Abstract
Infective spondylitis occurring concomitantly with mycotic aneurysm is rare. A retrospective record review was conducted in all cases of mycotic aneurysm from January 1995 to December 2004, occurring in a primary care and tertiary referral center. Spontaneous infective spondylitis and mycotic aneurysm were found in six cases (10.3% of 58 mycotic aneurysm patients). Neurological deficit (50% vs. 0; P < 0.001) is the significant clinical manifestation in patients with spontaneous infective spondylitis and mycotic aneurysm. The presence of psoas abscess on computed tomography (83.3% vs. 0; P < 0.001) and endplate destruction on radiography (50% vs. 0; P < 0.001) are predominated in patients with spontaneous infective spondylitis and mycotic aneurysm. Of these six patients, four with Salmonella infection received surgical intervention and all survived. Another two patients (one with Streptococcus pyogenes, another with Staphylococcus aureus) received conservative therapy and subsequently died from rupture of aneurysm or septic shock. Paravertebral soft tissue swelling, presence of psoas abscess and/or unclear soft tissue plane between the aorta and vertebral body in relation to mycotic aneurysm may indicate a concomitant infection in the spine. In contrast, if prevertebral mass is found in the survey of spine infection, coexisting mycotic aneurysm should be considered.
Keywords: Infective spondylitis, Mycotic aneurysm, Psoas abscess
Introduction
Infective spondylitis occurring with mycotic aneurysm are rare clinical entities and difficult to diagnose [7]. The initial presentations are non-specific (such as fever, chills, and back pain), thus the diagnosis of either spinal infection or mycotic aneurysm may be delayed and thereby resulting in hazardous complications or mortality [15].
Successful non-surgical treatment for mycotic aneurysm or spondylitis has been reported [1, 7, 11, 12]. However, the mortality rate of mycotic aneurysm with medical therapy alone has been high [11]. There is scanty treatment experience with regards to coexisting spondylitis and mycotic aneurysm [6, 7, 10, 13]. To assess the incidence and identify risk factors of coexisting infective spondylitis in patients with mycotic aneurysm, we conducted a retrospective study of patients with mycotic aneurysm at a medical center in Taiwan. The clinical characteristics, diagnostic image studies, and treatment outcomes of patients with spontaneous infective spondylitis and mycotic aneurysm were emphasized to clarify the treatment strategy.
Materials and methods
All patients hospitalized between January 1, 1995 and December 31, 2004 with a discharge diagnosis of mycotic aneurysm retrieved from the records of the Medical Coding Section of Chang Gung Memorial Hospital-Kaohsiung, a 2,500-bed facility serving as a primary care and tertiary referral center in Taiwan, were included in a compiled list of patients, and were subject to medical chart review. The diagnosis of mycotic aneurysm required meeting at least one of the following criteria: (1) in patients receiving surgical management, pus was macroscopically found in the aneurysm during operation; (2) aspirate or dissected tissue specimen of arterial wall or periaortic soft tissue with histopathologically confirmed purulent inflammation; and (3) in the presence of compatible clinical manifestations and finding on computed tomography (CT) or magnetic resonance imaging (MRI), the patients’ condition improved after medical treatment in cases without surgical intervention. Medical treatment was regarded as appropriate if the pathogen isolated from the abscess or blood was susceptible in vitro to the prescribed antibiotics. Demographics, clinical and laboratory characteristics, including sex, age, and clinical symptoms, underlying conditions, isolated organisms, and treatment modalities with outcome were collected. Patients were classified into mycotic aneurysm with or without spontaneous infective spondylitis. Variables from different groups were compared with each other. Mann–Whitney U test was used to assess the differences in continuous variables, while chi-square test or Fisher’s exact test was used to assess dichromatic variables between the two groups. A two-tailed P ≤ 0.05 was considered to be statistically significant.
We further focus on patients with coexisting mycotic aneurysm and infective spondylitis. Imaging studies, including radiography, CT, or MRI for the spine or aorta in these patients were analyzed. Functional outcomes of these patients were evaluated by the muscle strength of the lower legs, bladder/bowel function, and daily living activity, at a minimum of 2 years of follow-up.
Results
A total of 58 patients suffered from mycotic aneurysm were included during this period. There were 51 (87.9%) men and 7 (12.1%) women, with a mean age of 68.7 ± 8.2 years. Fifty (86.2%) patients were found to have underlying disease(s), and among the included patients, the leading underlying diseases were diabetes mellitus (36.2%), as well as renal disease and immunosuppressive agent user (each 31.0%). A variety of symptoms/signs were found in these patients, and the two leading ones were localized back pain in vicinity of the mycotic aneurysms (75.9%) and fever (68.9%). All 58 patients received appropriate medical treatment. Six (10.3%) mycotic aneurysm patients had coexisting infective spondylitis. No significant differences in demographics, underlying conditions, laboratory data, surgical intervention and in-hospital mortality between these two groups are found (Table 1). Only neurological deficit (lower limb weakness in two and paraplegia in one) is noted predominately in patients with spontaneous infective spondylitis and mycotic aneurysm (50% vs. 0; P < 0.001). In view of isolated organisms, Salmonella spp. are the most common pathogen in mycotic aneurysm with or without infective spondylitis (Table 2). Focus on the image feature for the clue of diagnosis of spontaneous infective spondylitis and mycotic aneurysm, psoas abscess on CT (83.3% vs. 0; P < 0.001) and endplate destruction on radiography (50% vs. 0; P < 0.001) are predominated (Table 2). The clinical details, image studies, surgical methods and outcome of these six patients with spontaneous infective spondylitis and mycotic aneurysm are summarized in Table 3. The involved vertebral levels were L3–5 in four patients; T12-L1 and T6-8 was in each one. Imaging study of CT or MRI revealed psoas abscess or paravertebral mass in all six patients, although radiography of the spine showed no bony lesion in a half of patients. For example, Fig. 1 shows infra-renal mycotic aneurysm with unclear soft tissue plane between aorta and L4 vertebra and bilateral paravertebral soft tissue swelling in Case 4. Conversely, a mycotic aneurysm which presented as a prevertebral mass was incidentally discovered in Case 1 while surveying for spine infection (Fig. 2). In terms of culprit, all six patients suffered from bacteremia (four Salmonella spp., one Streptococcus pyogenes and one Staphylococcus aureus), and tissue culture was positive in three patients (two Salmonella spp., and one Mycobacteriumtuberculosis).
Table 1.
Demographics, clinical manifestations, laboratory data and outcomes of 58 patients with mycotic aneurysm
| Variables | Mycotic aneurysm without infective spondylitis, N = 52 (%) | Mycotic aneurysm with infective spondylitis, N = 6 (%) | P level |
|---|---|---|---|
| Age (mean ± SD, year) | 70.7 ± 10.2 | 67.0 ± 6.2 | 0.81 |
| Male | 46 (88.9) | 5 (83.3) | 0.55 |
| Clinical features | |||
| Fever | 35 (66.7) | 5 (83.3) | 0.65 |
| Localized back pain | 38 (72.2) | 6 (100) | 0.32 |
| Heart failure | 8 (15.4) | 1 (16.7) | 1 |
| Pulsatile mass | 10 (19.2) | 2 (33.3) | 0.59 |
| Neurological deficit | 0 | 3 (50) | <0.001 |
| Underlying conditions | |||
| Renal disease | 17 (33.3) | 1 (16.7) | 0.65 |
| Immunosuppressive agent user | 17 (33.3) | 1 (16.7) | 0.65 |
| Hypertension | 11 (21.2) | 2 (33.3) | 0.61 |
| Diabetes mellitus | 19 (36.5) | 2 (33.3) | 1 |
| Heart disease | 8 (15.4) | 2(33.3) | 0.27 |
| Laboratory data | |||
| Leukocyte count (mm−3) | 9,694 ± 3,426 | 14,833 ± 9,559 | 0.17 |
| Erythrocyte sedimentation rate (mm/h) | 84.8 ± 23.2 | 86.1 ± 24.3 | 0.45 |
| Blood culture (positive) | 40 (76.9) | 6 (100) | 0.33 |
| Surgical intervention | 31 (59.6) | 4 (66.7) | 1 |
| Mortality (in hospital) | 16 (30.8) | 2 (33.3) | 1 |
Table 2.
Isolated microorganism and image features of 58 patients with mycotic aneurysm
| Variables | Mycotic aneurysm without infective spondylitis, N = 52 (%) | Mycotic aneurysm with infective spondylitis, N = 6 (%) | P level |
|---|---|---|---|
| Isolated microorganism | |||
| Gram-negative bacteria | |||
| Salmonella sp. | 18 (34.6) | 4 (66.6) | 0.19 |
| Klebsiella pneumoniae | 6 (11.6) | 0 | 1 |
| Escherichia coli | 2 (3.8) | 0 | 1 |
| Enterobacter sp. | 1 (1.9) | 0 | 1 |
| Burkholderia pseudomallei | 1 (1.9) | 0 | 1 |
| Gram-positive bacteria | |||
| Staphylococcus aureus | 6 (11.6) | 1 (16.6) | 0.56 |
| Viridans Streptococcus | 5 (9.6) | 0 | 1 |
| Staphylococcus saprophyticus | 1 (1.9) | 0 | 1 |
| Streptococcus group A | 1 (1.9) | 1 (16.6) | 1 |
| Streptococcus group D | 1 (1.9) | 0 | 1 |
| Propionibacterium sp. | 1 (1.9) | 0 | 1 |
| Image features | |||
| End-plate destruction on radiography | 0 | 3 (50) | <0.001 |
| Vertebral body collapse on radiography | 8 (15.4) | 2 (33.3) | 0.27 |
| Psoas abscess on computed tomography | 0 | 5 (83.3) | <0.001 |
Table 3.
Summary of clinical details, image studies, surgical methods and outcome of six patients with spontaneous infective spondylitis and mycotic aneurysm
| Case no. | Age (years)/sex | Underlying diseases | Clinical symptoms | Symptom duration | WBC count (mm3) | Lesion site | Radiography | CT or MRI | Pathogen | Surgical methodsb | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60/M | Diabetic mellitus | Back pain with fever | 8 weeks | 8,100 | T12–L1 | Endplate destruction (+) Vertebral body collapse (+) |
Prevertebral mass (+) left psoas abscess (+) | Salmonella choleraesuis | A + A′ + A″ + P | Survived, ambulation without aid |
| 2 | 50/M | Diabetic mellitus | Back pain with fever | 2 weeks | 12,800 | L3–L4 | Endplate destruction (−) Vertebral body collapse (−) |
Bilateral psoas abscess (+) | Salmonella choleraesuis | A + A′ | Survived, ambulation without aid |
| 3a | 79/M | CHF, Hypertension Adrenal insufficiency |
Back pain, abdominal pulsating mass, lower limb weakness (grade 3 of 5 motor strength) | 1 week | 12,600 | L3–L4 | Endplate destruction (−) Vertebral body collapse (−) |
Bilateral psoas abscess (+) |
Salmonella enteriditis Mycobacterium tuberculosis |
A + A′ + A″ + P | Survived, ambulation with wheelchair |
| 4 | 72/M | CHF, COPD | Back pain, fever, abdominal pulsating mass, lower limb weakness (grade 4 of 5 motor strength) | 3 weeks | 15,600 | L3–L4 | Endplate destruction (−) Vertebral body collapse (−) |
Bilateral psoas abscess (+) | Salmonella choleraesuis | A + A′ | Survived, ambulation with crutches |
| 5 | 81/M | Old stroke, COPD | Back pain, abdominal pain and fever | 4 weeks | 13,800 | L5 | Endplate destruction (+) Vertebral body collapse (+) |
Bilateral psoas abscess (+) | Streptococcus pyogenes | None | Died (rupture of aneurysm) |
| 6 | 59/F | ESRD, HCVD | Back pain, paraplegia and fever | 1 week | 12,500 | T7 | Endplate destruction (+) Vertebral body collapse (−) |
Paravertebral mass (+) | Staphylococcus aureus | None | Died (septic shock) |
Abbreviations: CHF congestive heart failure, COPD chronic obstructive pulmonary disease, CT computed tomography, ESRD end-stage renal disease, HCVD hypertensive cardiovascular disease, MRI magnetic resonance imaging, WBC white blood cell
aSalmonella enteriditis mycotic aneurysm and mycobacterium tuberculosis spondylitis
bSurgical methods: A: anterior approach for aneurysm resection, A′: A with psoas abscess debridement and sequestrectomy, A″: A′ + interbody fusion, P: posterior approach with internal instrumentation
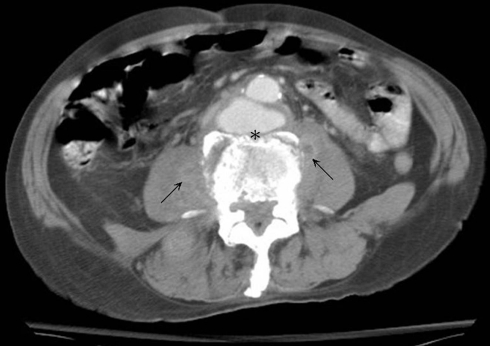
Fig. 1.
In Case 4, enhanced computed tomography of the abdomen showing infra-renal mycotic aneurysm, paravertebral soft tissue swelling (arrow) and unclear soft tissue plane between aorta and vertebra (asterisk)
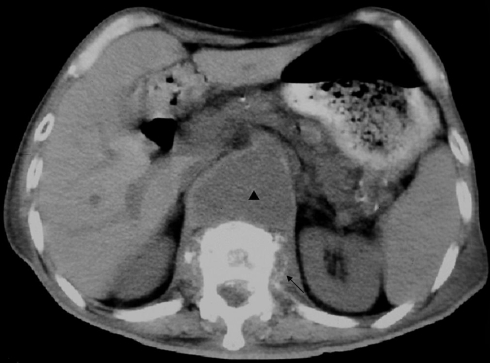
Fig. 2.
While surveying for spine infection in Case 1, non-enhanced spine computed tomography showing a prevertebral mass (arrowhead) and left psoas abscess (arrow)
Among these six patients, two did not receive surgical interventions because their unstable clinical conditions or the complexity of the mycotic aneurysm made surgery risky or non-feasible. They died of septic shock related to delayed diagnosis and high level lesion involved or aneurysm rupture separately. The choice of surgical procedures to achieve infection control depended on the patient’s general condition and consecutive imaging studies. On the basis of the preoperative impression of a retained mycotic aneurysm adhering to spondylitis in imaging studies, a surgical intervention with anterior approach for aneurysm resection, debridement, sequestrectomy, and interbody fusion of infected spine was performed to avoid massive bleeding during the initial management of vertebral damage (Case 1). He received posterior instrumented fusion later on and got satisfactory outcome. Conversely, when the patient underwent aneurysm resection, aggressive debridement of the tissue neighboring an aortic lesion would be performed (Cases 2, 3 and 4), although no vertebral bone destruction was seen in the initial images. The aortic bypass graft along with debridement of the paraaortic area was carried out without spinal fusion. A small piece of chipped bone in the footprint hole of the vertebral body was found and curetted during resection of the aortic aneurysmal wall. Case 2 patient experienced a smooth postoperative course. However, Case 4 patient underwent similar procedures but he suffered from persistent back pain, claudication, and focal kyphosis due to endplate erosion and collapse later. This experience of Case 4 patient emphasizes that further spinal intervention for stenosis and deformity should be undertaken after infection control. As we mentioned recently [3], Case 3 patient underwent aortic reconstruction of mycotic aneurysm, debridement of psoas abscess, and antibiotic therapy for Salmonella enteritidis bacteremia. However, he experienced persistent back pain, and decreased grade 3/5 muscle strength of the lower legs postoperatively. The follow-up MRI scan showed progression of L3–4 spondylitis and anterior columnar collapse. Eventually, he underwent sequestrectomy of L3–4, anterior interbody fusion and subsequently posterior instrumented fusion. The spinal tissue culture yielded Mycobacterium tuberculosis. Under this circumstance, the possibility of different etiologies should be considered especially in vulnerable patients with a poor clinical response to appropriate antimicrobial therapy.
Discussion
The clinical non-specificity of mycotic aneurysm or infective spondylitis makes diagnosis in the affected patients challenging. Although neurological deficit was statistically significant in patients with spontaneous infective spondylitis with mycotic aneurysm, this unique clinical characteristic always was noted in late stages of infective spondylitis [14]. Clinicians should keep in mind and not forget this rare clinical entity. The peculiar findings of image study in our report might be helpful for early diagnosis of coexisting infective spondylitis in patients with mycotic aneurysm.
Salmonella spp., the most common pathogen in mycotic aneurysm with or without infective spondylitis, contributes to exhibit a tendency to cause blood–borne infection in previously damaged arteries and invade the neighboring spine [4, 7, 11]. The primary site of infection in these patients with concurrent spondylitis and mycotic aneurysm may be either the aorta or the spine, with subsequent involvement of adjacent tissues. Once the process begins in the aorta, the infection can attach to the ulcerated artherosclerotic plaques, then deposit thrombi, or by contiguous spread from a retroperitoneal abscess. The vasa vasorum of the arterial wall will be destroyed, followed by contained rupture with repeated hemorrhage, increasing pressure and occlusion of lumbar arteries, which cause damage to the vertebrae [12]. Once the process develops in the spine, exudates that accumulate in the vertebral body can contaminate the intervertebral disc, destroy the endplate, encroach on the spinal canal, and spread through the anterior longitudinal ligament to involve adjacent tissues and result in psoas or paravertebral mass. Although MRI scan is the most sensitive, specific and accurate imaging tool for a prompt detection of infective spondylitis, bone or disc destruction and abscess formation may be obscured in the early stages of infection [2]. It might not easily distinguish prevertebral mass from pseudoaneurysm, which is secondary to rupture of the mycotic aneurysm [6, 13]. Enhanced CT for mycotic aneurysm can reveal prevertebral soft tissue involvement as periaortic fluid, gas, or hematomas, and abnormalities of saccular aneurysm with initial disruption and/or an unusual luminal shape. The presence of psoas abscess, paravertebral mass or unclear soft tissue plane between the aorta and vertebral body might suggest that spine is infected [8]. As the above results, spine MRI study should be performed in all mycotic aneurysm cases of suspected paravertebral infection. However, the extent of related bony destruction is better defined on CT images than on MRI, and only resorting to complete image studies can help surgeons to perform adequate debridement and reconstruction. Conversely, acknowledgment of the contained aortic aneurysm with enhanced CT study may help surgeons to avoid life-threatening hemorrhage during spinal operation.
Surgical treatment of pyogenic spondylitis is indicated when there is an evidence of osseous involvement, neurological deficits, and clinical unresponsiveness to antibiotics, refractory infection, or failure of biopsy to provide a firm diagnosis [5, 14]. There were no reports on surgical treatment guideline for coexisting infective spondylitis and mycotic aneurysm. In our study, patients who did not receive surgery died from uncontrolled sepsis or rupture of aneurysm. Whatever, Müller et al. [9] even described successful surgical treatment for mycotic aneurysm, resulting in poor clinical outcome because of the weakened health status of these vulnerable patients. Eradication of the infectious sources is the only way to successfully treat patients with concomitant infective spondylitis and mycotic aneurysm. Favorable results in the management of infective spondylitis and mycotic aneurysm are achieved with a circumscribed resection of the aorta, removal of all infected tissue including the bony lesion, replacement of a prosthetic interposition graft, and establishment of the spinal columns. It emphasized in selective reconstruction that both vascular and orthopedic surgeon should be available in the operation room to complete removal of aneurysm, vertebral debridement and reconstruction in one stage of infection eradication. In our experience, aggressive debridement of the vertebral body should be performed to obtain the pathogen if any evidence of vertebral invasion. If the spinal infection results in osseous instability, the addition of anterior interbody fusion is required. The decompression and posterior instrumented fusion were suggested as a staged procedure to achieve a better osseous stability after the infection had been controlled.
Conclusions
Spontaneous infective spondylitis and mycotic aneurysm is rare, easily overlooked and potentially fatal. Paravertebral soft tissue swelling, presence of psoas abscess and/or unclear soft tissue plane between the aorta and vertebral body in relation to mycotic aneurysm may indicate a concomitant infection in the spine. Aggressive surgical debridement and curettage of bone lesions are needed, although there may be normal findings in the initial spinal radiographs. If prevertebral mass was discovered on survey for spine infection, coexisting mycotic aneurysm should be considered to avoid massive bleeding during the management of vertebral destruction.
References
- 1.Chang IC. Salmonella spondylodiscitis in patients without sickle cell disease. Clin Orthop Relat Res. 2005;430:243–247. doi: 10.1097/01.blo.0000137561.82099.d5. [DOI] [PubMed] [Google Scholar]
- 2.Chang MC, Wu HT, Lee CH, et al. Tuberculous spondylitis and pyogenic spondylitis: comparative magnetic resonance imaging features. Spine. 2006;31(7):782–788. doi: 10.1097/01.brs.0000206385.11684.d5. [DOI] [PubMed] [Google Scholar]
- 3.Chen SH, Wong T, Kuo FY, et al. Tuberculous spondylitis and salmonella mycotic aneurysm in an immunocompromised patient. Bone Joint Surg Am. 2006;88(10):2275–2278. doi: 10.2106/JBJS.E.01121. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JI, Bartlett JA, Corey GR. Extra-intestinal manifestations of Salmonella infections. Medicine. 1987;66(5):349–388. doi: 10.1097/00005792-198709000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Dimar JR, Carreon LY, Glassman SD, et al. Treatment of pyogenic vertebral osteomyelitis with anterior debridement and fusion followed by delayed posterior spinal fusion. Spine. 2004;29(3):326–332. doi: 10.1097/01.BRS.0000109410.46538.74. [DOI] [PubMed] [Google Scholar]
- 6.Doita M, Marui T, Kurosaka M, et al. Contained rupture of the aneurysm of common iliac artery associated with pyogenic vertebral spondylitis. Spine. 2001;26(13):e303–e307. doi: 10.1097/00007632-200107010-00027. [DOI] [PubMed] [Google Scholar]
- 7.McHenry MC, Rehm SJ, Krajewski LP, et al. Vertebral osteomyelitis and aortic lesions: case report and review. Rev Infect Dis. 1991;13(6):1184–1194. doi: 10.1093/clinids/13.6.1184. [DOI] [PubMed] [Google Scholar]
- 8.Muckley T, Schutz T, Kirschner M, et al. Psoas abscess: the spine as a primary source of infection. Spine. 2003;28(6):e106–e113. doi: 10.1097/00007632-200303150-00021. [DOI] [PubMed] [Google Scholar]
- 9.Müller BT, Wegener OR, Grabitz K, et al. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg. 2001;33(1):106–113. doi: 10.1067/mva.2001.110356. [DOI] [PubMed] [Google Scholar]
- 10.Rubery PT, Smith MD, Cammisa FP, et al. Mycotic aortic aneurysm in patients who have lumbar vertebral osteomyelitis. a report of two cases. J Bone Joint Surg Am. 1995;77(11):1729–1732. doi: 10.2106/00004623-199511000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Santos EM, Sapico FL. Vertebral osteomyelitis due to Salmonellae: report of two cases and review. Clin Infect Dis. 1998;27(2):287–295. doi: 10.1086/514668. [DOI] [PubMed] [Google Scholar]
- 12.Soravia-Dunand VA, Loo VG, Salit IE. Aortitis due to Salmonella: report of 10 cases and comprehensive review of the literature. Clin Infect Dis. 1999;29(4):862–868. doi: 10.1086/520450. [DOI] [PubMed] [Google Scholar]
- 13.Sugawa M, Tanaka R, Nakamura M, et al. A case of infectious pseudoaneurysm of the abdominal aorta associated with infectious spondyliotis due to Klebsiella pneumoniae. Jpn J Med. 1989;28(3):402–405. doi: 10.2169/internalmedicine1962.28.402. [DOI] [PubMed] [Google Scholar]
- 14.Swanson AN, Pappou IP, Cammisa FP, et al. Chronic infections of the spine: surgical indications and treatments. Clin Orthop Relat Res. 2006;444:100–106. doi: 10.1097/01.blo.0000203447.44146.55. [DOI] [PubMed] [Google Scholar]
- 15.Woo SB, Cheng LC, Wong WC. Mycotic aortic aneurysm following treatment of pyogenic vertebral osteomyelitis. Asian Cardiovasc Thorac Ann. 2006;14(5):e102–e105. doi: 10.1177/021849230601400531. [DOI] [PubMed] [Google Scholar]