Abstract
Design of the experiment is to study the cross-sectional sample with retrospective information. The objective is to identify the types of physical activity associated with the decreased occurrence of low-back pain (LBP) in schoolchildren. Physical activity may be hypothesized to possess a potential for LBP prevention. The possible connection between LBP and specific sports activities is however sparsely documented. A total of 546, 15- to 16-year-old schoolchildren filled a questionnaire on current physical activities and LBP occurrence and severity. In multiple logistic regressions, the association of LBP with exposure variables was corrected for body height and weight (data from school health service files) and for anthropometric and school furniture parameters. More than half of the children reported pain or discomfort in the low-back region during the preceding 3 months, and 1/4 experienced a decreased functioning or need of care because of LBP. LBP correlated with physical inactivity, e.g. time spent on homework and hours watching TV or video, and with a series of sports activities, e.g. jogging, handball playing and gymnastics. Among sports activities, only swimming and the number of hours per week participating in soccer were associated with a decreased LBP prevalence. With the exception of swimming and soccer, the types of sport reported by this schoolchild population do not offer themselves for consideration as tools for LBP prevention. Based on the associations found with indicators of physical inactivity, attempts to motivate the children to increase their general physical activity level should be considered for trial.
Keywords: Adolescents, Low-back pain, Physical activity, Prevention, Health promotion
Background
Low-back pain (LBP) is frequent among schoolchildren [1–8]. The occurrence increases with age, especially in the early teen-years [1, 2, 9, 10] so that, by age 14–17, 11–71% will have experienced at least one episode of LBP [1–5, 9–11]. Recurrent LBP during childhood seems to occur among 5–19% of all children [4, 5, 9], and LBP in childhood may be a forerunner of LPB in adult life [12]. Findings concerning an association with gender have been ambiguous [1, 2, 5, 7–14].
In the light of the frequency of LBP and its eventual long-term consequences, preventive efforts should have the high priority. Mapping the etiology or at least a few risk indicators will be a prerequisite for the development of effective primary prevention. Moreover, the etiology is largely unknown and few risk factors have been identified, so that the etiology of LBP in childhood is still a matter of debate.
A family history of LBP seems to increase the risk [2, 4, 10, 12, 13, 15, 16], as does an accelerated growth rate [17], physically demanding occupational activity [5, 17], smoking [1, 5, 17, 18], and some psychological traits, e.g. depression and somatization [19, 20]. There are no clear associations with biological parameters such as body length [17, 21–23]; weight [22–24]; Body Mass Index [5, 14, 22, 25]; length of the trunk [21, 23–25]; vertebral column mobility [5, 10, 23, 26, 27] and radiological changes of the vertebral column [12, 22, 28–32]. Also the findings concerning the association between LBP and general muscle strength [22, 27, 33, 34] and more specifically, hip flexor muscle flexibility [17, 26, 35] have been ambiguous.
Thus there is still room for hypothesizing LBP occurrence to depend on the amount and the intensity of physical activity. This hypothesis is attractive from the point of view of prevention, as physical activity is amenable to behavioral intervention, and physical training programs at school and leisure activities may be adjusted to aim at strengthening the back. Moreover, physical inactivity and intensive sports activity have been found to be associated with LBP in some [1, 5, 22, 34–36], but not all studies [7, 10, 16, 17, 23, 33]. So far, research on possible associations with specific types of sport is however sparse [13, 37]. Accordingly, the aim of the present study was to investigate the relationship between LBP in schoolchildren and measures of physical activity and inactivity.
Materials and methods
The present study includes schoolchildren in the 9th grade of 14 public schools in the municipality of Aarhus, a Danish provincial town (population, about 300,000). Schools were sampled based on: (1) a 9th grade school quotient of at least 40 children per school, and (2) the availability of data from the school health service on body height and weight. Based on these criteria, 14 out of 26 eligible schools accepted to participate. The schoolchildren were informed in writing about the investigation and about the anonymity of the questionnaire. Participation was voluntary. All schoolchildren present on the day of data collection participated, in total 555. Nine children with a medically verified chronic back disorder were excluded, leaving 546 children for analysis.
Participants completed a questionnaire on LBP occurrence during the previous 3 months (period prevalence) including the intensity and duration of LBP and pain coping behavior, e.g. reduced daily activity and care seeking. LBP was defined as pain or discomfort in the low-back region, from the lower rib curvature to the lower part of the seat region, as visualized by a drawing shown in the questionnaire. Menstrual pain was specifically excluded from attention. The questionnaire asked about the overall physical activity during school and leisure hours and about specific sports activities; the weekly number of hours spent on specific sports activities; membership of sports clubs; participation in competition sport; watching TV or video; working at the computer or doing homework; transport of the school bag; vocational activity; smoking; school furniture and furniture at home. Questionnaires were completed class-wise during school lessons, in the presence of one of the authors (B.S.), during 11 January 2002–30 April 2002. Data on body height and weight were collected from the school health service files. The questionnaire was tested in a pilot study including 17 schoolchildren in 9th grade.
In the statistical analyses, medians were computed for continuous variables [38]. To identify factors with impact on LBP prevalence, multiple logistic regression was applied, yielding odds ratios (OR) estimating the relative risk associated with a risk factor for either LBP or function-limiting LBP. For example, the relative risk of LBP for jogging would be p(LBPjogging)/p(LBPnot jogging). A value >1.0 would indicate that jogging makes LBP more probable than not jogging. The OR of a single factor was derived from contingency table analyses that used Pearson’s χ2 to test the significance. Regression models were reduced by the use of backward elimination of variables with the χ2 distributed –2ln (likelihood ratio) as significance test for variable inclusion. Regression model fit was estimated by use of the Hosmer and Lemeshow statistic. Statistical significance required that the p-value be less than 0.05. Results were reported for the whole group and for gender-specific analyses. To reduce the risk of multi-collinearity, multiple regressions were performed separately for dichotomized and continuous predictors indicating the presence/absence, and amount of sports activity and physical inactivity. In the present context, data on furniture are exclusively used for confounder correction. The project complies with the Helsinki II declaration, and the database was approved by the National Data Inspectorate.
Results
Participation
Seventy-eight children (12.3%) did not participate because of absence from school on the day of questionnaire completion. Non-participants differed from participants concerning the body weight (mean 63.8 kg in non-participants versus 60.5 kg in participants) and Body Mass Index (mean 21.8 vs 20.6 kg/m2). In one of the schools, absenteeism and, consequently, project non-participation was more outspoken than in other schools (25.7 vs 11.7%). The age span was 14–17 years in the 546 respondents; 97.8% were 15 or 16 years of age and 53.3% were boys.
Physical activity profile
During the preceding 3 months, 85.7% of respondents engaged in leisure time sports activities (median, 5 h/week in children engaging in sports). Some children participated in more than one type of sports activity. The most prevalent types of sports were soccer (32.8%), jogging (28.8%) and biking (20.1%) (Table 1). Children playing soccer, handball or basketball, or who engaged in fighting, riding, playing golf or sailing, spent at least 3 h/week (median) on these. Only 1–6 children participated in squash, volleyball, athletics, sailing and rowing.
Table 1.
Types of sport performed during leisure time (preceding 3 months) in 546 schoolchildren
| Type of sport | Children | No. of hours per week | ||
|---|---|---|---|---|
| No. | (%) | Mediana | Range | |
| Soccer | 179 | 32.8 | 3.0 | 0–15 |
| Jogging | 157 | 28.8 | 1.0 | 0–25 |
| Biking | 110 | 20.1 | 2.0 | 1–12 |
| Dance | 64 | 11.7 | 2.0 | 1–20 |
| Handball | 61 | 11.2 | 3.0 | 1–9 |
| Badminton | 47 | 8.6 | 2.0 | 0–10 |
| Swimming | 42 | 7.7 | 2.0 | 0–10 |
| Fighting | 41 | 7.5 | 3.0 | 1–14 |
| Basketball | 36 | 6.6 | 3.0 | 1–13 |
| Gymnastics | 35 | 6.4 | 2.0 | 1–13 |
| Riding | 24 | 4.4 | 3.5 | 1–22 |
| Scouting | 19 | 3.5 | 2.0 | 1–15 |
| Golf | 17 | 3.1 | 4.0 | 1–30 |
| Tennis | 15 | 2.7 | 2.0 | 1–7 |
| Table tennis | 13 | 2.4 | 1.0 | 0–15 |
| Shooting | 10 | 1.8 | 2.5 | 1–7 |
| Other | 104 | 19.0 | 3.0 | 0–30 |
aIn children performing the specific type of sport
The day before questionnaire completion, 41.6% walked to school, 33.0% biked, 14.3% took the bus, and 10.3% were transported by car and 2.6% by motorbike. On the same day, only 15.2% made sports, preferably ball-play, during school breaks.
During the 2 days preceding questionnaire completion, the children spent a median of 2 h/day watching TV or video, 1 h at the computer and 1 h of homework. During the preceding weekend, a total of 4 h (median) were spent watching TV or video, 1 h at the computer, and 1 h doing homework.
LBP and LBP coping behavior
As much as 64.8% had ever experienced one or more episodes of LBP, and 60.3 and 51.3% reported LBP during the preceding 12 and 3 months, respectively. Nearly 1/4 (24.2%) reported LBP during the preceding 3 months, which had limited their daily activities or resulted in contact with the health care system. Thus, 5.9% had contacted their general practitioners, 0.7% had been treated at an outpatient clinic, 1.1 and 2.7% had visited a medical specialist or a physiotherapist, respectively; and 1.3% saw a chiropractor, 3.7% another type of therapist and 0.4% had been hospital inpatients.
Physical activity and LBP
Based on bivariate analysis, LBP was found to be un-associated with age and gender. In general, LBP and sports activities during leisure time were un-associated (OR 1.1, p = 0.625), and LBP was furthermore unassociated with hours per week spent on sports activity (OR 1.0 h–1, p = 0.133). Transport to school by car and some activities during school breaks correlated positively with LBP (Table 2).
Table 2.
Low-back pain (LBP) during the preceding 3 months, by physical activity (based on bivariate analysis) in 546 schoolchildren
| Physical activity | LBP | Function-limiting LBPa | ||||
|---|---|---|---|---|---|---|
| (%) | OR | p-value | (%) | OR | p-value | |
| Type of sport | ||||||
| Jogging | 58.6 | 1.5 | 0.030 | 31.2 | 1.7 | 0.015 |
| Handball | 67.2 | 2.1 | 0.008 | 39.3 | 2.3 | 0.003 |
| Swimming | 40.5 | 0.6 | 0.145 | 7.1 | 0.2 | 0.007 |
| Gymnastics | 60.0 | 1.5 | 0.286 | 40.0 | 2.2 | 0.024 |
| Riding | 75.0 | 3.0 | 0.017 | 20.8 | 0.8 | 0.696 |
| Scouting | 36.8 | 0.5 | 0.200 | 0.0 | 0.8 | 0.012 |
| Transport to school | ||||||
| By carb | 66.1 | 2.0 | 0.019 | 37.5 | 2.0 | 0.014 |
| Activities during school breaks | ||||||
| Sitting talkingb | 54.0 | 1.5 | 0.047 | 25.3 | 1.2 | 0.329 |
| Standing talkingb | 58.1 | 2.0 | 0.000 | 28.1 | 1.8 | 0.007 |
Only activities significantly associated with LBP are presented
aLBP resulting in disturbed sleep at night, school absence, prohibition of normal leisure activities, or care seeking
bThe preceding day
Based on bivariate analysis, LPB as well as function-limiting LBP were positively associated with time spent on handball (in both cases, OR was 1.2 h/week; p = 0.017 and p = 0.021, respectively). Also the time spent on gymnastics was associated with function-limiting LBP (OR 1.4 h–1, p = 0.030). Concerning indicators of amount of physical inactivity, the number of hours watching TV or video, the preceding weekend or doing homework the day before questionnaire completion correlated positively with LBP (OR 1.1 h–1 watching TV, p = 0.026, and OR 1.1 h–1 doing homework, p = 0.018), and the number of hours watching TV or video 2 days before questionnaire completion was positively associated with function-limiting LBP (OR 1.2 h–1, p = 0.013).
In multiple logistic regressions, LBP was found to be positively associated with jogging, handball, gymnastics and riding, and negatively with swimming and hours spent playing soccer (Tables 3, 4). Handball was associated with LBP as well as function-limiting LBP, both as indicated by the mere activity and by the number of hours per week. Also jogging and gymnastics were associated with function-limiting LBP, whether indicated by binary variables or by the number of hours. Negative associations were found between swimming and function-limiting LBP (Table 3) and between the number of hours per week playing soccer and function-limiting LBP (Table 4). The number of hours per week swimming was borderline significantly negatively associated with LBP (OR 0.63, p = 0.065). LBP was furthermore positively associated with a series of indicators of inactivity such as transport to school by car, standing talking during school breaks (Table 3), and time spent watching TV or video or doing homework (Table 4, Fig. 1). Analyses conducted separately for girls and boys did not reveal any gender difference.
Table 3.
Significant (p < 0.05) physical activity risk estimates (OR odds ratio, with 95% confidence limits by multiple logistic regression) of low-back pain (LBP) during the preceding 3 months in 546 schoolchildren
| Dependent variable | Significant predictors | ORa | p-value | 95% CL |
|---|---|---|---|---|
| LBP | Sports activity indicators | |||
| Handball playingb | 2.35 | 0.005 | 1.29–4.28 | |
| Ridingb | 3.46 | 0.015 | 1.28–9.35 | |
| Physical inactivity indicators | ||||
| Transport to school by carc | 2.14 | 0.019 | 1.13–4.04 | |
| Standing talking during school breakc | 1.87 | 0.001 | 1.28–2.73 | |
| Function-limiting LBPd | Sports activity indicators | |||
| Swimmingb | 0.19 | 0.009 | 0.06–0.67 | |
| Joggingb | 1.59 | 0.046 | 1.01–2.49 | |
| Handball playingb | 2.17 | 0.010 | 1.20–3.94 | |
| Gymnasticsb | 2.23 | 0.043 | 1.03–4.84 | |
| Physical inactivity indicators | ||||
| Transport to school by carc | 1.94 | 0.037 | 1.04–3.60 | |
| Standing talking during school breakc | 1.61 | 0.039 | 1.03–2.53 | |
| Doing homework during school breakc | 1.87 | 0.038 | 1.03–3.37 | |
aOR adjusted for age and gender, Body Mass Index, physical activity and inactivity indicators, weight and transport of schoolbag, school, smoking, school furniture, furniture at home and the other predictors of the table. Model fits, both p > 0.6
bThe preceding 3 months
cThe preceding day
dLBP resulting in disturbed sleep at night, absence from school, prohibition of normal leisure activities, or care seeking
Table 4.
Significant (p < 0.05) physical activity per hour risk estimates (OR odds ratio, with 95% confidence limits by multiple logistic regression) of low-back pain (LBP) during the preceding 3 months in 546 schoolchildren
| Dependent variable | Significant predictors | Per hour | ||
|---|---|---|---|---|
| ORa | p-value | 95% CL | ||
| LBP | Sports activity indicators | |||
| Handball playingb | 1.21 | 0.007 | 1.06–1.39 | |
| Physical inactivity indicators | ||||
| Watching TV or videoc | 1.07 | 0.014 | 1.01–1.14 | |
| Doing homework/reading other things | ||||
| The preceding day | 1.27 | 0.001 | 1.11–1.45 | |
| The preceding weekend | 0.94 | 0.034 | 0.89–1.00 | |
| Function-limiting LBPe | Sports activity indicators | |||
| Playing soccerb | 0.90 | 0.042 | 0.81–1.00 | |
| Joggingb | 1.19 | 0.005 | 1.05–1.34 | |
| Handball playingb | 1.16 | 0.029 | 1.02–1.32 | |
| Gymnasticsb | 1.51 | 0.030 | 1.04–2.18 | |
| Physical inactivity indicators | ||||
| Watching TV or videod | 1.18 | 0.007 | 1.05–1.34 | |
| Doing homework/reading other things | ||||
| 2 days before investigation | 1.17 | 0.047 | 1.00–1.35 | |
| The preceding weekend | 0.89 | 0.020 | 0.81–0.98 | |
aOR adjusted for age and gender, Body Mass Index, physical activity and inactivity indicators, weight and transport of schoolbag, school, smoking, school furniture, furniture at home and the other predictors of the table. Model fits, both p > 0.6
bHours per week within the preceding 3 months
cHours the preceding weekend
dHours two days before the investigation
eLBP resulting in disturbed sleep at night, absence from school, prohibition of normal leisure activities, or care seeking
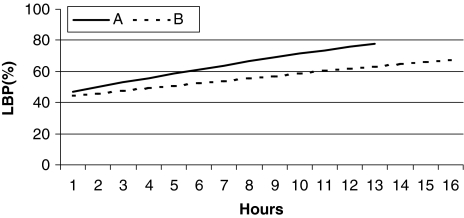
Fig. 1.
Estimated 3-month period prevalence (estimates based on logistic regression; both model fits, P > 0.4) of low-back pain (LBP) by a hours preparing to school on the preceding day and b hours watching TV or video during the preceding weekend, in 546 schoolchildren
Discussion
The only type of sport found to correlate with a reduced LBP occurrence in the present period prevalence study was swimming, whereas other types of sports, jogging, handball, gymnastics and riding, were associated with an increased period prevalence risk. The amount of physical activity, however, were ambiguous, as the LBP risk decreased by the number of hours playing soccer, whereas it increased by the number of hours jogging, playing handball and doing gymnastics. In addition, several indicators of physical inactivity took a negative role, e.g. being passively transported to school, not being physically active during school breaks, and the daily number of physically passive hours spent watching TV or video. The number of hours doing homework the day before the investigation was associated with LBP but inversely associated with the number of hours doing homework during the preceding weekend. A possible explanation to this apparent paradox could be that some schoolchildren may plan to do their homework during the weekend and therefore achieved a reduced stress level on weekdays.
As the observation of LBP incidence is time-consuming and probably can be performed only with modest validity, the present study, like most other studies on LBP etiology, is based on the observation of LBP period prevalence. Considering this being the best choice from a pragmatic point of view and with the object of reducing imprecision caused by memory failure, we chose a parallel 3-month perspective for indicators of physical activity and LBP occurrence. However, some indicators of physical activity focused on the very near past, e.g. the day or the weekend before questionnaire completion.
Prevalence studies regarding LBP involve some “chicken-or-egg”-questions, i.e. indicators actually may denote consequences rather than causes of LBP, or they may include at least an element of LBP impact. Standing talking during the school breaks thus may denote an example of physical inactivity associated with an increased LBP as well as a pain coping reaction. This also counts for other inactivity indicators. Being transported inactively to school has previously been demonstrated to be associated with LBP [16], and in another study LBP was observed more frequently in children who did not walk to school [39]. Also other authors have found LBP to be positively associated with time spent on watching TV [1, 37]. It is well documented that physical inactivity has biochemical consequences and may exert heavy impact on the connective tissue of the locomotor system [40]. Therefore, the association between the reported inactivity and LBP seems relevant.
The most prominent finding concerning leisure time sports activity seems to be the lack of an overall association with LBP, whether negative or positive. Consequently, it is not possible to conclude that, e.g. in general more sports activity will result in less LBP. This is in agreement with previous research [7, 10, 16, 17, 23, 33]. In a recent Danish study, no association was found between directly observed physical activity and LBP [41]. This contrasts the association between the amount of leisure time physical activity and the relatively low occurrence of recurrent low-back pain found in males participating in a 25-year follow-up study [42]. Moreover, inferring about the possible role of specific sports activities may be even more problematic. Children with LBP would not be expected to possess a tendency to choose, e.g. team handball (with sudden and often rather violent movements) or jogging (with repeated pushes of the locomotor system, including the spine), why these associations could hardly be the result of selection bias. Furthermore, these two types of sport have been shown to be associated with pain in other regions, e.g. the knee [43]. Parallel etiologic mechanisms may be active as concerns riding and gymnastics.
In a previous study [13], associations between participation in volleyball and LBP have been found. The present study cannot highlight this question, as few children reported participation in volleyball.
In the present study, swimming was found to correlate with a relatively low-LBP period prevalence. This could, in theory, be a result of healthy children selecting this type of sport. However, swimming activates the muscles of the trunk, e.g. the erector muscles of the spine, so that there is a basis for considering swimming a tool for LBP prevention. The inverse correlation between hours playing soccer and LBP could denote a selection of healthy children playing soccer during many hours. Soccer has been found to be positively associated with chronic LBP [44], whereas other types of physical activities, such as regular walking and bicycling, have been found to be associated with less LBP [37].
In summary, with the exception of the case of swimming, the present research does not provide an observational basis for suggesting more sports activity with the aim of preventing LBP. On the other hand, the associations between inactivity measures and LBP may point to the desirability in avoiding physical inactivity in general and, conversely in promoting activity during and after school hours. Increased physical activity should, of course, not include activities shown to correlate positively with LBP. Before implementation, such low-back health promotion programs should be tested by use of randomized controlled trials conducted in relevant school populations.
Acknowledgments
The project received financial support from the Danish Physiotherapists’ Research Foundation and the Health Council of The Municipality of Aarhus, Denmark.
References
- 1.Balagué F, Dutoit G, Waldburger M. Low back pain in schoolchildren. Scand J Rehab Med. 1988;20:175–179. [PubMed] [Google Scholar]
- 2.Balagué F, Nordin M, Skovron ML, Dutoit G, Yee A, Waldburger M. Non-specific low back pain among schoolchildren: a field survey with analysis of some associated factors. J Spinal Disord. 1994;7:374–379. [PubMed] [Google Scholar]
- 3.Burton AK, Clarke RD, McClune TD, Tillotson KM. The natural history of low back pain in adolescents. Spine. 1996;21:2323–2328. doi: 10.1097/00007632-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Duggleby T, Kumar S. Epidemiology of juvenile low back pain: a review. Disabil Rehabil. 1997;19:505–512. doi: 10.3109/09638289709166043. [DOI] [PubMed] [Google Scholar]
- 5.Harreby M, Nygaard B, Jessen T, Larsen E, Storr-Paulsen A, Lindahl A, Fisker I, Lægaard E. Risk factors for low back pain in a cohort of 1389 Danish school children: an epidemiologic study. Eur Spine J. 1999;8:444–450. doi: 10.1007/s005860050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leboeuf-Yde C, Kyvik KO. At what age does low back pain become a common problem. Spine. 1998;23:228–234. doi: 10.1097/00007632-199801150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Taimela S, Kujala UM, Salminen JJ, Viljanen T. The prevalence of low back pain among children and adolescents. Spine. 1997;22:1132–1136. doi: 10.1097/00007632-199705150-00013. [DOI] [PubMed] [Google Scholar]
- 8.Wedderkopp N, Leboeuf-Yde C, Andersen LB, Froberg K, Hansen HS. Back pain reporting pattern in a Danish population-based sample of children and adolescents. Spine. 2001;26:1879–1883. doi: 10.1097/00007632-200109010-00012. [DOI] [PubMed] [Google Scholar]
- 9.Olsen TL, Anderson RL, Dearwater SR, Kriska AM, Cauley JA, Aaron DJ, LaPorte RE. The epidemiology of low back pain in an adolescent population. Am J Public Health. 1992;82:606–608. doi: 10.2105/AJPH.82.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salminen JJ (1984) The adolescents back. A field survey of 370 Finnish schoolchildren. Acta Paediatr Scand 1–122 [PubMed]
- 11.Salminen JJ, Pentti J, Terho P. Low back pain and disability in 14-year-old schoolchildren. Acta Paediatr. 1992;81:1035–1039. doi: 10.1111/j.1651-2227.1992.tb12170.x. [DOI] [PubMed] [Google Scholar]
- 12.Harreby M, Neergaard K, Hesselsøe G, Kjer J. Are radiologic changes in the thoracic and lumbar spine of adolescents risk factors for low back pain in adults. Spine. 1995;20:2298–2302. doi: 10.1097/00007632-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Balagué F, Troussier B, Salminen JJ. Non-specific low back pain in children and adolescents: risk factors: a review. Eur Spine J. 1999;8:429–438. doi: 10.1007/s005860050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viry P, Creveuil C, Marcelli C. Nonspecific back pain in children. Rev Rhum. 1999;66:381–388. [PubMed] [Google Scholar]
- 15.Balagué F, Nordin M. Back pain in children and teenagers. Baillières Clin Rheumatol. 1992;6:575–593. doi: 10.1016/S0950-3579(05)80128-1. [DOI] [PubMed] [Google Scholar]
- 16.Gunzburg R, Balagué F, Nordin M, Szpalski M, Duyck D, Bull D, Mélot C. Low back pain in a population of school children. Eur Spine J. 1999;8:439–443. doi: 10.1007/s005860050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman DE, Shrier I, Rossignol M, Abenhaim L. Risk factors for the development of low back pain in adolescence. Am J Epidemiol. 2001;154:30–36. doi: 10.1093/aje/154.1.30. [DOI] [PubMed] [Google Scholar]
- 18.Feldman DE, Rossignol M, Shrier I, Abenhaim L. Smoking. A risk factor for development of low back pain in adolescents. Spine. 1999;24:2492–2496. doi: 10.1097/00007632-199912010-00011. [DOI] [PubMed] [Google Scholar]
- 19.Balagué F, Skovron ML, Nordin M, Dutoit G, Waldburger M. Low back pain in schoolchildren. A study of familial and psychological factors. Spine. 1995;20:1265–1270. doi: 10.1097/00007632-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Gent Cv, Dols JJCM, Rover CMd, Sing RAH, Vet HCWd. The weight of schoolbags and the occurrence of neck, shoulder, and back pain in young adolescents. Spine. 2003;28:916–921. doi: 10.1097/00007632-200305010-00014. [DOI] [PubMed] [Google Scholar]
- 21.Nissinen M, Heliövaara M, Seitsamo J, Alaranta H, Poussa M. Anthropometric measurements and the incidence of low back pain in a cohort of pubertal children. Spine. 1994;19:1367–1370. doi: 10.1097/00007632-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Salminen JJ, Erkintalo M, Laine M, Pentti J. Low back pain in the young. Spine. 1995;20:2101–2108. doi: 10.1097/00007632-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Widhe T. Spine: posture, mobility and pain. A longitudinal study from childhood to adolescence. Eur Spine J. 2001;10:118–123. doi: 10.1007/s005860000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairbank JCT, Pynsent PB, Poortvliet JAV, Phillips H. Influence of anthropometric factors and joint laxity in the incidence of adolescent back pain. Spine. 1984;9:461–464. doi: 10.1097/00007632-198407000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Harreby M, Kjer J, Hesselsøe G, Neergaard K. Epidemiological aspects and risk factors for low back pain in 38-year-old men and women: a 25-year prospective cohort study of 640 school children. Eur Spine J. 1996;5:312–318. doi: 10.1007/BF00304346. [DOI] [PubMed] [Google Scholar]
- 26.Kujala UM, Taimela S, Salminen JJ, Oksanen A. Baseline anthropometry, flexibility and strength characteristics and future low back pain in adolescent athletes and nonathletes. Scand J Med Sci Sports. 1994;4:200–205. doi: 10.1111/j.1600-0838.1994.tb00426.x. [DOI] [Google Scholar]
- 27.Sjölie AN, Ljunggren AE. The significance of high lumbar mobility and low lumbar strength for current and future low back pain in adolescents. Spine. 2001;26:2629–2636. doi: 10.1097/00007632-200112010-00019. [DOI] [PubMed] [Google Scholar]
- 28.Erkintalo MO, Salminen JJ, Alanen AM, Paajanen HEK, Kormano MJ. Development of degenerative changes in the lumbar intervertebral disk: results of a prospective MR imaging study in adolescents with and without low back pain. Radiology. 1995;196:529–533. doi: 10.1148/radiology.196.2.7617872. [DOI] [PubMed] [Google Scholar]
- 29.Salminen JJ, Erkintalo-Tertti MO, Paajanen HEK. Magnetic resonance imaging findings of lumbar spine in the young: correlation with leisure time physical activity, spinal mobility, and trunk muscle strength in 15-year-old pupils with or without low back pain. J Spinal Disord. 1993;6:386–391. [PubMed] [Google Scholar]
- 30.Swärd L, Hellstrom M, Jacobsson B, Pëterson L. Back pain and radiologic changes in the thoraco-lumbar spine of athletes. Spine. 1990;15:124–129. doi: 10.1097/00007632-199002000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Tertti MO, Salminen JJ, Paajanen HEK, Terho PH, Kormano MJ. Low back pain and disk degeneration in children: a case-control MR imaging study. Radiology. 1991;180:503–507. doi: 10.1148/radiology.180.2.1829844. [DOI] [PubMed] [Google Scholar]
- 32.Tertti M, Paajanen H, Kujala UM, Alanen A, Salmi TT, Kormano M. Disc degeneration in young gymnasts. Am J Sports Med. 1990;18:206–208. doi: 10.1177/036354659001800216. [DOI] [PubMed] [Google Scholar]
- 33.Balagué F, Damidot P, Nordin M, Parnianpour M, Waldburger M. Cross-sectional study of the isokinetic muscle trunk strength among school children. Spine. 1993;18:1199–1205. doi: 10.1097/00007632-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Newcomer K, Sinaki M. Low back pain and its relationship to back strength and physical activity in children. Acta Paediatr. 1996;85:1433–1439. doi: 10.1111/j.1651-2227.1996.tb13948.x. [DOI] [PubMed] [Google Scholar]
- 35.Kujala UM, Salminen JJ, Taimela S, Oksanen A, Jaakkola L. Subject characteristics and low back pain in young athletes and nonathletes. Med Sci Sports Exerc. 1992;24:627–632. [PubMed] [Google Scholar]
- 36.Kujala UM, Taimela S, Viljanen T. Leisure physical activity and various pain symptoms among adolescents. Br J Sports Med. 1999;33:325–328. doi: 10.1136/bjsm.33.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjölie AN. Associations between activities and low back pain in adolescents. Scand J Med Sci Sports. 2004;4:352–359. doi: 10.1111/j.1600-0838.2004.377.x. [DOI] [PubMed] [Google Scholar]
- 38.Armitage P, Berry G. Statistical methods in medical research. Oxford: Blackwell; 1994. [Google Scholar]
- 39.Szpalski M, Gunzburg R, Balagué F, Nordin M, Mélot C. A 2-year prospective longitudinal study on low back pain in primary school children. Eur Spine J. 2002;11:459–464. doi: 10.1007/s00586-002-0385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 41.Wedderkopp N, Leboeuf-Yde C, Andersen LB, Froberg K, Hansen HS. Back pain in children: no association with objectively measured level of physical activity. Spine. 2003;28:2019–2024. doi: 10.1097/01.BRS.0000083238.78155.31. [DOI] [PubMed] [Google Scholar]
- 42.Mikkelsson LO, Nupponen H, Kaprio J, Kautiainen H, Mikkelsson M, Kujala UM. Adolescent flexibility, endurance strength, and physical activity as predictors of adult tension neck, low back pain, and knee injury: a 25-year follow up study. Br J Sports Med. 2006;40:107–113. doi: 10.1136/bjsm.2004.017350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn T, Foldspang A. Prevalent knee pain and sport. Scand J Soc Med. 1998;26:44–52. doi: 10.1177/14034948980260011001. [DOI] [PubMed] [Google Scholar]
- 44.Bejia Low back pain in a cohort of 622 Tunisian schoolchildren and adolescents: an epidemiological study. Eur Spine J. 2005;14:331–336. doi: 10.1007/s00586-004-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]