Abstract
Limited information is available regarding the role of endogenous Glial cell line-derived neurotrophic factor (GDNF) in the spinal cord following transection injury. The present study investigated the possible role of GDNF in injured spinal cords following transection injury (T9–T10) in adult rats. The locomotor function recovery of animals by the BBB (Basso, Beattie, Bresnahan) scale score showed that hindlimb support and stepping function increased gradually from 7 days post operation (dpo) to 21 dpo. However, the locomotion function in the hindlimbs decreased effectively in GDNF-antibody treated rats. GDNF immunoreactivty in neurons in the ventral horn of the rostral stump was stained strongly at 3 and 7 dpo, and in the caudal stump at 14 dpo, while immunostaining in astrocytes was also seen at all time-points after transection injury. Western blot showed that the level of GDNF protein underwent a rapid decrease at 7 dpo in both stumps, and was followed by a partial recovery at a later time-point, when compared with the sham-operated group. GDNF mRNA-positive signals were detected in neurons of the ventral horn, especially in lamina IX. No regenerative fibers from corticospinal tract can be seen in the caudal segment near the injury site using BDA tracing technique. No somatosensory evoked potentials (SEP) could be recorded throughout the experimental period as well. These findings suggested that intrinsic GDNF in the spinal cord could play an essential role in neuroplasticity. The mechanism may be that GDNF is involved in the regulation of local circuitry in transected spinal cords of adult rats.
Keywords: GDNF, Spinal cord transection, Immunohistochemistry, In situ hybridization, Western blot, Antibody, Rats
Introduction
Spinal cord injury (SCI), a common result of car accidents, high-altitude falls and crashes, and other violent injuries is increasing yearly. It has been reported that there are about 15–40 traumatic SCI cases per million persons annually population worldwide [1]. Once the spinal cord is damaged, it usually results in severe neurologic dysfunction and disability. Over the past several years, many extensive experiments have been done in order to find a way to promote the repair of the injured spinal cord. However, no good therapeutic approaches have yet been found. Recently, it has been shown that delivery of exogenous neurotrophic factors (NTFs) to the injured spinal cord has beneficial effects in improving functional recovery [2], indicating the potential value of NTFs in the treatment of SCI.
Glial cell line-derived neurotrophic factor (GDNF), a distant member of the transforming growth factor-β (TGF-β) family, was originally purified using an assay based on its ability to maintain the survival and function of embryonic ventral midbrain dopaminergic neurons in vitro [3]. GDNF supports neural survival in several neuronal populations, including midbrain dopamine neurons and motoneurons in the central nervous system. It promotes the survival and the differentiation of many peripheral neurons, such as sympathetic, parasympathetic, and sensory neurons [3–10]. Following injury, GDNF showed a benefit effect to rescue motoneurons degeneration after spinal root avulsion and distal nerve axotomy [11], promotion of axonal regeneration [12] and enhancing regeneration of dorsal roots (DR) into the adult rat spinal cord [13]. In addition, the transplantation of GDNF-producing cells greatly enhances the survival of spinal cord motorneurons [11, 14, 15] and regeneration of several spinal systems, including dorsal column sensory, and regionally projecting propriospinal pathways [16, 17] after SCI. The biological action of GDNF is mediated by a two-component receptor GFRα-1 and protein tyrosine kinase Ret [18–21]. The GFRα-1 receptors expressed on a variety of neurons that project into the spinal cord, including supraspinal neurons, dorsal root ganglia, and local neurons. GDNF mRNAs are widely distributed in a variety of neuronal and non-neuronal tissues of embryos and adults [4, 22–25].
Although exogenous GDNF is available partially to promote propriospinal axonal regeneration and locomotion functional recovery, the role of intrinsic GDNF in injured spinal cords is largely unknown. Therefore, the present study investigated the possible role of GDNF at various time intervals following spinal cord transection injury in adult rats, so as to gain insights into the contribution of endogenous GDNF in spinal neuroplasticity.
Materials and methods
Characterization of antibodies
To localize GDNF protein in the spinal cord, an affinity-purified rabbit polyclonal antibody (D-20, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used in this study. The specificity of antibody for GDNF was confirmed by Western blots using rat spinal cord homogenates.
Preparation of probe for in situ hybridization
For detection of GDNF cellular expression in situ, we used Digoxigenin-labeled oligonucleotide probe designed by Primer premier 5.0 package which was complementary to the rats GDNF gene sequence (33 mer, 5′-GCCCTACTTTGTCAC–TCACCAGCCTTCTATTTC-3′). This antisense DNA single-stranded oligonucleotide probe was synthesized by Takara Biotechnology Company.
Animal grouping and surgery
Sixty-five adult Sprague-Dawley rats of either sex (weighing 200–220 g) were obtained from the Animal Experiment Center of Sichuan University of Medical Sciences. Fifty rats among all animals, designated as sham-operated group and spinal cord transection group that animals were allowed to survive 3, 7, 14 and 21 days post operation (dpo), were used for immunohistochemistry and in situ hybridization (n = 5 in each group), and Western blot analysis (n = 5 in each group) respectively. Another five rats were intraperitoneally injected anti-GDNF solution (2.5 ml/kg) once every 2 days until 21 dpo, designed as antibody neutralization group. Five animals, subjected to cord transaction, were injected with distilled water as the distilled water group. The last five operated rats were used to investigate corticospinal tract sprouting. Every effort was taken to reduce the number of animals and suffering during the experiments. All the experiments using animals were carried out according to the guidelines of the NIH Guide for Care and Use of Laboratory Animal. For the cord transection operation, the rats were anesthetized with 3.6% Chloral Hydrate (1 ml/100 g). A midline incision was made in the back, then the T7–T8 spinous processes and the vertebral laminae were removed to expose the spinal cord at T9–T10 level. The cord was transected between T9 and T10 segments with a pair of microscissors. After surgery, the superficial back muscles and the skin were sutured along the midline.
Behavior tests
All rats in each group were evaluated using the BBB (Basso, Beattie, Bresnahan) scale score [26]. The BBB score for evaluation of hindlimbs function after transected injury ranges from zero, which corresponds to flaccid paralysis, to 21, which is normal gait. Animals were allowed to walk around freely in a circular field (1.2 m in diameter) for 4 min while movements of the hindlimbs were closely observed. Ranking according to the scoring system described by Basso et al. [26] includes frequency and quality of hindlimb movement as well as forelimb/hindlimb coordination.
Measurement of somatosensory evoked potentials (SEP)
The right peroneal nerve of the rats was dissected and a stimulation electrode was placed on it while a hole (3–4 mm in diameter) was made on the skull (2 mm to the left of the midline and 2 mm in front of the fonticulus posterior). An electrode was placed on the dura of brain cortex to record the SEP. The reference electrode was inserted at the nose epithelium. A ground electrode was inserted at the tail. The stimulus intensity was set high enough to produce a marked muscle twitch in the hind limb, about 1.1 mA, the amplitude was 0.2 ms and the frequency 3 Hz. The SEP Tracings represented the average of 200 responses.
Immunohistochemistry
After anesthesia with 3.6% Chloral Hydrate (1 ml/100 g), rats were perfused with 500 ml of cold phosphate-buffered saline (PBS) for 5 min and 500 ml cold 4% paraformaldehyde solution for 30 min. The T9 and T10 spinal cord segments in the sham-operated group, and the cord stumps rostral and caudal to the injury site in the experimental group were harvested. This was followed by a postfix for 6–12 h, then immersed in 0.1 M PBS containing 20% sucrose overnight. Sections of 20 μm thickness were cut on a freezing microtome and used for freefloating GDNF immunostaining, then rinsed with 0.01 M PBS and soaked in PBS containing 3% H2O2 for 30 min at room temperature to quench the endogenous peroxidase activity. They were immersed in PBS containing 5% goat serum and 0.3% TritonX-100 solution at room temperature for 2 h, then incubated at 4°C for 24 h with anti-GDNF (1:1,000, Chemicon) rabbit polyclonal antibody. Sections were incubated with biotinylated goat anti-rabbit IgG antibody (1:100 dilution) for 1.5 h at room temperature, and by reaction with avidin-biotinylated peroxidase complexes (1:250, ABC Elite, Vector Labs). GDNF immunoreactivity was visualized with brown staining using diaminobenzidine (DAB) and H2O2 as substrate for 5 min, and observed with a light microscope. Negative control experiments in which PBS was substituted for the primary antibody were performed to ascertain the specificity of antibody staining.
In situ hybridization
Sections from sham-operated rats of 25 μm thickness were also cut on a freezing microtome and used for in situ hybridization. Sections were fixed in 4% paraformaldehyde in 0.1 M PBS, pH 7.2 (all treatments were performed at room temperature unless otherwise indicated), and further treated with 0.3% TritonX-100 solution for 10 min and proteinase K (5 μg/ml) at 37°C for 25 min, refixed with 4% paraformaldehyde for 5 min, repeatedly immersed in 0.1 M PBS, then acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0) to prevent non-specific binding of the probes. The sections were washed with 2× SSC (pH 7.0) and then prehybridized in a hybridization solution (50% formamide, 10% dextran sulfate, 1× Denhardt’s solution, 0.2 mg/ml Herring sperm DNA, and 10 mM dithiothreitol) without probes at 37°C for 2 h before hybridization, then hybridized in 100 μl hybridization solution containing 1 μl probes at 37°C for 12–16 h in a moist chamber. This was followed by washing in decreasing concentrations of SSC, from 4× SSC (pH 7.0) at 37°C for 20 min, 2× SSC (pH 7.0) at 42°C for 20 min, 1× SSC (pH 7.0) at 48°C for 20 min and ending with 0.5× SSC (pH 7.0) at 50°C for 20 min. Then sections were incubated at 37°C in 1% blocking buffer (Roche) for 1 h, subsequently reacted in 1:1,000 sheep anti-digoxygenin-alkaline phosphatase (AP) antibody in 1% blocking buffer at 4°C overnight. Lastly, AP activity was detected using nitroblue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP) substrate (Roche). The sections were visualized with blue and purple sedimentation then observed with a light microscope.
GFAP/GDNF double-label immunohistochemistry
Two-color immunohistochemical staining for simultaneous detection of glial fibrillary acidic protein (GFAP)/GDNF expression was performed as described above. Briefly, sections were stained with anti-GDNF rabbit polyclonal antibody (1:1,000), and using DAB solutions as a substrate. This was followed by second incubation with anti-GFAP rabbit polyclonal antibody (1:1,000, AB5804, Chemicon), and finally, reacted with TMB solutions. Double labeling showed a combination of brown and blue product.
Western blotting
The spinal cord from the rostral and caudal stumps was obtained. After carefully removing the spinal meninges, the cords were homogenized on ice in a Lysis Buffer containing 0.05 M Tris–HCl (pH 7.4, Amresco), 0.5 M EDTA (Amresco), 30% TritonX-100 (Amresco), NaCl (Amresco), 10% SDS (Sigma) and 1 mM PMSF (Amresco), then centrifuged at 12,000g for 30 min. The supernatant was obtained and stored at −80°C for late use. Protein concentration was assayed with BCA reagent (Sigma, St. Louis, MO, USA). A 20 μl aliquot of the samples was loaded on to each lane and electrophoresed on 12% SDS-polyacrylamide gel (SDS-PAGE) for 2.5 h at a constant voltage of 120 V. Proteins were transferred from the gel to a nitrocellulose membrane for 435 min at 24 V. The membrane was blocked with phosphate-buffered saline containing 0.05% Tween-20 (PBST) with 10% non-fat dry milk overnight at 4°C. The membrane was rinsed with PBST and incubated with the primary antibody for GDNF (1:1,000) at 4°C. The membrane was incubated with a HRP-conjugated goat anti-rabbit IgG (1:5,000; Vector Laboratories, CA) for 2 h at room temperature. The membrane was developed in ECM kit and exposed against X-ray film in a darkroom. Densitometry analysis for the level of GDNF protein was performed by Bio-Gel Imagining system equipped with Genius synaptic gene tool software. β-actin (the primary antibody, 1:1,000, the secondary antibody, 1:2,000; Santa Cruz Biotechnology) was used as an internal control.
BDA anterograde tracing
At 14 dpo, the animals for this part were anesthetized and fixed in a David Kopf Instruments (Tujunga, CA) stereotaxic head-holder device. Burr holes were made in the dorsal cranium, and biotinylated dextran amine (BDA) (10% BDA solution, Molecular Probes) was microinjected into eight sites at a depth of 0.7 mm from the cortical surface (0.5 μl/site) to cover the hindlimb region. Animals were then sacrificed 2 weeks later to allow sufficient time for axonal transport of BDA in corticospinal tract. The spinal cords were removed and postfixed at 3 days in cold 4% paraformaldehyde in 0.1 M PBS (pH 7.2). Transverse sections (30 μm) of spinal cord at the injury site and neighboring rostral and caudal parts to the injury site were processed for the presence of BDA-labeled axons by incubation in avidin-HRP (Molecular Probes). Lastly, DAB stain was performed to visualize the positive fiber, as brown color staining.
GDNF antibody neutralization
After 14 dpo, each rat was intraperitoneally injected with 0.5 ml (30 mg/ml, 30 mg of anti-GDNF diluted in 1 ml of distilled water) anti-GDNF solution once every 2 days until 21 dpo. GDNF antibody was the distilled water replace as control in another five rats. The locomotion in hindlimbs by BBB score was evaluated at 3, 7, 14, and 21 dpo.
Statistical analysis
All data were expressed as the mean ± S.E.M. They were analyzed using One-way ANOVA and LSD-q test by SPSS software package. The statistical significance was defined as P < 0.05.
Results
Behavior tests
The BBB score for locomotor function in sham-operated rats hindlimbs was 21. Compared with the sham-operation group, the BBB score of animals in the group with only the cord transection and distilled water group increased gradually from 7 to 21 dpo. A significant decrease in the BBB score for the GDNF-antibody treated group was observed (P < 0.05) (Table 1).
Table 1.
Mean values of BBB scores in cord transected rats (mean ± S.E.M)
| Group | 3 Days | 7 Days | 14 Days | 21 Days |
|---|---|---|---|---|
| Sham-operation group | 5 ± 0.6 | 21 ± 0 | 21 ± 0 | 21 ± 0 |
| Transection group | 0 ± 0 | 0.8 ± 0.3 | 2.4 ± 0.7 | 3.6 ± 0.5 |
| Distilled water group | 0 ± 0 | 0.6 ± 0.7 | 2.0 ± 0.4 | 3.3 ± 0.3 |
| GDNF-antibody treated group | 0 ± 0 | 0.2 ± 0.4 | 0.6 ± 0.3 | 0.9 ± 0.5 |
BBB score in rats subjected to the sham operation was constant. Following transection injury, the BBB score of animals increased significantly from 7 to 21 dpo (P < 0.05). There had no statistical significance in BBB score between cord transection group and distilled water group (P > 0.05), while a significant decrease of BBB score in GDNF-antibody treated group was observed (P < 0.05)
Somatosensory evoked potentials (SEP)
Evoked potentials are the electrical signals generated by the nervous system in response to sensory stimuli. Somatosensory evoked potentials consist of a series of waves that reflect sequential activation of neural structures along the somatosensory pathways following electrical stimulation of peripheral nerves. SEP (P1N1P2 type) was recorded in the control rats and shown in Fig. 1. Throughout the experimental period, no SEP could be recorded in rats subjected to cord transection.
Fig. 1.
Somatosensory evoked potential was recorded in normal adult rats (P1N1P2 type). The latency of SEP: P1 (10.3 ± 0.26) ms, N1 (8.81 ± 0.34) ms, P2 (15.5 ± 0.43) ms, amplitude: P1 (2.34 ± 0.02), N1 (16.3 ± 0.14), P2 (−5.06 ± 0.05)
The GDNF immunostained intensity changes
In the control group, GDNF immunoreactivity was observed in the cytoplasm and nuclei of the neurons from the ventral horn in the gray matter (Fig. 3a). Most of the small and medium-sized neurons in the intermediate zone and dorsal horn were stained faintly. GDNF positive profiles (Fig. 3b) simultaneously labeled with GFAP, were present in the white matter (Fig. 2b). In the negative control group, no specific immunopositive staining was detected (Fig. 2a).
Fig. 3.
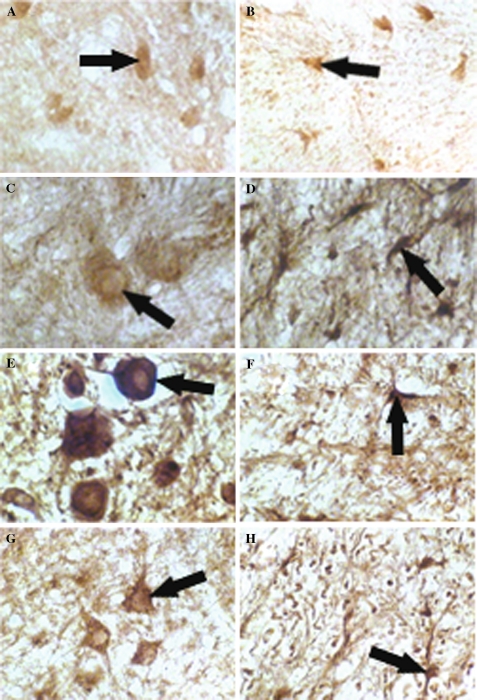
Under higher magnification (400×), GDNF immunoreactivity (IR) was observed in neurons from the ventral horn of the gray matter in the normal spinal cord (a, arrow). GDNF (IR) in astrocytes was also seen in white matter (b, arrow). In the rostral stump, strong GDNF IR was detected in neurons from the ventral horn at 3 and 7 dpo (c, e, 400×, arrows). Moderate to intense immunostained neurons were labeled at 14 dpo (g, 400×, arrow). Intensely immunostained IR in astrocytes of the white matter were observed from 3 to 14 dpo (d, f, h, 400×, arrows)
Fig. 2.
In the negative control group, no specific immunopositive staining was detected (a). GDNF positive profiles, simultaneously label-GFAP, were present in the white matter (b)
In the rostral stump, strong immunostaining for GDNF was observed in neurons from the ventral horn at 3 and 7 dpo (Fig. 3c, d; Table 2). Moderate to intense immunostained neurons were seen at 14 and 21 dpo, as shown in Figs. 3g, 4a and Table 2. In the white matter, astrocytes showed strong immunoreactivity of GDNF from 3 to 21 dpo (Figs. 3d–f, 4b; Table 2).
Table 2.
GDNF immunostaining intensity in neurons and astrocytes
| Normal | 3 Days | 7 Days | 14 Days | 21 Days | ||
|---|---|---|---|---|---|---|
| Neurons | + | +++ | +++ | ++∼+++ | ++∼+++ | |
| Rostral part | Astrocytes | ++ | +++ | +++ | +++ | +++ |
| Caudal part | Neurons | + | +∼++ | ++ | +++ | ++ |
| Astrocytes | ++ | ++ | ++ | +++ | +++ |
Intensity: + weak, ++ moderate, +++ strong
Neurons in the ventral horn of the rostral stump was strongly stained for GDNF at 3 and 7 dpo, and in the caudal stump at 14 dpo. The GDNF positive staining in astrocytes was also stronger at all time-points after transection injury than seen in control group
Fig. 4.
In the rostral stump, moderate to intense immunostained neurons were labeled at 21 dpo (a, 400×, arrow). Intensely immunostained IR in astrocytes of the white matter were also observed at 21 dpo (b, 400×, arrow). In the caudal stump, immunostained products were detected in neurons of the gray matter at 3 and 7 dpo (c, d, 400×, arrows). Strong immunostained IR for GDNF in neurons was observed at 14 dpo (e, 400×, arrow). Under higher magnification, immunoreactive structures of GDNF were localized in both cytoplasm and nuclei at 21 dpo (g, 400×, arrow), GDNF IR in astrocytes of the white matter was intensely immunostained at 14 and 21 dpo (f, h, 400×, arrows)
In the gray matter of the caudal stump, moderately immunostained neurons were detected at 3, 7 and 21 dpo, and more so at 14 dpo (Fig. 4c–e, g; Table 2). The subcellular localization for GDNF was observed both in cytoplasm and nuclei at 21 dpo (Fig. 4g). At both 14 and 21 dpo, astrocytes in the white matter strongly stain with GDNF immunoreactivity as shown in Fig. 4f, h and Table 2.
Localization of GDNF mRNA in spinal cord of rats
Signals of hybridization for GDNF mRNA were markedly detected in the Central canal and lamina III, IV and V, in which small neurons were moderately NBT/BCIP stained (Fig. 5a). Medium-sized neurons were seen in the field of the intermediomedial nucleus and nucleus dorsalis (Fig. 5a). In the ventral horn, GDNF mRNA-positive large neurons were also observed in lamina IX (Fig. 5b). A few scattered glial cells were probe-labeled with light density in white matter (Fig. 5c).
Fig. 5.
In the spinal cord of adult rats, GDNF mRNA-positive product were markedly detected in neurons from the lamina III, IV and V and the central canal (a). Medium-sized neurons were seen in the field of intermediomedial nucleus and Nucleus dorsalis. In the ventral horn, GDNF mRNA-positive large neurons were also observed in lamina IX. Under higher magnification (200×), positive products with blue and purple staining were localized in the cytoplasm (b, arrows), also in white matter scattered glial cells were probe-labeled with light density (c, arrows)
Western blotting
A single positive band was observed at a molecular weight of about 34,000, which corresponds to the molecular weight of GDNF.
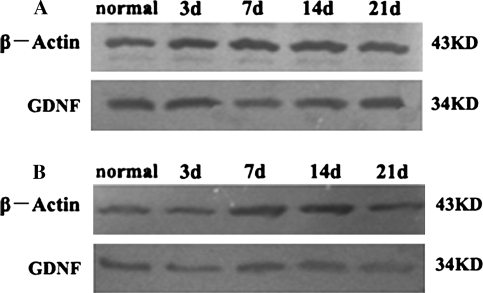
In the rostral stump, the level of GDNF protein increased at 3 dpo, when compared with the control group (P < 0.05), then decreased quickly to its lowest level at 7 dpo (P < 0.05) which was less than that of the control level, and then began to increase again at 14 dpo. However, GDNF protein was higher than what was seen in the control group at 21 dpo (Figs. 6a, 7). In the caudal stump, GDNF protein immediately decreased at 3 dpo, reached its lowest level at 7 dpo (P < 0.05), then increased from 14 to 21 dpo, but it did not go back to the control level (Figs. 6b, 7).
Fig. 6.
In the rostral stump, GDNF protein level increased at 3 dpo (P < 0.05), then reached its lowest point at 7 dpo which was below the control group (P < 0.05), then began to recover at 14 dpo, but it was less than that of normal level. This was followed by a continuous increase higher than the control group at 21 dpo (a). In the caudal stump, GDNF protein decreased at 3 dpo, reached its lowest point at 7 dpo (P < 0.05), then started to increase at 14 dpo, but it was lower than the normal level at 21 dpo (b)
Fig. 7.
GDNF levels were significantly different from each other (P < 0.05), except for the comparison between the 21 dpo and normal group, as well as the 21 and 3 dpo group in the rostral stump. The groups were significantly different from each other (P < 0.05), except for the comparison between the 3 and 14 dpo group in the caudal stump
BDA tracing
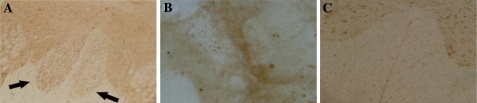
BDA-positive axons which stained as brown silkiness were detected in rostral cord sections of the injury site (Fig. 8a), but not in the field of scar tissue (Fig. 8b), and in the caudal segment near the injury site (Fig. 8c).
Fig. 8.
BDA-positive axons staining as brown silkiness were detected in rostral cord sections near the injury site (a), but not in the field of scar tissue (b), and caudal cord sections near the injury site (c)
Discussion
In the present study, GDNF immunoreactive products in neurons from the gray matter and in astrocytes from the white matter of the spinal cord were observed in the sham-operation group. This indicates that GDNF could be involved in either the physiological function or the maintenance of survival for all kinds of neurons and astrocytes. It has been shown that GDNF mRNAs widely distributed in a variety of neuronal and non-neuronal tissues of embryos and adults [4, 23–25]. In addition, GDNF mRNA positive signals were found in the ventral horns, the field of nucleus dorsalis, and the dorsal horns in the spinal cords of adult rats [2, 16, 27] and in the dorsal root ganglia in newborn rats [23]. Therefore, it raised the possibility that GDNF was synthesized by cells of the spinal cord and the GDNF expression may be changed after lesion, which implies that GDNF plays a potential role in both physiological and pathological conditions.
Following cord transection, rats exhibited spinal cord dysfunction throughout the experimental period. There was an absence of SEP in surface recordings, indicating a loss of sensory function in the hindlimbs following transection injury. Comparatively, locomotion function recovery was observed from 7 to 21 dpo, which was parallel to the improvement in BBB score. It showed that locomotor functional plasticity in the hindlimbs of rats did occur following SCI. As corticospinal tract regeneration was not seen in the caudal part near the injury site and scar tissues, it appears impossible to reestablish connectivity between the areas rostral and caudal of the injury site. The possible reason for locomotor function improvement may be related to the modulation of spinal circuit activity from endogenous neurotrophins. In the present study, BBB scores in the GDNF-antibody treated rats was inferior to that of the group not receiving the antibody treatment, indicating intrinsic GDNF may be available for improving locomotor function recovery to some extent in the hindlimbs of rats [15, 28]. It has been reported that GDNF can promote the repair or survival of spinal motoneurons [4, 7, 29–32]. Several other studies have shown that the administration of GDNF could promote axonal regeneration, and enhance locomotion functional recovery [2, 15, 30, 33–36].
GDNF immunoreactivity products in neurons from the ventral horn of the rostral stump were strongly stained at 3 and 7 dpo, and in the caudal stump at 14 dpo, suggesting that the GDNF expression in neurons was up-regulated following injury. This may be related to the locomotion functional recovery of hindlimbs. Glazner et al. [37] showed that strong labeling for GDNFR-α and c-ret mRNA was observed in large neurons from the ventral horn. Our results also supported the mRNA expression for GDNF in neurons of the ventral horn, as well as astrocytes in white matter using in situ hybridization. RET expression was unregulated following peripheral axotomy in alpha-motoneurons [38], and topical application of GDNF 30 min after SCI significantly improved motor function and reduced blood–spinal cord barrier (BSCB) breakdown, edema formation, and cell injury at 5 h has been reported [39]. Therefore, GDNF is available to assist in locomotion functional plasticity by exerting behavioral and anatomic neuroprotection following SCI.
In the present study, the expression patterns of endogenous GDNF indicated its possible role in maintaining motorneuron survival because GDNF mRNA-positive products were detected in neurons from the ventral horn by in situ hybridization. Of course, GDNF retrograde axonal transport from a target tissue to neuronal cell bodies [40, 41] is not absolutely excluded. It has been reported that GDNF produced by skeletal muscle is taken up at the nerve terminals and retrogradely transported to motoneurons of the ventral horn by axons [30].
Comparing the results of immunostaining, the GDNF level varied throughout the spinal cord at 3 dpo. In the caudal stump, GDNF showed a significant decrease. This was probably due to the transection injury that triggers a process destructive to ascending and descending tracts conduction and that extends tissue loss. Differently, a rapid up-regulation of GDNF was observed in the rostral stump. This suggested that GDNF had accumulated around the transection site due to interruption of its transport within the axons. Satake et al. [42] reported GDNF transcription began to increase within 30 min after injury and peaked within 3 h after spinal cord contusion injury. Interestingly, the level of GDNF protein reached its lowest point at 7 dpo in both stumps, probably due to the consequences of cell death or apoptosis and/or interruption of the spinal cord pathways. Subsequently, GDNF protein levels gradually started to recover and surpassed the control level in the rostral stumps at 21 dpo, which may be explained by (1) GDNF transport within axons was strengthened, (2) inflammation leads to the increase of GDNF synthesis [36], and (3) induction of de novo synthesis of GDNF by neurons and/or non-neuronal cells.
Noticeably, the GDNF positive cells in the white matter were labeled by GFAP simultaneously, which identified the labeled cells as astrocytes. Indeed, an association between reactive astrocytes and regenerating nerve fibers in the white matter of the central nervous system in vivo has been reported [43, 44]. Also, increased GFR-1 in astrocytes of degenerating white matter in adult rat spinal cords after mechanical injury has been shown [2]. These results indicated that GDNF expression in astrocytes may be involved in neuroplasticity following spinal cord transection injury.
The present study provides new evidence to understand the role of intrinsic GDNF in the rostral and caudal stumps of injured spinal cords. It suggests that GDNF plays an essential role in the neuroplasticity of the local circuitry, especially in the caudal stump of the transected spinal cords.
Acknowledgements
We thank Kevin J. Foehrkolb and Yates Lee M.D. for their invaluable comments in the writing of this manuscript.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s11064-008-9786-6
Reference
- 1.Lim PA, Tow AM (2007) Spinal cord injury (SCI) often results in significant neurologic dysfunction and disability. An annual incidence of 15 to 40 traumatic SCI cases per million population has been reported worldwide, and a conservative estimate for Singapore would be 23 cases per million. Ann Acad Med Singap 36(1):49–57 17285186
- 2.Widenfalk J, Lundströmer K, Jubran M, Brené S, Olson L (2001) Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci 21(10):3457–3475 [DOI] [PMC free article] [PubMed]
- 3.Lin L-FH, Doherty DH, Lile J, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132 [DOI] [PubMed]
- 4.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simpson LC, Moffet B, Vandlen RA, Koliatsos VE, Rosenthal A (1994) GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science 266:1062–1064 [DOI] [PubMed]
- 5.Beck K, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen R, Rosenthal A, Hefti F (1995) Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature 373:339–341 [DOI] [PubMed]
- 6.Buj-Bello A, Buchman VL, Horton A, Rosenthal A, Davies AM (1995) GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron 15:821–828 [DOI] [PubMed]
- 7.Li LX, Wu WT, Lin LFH, Lei M, Oppenheim RW, Houenou LJ (1995) Rescue of adult mouse motoneurons from injury-induced cell death by glial cell line-derived neurotrophic factor. Proc Natl Acad Sci USA 92:9771–9775 [DOI] [PMC free article] [PubMed]
- 8.Tomac A, Lindqvist E, Lin L-F, Ögren S, Young D, Hoffer B, Olson L (1995) Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373:335–339 [DOI] [PubMed]
- 9.Airaksinen MS, Titievsky A, Saarma M (1999) GDNF family neurotrophic factor signaling: four masters, one servant?. Mol Cell Neurosci 13:313–325 [DOI] [PubMed]
- 10.Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394 [DOI] [PubMed]
- 11.Yuan Q, Wu W, So KF, Cheung AL, Prevette DM, Oppenheim RW (2000) Effects of neurotrophic factors on motoneuron survival following axonal injury in newborn rats. Neuroreport 11(10):2237–2241 [DOI] [PubMed]
- 12.Dolbeare D, Houle JD (2003) Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line-derived neurotrophic factor (GDNF). J Neurotrauma 11:1251–1261 [DOI] [PubMed]
- 13.Iwakawa M, Mizoi K, Tessler A, Itoh Y (2001) Intraspinal implants of fibrin glue containing glial cell line-derived neurotrophic factor promote dorsal root regeneration into spinal cord. Neurorehabil Neural Repair 15(3):173–182 [DOI] [PubMed]
- 14.Albeck DS, Hoffer BJ, Quissell D, Sanders LA, Zerbe G, Granholm AC (1997) A non-invasive transport system for GDNF across the blood–brain barrier. Neuroreport 8(9–10):2293–2298 [DOI] [PubMed]
- 15.Watabe K, Ohashi T, Sakamoto T, Kawazoe Y, Takeshima T, Oyanagi K, Inoue K, Eto Y, Kim SU (2000) Rescue of lesioned adult rat spinal motoneurons by adenoviral gene transfer of glial cell line-derived neurotrophic factor. J Neurosci Res 60(4):511–519 [DOI] [PubMed]
- 16.Blesch A, Tuszynski MH (2003) Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol 467(3):403–417 [DOI] [PubMed]
- 17.Iannotti C, Li H, Yan P, Lu X, Wirthlin L, Xu XM (2003) Glial cell line-derived axonal regeneration and enhance myelination after spinal cord injury. Exp Neurol 183(2):379–393 [DOI] [PubMed]
- 18.Jing SQ, Spencer T, Miller K, Hopkins C, Trowbridge IS (1990) Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol 110:283–294 [DOI] [PMC free article] [PubMed]
- 19.Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Suvanto P, Wartiovaara K, Smith D, Ponder B, Costantini F, Saarma M, Sariola H, Pachnis V (1996) Glial cell line-derived neurotrophic factor signaling through the Ret receptor tyrosine kinase. Nature 381:789–792 [DOI] [PubMed]
- 20.Treanor J, Goodman L, Desauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, Phillips HS, Goddard A, Moore MW, Bujbello A, Davies AM, Asai N, Takahashi M, Vandlen R, Henderson CE, Rosenthal A (1996) Characterization of a multicomponent receptor for GDNF. Nature 382:80–83 [DOI] [PubMed]
- 21.Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumäe U, Sariola H, Saarma M, Ibáñez CF (1996) Functional receptor for glial cell line-derived neurotrophic factor encoded by the c-ret proto-oncogene product. Nature 381:785–789 [DOI] [PubMed]
- 22.Schaar DG, Sieber BA, Dreyfus CF, Black IB (1993) Regional and cell-specific expression of GDNF in rat brain. Exp Neurol 124:368–371 [DOI] [PubMed]
- 23.Springer JE, Mu X, Bergmann LW, Trojanowski JQ (1994) Expression of GDNF mRNA in rat and human nervous tissue. Exp Neurol 127(2):167–170 [DOI] [PubMed]
- 24.Trupp M, Ryden M, Joernvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF (1995) Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol 130:137–148 [DOI] [PMC free article] [PubMed]
- 25.Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM, Milbrandt J (1996) Neurturin, a relative of glial-cell-line-derived neurotrophic factor. J Nature 384:467–470 [DOI] [PubMed]
- 26.Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1–21 [DOI] [PubMed]
- 27.Nosrat CA, Tomac A, Lindqvist E, Lindskog S, Humpel C, Stromberg I, Ebendal T, Hoffer BJ, Olson L (1996) Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res 286(2):191–207 [DOI] [PubMed]
- 28.Cheng H, Wu JP, Zeng SFT (2002) Neuroprotection of glial cell line-derived neurotrophic factor in damaged spinal cords following contusive injury. J Neurosci Res 69(3):397–405 [DOI] [PubMed]
- 29.Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S (1995) Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature 373:344–346 [DOI] [PubMed]
- 30.Yan Q, Mathson C, Lopez OT (1995) In vivo neurotrophic factor of GDNF on neonatal and adult facial motor neurons. Nature 373(6512):341–344 [DOI] [PubMed]
- 31.Unsicker K (1996) GDNF: a cytokine at the interface of TGF-betas and neurotrophins. Cell Tissue Res 286:175–178 [DOI] [PubMed]
- 32.Junger H, Varon S (1997) Neurotrophin-4 (NT-4) and glial cell line-derived neurotrophic factor (GDNF) promote the survival of corticospinal motor neurons of neonatal rats in vitro. Brain Res 762:56–60 [DOI] [PubMed]
- 33.Lu KW, Chen ZY, Jin DD, Hou TS, Cao L, Fu Q (2002) Cationic liposome-mediated GDNF gene transfer after spinal cord injury. J Neurotrauma 19(9):1081–1090 [DOI] [PubMed]
- 34.Lu KW, Chen ZY, Hou TS (2003) Protective effect of liposome mediated glial cell line-derived neurotrophic factor gene transfer in vivo on motoneurons following spinal cord injury in rats. Sheng Li Xue Bao 55(3):349–354 12817305
- 35.Cao L, Liu L, Chen ZY, Wang LM, Ye JL, Qiu HY, Lu CL, He C (2004) Olfactory ensheathing cells genetically modified to secrete GDNF to promote spinal cord repair. Brain 127:535–549 [DOI] [PubMed]
- 36.Hashimoto M, Nitta A, Fukumitsu H, Nomoto H, Shen L, Furukawa S (2005) Inflammation-induced GDNF improves locomotor function after spinal cord injury. Neuroreport 16(2):99–102 [DOI] [PubMed]
- 37.Glazner GW, Mu XJ, Springer JE (1998) Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol 391:42–49 [DOI] [PubMed]
- 38.Jongen JL, Jaarsma D, Hossaini M, Natarajan D, Haasdijk ED, Holstege JC (2007) Distribution of RET immunoreactivity in the rodent spinal cord and changes after nerve injury. J Comp Neurol 500(6):1136–1153 [DOI] [PubMed]
- 39.Sharma HS (2006) Post-traumatic application of brain-derived neurotrophic factor and glia-derived neurotrophic factor on the rat spinal cord enhances neuroprotection and improves motor function. Acta Neurochir Suppl 96:329–334 [DOI] [PubMed]
- 40.Mohajeri MH, Figlewicz DA, Bohn MC (1999) Intramuscular grafts of myoblasts genetically modified to secrete glial cell line-derived neurotrophic factor prevent motoneuron loss and disease progression in a mouse model of familial amyotrophic lateral sclerosis. Hum Gene Ther 10:1853–1866 [DOI] [PubMed]
- 41.Alisky JM, Davidson BL (2000) Gene therapy for amyotrophic lateral sclerosis and other motor neuron diseases. Hum Gene Ther 11:2315–2329 [DOI] [PubMed]
- 42.Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K (2000) Up-regulation of glial cell line-derived neurotrophic factor (GDNF) following traumatic spinal cord injury. Neuroreport 11(17):3877–3881 [DOI] [PubMed]
- 43.Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J (1997) Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390:680–683 [DOI] [PubMed]
- 44.Davies SJ, Goucher DR, Doller C, Silver J (1999) Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci 19:5810–5822 [DOI] [PMC free article] [PubMed]