Abstract
Degenerative spondylolisthesis (DS) is a disorder that causes the slip of one vertebral body over the one below due to degenerative changes in the spine. Lumbar DS is a major cause of spinal canal stenosis and is often related to low back and leg pain. We reviewed the symptoms, prognosis and conservative treatments for symptoms associated with DS. PubMed and MEDLINE databases (1950–2007) were searched for the key words “spondylolisthesis”, “pseudospondylolisthesis”, “degenerative spondylolisthesis”, “spinal stenosis”, “lumbar spine”, “antherolisthesis”, “posterolisthesis”, “low back pain”, and “lumbar instability”. All relevant articles in English were reviewed. Pertinent secondary references were also retrieved. The prognosis of patients with DS is favorable, however, those who suffer from neurological symptoms such as intermittent claudication or vesicorectal disorder, will most probably experience neurological deterioration if they are not operated upon. Nonoperative treatment should be the initial course of action in most cases of DS, with or without neurologic symptoms. Treatment options include use of analgesics and NSAIDs to control pain; epidural steroid injections, and physical methods such as bracing and flexion strengthening exercises. An up-to-date knowledge on diagnosis and prevention of lumbar DS can assist in determination of future research goals. Additional studies are required to establish treatment protocols for the conservative treatment of DS.
Keywords: Degenerative spondylolisthesis, Diagnosis, Treatment, Lumbar spine
Introduction
Degenerative spondylolisthesis (DS) is a disorder that causes the slip of one vertebral body over the one below. It differs from spondylolytic spondylolisthesis by the absence of a pars interarticularis defect (spondylolysis), i.e., in DS the whole upper vertebra (vertebral body and posterior part of the vertebra including neural arch and processes) slips relative to the lower vertebra. In a congenital spondylolisthesis, sometimes due to dysplastic facets with intact pars interarticularis the whole upper vertebra, can slip forward and cause spinal stenosis with potential impingement of cauda equine or spinal nerve roots. However, symptoms in this type of listhesis usually develop during the adolescent growth period, and not as in DS, where symptoms develop in patients older than 40. DS can be found in classifications of spondylolisthesis, spinal stenosis, and segmental instability, indicating that the clinical presentation is varied [19]. The degree of slip is usually mild, with a mean slip of 14% reported in a study of 200 patients [50]. As the neural arch is intact, even a small progression in the slip can result in cauda equina compression. The focus of this review is symptoms and treatments of DS because it has traditionally been considered as one of the major causes of low back pain (LBP) among the elderly and is a major cause of spinal canal stenosis related to low back and leg pain [1, 16, 19, 24]. Other types of spondylolisthesis and surgical treatments will not be discussed.
Methods
PubMed and MEDLINE databases (1950–2007) were searched for the key words “spondylolisthesis”, “degenerative spondylolisthesis”, “spinal stenosis”, “lumbar spine”, “antherolisthesis”, “posterolisthesis”, “low back pain”, “lumbar instability”, “treatment”, “exercises”, “bracing”, “imaging”, and “pseudospondylolisthesis”. All relevant articles in English were reviewed. Pertinent secondary references were also retrieved. We critically analyzed all the published materials. We are aware that this traditional approach to narrative reviews has much more potential for bias than systematic reviews or meta-analyses; however, we endeavored to be inclusive and open-minded. We also consulted experts in orthopedic surgery, rheumatology and radiology to produce this narrative review on lumbar degenerative spondylolisthesis.
Results
Degenerative spondylolisthesis is the result of longstanding intersegmental instability at the lumbar motion segment [15]. Patients presenting with DS generally are older than 50 years and may have any combination of LBP, neurogenic claudication, vesicorectal disorder, and radiculopathy [59]. The etiology of DS is multifactorial, and it is interlinked with other pathologies, such as, for example, disk degeneration, facet joint osteoarthritis and spinal stenosis. The major local reasons that probably lead to the development of degenerative vertebral slippage are: (1) arthritis of the facet joints with loss of their normal structural support; (2) malfunction of the ligamentous stabilizing component, probably due to hyperlaxity; and (3) ineffectual muscular stabilization. There are controversial evidences about involvement if disc degeneration in etiology of DS. The general belief today is that disc degeneration leads to segmental instability in the sagittal plane and may result in DS [51]. As in other degenerative disorders of the spine, potential risk factors can comprise increasing age >50, female sex, pregnancies, African–American ethnicity, generalized joint laxity, and anatomical predisposition (sagittally oriented facet joints, hyperlordosis, high pelvic incidence).
Degenerative spondylolisthesis occurs mostly at the L4–5 level [15, 19] as opposed to its isthmic counterpart, which occurs most often at the lumbosacral level (L5–S1). The L4–L5 vertebral space is affected 6–9 times more commonly than other spinal levels. The reason for this specific location is mainly the ilio-lumbar ligaments that strongly keep L5 on its anatomical position [2].
Symptoms associated with degenerative spondylolisthesis
The most probable sources for signs and symptoms related to DS are: (1) degenerated and subluxated facet joints; (2) segmental instability that cause tension of facet joint capsule and ligaments as well as overuse of stabilization muscles; and (3) spinal stenosis and intervertebral foramen stenosis. Lumbar DS is a major cause of spinal canal stenosis and is often related to low back and leg pain [24, 36]. The most common complaint of patients with DS is back pain. Often the pain has been episodic and recurrent for many years [19]. As is the case with all mechanical back pain, patients usually report that their symptoms vary as a function of mechanical loads (such as in going from supine to erect position) imposed, and pain frequently worsens over the course of the day. Radiation into the posterolateral thighs is also common and is independent of neurologic signs and symptoms. The advent of leg symptoms is the most common reason why patients and referring physicians become truly concerned and seek specialized medical attention. The pain may be diffuse in the lower extremities, involving the L5 and/or L4 roots unilaterally or bilaterally. One of the most characteristic symptoms of DS with stenosis is leg pain that shifts from side to side. One time the patient will complain of neurogenic pain in one leg and another time will complain of the opposite leg. Monoradiculopathy is the less common type of leg pain; when present, it is the result of entrapment of the L5 root in the lateral recess. Another common pain presentation is that of neurogenic claudication. Symptoms of neurogenic claudication that cause the patient to stop and sit after less than two blocks of walking usually correspond to the time, when the patient consents to surgery. These symptoms of spinal stenosis are reported by 42–82% of patients who seek help from orthopedists [18]. Additional complaints include cold feet, altered gait, and “drop episodes,” wherein the patient unexpectedly falls while walking [18].
With extreme stenosis, interference with bladder and bowel control can occur, as was reported by Kostuik et al. [32] in their patients. Unlike the acute and often devastating bladder and bowel symptoms of cauda equina syndrome in lumbar disk herniation, spinal stenosis often has an insidious and subtle presentation. Stenotic symptoms are the result of mechanical and vascular factors. As the slip progresses, facet hypertrophy, buckling of the ligamentum flavum, and diffuse disk bulging contribute with the forward displacement to compression of the cauda equina. As in all stenotic conditions, the relief of symptoms that follows forward spinal flexion is thought to be related to the increase in the anteroposterior dimensions of the spinal canal that occurs in that posture. At the extreme, patients may report the need to sleep in the fetal position to relieve leg symptoms. The significant vascular component in complaints of leg pain may lead to another manifestation, restless legs syndrome, sometimes called “vespers curse” [33]. In this condition, patients are awakened by aching pain in the calves, restlessness, an irresistible urge to move the legs, and fasciculations. This syndrome is reported to be exacerbated by congestive heart failure, which, in turn, may increase pressure in the arteriovenous anastomoses that characterize the lumbar nerve-root microcirculation. Other associated neurological symptoms, such as numbness and weakness, are variably present.
Diagnostic modalities
The primary role of imaging studies is to confirm the clinical diagnosis of DS, although advanced imaging studies are also essential for preoperative planning. The plain radiographic features include the essential finding on a lateral view of forward displacement of L4 on L5 or, more rarely, L5 on S1 or L3 on L4 in the presence of an intact neural arch. Defect of pars interarticularis (which has the appearance of a Scottie dog with a collar) that can be seen on lateral or bilateral oblique views helps to distinguish between DS and isthmic spondylolisthesis. Since in DS the neural arch is intact, the spinous process moves forward with the vertebral body. This results in malalignment of the spinous processes, which can be identified on lateral radiographs [7]. The additional findings are usually consistent with a long-standing degenerative process and include disk-space narrowing, vacuum sign, endplate sclerosis, peridiscal osteophytes, and facet sclerosis and hypertrophy. The anteroposterior radiograph often, but not always, demonstrates the accompanying hemisacralization of L5 [19].
Additional imaging studies may be warranted depending on the patient’s presentation and the clinical findings. Factors that speak about the need for further imaging include significant and progressing neurologic claudication or radiculopathies and the clinical suspicion that another condition, such as metastatic disease, may be causative. An absolute indication is the presence of bladder or bowel complaints [7].
The imaging alternatives include computed tomography (CT), myelography, contrast material-enhanced CT, and magnetic resonance imaging (MRI). CT of the spine can be performed with or without intrathecal contrast enhancement. Axial images are obtained in a plane parallel to the disk spaces at each level imaged. Sagittal reconstruction images are also obtained by using post-acquisition processing software. Bone window (e.g., 1,500/300 HU) and soft-tissue window (e.g., 300/30 HU) settings are used. CT shows the alignment of the facet joints and their degenerative changes. Asymmetrical slip of the facets results in a rotational component to the spondylolisthesis. In patients with signs and symptoms consistent with spinal stenosis, MRI or postmyelographic CT is needed to confirm neural element compression [26]. Until the advent of MR imaging, the most widely utilized radiologic technique for evaluating spinal stenosis was myelography in combination with CT. On the myelogram, nerve root entrapment in the lateral recess or central canal stenosis is demonstrated by the level of cutoff of contrast material. The postmyelographic CT images can then be used to identify the bone or soft tissue at each level that must be removed for decompression. There are several drawbacks in using CT imaging. One is relatively high dose of radiation, and also the fact that usually only the three lower segments are depicted and the possible stenosis above these levels will not be visualized.
The role of CT and CT myelography in the assessment of neurological symptoms in DS has been largely replaced by MRI. MRI is a noninvasive technique that can also define vertebral slippage and neural element compression through cross-sectional axial and sagittal imaging. This very sensitive method of evaluation should be used to confirm a clinical diagnosis of DS. Regardless of the imaging study chosen, the typical findings are a significant constriction of the cauda equina associated with a diminished cross-sectional area of the vertebral canal, apparent thickening and buckling of the ligamentum flavum, and hypertrophy of adjacent facet joints. All of these factors contribute to the symptoms of spinal stenosis [19]. The facet joint degenerative changes are shown by the presence of osteophytes and cartilage loss. Stenosis in the inferior aspect of the lateral recess (intervertebral foramen) is caused by the ventral slip of the superior articular processes. In the case of L4/5 DS, this results in compression of the L5 nerve roots at the level of the L5 pedicle. As a result of the slip, the intervertebral foramen at the involved level assumes a more horizontal configuration, resulting in reduced foraminal height. This, together with bulging of the degenerated disc into the foramen, may result in foraminal stenosis. In this case L4/5 DS can cause L4 root compression [7].
Sometimes, in a case of dynamic DS the vertebral slipping cannot be seen on the standard supine radiographs or MRI. Jayakumar et al. [28] in case series have shown that axial loaded MRI identified occult dynamic DS, which correlated with the clinical picture but was not shown on initial conventional MRI or plain radiography. The presence of facet joint synovial cysts (facet ganglia), which have a recognized association with degenerative spondylolisthesis, can also cause narrowing of the lateral recesses and are well shown on MRI [3].
Additional studies that may be selected include technetium bone scanning, particularly when a metastatic tumor is suspected, and electrodiagnostic studies if a systemic neurologic disorder is a possibility.
Grading of spondylolisthesis
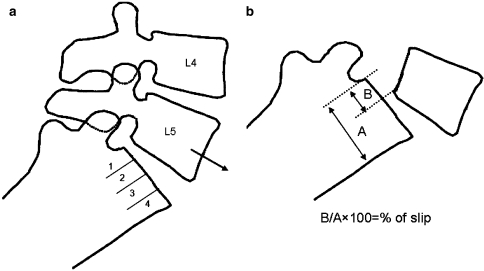
Regardless of the imaging method and the type of spondylolisthesis, the forward slip of the vertebra above can be measured by one of two methods [62]. The first is the method of Meyerding [40]. The anteroposterior (AP) diameter of the superior surface of the lower vertebral body is divided into quarters and a grade of I–IV is assigned to slips of one, two, three or four quarters of the superior vertebra, respectively. The second method, first described by Taillard [57], expresses the degree of slip as a percentage of the AP diameter of the top of the lower vertebra (Fig. 1). Complete slip of L5 on S1 is termed spondyloptosis. The second method is favored by most authors as it is more accurately reproducible [7]. Measurement of the slip and its apparent progression, however, should be viewed with caution. Studies have shown that there can be inter- and intra-observer error of up to 15%. This variation can increase if there is an element of rotation. Therefore, only a progression of greater than 20% slip can be reliably assessed [10, 11].
Fig. 1.
Scheme of spondylolisthesis grading methods: a Meyerding; b Taillard
Prognosis
The natural history of DS generally is favorable. Only 10–15% of patients seeking treatment eventually will have surgery [45]. In a long term follow-up study of 145 non-surgically managed patients [38] with DS progression of vertebral slippage was observed in 34% of patients. There was no correlation between changes in clinical symptoms and progression of spondylolisthesis. The intervertebral spaces of the slipped segments were decreased significantly in size during follow-up examination in patients in whom no progression was found. LBP improved following a decrease in the total intervertebral space size. The development of osteoarthritic spurs, hypertrophy and ossification of the intervertebral ligaments, and facet arthrosis may lead to secondary stabilization that prevents slip progression. Seventy-six percent of patients who had no neurological deficits at initial examination remained without neurological deficit after 10 years of follow-up. Eighty-three percent of the patients who had neurological symptoms at the baseline (N = 35), such as intermittent claudication or vesicorectal disorder, at initial examination and refused surgery experienced neurological deterioration. The final prognosis for these patients was very poor. However, Johnsson et al. [29] followed-up 32 untreated patients with clinical symptoms and myelographically confirmed spinal stenosis (nine of them with DS) for an average of 49 months. About 75% of the patients had spinal claudication. In the follow-up survey, the same number of patients had claudication, but the symptoms were milder. In estimation by visual analog scale, symptoms in 70% of the cases were unchanged, 15% showed improvement, and 15% worsened. No proof of severe deterioration was found after 4 years.
Treatment options
The study of the natural history [38] showed that back pain improved as the disc space was collapsed and progression of the slippage occurred only in 34% of the cases. Seventy-six percent of the patients who were initially neurologically intact did not deteriorate over time and these patients may be treated conservatively. Conversely, most patients (83%) with history of neurogenic claudication or vesicorectal symptoms deteriorated with poor final outcome and these patients should preferably have surgical treatment [38]. Recent study of Weinstein et al. [61] found that patients with DS and spinal stenosis treated surgically showed substantially greater improvement in pain and function during a period of 2 years than patients treated nonsurgically.
A review by Herkowitz et al. [24] suggested that the indications for surgical treatment are:
Persistent or recurrent back and/or leg pain or neurogenic claudication, with significant reduction of quality of life, despite a reasonable trial of non-operative treatment (a minimum of 3 months).
Progressive neurological deficit.
Bladder or bowel symptoms.
There are a number of publications about surgical treatment of signs related to DS [14, 21, 30, 37, 39, 47, 61] including the recent comprehensive review of Sengupta and Herkowitz [51]. In the present review we concentrated on conservative treatment options and would encourage those who are interested in surgery to read these other articles.
Nonoperative treatment is the mainstay of treatment for LBP and should be the initial course of action in most cases of spondylolisthesis, with or without neurologic symptoms [59]. There are, however, no prospective, randomized clinical trials establishing an optimal nonoperative treatment protocol. According to Vibert et al. [59] most physicians begin with a 1- to 2-day period of rest followed by a short course of anti-inflammatory medications, if they are not contraindicated for gastrointestinal reasons. If symptoms persist beyond 1–2 weeks, physical therapy can be applied. Stationary bicycling is an excellent exercise because it promotes spine flexion, deconstriction of the thecal sac, and allows for more exercise before the development of neurogenic claudication is present. Furthermore, bicycling allows the patient to avoid the wear and tear associated with impact aerobic exercise such as running. Swimming, walking, and elliptical machines are other good alternatives for cardiovascular exercise [59], albeit there is no evidence of their value for DS.
Frymoyer [19] more than two decades ago suggested a similar treatment program: (1) nonsteroidal anti-inflammatory drugs (NSAIDs) (in the elderly, there should be careful monitoring for gastrointestinal complaints and melena); (2) encouragement of aerobic conditioning, on the premise that this exercise may improve arterial circulation to the cauda equina (because walking often aggravates symptoms, a stationary bicycle is a good alternative, particularly if the handlebars and seat are set up to allow the forward-flexed posture); (3) weight reduction, although this strategy often minimally affects neurologic complaints; and (4) careful management of osteoporosis.
Taking into account the gastrointestinal side effects of NSAIDs, cardiovascular side effects of COX-2 inhibitors and the fact that the DS population comprised mostly from elderly people, acetaminophen or other not-NASID analgesics should be the first choice for initial management of DS. NSAIDs can be part of the initial management of symptomatic DS, however, they are equally efficacious compared to acetaminophen, which only provides pain relief [58]. At lower doses, the analgesic effect reduces musculoskeletal pain; at higher doses, NSAIDs provide an anti-inflammatory effect on nerve root and joint irritation. Unfortunately, many elderly patients cannot tolerate the gastrointestinal and renal side effects. There is no evidence that one non-steroidal agent is more effective for LBP than another, but cyclooxygenase (COX)-2 selective agents had been recommended in older individuals because of fewer gastrointestinal side effects. Another option is enteric-coated aspirin, which may be as effective, at lower cost and with fewer gastrointestinal side effects. Patients taking any of these medicines should have their hepatic and renal function monitored.
For pain inadequately controlled with acetaminophen or NSAIDs, opioids and muscle relaxants are commonly prescribed for LBP even though they have not been shown to be more effective than acetaminophen and NSAIDs in well-controlled studies [58]. Because the main symptoms of DS are caused by spinal stenosis and degenerative changes in facet joints, the use of muscle relaxants makes less inherent sense, although there is no evidence arguing that the relative risks and benefits favor other agents.
Vilbert et al. [59] suggested that if patients fail a reasonable course of therapy (4–6 weeks), they may benefit in the short term from a course of epidural steroid injections (ESI). ESI involves delivery of a corticosteroid preparation, such as methylprednisolone, around the stenotic cauda equina and nerve roots in order to relieve LBP, lower extremity pain related to radiculopathy and neurogenic claudication. No authors have evaluated the effectiveness of epidural steroid injections in patients with DS alone. However, authors of a long-term follow-up study of patients having a course of injections failed to reveal a lasting benefit (beyond 3 years) in patients with degenerative disc disease, herniated discs, lumbar radicular pain, or spinal stenosis [8, 9, 20, 49, 60]. However, a two studies of patients with spinal stenosis [12, 48] demonstrated a significant (P < 0.05) improvement in short-term benefits (decreasing of pain, improvement of functional measures and lower operative rate) after ESI. In a study restricted to patients with symptomatic lumbar stenosis, Hoogmartens and Morelle [27] found that 48% of patients treated with ESI demonstrated functional improvement from their preinjection status approximately 2 years after treatment. However, the patients were evaluated retrospectively and were not compared with a control group. Furthermore, the authors conceded that the improvement rate was close to that of the placebo effect. Nevertheless, they suggested that ESI is a good alternative to surgical treatment in older patients with medical comorbidities.
Physical rehabilitation methods
Physiotherapy is the most common method used to apply non-operative treatment of symptoms associated with DS. Therapeutic protocols may include the use of modalities for pain relief, bracing, exercise, ultrasound, electrical stimulation, and activity modification [13, 44, 55, 56]. Unfortunately, some of the evidences for effectiveness of physical rehabilitation methods are coming from case reports [13, 44] and cannot be generalized to the rest of the population. Physiotherapy treatment is recommended to reduce pain [56], to restore range of motion and function, and to strengthen and stabilize the spine [17, 23] and restore mobility of the neural tissue [6]. However, no study has yet shown their usefulness in patients with DS. Further, we review studies that mostly investigated treatment options for other types of spondylolisthesis, spinal segmental instability and spinal stenosis in attempt to understand their rationale and to apply it to treatment of DS.
Bracing
We did not find any studies that specifically evaluated brace treatment for symptoms associated with DS. However, Prateepavanich et al. [46] evaluated the effectiveness of a lumbosacral corset in a self controlled comparative study on 21 patients (mean age 62.5) with symptomatic degenerative lumbar spinal stenosis (neurogenic claudication). Patients treated with the corset showed a statistically significant improvement in walking distance and decrement of pain score in daily activities in comparison with patients who did not wear the corset. Because most patients with symptomatic DS suffer from neurogenic claudication, use of bracing needs to be examined for treatment of patients with DS. The other rationale to use bracing in patients with DS is to decrease segmental spinal instability, although it is not a main pain generator in DS. Bell et al. [5] showed that adolescents with grade I and II isthmic spondylolisthesis who received brace treatment for 25 month were pain-free and none had demonstrated a significant increase in slip percent. In addition, patients with lateral recess stenosis with impingement of the nerve root can potentially benefit from a brace that prevents rotation.
Spratt et al. [54] evaluated the influence of combined treatment of bracing, exercises and education controlling either flexion or extension postures on patients with radiographic instability. Fifty-six patients meeting strict study inclusion and radiographic evaluation criteria were assigned to a bracing treatment (flexion, extension, placebo-control) according to a randomization scheme, designed to ensure equal representation of translation categories (retrolisthesis, normal, spondylolisthesis) across treatment groups, and assessed at admission and 1-month follow-up. This study did not make any conclusions about effectiveness of bracing for instability, but authors found that brace treatments (that was done in combination with exercises) were not shown to reduce patient range of motion or lessen trunk strength.
Flexion/extension strengthening exercises
Sinaki et al. [53] divided 48 patients with LBP secondary to spondylolisthesis into two groups: those doing flexion and those doing extension back strengthening exercises. All patients received instructions on posture, lifting techniques, and the use of heat for relief of symptoms. After 3 months, only 27% of patients who were instructed in flexion exercises had moderate or severe pain and only 32% were unable to work or had limited their work. Of the patients who were instructed in extension exercises, 67% had moderate or severe pain and 61% were unable to work or had limited their work. At 3-year follow-up, only 19% of the flexion group had moderate or severe pain and 24% were unable to work or had limited their work. The respective figures for the extension group were 67 and 61%. The overall recovery rate after 3 months was 58% for the flexion group and 6% for the extension group. At 3-year follow-up these figures improved to 62% for the flexion group and dropped to 0% for the extension group. On the basis of these findings, Sinaki et al. [53] suggested that if a conservative treatment program is elected, back flexion or isometric back strengthening exercises should be considered. In a study of Gramse et al. [22] 47 patients with symptomatic back pain secondary to spondylolisthesis who were not surgical candidates were instructed in a treatment program that included flexion or extension or combined flexion-extension exercises. They found that patients treated with flexion-type exercises were less likely to require use of back supports, require job modification, or limit their activities because of pain. To some extent the results of these studies may be explained by results of Penning and Wilmink [43] study, who found narrowing of the spinal canal in extension and widening of the canal with relief of nerve root involvement in flexion.
Specific muscular and biomechanical impairments have been identified in people with spinal stenosis, including paraspinal muscle denervation [34] and trunk extensor muscle function [31]. Such findings suggest that non-surgical physical interventions possibly should include exercises specifically directed toward the spinal extensor muscle group, but taking into account results of Sinaki et al. [53] study it should be done with great caution and without actual extension at the spine (e.g., isometric exercise). There have been no reports in the literature of exercise regimens that have targeted the spinal extensor muscle group in those with spinal stenosis [4].
Stabilization exercises
O’Sullivan et al. [42] found that individuals with chronic LBP and a radiological diagnosis of spondylolysis or spondylolisthesis who underwent a 10-week specific exercise treatment program involving the specific training of the deep abdominal muscles, with co-activation of the lumbar multifidus proximal to the pars defects showed a statistically significant reduction in pain intensity and functional disability levels, which was maintained at 30-month follow-up. The control group that received treatment as directed by their treating practitioner showed no significant change in these parameters after intervention or at follow-up. Lindgren et al. [35] found that exercise therapy in patients with chronic low back pain and segmental instability symptoms can improve strength and electromyographic parameters of paraspinal muscles, but not change the radiographic signs of instability.
Hicks et al. [25] developed a clinical rule to predict treatment response to a stabilization exercise program for patients with LBP. They found that the most important variables for success of stabilization exercises were age, straight-leg raise, prone instability test, aberrant motions, lumbar hypermobility, and fear-avoidance beliefs. The preliminary prediction rule for success with stabilization treatment contained four variables: positive prone instability test, aberrant movements present, average SLR greater than 91°, and age greater than 40 years old.
Combined treatment
As we mentioned before, symptoms associated with spinal stenosis are main complain of patients with DS. Simotas et al. [52] report on a case series of 49 patients treated non-operatively for spinal stenosis. In addition to pharmacologic intervention that may have included oral analgesics and ESI, the intervention consisted of therapeutic exercise (postural instruction, lumbopelvic mobilization exercises, and a flexion-based exercise program). After 3 years, nine of 49 patients (18%) had surgical intervention. Five patients (10%) reported their condition to be worse, and the remaining 35 patients (71%) either reported no deterioration in their condition or reported improvement (slight or sustained). The authors conclude that aggressive nonoperative treatment for spinal stenosis remains a reasonable option.
Spinal manipulation
Spinal manipulation is an alternative treatment often pursued by patients. No randomized clinical trials of patients with spondylolisthesis or spinal stenosis have been done. We found only one study [41] that evaluated effectiveness of spinal manipulative therapy for LBP by comparing two groups of patients: a small group (25) of patients with lumbar spondylolisthesis and a larger group (260) of patients without spondylolisthesis. This study showed that the results of manipulative treatment are not significantly different in patients with or without lumbar spondylolisthesis. Patients may have some short-term pain relief from chiropractic manipulation, but no long term benefit has been proven.
Discussion
Degenerative spondylolisthesis is a complex multifactorial problem. Although, DS is a common diagnosis in aging individuals, there is little empiric evidence to support many of the common nonsurgical interventions for symptomatic individuals. Because of limitations with the existing literature that have been highlighted, current practice recommendations require incorporating findings from available studies into existing clinical and biologic paradigms in order to provide a rational basis for treatment recommendations. In addition, the absence of consensus guidelines from national or international organizations, the treatment of DS remains highly dependent on patient and physician expectations and preferences. Despite many surgical options exist for the treatment of DS, it generally is agreed that in most cases nonoperative treatment should be attempted before surgical intervention is pursued. Of the nonoperative options, none are conclusively superior to the others and all have a role in the treatment of symptomatic patients. For patients with DS, nonsurgical treatments should focus on patient education, medications to control pain, flexion strengthening and stabilizing exercises and physical and cognitive treatments to regain or maintain activities of daily living. Specific aims of nonsurgical treatments should focus of improvement of spinal segmental stability and reliving neurological symptoms that caused by spinal stenosis associated with DS. For many patients, several nonsurgical treatments may be used sequentially or in combination depending on the severity of symptoms and their change with time. However, in our opinion exercises should be recommended to be done on the daily basis.
An up-to-date knowledge on diagnosis and prevention of lumbar DS can assist in determination of future research goals. Additional studies are required to establish treatment protocols for the conservative treatment of DS.
Acknowledgments
L. K. is supported by an Arthritis Foundation Postdoctoral Grant.
References
- 1.Andersson GBJ. Epidemiology of spinal stenosis. In: Weinstein JN, Gordon SL, editors. Low back pain: a scientific and clinical overview. Rosemont: American Academy of Orthopaedic Surgeons; 1996. pp. 637–641. [Google Scholar]
- 2.Aihara T, Takahashi K, Yamagata M, Moriya H, Tamaki T. Biomechanical functions of the iliolumbar ligament in L5 spondylolysis. J Orthop Sci. 2000;5:238–242. doi: 10.1007/s007760050158. [DOI] [PubMed] [Google Scholar]
- 3.Apostolaki E, Davies AM, Evans N, Cassar-Pullicino VN. MR imaging of lumbar facet joint synovial cysts. Eur Radiol. 2000;10:615–623. doi: 10.1007/s003300050973. [DOI] [PubMed] [Google Scholar]
- 4.Atlas SJ, Delitto A. Spinal stenosis: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:198–207. doi: 10.1097/01.blo.0000198722.70138.96. [DOI] [PubMed] [Google Scholar]
- 5.Bell DF, Ehrlich MG, Zaleske DJ. Brace treatment for symptomatic spondylolisthesis. Clin Orthop Relat Res. 1988;236:192–198. [PubMed] [Google Scholar]
- 6.Butler D, Gifford L. The concept of adverse mechanical tension in the nervous system. Part 2: examination and treatment. Physiotherapy. 1989;75:629–636. doi: 10.1016/S0031-9406(10)62375-9. [DOI] [PubMed] [Google Scholar]
- 7.Butt S, Saifuddin A. The imaging of lumbar spondylolisthesis. Clin Radiol. 2005;60:533–546. doi: 10.1016/j.crad.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Butterman GR. Treatment of lumbar disk herniation: Epidural steroid injection compared with discectomy. A prospective, randomized study. J Bone Joint Surg. 2004;86A:670–679. [PubMed] [Google Scholar]
- 9.Cuckler JM, Bernini PA, Wiesel SW, Booth RE, Jr, Rothman RH, Pickens GT. The use of epidural steroids in the treatment of lumbar radicular pain: a prospective, randomized, doubleblind study. J Bone Joint Surg Am. 1985;67:63–66. [PubMed] [Google Scholar]
- 10.Danielson B, Frennerd K, Irstam L. Roentgenologic assessment of spondylolisthesis. I: a study of measurement variations. Acta Radiol. 1988;29:345–351. [PubMed] [Google Scholar]
- 11.Danielson B, Frennerd K, Selvik G, Irstram L. Roentgenologic assessment of spondylolisthesis. II: an evaluation of progression. Acta Radiol. 1989;30:65–68. doi: 10.3109/02841858909177460. [DOI] [PubMed] [Google Scholar]
- 12.Dilke TFW, Burry HC, Grahame R. Extradural corticosteroid injection in the management of lumbar nerve root compression. BMJ. 1973;2:635–637. doi: 10.1136/bmj.2.5867.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fellander-Tsai L, Micheli LJ. Treatment of spondylolysis with external electrical stimulation and bracing in adolescent athletes: a report of two cases. Clin J Sport Med. 1998;8:232–234. doi: 10.1097/00042752-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807–2812. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald J, Newman PH. Degenerative spondylolisthesis. J Bone Joint Surg Br. 1976;58:184–192. doi: 10.1302/0301-620X.58B2.932080. [DOI] [PubMed] [Google Scholar]
- 16.Friberg O. Instability in spondylolisthesis. Orthopedics. 1991;1:463–466. doi: 10.3928/0147-7447-19910401-11. [DOI] [PubMed] [Google Scholar]
- 17.Fritz JM, Erhard RE, Hagen BF. Segmental instability of the lumbar spine. Phys Ther. 1998;78:889–896. doi: 10.1093/ptj/78.8.889. [DOI] [PubMed] [Google Scholar]
- 18.Frymoyer JW. Degenerative spondylolisthesis. In: Andersson GBJ, McNeill TW, editors. Lumbar spinal stenosis. St Louis: Mosby Year Book; 1992. [Google Scholar]
- 19.Frymoyer JW. Degenerative spondylolisthesis: diagnosis and treatment. J Am Acad Orthop Surg. 1994;2:9–15. doi: 10.5435/00124635-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Fukusaki M, Kobayashi I, Hara T, Sumikawa K. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin J Pain. 1998;14:148–151. doi: 10.1097/00002508-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis. Cochrane Database Syst Rev. 2005;2:CD001352. doi: 10.1002/14651858.CD001352.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Gramse RR, Sinaki M, Ilstrup DM. Lumbar spondylolisthesis: a rational approach to conservative treatment. Mayo Clin Proc. 1980;55:681–686. [PubMed] [Google Scholar]
- 23.Hall CM, Brody LT. Therapeutic exercise: moving toward function. Philadelphia: Lippincott; 1999. pp. 344–345. [Google Scholar]
- 24.Herkowitz HN. Spine update: degenerative lumbar spondylolisthesis. Spine. 1995;20:1084–1090. doi: 10.1097/00007632-199505000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Hicks GE, Fritz JM, Delitto A, McGill SM. Preliminary development of a clinical prediction rule for determining which patients with low back pain will respond to a stabilization exercise program. Arch Phys Med Rehabil. 2005;86:1753–1762. doi: 10.1016/j.apmr.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Hilibrand AS, Rand N. Degenerative lumbar stenosis: diagnosis and management. J Am Acad Orthop Surg. 1999;7:239–249. doi: 10.5435/00124635-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Hoogmartens M, Morelle P. Epidural injection in the treatment of spinal stenosis. Acta Orthop Belg. 1987;53:409–411. [PubMed] [Google Scholar]
- 28.Jayakumar P, Nnadi C, Saifuddin A, Macsweeney E, Casey A. Dynamic degenerative lumbar spondylolisthesis: diagnosis with axial loaded magnetic resonance imaging. Spine. 2006;31:E298–E301. doi: 10.1097/01.brs.0000216602.98524.07. [DOI] [PubMed] [Google Scholar]
- 29.Johnsson KE, Rosen I, Uden A. The natural course of lumbar spinal stenosis. Clin Orthop. 1992;279:82–86. [PubMed] [Google Scholar]
- 30.Kanayama M, Hashimoto T, Shigenobu K, Oha F, Ishida T, Yamane S. Non-fusion surgery for degenerative spondylolisthesis using artificial ligament stabilization: surgical indication and clinical results. Spine. 2005;30:588–592. doi: 10.1097/01.brs.0000154766.74637.5e. [DOI] [PubMed] [Google Scholar]
- 31.Keller TS, Szpalski M, Gunzburg R, Spratt KF. Assessment of trunk function in single and multi-level spinal stenosis: a prospective clinical trial. Clin Biomech (Bristol, Avon) 2003;18:173–181. doi: 10.1016/S0268-0033(02)00190-0. [DOI] [PubMed] [Google Scholar]
- 32.Kostuik JP, Harrington I, Alexander D, Rand W, Evans D. Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg Am. 1986;68:386–391. [PubMed] [Google Scholar]
- 33.LaBan MM, Viola SL, Femminineo AF, Taylor RS. Restless legs syndrome associated with diminished cardiopulmonary compliance and lumbar spinal stenosis: a motor concomitant of “vespers curse.”. Arch Phys Med Rehabil. 1990;71:384–388. [PubMed] [Google Scholar]
- 34.Leinonen V, Maatta S, Taimela S, Herno A, Kankaanpaa M, Partanen J, Hanninen O, Airaksinen O. Paraspinal muscle denervation, paradoxically good lumbar endurance, and an abnormal flexion-extension cycle in lumbar spinal stenosis. Spine. 2003;28:324–331. doi: 10.1097/00007632-200302150-00003. [DOI] [PubMed] [Google Scholar]
- 35.Lindgren K, Sihvonen T, Leino E, Pitkanen M. Exercise therapy effects on functional radiographic findings and segmental electromyographic activity in lumbar spine instability. Arch Phys Med Rehabil. 1993;74:933–939. [PubMed] [Google Scholar]
- 36.Magora A, Schwartz A. Relation between low back pain syndrome and X-ray findings. Lysis and olisthesis. Scand J Rehabil Med. 1980;12:47–52. [PubMed] [Google Scholar]
- 37.Mardjetko SM, Connolly PJ, Shott S. Degenerative lumbar spondylolisthesis: a meta-analysis of literature 1970–1993. Spine. 1994;19(suppl):S2256–S2265. doi: 10.1097/00007632-199410151-00002. [DOI] [PubMed] [Google Scholar]
- 38.Matsunaga S, Ijiri K, Hayashi K. Nonsurgically managed patients with degenerative spondylolisthesis: a 10- to 18-year follow-up study. J Neurosurg. 2000;93(Suppl):194–198. doi: 10.3171/spi.2000.93.2.0194. [DOI] [PubMed] [Google Scholar]
- 39.McAfee PC, DeVine JG, Chaput CD, Prybis BG, Fedder IL, Cunningham BW, Farrell DJ, Hess SJ, Vigna FE. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine. 2005;30(Suppl):S60–S65. doi: 10.1097/01.brs.0000155578.62680.dd. [DOI] [PubMed] [Google Scholar]
- 40.Meyerding HW. Spondyloptosis. Surg Gynaecol Obstet. 1932;54:371–377. [Google Scholar]
- 41.Mierau D, Cassidy JD, McGregor M, Kirkaldy-Willis WH. A comparison of the effectiveness of spinal manipulative therapy for low back pain in patients with and without spondylolisthesis. J Manipulative Physiol Ther. 1987;10:49–55. [PubMed] [Google Scholar]
- 42.O’Sullivan PB, Phyty GD, Twomey LT, Allison GT. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine. 1997;22:2959–2967. doi: 10.1097/00007632-199712150-00020. [DOI] [PubMed] [Google Scholar]
- 43.Penning L, Wilmink JT. Posture-dependent bilateral compression of L4 or L5 nerve roots in facet hypertrophy: a dynamic CT-myelographic study. Spine. 1987;12:488–500. doi: 10.1097/00007632-198706000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Pettine KA, Salib RM, Walker SG. External electrical stimulation and bracing for treatment of spondylolysis. A case report. Spine. 1993;18:436–439. doi: 10.1097/00007632-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Postacchini F, Cinotti G, Perugia D. Degenerative lumbar spondylolisthesis II. Surgical treatment. Ital J Orthop Traumatol. 1991;17:467–477. [PubMed] [Google Scholar]
- 46.Prateepavanich P, Thanapipatsiri S, Santisatisakul P, Somshevita P, Charoensak T. The effectiveness of lumbosacral corset in symptomatic degenerative lumbar spinal stenosis. J Med Assoc Thai. 2001;84:572–576. [PubMed] [Google Scholar]
- 47.Resnick DK, Choudhri TF, Dailey AT, Groff MW, Khoo L, Matz PG, Mummaneni P, Watters WC, 3rd, Wang J, Walters BC, Hadley MN. American association of neurological surgeons/congress of neurological surgeons guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: fusion in patients with stenosis and spondylolisthesis. J Neurosurg Spine. 2005;2:679–685. doi: 10.3171/spi.2005.2.6.0679. [DOI] [PubMed] [Google Scholar]
- 48.Riew KD, Yin Y, Gilula L, Bridwell KH, Lenke LG, Lauryssen C, Goette K. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am. 2000;82-A:1589–1593. doi: 10.2106/00004623-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Rosen CD, Kahanovitz N, Bernstein R, Viola K. A retrospective analysis of the efficacy of epidural steroid injections. Clin Orthop Relat Res. 1988;228:270–272. [PubMed] [Google Scholar]
- 50.Rosenberg NJ. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am. 1975;57:467–474. [PubMed] [Google Scholar]
- 51.Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine. 2005;30(Suppl):S71–S81. doi: 10.1097/01.brs.0000155579.88537.8e. [DOI] [PubMed] [Google Scholar]
- 52.Simotas AC, Dorey FJ, Hansraj KK, Cammisa F., Jr Nonoperative treatment for lumbar spinal stenosis. Clinical and outcome results and a 3-year survivorship analysis. Spine. 2000;25:197–203. doi: 10.1097/00007632-200001150-00009. [DOI] [PubMed] [Google Scholar]
- 53.Sinaki M, Lutness MP, Ilstrup DM, Chu CP, Gramse RR. Lumbar spondylolisthesis: retrospective comparison and three-year follow-up of two conservative treatment programs. Arch Phys Med Rehabil. 1989;70:594–598. [PubMed] [Google Scholar]
- 54.Spratt KF, Weinstein JN, Lehmann TR, Woody J, Sayre H. Efficacy of flexion and extension treatments incorporating braces for low-back pain patients with retrodisplacement, spondylolisthesis, or normal sagittal translation. Spine. 1993;18:1839–1849. doi: 10.1097/00007632-199310000-00020. [DOI] [PubMed] [Google Scholar]
- 55.Stasinopoulos D. Treatment of spondylolysis with external electrical stimulation in young athletes: a critical literature review. Br J Sports Med. 2004;38:352–354. doi: 10.1136/bjsm.2003.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szpalski M, Gunzburg R, Pope MH. Lumbar segmental instability. Philadelphia: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 57.Taillard W. Le spondylolisthesis chez l’enfant et l’adolescent. Acta Orthop Scand. 1954;24:115–144. doi: 10.3109/17453675408988556. [DOI] [PubMed] [Google Scholar]
- 58.Tulder MW, Scholten RJ, Koes BW, Deyo RA. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane collaboration back review group. Spine. 2000;25:2501–2513. doi: 10.1097/00007632-200010010-00013. [DOI] [PubMed] [Google Scholar]
- 59.Vibert BT, Sliva CD, Herkowitz HN. Treatment of instability and spondylolisthesis: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:222–227. doi: 10.1097/01.blo.0000200233.99436.ea. [DOI] [PubMed] [Google Scholar]
- 60.Wang JC, Lin E, Brodke DS, Youssef JA. Epidural steroid injections for the treatment of symptomatic lumbar herniated discs. J Spinal Disord Tech. 2002;15:269–272. doi: 10.1097/00024720-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, Birkmeyer NJ, Hilibrand AS, Herkowitz H, Cammisa FP, Albert TJ, Emery SE, Lenke LG, Abdu WA, Longley M, Errico TJ, Hu SS. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiltse LL, Winter RB. Terminology and measurement of spondylolisthesis. J Bone Joint Surg. 1983;65A:768–772. [PubMed] [Google Scholar]