitats is supported, but only for migratory species. This is remarkable, given that the reduction in gene flow between locally adapting populations requires an extra step in these species. In non-migrants, all that is needed to mate with a locally adapted partner is not to disperse. In migrants, however, all individuals migrate away from the non-breeding grounds to breed, often to the other end of the globe. To mate with a partner from the same non-breeding grounds, the non-breeding populations must either have segregated breeding grounds or have a very sophisticated individual recognition system. There is good evidence for a number of coastally wintering migrants that the former is true (Wennerberg 2001; Atkinson et al. 2005; Buehler et al. 2006), whilst the latter has not been investigated.
I suggest that the predictability of coastal non-breeding grounds favours rigid migratory pathways to and from the non-breeding grounds. Returning to the same non-breeding ground each year would save individuals from wasting time on searching for suitable non-breeding areas and allow them to benefit from learned aspects of local conditions (such as food distribution). The rigidity of the migratory pathways could then result in spatial segregation on the breeding grounds because individuals would be genetically predisposed to favour the same breeding grounds each year. The formation of distinct sub-species would then be a by-product of the spatially discontinuous distribution of non-breeding grounds. By contrast, many of the species that spend the non-breeding season on inland habitats have to track spatially and temporally variable resources (Roshier et al. 2001, 2002), which should select for flexible movement patterns during the non-breeding season. This could translate into flexible migration patterns, perhaps through a genetic correlation between migratory and non-migratory movements, and flexible movement patterns on the breeding grounds. If true, this would promote gene flow and inhibit diversification.
Alternatively, the divergent ecology of different coastal non-breeding grounds may actively select for assortative mating of corresponding ecomorphs. For example, it may be costly for waders wintering on tropical mudflats to pair with those that winter on temperate mudflats because the resultant hybrids would be poorly adapted to either habitat. Support for this interpretation comes from the fact that many sub-species of coastal waders differ from each other in bill length, which is an ecologically relevant trait (Engelmoer and Roselaar 1998). By contrast, the unpredictability of inland wetlands would not allow specialisation to the specific conditions presented by any particular area of habitat.
The question remains why the difference in diversification between species occupying coastal and inland habitats is not reflected in the non-migratory species that breed there? Given that migrant species often greatly outnumber resident species at individual feeding areas during the non-breeding season, migrant species may have greater effective population sizes per non-breeding area than non-migrants. This would allow more efficient response to selection and increase the potential for local adaptive change in migrants relative to non-migrants. Given the arguments above, such change is most likely in coastal areas.
Acknowledgements
I thank Martine Maan, David Roshier and Danny Rogers for their constructive comments on earlier versions of this paper. Apologies to Bertus de Lange for spoiling his copy of HBW. My work is supported by a Veni grant from The Netherlands Organisation for Scientific Research.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
S1. Appendix
References
- Abouheif E (1999) A method for testing the assumption of phylogenetic independence in comparative data. Evol Ecol Res 1:895–909
- Atkinson PW, Baker AJ, Bevan RM, Clark NA, Cole KB, Gonzalez PM, Newton J, Niles LJ, Robinson RA (2005) Unravelling the migration and moult strategies of a long-distance migrant using stable isotopes: Red Knot Calidris canutus movements in the Americas. Ibis 147:738–749 [DOI]
- Beath KJ (2001) GLMStat user manual, version 5.5. Available at: http://www.glmstat.com/
- Buehler DM, Baker AJ (2005) Population divergence times and historical demography in Red Knots and Dunlins. Condor 107:497–513 [DOI]
- Buehler DM, Baker AJ, Piersma T (2006) Reconstructing paleoflyways of the late Pleistocene and early Holocene Red Knot Calidris canutus. Ardea 94:485–498
- Del Hoyo J, Elliott A, Sargatal J (1996) Handbook of the birds of the world: hoatzin to auks, vol 3. Lynx Edicions, Barcelona
- Engelmoer M, Roselaar CS (1998) Geographical variation in waders. Kluwer, Dordrecht
- Funk DJ, Nosil P, Etges WJ (2006) Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc Nat Acad Sci U S A 103:3209–3213 [DOI] [PMC free article] [PubMed]
- Gavrilets S (2004) Fitness landscapes and the origin of species. Princeton University Press, Princeton
- Phillimore AB, Orme CDL, Davies RG, Hadfield JD, Reed WJ, Gaston KJ, Freckleton RP, Owens IPF (2007) Biogeographical basis of recent phenotypic divergence among birds: a global study of subspecies richness. Evolution 61:942–957 [DOI] [PubMed]
- Piersma T (1997) Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80:623–631 [DOI]
- Piersma T (2003) “Coastal” versus “inland” shorebird species: interlinked fundamental dichotomies between their life- and demographic histories? Wader Study Group Bulletin 100:5–9
- Piersma T, van Aelst R, Kurk K, Berkhoudt H, Maas LRM (1998) A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics? Proc R Soc Lond B 265:1377–1383 [DOI]
- Piersma T, De Goeij P, Tulp I (1993a) An evaluation of intertidal feeding habitats from a shorebird perspective: towards relevant comparisons between temperate and tropical mudflats. Neth J Sea Res 31:503–512 [DOI]
- Piersma T, Koolhaas A, Dekinga A (1993b) Interactions between stomach structure and diet choice in shorebirds. Auk 110:552–564
- Roshier DA, Robertson AI, Kingsford RT (2002) Responses of waterbirds to flooding in an arid region of Australia and implications for conservation. Biol Conserv 106:399–411 [DOI]
- Roshier DA, Whetton PH, Allan RJ, Robertson AI (2001) Distribution and persistence of temporary wetland habitats in arid Australia in relation to climate. Austral Ecology 26:371–384 [DOI]
- Thomas GH, Wills MA, Székély T (2004) A supertree approach to shorebird phylogeny. BMC Evol Biol 4:28 [DOI] [PMC free article] [PubMed]
- Webster MS, Parra PP, Haig SM, Bensch S, Holmes RT (2002) Links between worlds: unravelling migratory connectivity. Trends Ecol Evol 17:76–83 [DOI]
- Wenink PW, Baker AJ, Tilanus MGJ (1993) Hypervariable-control-region sequences reveal global population structuring in a long-distance migrant shorebird, the Dunlin (Calidris alpina). Proc Nat Acad Sci U S A 90:94–98 [DOI] [PMC free article] [PubMed]
- Wennerberg L (2001) Breeding origin and migration pattern of dunlin (Calidris alpina) revealed by mitochondrial DNA analysis. Mol Ecol 10:1111–1120 [DOI] [PubMed]