Abstract
Peridural fibrosis is one of the more frequent complications of lumbar surgery. Nonsteroidal anti-inflammatory drugs inhibit the inflammatory and fibroblastic response. We performed lumbar laminectomies in 24 rabbits, divided into two groups. The experimental group received 5 mg/kg/day of aceclofenac for 7 days and the control group received 1 cm3 of physiological saline. The samples were stained using immunohistochemical methods. The cellular populations in the inflammatory reaction and the thickness of the fibrous membrane were quantified. The mean of the fibrous area was always less in the rabbits of the experimental group compared to controls (47% less at 2 weeks and 41% less at 4 weeks). We observed an 8% decrease in the number of fibroblasts with antivimentin monoclonal antibodies in the experimental group. In this model, aceclofenac inhibits the presence of inflammatory cells in the fibrous scar in the early stages and reduces the extension of adhesions without adverse reactions.
Keywords: Prevention, Nonsteroidal drugs, Peridural fibrosis, Laminectomy, Epidural scar formation
Introduction
Peridural fibrosis consists of the formation of extradural fibrous tissue, which produces an adherence of the dura mater and the nerve roots to the erector muscles of the spinal column in the posterior part and to the disc and vertebral body in the anterior part. This scar formation may be compressive and at the same time restricts the mobility of the nerve root that is most vulnerable against new discal protrusions and favors the presence of a stenosis of the neural canal. Even today, the most accepted etiopathogenic theory is that proposed by LaRocca and MacNab [18], who suggest that the peridural fibrosis originates from the bleeding surface of the deep layer of the posterior paravertebral muscles. These authors baptized this layer as “laminectomy membrane” which would cover the defect created by the bone resection in an attempt to replace the empty space by means of the formation of fibrous tissue would aim to reconstitute the removed lamina and which would extend within the neural canal.
When faced with an established peridural fibrosis treatment is limited, no effective treatment is known to cure peridural fibrosis [5]. The prescription of analgesics, physiotherapeutic measures or anti-inflammatory medication has been considered only palliative for painful periods. There is no consensus on the effectiveness of surgery. Some authors rule out this possibility [4, 17, 23] and others recommend, such as, a last resort in patients with incapacitating pain [18, 20, 22].
Most authors believe the best way of avoiding the appearance of peridural fibrosis is to prevent its formation [2, 11, 12, 14]. Meticulous surgery, as a preventive intraoperative measure, has been suggested [6, 25] with careful hemostasis and the placement of physical [11, 21] and chemical [4, 9, 13] barriers between the paravertebral musculature and the dura. The nonsteroidal anti-inflammatory drugs (NSAIDs) are the examples of systemic chemical barrier. They present an advantage over the physical barriers of not introducing foreign bodies, which may increase the inflammatory response. Moreover, NSAIDs inhibit cyclooxygenase, which is responsible for the synthesis of prostaglandins (biological mediators capable of triggering reactions of vasodilation and chemotaxis). The beneficial effect of the NSAIDs in the prevention of the calcifications of the soft tissues, heterotopic ossifications and adherences is well-known [10, 20].
The question we intended to address was if a commonly used European NSAID, aceclofenac (Almirall Prodesfarma, Barcelona, Spain), administered from the time of intervention would inhibit fibroblastic proliferation after performing a laminectomy in experimental animals.
Materials and methods
An experimental study was made in 24 New Zealand white (NZW) rabbits, in which, a laminectomy was performed in the lamina L4. The 24 animals were equally divided into two experimental groups, which were killed at weeks 2 and 4, respectively (six in each group), with their corresponding control groups. To the control group, 1 cm3 of physiological saline was injected and to the animals of the experimental group, 5 mg/kg/day of aceclofenac was injected intramuscularly, from the day of intervention to the seventh postoperative day. Approval was obtained from the Animal Research Committee before any animal studies were begun.
Under adequate anesthesia and antibiotic protection, a longitudinal incision of 4 cm was made in a posterior line between L3 and L5; the fascia was incised in order to expose the extreme of the spinous processes. The paraspinal muscles were detached subperiosteally from the spinous processes and the laminas, and retracted with an autostatic separator. Following a meticulous technique in order not to damage the spinal cord [23], the spinous processes of the caudal vertebra, the ligamentum flavum, inferior articular processes and the third distal part of the lamina of the cranial vertebra were resected until achieving an exposure of the dura of 4 × 8 mm. A drainage was used for 24 h.
The animals were killed at weeks 2 and 4 by means of an overdose of intravenous sodium pentobarbital (60 mg/kg). The spinal column was resected in bloc between L3 and L5. This was fixed with formalin at 10% for 24 h and decalcified in formic acid for 3 weeks.
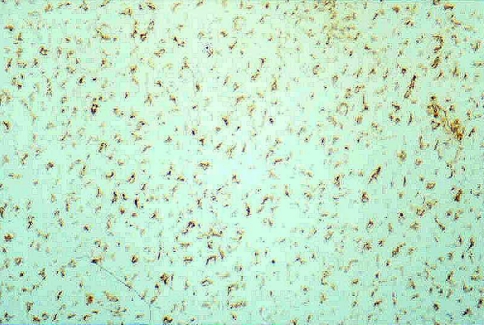
We obtained systematically four histological sections of the entire transverse diameter of the spinal canal from each animal. Each section was stained with hematoxylin-eosin (H&E), Masson’s trichrome and immunohistochemical methods. The streptavidin-biotin-peroxidase was used for the detection of antigens, and the enzymatic marking was made with horseradish peroxidase. The substrate was hydrogen peroxide and the chromogen was DAB (diamino-benzidine). This chromogen marks the fibroblasts in brown color (Fig. 1). The monoclonal antivimentin antibody produced in rabbit (clone V9 of Biogenex®) was employed. In each section, the following measures were made: the area of the fibrous membrane (mm2), the density of the fibroblasts per square millimetre and the density of the inflammatory cells per square millimetre. The adherences were graded into 0 (no adherence was seen between the dura and the fibrosis), 1 (adherence less than one-third of the surface of the dura), 2 (between one and two-third) and 3 (greater than two-third).
Fig. 1.
This figure shows the fibroblasts as positive-vimentin cells in brown color (original magnification, ×250)
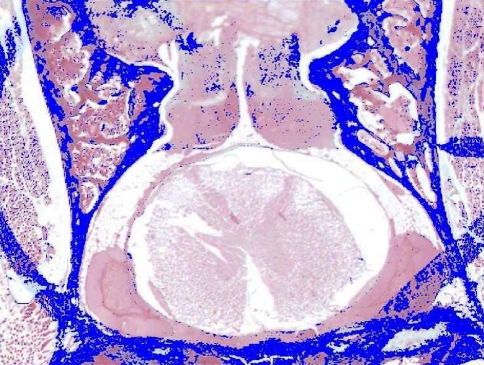
For the quantification of the area of the fibrous membrane, the histological preparations stained with Masson’s trichrome were focused by a digital Leica DC 100 camera (Leica, Solms, Germany) and with a Micro-Nikon macro-lens of 55 mm. The samples were analyzed using a Leica QWIN image processor. The detection of the area of fibrosis was made according to the intensities of the green color of Masson’s staining, this being reflected in false color for better visualization (Fig. 2). A systematic selection was performed using an optic pencil for separating the regions of fibrosis from other areas that presented a tonicity similar to the green color, and proceeding finally to the quantification of the area of fibrosis in square millimetre.
Fig. 2.
We can observe the detection of the area of fibrosis, in false color (orientation: posterior region is at the top of this figure)
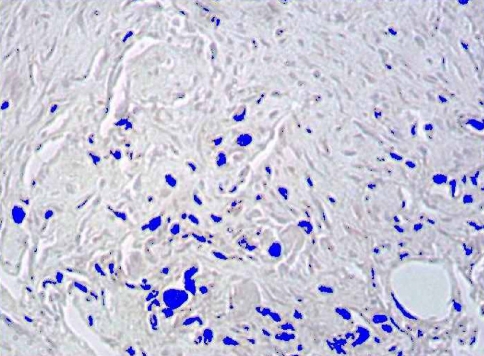
In order to quantify the number of fibroblasts, the immunohistochemical preparations were digitalized with a Leica DC 100 camera connected to a Leica DMR-XA microscope and analyzed by means of a Leica QWIN image processor. The capture was performed by means of digitizing in real color, the mentioned regions of fibrosis, thus the range of color was established. At this time an automatic detection was made, according to the color, of all the cytoskeletal cells. The computer program interprets brown as a mixture of the basic red, blue and green colors in a range between 0 and 255 (Fig. 3).
Fig. 3.
We performed the automatic detection of the fibroblasts, in false color
Finally, we quantified the inflammatory cells (lymphocytes, polymorphonuclear cells and macrophages). The histological preparations were stained with H&E and Masson’s trichrome, and examined with a Reichert microscope and analyzed using an Olympus WH 10 × 3 reticle.
The histological and immunohistochemical examinations were performed in a blinded manner. Two persons making the examinations were unaware if the specimen was from study or control group.
The analysis of data was made with descriptive and inferential statistics. As the sample size was small, the non-parametric Mann–Whitney U test was employed for the continuous quantitative variables (area of the fibrous membrane, density of the fibroblasts and density of the inflammatory cells) and the χ2 for qualitative variables (fibrous adherence).
Results
No superficial or deep infection was seen. In no case, complications in the surgical wound were seen. At day 7 the skin sutures were removed.
The histological findings at week 2 in the control group consisted of an extensive hematoma that filled the laminectomy and was found in contact with the dura. A smaller adherence of the hematoma to the dura (adherence less than one-third of the surface of the dura) was seen in the experimental group at week 2, with similar cellular lines. In the control group at week 4, the hematic cell infiltrate was progressively replaced with fibrous tissue and in the areas near the resected lamina, a metaplasia to the chondroid tissue and bone were observed in the experimental group at week 4, the hematoma had been progressively replaced by fibrous tissue.
The mean fibrous surface was less (p < 0.05) in the rabbits of the experimental group compared to the controls, both at weeks 2 and 4 (Table 1).
Table 1.
Total fibrous surface area (mm2)
| Time (weeks) | Study group (mean ± SD) | Control group (mean ± SD) | p value |
|---|---|---|---|
| 2 | 2.9241 ± 2.0185 | 6.2116 ± 3.2129 | 0.046 |
| 4 | 1.3875 ± 1.0353 | 3.2958 ± 1.8604 | 0.041 |
SD standard deviation
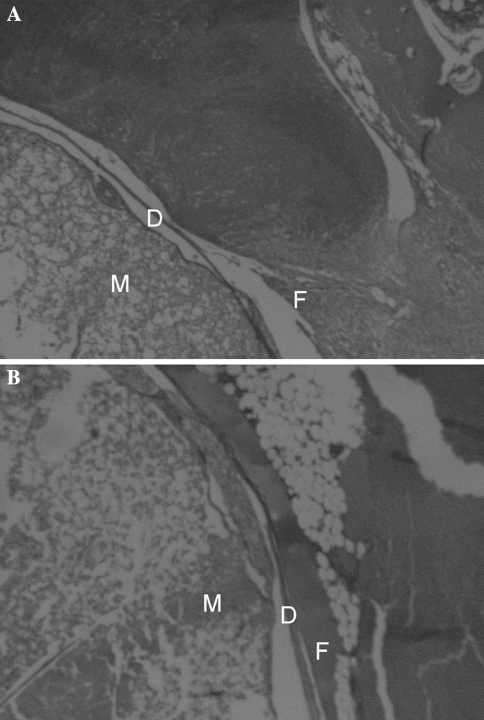
In more than 70% of the samples, the adherence of the peridural fibrosis to the dura was, as a minimum, of moderate size (between one and two-third of the surface of the dura) and at least one-third of the samples had considerable proportions (greater than two-third of the surface of the dura). Adherence was less (p = 0.04) in the rabbits of the experimental groups (Fig. 4). At week 2, statistically significant differences were seen in the adherence grade 3 between control and study groups.
Fig. 4.
a We can see the fibrous adherence to the dura in the study group at 4 weeks (stain, hematoxylin and eosin; original magnification, ×40). M spinal marrow, D dura mater and F fibrosis. b We can observe the fibrous adherence to the dura in the control group at 4 weeks (stain, hematoxylin and eosin; original magnification, ×40). M spinal marrow, D dura mater and F fibrosis
We observed a smaller number (p = 0.08) of fibroblasts in the experimental groups with a progressive decrease (p = 0.25) over time between weeks 2 and 4 (Table 2).
Table 2.
Cellular density of the fibroblasts (mm2)
| Time (weeks) | Study group (mean ± SD) | Control group (mean ± SD) | p value |
|---|---|---|---|
| 2 | 2693 ± 415 | 3377 ± 802 | 0.08 |
| 4 | 1536 ± 930 | 2203 ± 1012 | 0.25 |
SD standard deviation
In the study groups, a smaller number of inflammatory cells than the control groups were seen (Table 3). No differences were observed in the types of inflammatory cells.
Table 3.
Density of inflammatory cells (mm2)
| Time (weeks) | Study group (mean ± SD) | Control group (mean ± SD) | p value |
|---|---|---|---|
| 2 | 465 ± 95 | 578 ± 87 | 0.04 |
| 4 | 205 ± 94 | 281 ± 73 | 0.14 |
SD standard deviation
Discussion
From the time of the first studies on peridural fibrosis by LaRocca and MacNab [18], different physical barriers have been proposed. These barriers impede the migration of the fibroblasts from the musculature to the neural and the paraneural structures. With this aim, many materials have been employed, with biological capacity or inert, resorbable or nonresorbable, solid or fluid, but none have been found to be entirely effective [5, 7, 10, 12, 14, 15, 24, 26]. Recently, an expandable polytetrafluroethylene membrane (ePTFE) [7] and an ADCON-L (bioresorbable gelatin and polyglycan gel) [10] have been recommended.
Some authors [1, 4, 17] have suggested a mechanical role for the epidural fat, which protects and facilitates the movement of the dural sac in the bone canal. However, this fat is not without complications, such as, infection and hematoma in the donor area [4]. Kuivila et al. [17] mention other inconveniences of the fat graft such as necrosis, atrophy and even compression on the cauda equina [27].
Other authors [20] studied some complications of the physical barriers, concluding in a prospective study, that the placement of fat barriers or gel foam on the roots and dura after a discectomy had no effect on the clinical result, functional status, or MRI findings. The interposition membrane produced no beneficial effects. Bellen [2] on the other hand found monoradicular motor paralysis produced by hematoma on the third postoperative day, this being attributed to the use of gelfoam as hemostatic agent. Le et al. [19] have published their experience with the use of ADCON-L. In four patients, it can be seen that this material exacerbated the leak of cephalorachidian fluid from microscopic durotomies unrecognized at the time of surgery.
Due to the controversial results of physical and mechanical barriers, attempts have been made to apply chemical barriers such as corticoids, activators of tissue plasminogen [2], mitomycin C, urokinase [9] and elastase. However, these are no longer used [2, 4, 5, 14].
The NSAIDs inhibit the synthesis of prostaglandins, thereby decreasing the permeability of the capillaries, inhibiting the inflammatory infiltration, and controlling fibroblastic hyperplasia and formation of granulation tissue. Aceclofenac is a widely used NSAID in musculoskeletal pathology due to its analgesic and anti-inflammatory properties and is well tolerated when used for short periods. It is a derivative of phenylacetic acid, with a structure similar to other NSAIDs. Its main action mechanism is the inhibition of cyclooxygenase and therefore of the synthesis of prostaglandins but it also modifies other cellular processes such as the synthesis of leukotrienes, generation of superoxides, release of enzymes by lysosomes, aggregation and adhesion of neutrophils, and the functions of the cellular membrane [16].
We chose the dose based upon the studies of Brogden [3]. Agreement exists that the appropriate dose is 5 mg/kg and that the route of administration has no clinical relevance [8]. This dose was administered for only 1 week in order to evaluate the early inhibitory effect of the fibrosis and to avoid possible adverse reactions.
The immunohistochemical techniques permit the detection and identification of biomolecular components, which are the integral parts of cells and tissues, by means of the study of the reaction between these components and the corresponding polyclonal and monoclonal antibodies. In our study, this technique facilitated the identification of the fibroblasts such as positive-vimentin cells.
The mean of the fibrous surface was less in the rabbits of the experimental groups compared to controls, at both weeks 2 and 4, but the difference was significant only when the two time groups were combined, perhaps because the sample size was small.
We have noticed a smaller number of all the inflammatory cells but different only in early stages. The types of inflammatory cells were similar in both groups, as He et al. [13] also affirm, after administering ketoprophene in rats.
We have shown that peridural fibrosis can be developed experimentally in the rabbit after lumbar laminectomy and that a NSAID is capable both of reducing the fibrous area and of inhibiting the presence of inflammatory cells in the fibrous scarring. The immunohistochemical techniques help to quantify them. We consider that aceclofenac, when administered early, may be a useful drug for preventing the formation of the peridural fibrosis.
References
- 1.Beaujeux R, Wolfram-Gabel R, Kehrli P, et al. Posterior lumbar epidural fat as a functional structure? Histologic specificities. Spine. 1997;22:1264–1268. doi: 10.1097/00007632-199706010-00021. [DOI] [PubMed] [Google Scholar]
- 2.Bellen P. Prevention of peridural fibrosis following laminectomy. Apropos of a case of monoradicular paralysis due to an intracanalar hematoma on Gelfoam [in French] Acta Orthop Belg. 1992;58:236–239. [PubMed] [Google Scholar]
- 3.Brogden RN, Wiseman LR. Aceclofenac: a review of its pharmacodynamic properties and therapeutic potential in the treatment of rheumatic disorders and in pain management. Drugs. 1996;52:113–124. doi: 10.2165/00003495-199652010-00008. [DOI] [PubMed] [Google Scholar]
- 4.Cabezudo JM, López A, Bacci F. Symptomatic root compression by a free fat transplant after hemilaminectomy: case report. J Neurosurg. 1985;63:633–635. doi: 10.3171/jns.1985.63.4.0633. [DOI] [PubMed] [Google Scholar]
- 5.Ceviz A, Arslan A, Ak HE, Inalöz S. The effect of urokinase in preventing the formation of epidural fibrosis and/or leptomeningeal arachnoiditis. Surg Neurol. 1997;47:124–127. doi: 10.1016/S0090-3019(96)00038-9. [DOI] [PubMed] [Google Scholar]
- 6.Cokluk C, Aydi K. Experimental rabbit hemilaminotomy model in the evaluation of peridural fibrosis: a minimally invasive peridural fibrosis model. Minim Invasive Neurosurg. 2005;48:235–239. doi: 10.1055/s-2005-870909. [DOI] [PubMed] [Google Scholar]
- 7.DiFazio FA, Nichols JB, Pope MH, Frymoyer JW. The use of expanded polytetrafluoroethylene as an interpositional membrane after lumbar laminectomy. Spine. 1995;20:986–991. doi: 10.1097/00007632-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Dimar JR, Ante WA, Zhang YP, Glassman SD. The effects of nonsteroidal anti-inflammatory drugs on posterior spinal fusions in the rat. Spine. 1996;21:1870–1876. doi: 10.1097/00007632-199608150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Dogulu F, Kurt G, Emmez H, et al. Topical mitomycin C-induced inhibition of postlaminectomy peridural fibrosis in rabbits. J Neurosurg. 2003;99(Suppl 1):76–79. doi: 10.3171/spi.2003.99.1.0076. [DOI] [PubMed] [Google Scholar]
- 10.Einhaus SL, Robertson JT, Dohan FC, Jr, Wujek JR, Ahmad S. Reduction of peridural fibrosis after lumbar laminotomy and discectomy in dogs by a resorbable gel (ADCON-L) Spine. 1997;22:1440–1446. doi: 10.1097/00007632-199707010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Golan A, Maymon R, Winograd I, Bukovsky I. Prevention of post-surgical adhesion formation using aspirin in a rodent model: a preliminary report. Hum Reprod. 1995;10:1797–1800. doi: 10.1093/oxfordjournals.humrep.a136177. [DOI] [PubMed] [Google Scholar]
- 12.Griffet J, Bastiani F, Hofman P, Argenson C. Prevention of scar formation by polyglactin 910 (Vicryl) mesh after lumbar laminectomy in the rat [in French] Rev Chir Orthop Reparatrice Appar Mot. 1992;78:365–371. [PubMed] [Google Scholar]
- 13.He Y, Revel M, Loty B. A quantitative model of post-laminectomy scar formation: effects of a nonsteroidal anti-inflammatory drug. Spine. 1995;20:557–563. doi: 10.1097/00007632-199503010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Henderson R, Weir B, Davis L, Mielke B, Grace M. Attempted experimental modification of the postlaminectomy membrane by local instillation of recombinant tissue-plasminogen activator gel. Spine. 1993;18:1268–1272. doi: 10.1097/00007632-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Herculano MA, Tella OI, Jr, Prandini MN, Seixas Alves MT. Inhibition of peridural fibrosis after laminectomy using biological sheet in rat model. Arq Neuropsiquiatr. 2006;64:259–263. doi: 10.1590/s0004-282x2006000200016. [DOI] [PubMed] [Google Scholar]
- 16.Keller J, Schumacher B, Lind M. Effect of local prostaglandin E2 on periosteum and muscle in rabbits. Acta Orthop Scand. 1992;63:623–627. doi: 10.1080/17453679209169722. [DOI] [PubMed] [Google Scholar]
- 17.Kuivila TE, Berry JL, Bell GR, Steffee AD. Heparinized materials for control of the formation of the laminectomy membrane in experimental laminectomies in dogs. Clin Orthop Relat Res. 1988;236:166–174. [PubMed] [Google Scholar]
- 18.LaRocca H, MacNab I. The laminectomy membrane: studies in its evolution, characteristics, effects and prophylaxis in dogs. J Bone Joint Surg. 1974;56B:545–550. [PubMed] [Google Scholar]
- 19.Le AX, Rogers DE, Dawson EG, et al. Unrecognized durotomy after lumbar discectomy: a report of four cases associated with the use of ADCON-L. Spine. 2001;26:115–117. doi: 10.1097/00007632-200101010-00020. [DOI] [PubMed] [Google Scholar]
- 20.MacKay MA, Fischgrund JS, Herkowitz HN, et al. The effect of interposition membrane on the outcome of lumbar laminectomy and discectomy. Spine. 1995;20:1793–1796. doi: 10.1097/00007632-199508150-00008. [DOI] [PubMed] [Google Scholar]
- 21.Nakano M, Matsui H, Miaki K, Tsuji H. Postlaminectomy adhesion of the cauda equina: inhibitory effects of anti-inflammatory drugs on cauda equina adhesion in rats. Spine. 1998;23:298–304. doi: 10.1097/00007632-199802010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Robertson JT, Meric AL, Dohan FC, Jr, et al. The reduction of postlaminectomy peridural fibrosis in rabbits by a carbohydrate polymer. J Neurosurg. 1993;79:89–95. doi: 10.3171/jns.1993.79.1.0089. [DOI] [PubMed] [Google Scholar]
- 23.Senegas J, Vital JM, Riojas A, Richard O, Caille JM. Diagnosis of recurrent herniated disk using discographic scanning [in French] Rev Chir Orthop Reparatrice Appar Mot. 1988;74(Suppl 2):91–94. [PubMed] [Google Scholar]
- 24.Tatsui CE, Martinez G, Li X, Pattany P, Levi AD. Evaluation of DuraGen in preventing peridural fibrosis in rabbits. J Neurosurg Spine. 2006;4:51–59. doi: 10.3171/spi.2006.4.1.51. [DOI] [PubMed] [Google Scholar]
- 25.Toh E, Mochida J. Histologic analysis of the lumbosacral nerve roots after compression in young and aged rabbits. Spine. 1997;22:721–726. doi: 10.1097/00007632-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 26.Topsakal C, Akpolat N, Erol FS, et al. Seprafilm superior to Gore-Tex in the prevention of peridural fibrosis. J Neurosurg. 2004;101:295–302. doi: 10.3171/jns.2004.101.2.0295. [DOI] [PubMed] [Google Scholar]
- 27.Yamagami T, Matsui H, Tsuji H, Ichimura K, Sano A. Effects of laminectomy and retained extradural foreign body on cauda equina adhesion. Spine. 1993;18:1774–1781. doi: 10.1097/00007632-199310000-00010. [DOI] [PubMed] [Google Scholar]