Abstract
Angiopoietin-1 has powerful vascular protective effects, suppressing plasma leakage, inhibiting vascular inflammation and preventing endothelial death. Pre-clinical studies indicate that Ang1 may be therapeutically useful in a number of situations, including treatment of oedema, endotoxaemia and transplant arteriosclerosis. However, the ligand has also been implicated in vessel remodelling, induction of angiogenesis and pulmonary hypertension, indicating that strategies to minimize any deleterious effects whilst optimizing vessel protection are likely to be needed. This Review surveys the published data on vascular protective effects of Ang1 and highlights the therapeutic potential of this ligand, as well as possible limitations to its use. We also consider the data on Ang1 receptors and speculate on how to maximize therapeutic benefit by targeting the Tie receptors.
Keywords: angiopoietin, endothelial, Tie1, Tie2, integrin
INTRODUCTION
Angiopoietin-1 (Ang1) is an oligomeric secreted glycoprotein and a member, with Ang2 and Ang3/4, of the angiopoietin family of growth factors. These ligands bind Tie2, one of two receptor tyrosine kinases, the other being Tie1, that make up the Tie family of receptors expressed primarily in vascular endothelium. The angiopoietin and Tie families have primary roles in the latter stages of vascular development and in adult vasculature where they control remodelling and stabilization of vessels. Ang1 is required for correct organization and maturation of newly formed vessels, and promotes quiescence and structural integrity of adult vasculature. The importance of Ang1 for developmental angiogenesis is illustrated in transgenic mice deficient in the ligand. These animals die at about embryonic day 12.5 and although the vasculature has formed, it displays decreased complexity with dilated vessels, diminished branching and reduced numbers of small vessels 1. In addition, blood vessels in the Ang1−/− mice have fewer endothelial cells, defects in association of endothelia with extracellular matrix and vessel rupture 1. A key role for Tie2 in transducing these Ang1 signals is indicated by the phenotype of Tie2−/− mice which have many similarities with Ang1−/− mice, including decreased sprouting, dilated vessels, lack of periendothelial support and vessel rupture 2-5. A summary of the phenotypes of these and other Tie and angiopoietin transgenic mice is shown in Table 1. The pro-stabilizing effects of Ang1 will be discussed later, but are clearly demonstrated in studies in which Ang1 is directly administered or overexpressed leading to marked improvements in vascular integrity in both growing and adult mice.
Table 1.
Phenotypes of transgenic mice lacking or overexpressing Tie receptors and Angiopoietins
| Gene | Vascular Phenotype | Lymphatic or haematopoietic Phenotype |
|---|---|---|
| Tie1−/− | Die E13.5 onwards. Microvessel haemorrhage, oedema, rupture. Small hearts with endocardial defects 3,4,77 Tie1−/− EC lost from chimaeric WT/Tie1−/− mice 110. |
|
| Tie2−/− | Die E9.5 onwards. Poor remodelling, decreased branching, dilated vessels. Fewer EC, rounded EC, lack perivascular cells. Haemorrhage, vessel rupture, endocardial defects 2-5. |
|
| Double Tie1−/− /Tie2−/− |
Similar Tie2−/− but more severe. Tie1−/− embryo'ssensitive to Tie2 gene dosage 5. Tie1−/−/Tie2−/− EC absent from capillaries of adult chimaeric WT/double knockout mice 5. |
Tie1−/−/Tie2−/− cells have reduced capacity to contribute to haematopoiesis in adult, but not foetus 111, Tie1−/− cells can contribute to haematopoiesis 110. |
| Ang1−/− | Die E12.5. Similar to Tie2−/−. Dilated vessels, EC rounded with poor interaction with matrix/periendothelial cells. Few perivascular cells 1. |
|
| Ang1 overexpression |
Overexpression in skin increases number & branching of vessels & enlarges vessels 82,112. |
Lymphatic hyperplasia in skin of mice overexpressing Ang1 in epidermis97. |
| Ang2−/− | Die by P14. Failure to remodel & regress hyaloid vasculature, abnormal outgrowth of retinal capillaries & lack of ischaemia- induced neovascularization 113,114. |
Defects in lymphangiogenesis - disorganization, hypoplasia in dermal & intestinal lymphatics that can be rescued by Ang1 113. |
| Ang2 overexpression |
Overexpression in EC. Die at E9.5-10.5. Similar to Tie2−/− & Ang1−/−, but more severe. Rounded EC, poor interaction with matrix, endocardial defects 6. |
Unusually for a group of ligands, the angiopoietin family have both agonist and antagonist members. Ang1 and Ang2 are best characterized in this respect and can have opposing effects on receptor activation, with Ang1 stimulating Tie2 and Ang2 capable of antagonizing this 6. Consistent with these effects at the receptor level, genetic evidence indicates that Ang2 can counteract Ang1 activity. Ang2 overexpression in transgenic mice leads to embryonic death with a phenotype similar to Ang1 or Tie2 deletion 6. Ang2 also disrupts endothelial monolayer interaction with smooth muscle cells in culture 7. Thus, signalling through Tie2 appears to depend on the balance between Ang1 and Ang2. There is now strong evidence to support the concept that Ang1 provides a basal signal to promote and maintain quiescence and integrity of the endothelium in mature vessels, while Ang2 induced by hypoxia or other activators suppresses the effects of Ang1 resulting in vessel destabilization. The effects mediated by Ang2 thus allow vessel growth or regression depending on which other endothelial effectors are also present 8.
In recent studies, Ang1 has been shown to have potent effects on adult vessels including promotion of vessel survival, inhibition of vascular leakage and suppression of inflammatory gene expression. It is not surprising; therefore, that this ligand is being assessed in preclinical studies and shows promise for therapeutic use in a range of pathologies including sepsis, stroke, transplant arteriosclerosis and diabetic retinopathy. In addition to promoting vessel stability, Ang1 also stimulates vessel remodelling and angiogenesis, and has been implicated in pulmonary hypertension. These latter activities suggest therapeutic application may require strategies to minimize the possibility of potentially deleterious effects whilst optimizing vessel protective effects. Being able to manipulate the effects of Ang1, directly or indirectly, for therapeutic benefit will require an understanding of the receptors and signalling pathways used by the ligand. Although Tie2 is the best established receptor for Ang1, there is emerging data showing the ligand may also signal through the related tyrosine kinase Tie1 9. Furthermore, certain integrins, such as α5β1 have been found to bind Ang1 and may transduce some of its cellular effects 10,11. This preliminary data suggests that, in addition to Tie2, two other receptors may participate in Ang1 signalling. Differences in signalling motifs and mechanisms between Tie2, Tie1 and integrins further suggest that the receptors may mediate distinct cellular actions of Ang1. This diversity could provide an opportunity to promote specific desired activities at the cellular or in vivo level by targeting particular Ang1 receptors.
Here we review the potential therapeutic importance and applications of Ang1 as well as possible limitations to its use that need to be addressed.
THE ANGIOPOIETIN FAMILY
The first member of the angiopoietin family to be discovered, Ang1, was identified by its ability to bind Tie2 extracellular domain 12. Subsequently, low stringency screening was used to clone Ang2, 3 and 4 6,13. The best-characterized members of the family are Ang1 and Ang2 and these two ligands share approximately 60% amino acid identity 6. Ang1 and Ang2 bind to Tie2 with similar affinities; however, whereas Ang1 is an agonist the ability of Ang2 to activate Tie2 appears to depend on the cell type and context. As indicated above, in endothelial cells Ang2 is largely unable to activate Tie2 6, although if the ligand is present at high concentrations or for prolonged periods it can stimulate the receptor 14,15. In non-endothelial cells transfected to express Tie2, Ang2 acts as an agonist 6. Mouse Ang3 and human Ang4 are orthologues and represent the third member of the Ang family 13. Ang4 activates Tie2 in human endothelial cells and Ang3 is an agonist for the receptor in mouse endothelium 13,16.
The angiopoietins share a similar overall structure with a short amino-terminal motif followed by a coiled-coil domain and carboxy-terminal fibrinogen-like domain 6,12,13. Binding of the ligands to Tie2 is mediated by their fibrinogen-like domains 17. The precise residues required for Tie2 interaction have yet to be defined, though based on the crystal structure of the Ang2 fibrinogen domain and preliminary amino acid substitution analysis a region in the carboxy-terminal half of the domain appears to be important 18. The coiled-coil sequences of the angiopoietins are responsible for homo-oligomerization of the ligands 17. Native Ang1 exists as trimeric, tetrameric and pentameric homo-oligomers that cluster into multimeric structures via cysteines within the amino-terminal motif 19,20. This multimerization is essential for activation of Tie2 in endothelium and Ang1 must present as a tetramer or higher order structure to stimulate the receptor 19,20. Interestingly, Tie2 transfected into non-endothelial cells can be activated by dimeric Ang1 suggesting that in endothelial cells co-receptors or regulators are present that require the ligand to assume higher order multimers in order to be activating 20. Native Ang2 exists as disulphide-linked homodimers and recombinant Ang2 has been reported as trimeric, tetrameric and pentameric oligomers 20. In contrast to Ang1, there appears to be little multimerization of Ang2 17,19,20. This incapacity to form higher order multimers would prevent Ang2 from activating Tie2 and likely contributes to the ability of Ang2 to act as an antagonist. However, even multimeric constructs of the Ang2 fibrinogen domain are unable to activate endothelial Tie2 indicating determinants in the receptor-binding domain of this ligand also prevent it from activating Tie2 19,20.
Ang1 is widely expressed and is present in periendothelial cells in quiescent vasculature 12,21. In contrast Ang2 expression occurs in areas of endothelial activation and angiogenesis, for example in ovary and tumour vessel endothelia where it coincides with vessel destabilization during angiogenesis 6,22. Ang2 expressed in endothelium is stored within Weibel-Palade bodies and rapidly released following stimulation with thrombin and other agonists 23. A broad range of factors has been reported to modulate Ang2 expression, including hypoxia, vascular endothelial growth factor (VEGF), angiotensin II, and leptin 24. This pattern of expression and regulation of Ang1 and Ang2 is consistent with the concept that Ang1 provides a constitutive tonic signal to promote quiescence of the endothelium and this is modified by the more actively regulated antagonist Ang2 8.
Much less is known about the expression patterns of Ang3/4. There is relatively little data on the sites of expression of Ang4. Endothelial cells are known to express the ligand and it is upregulated by hypoxia and VEGF 25,26. Tissue distribution of Ang4 has not been studied in depth, though lung appears to express relatively high levels 13.
ANGIOPOIETIN-1 RECEPTORS
Tie2
Ang1 binds and signals through the receptor tyrosine kinase Tie2 12. This receptor and its close relative Tie1 share a similar overall structure with an extracellular domain comprising of an immunoglobulin (Ig)-like motif followed by three EGF-homology domains a second Ig motif and three fibronectin type III repeats 27-29. The intracellular domains of Tie1 and Tie2 are tyrosine kinases with a short kinase insert region and carboxy-terminal tail 27-29. Overall amino acid sequence identity between Tie1 and Tie2 intracellular domains is 76%. The primary sequences of Tie ectodomains are less conserved than intracellular portions of the receptors and have an amino acid identity of 31%. The region of Tie2 responsible for Ang1 binding is in the amino-terminal 360 amino acids of the receptor ectodomain, encompassing the first Ig motif and EGF-homology regions 30.
Tie1
Recent data indicates that in addition to the established receptor Tie2, Ang family members may signal through Tie1. Ang1 can induce Tie1 phosphorylation in endothelial cells 9, but the mechanism for this activation still requires clarification. It is likely that either the ligand interacts with Tie1 to induce activation, or that it activates Tie1 indirectly via another Ang1 receptor. Activation of Tie1 indirectly would be consistent with the finding that Ang1 does not appear to bind recombinant soluble Tie1 extracellular domain 12. However, it is also possible that the conformation of soluble ectodomain is different from the conformation of the full-length Tie1 presented in a cellular context, or indeed that additional membrane components are required to enable cellular Tie1 to bind Ang1. Clearly, it will be important to define the mechanism by which Ang1 activates Tie1. Tie2 greatly enhances Ang1-activated Tie1 phosphorylation, probably via the interaction between the two receptors that has previously been reported 9,31-33. Ang2 appears to antagonize the effects of Ang1 on Tie1 phosphorylation 9. Thus, Ang2 could act to limit Ang1 signalling through Tie1 as it does with Tie2.
Integrins
In addition to Tie receptors, Ang1, as well as Ang2, has been found to bind integrins. Experiments with blocking antibodies, as well as cells deficient in certain integrins, suggest Ang1 can bind several different integrins, including α2β1, α5β1, αvβ3 and αvβ5 10,11,34,35. Ang1 has been shown to bind directly to α5β1 34,35 via the receptor binding fibrinogen-like domain of Ang1 34, but the precise motif within this domain has not been defined. The interaction is inhibited by RGD-peptides 10,11, however the fibrinogen-like domain of Ang1 does not contain an RGD motif, or any known integrin binding site 10. Nevertheless, it has been suggested that within this domain a conserved QHREDGS sequence, which is similar to integrin-binding sequences in fibrinogen and fibronectin, could mediate Ang binding 10. Monomeric fibrinogen-like domains appear to bind α5β1 and Tie2 with similar low affinity 34, but the relative affinities of interaction of integrins and Tie2 with Ang1 in its natural multimerization state remain to be defined.
The possible signalling and cellular functions associated with Ang1 activation of Tie1 or integrins are discussed below. However, it is clear much work remains to be done to confirm and expand the data on Ang1 action through these receptors.
CELLULAR EFFECTS AND SIGNALLING OF ANGIOPOIETIN-1
Apoptosis
The most extensively studied cellular effect of Ang1 is its ability to inhibit endothelial cell apoptosis. The ligand suppresses apoptosis in a variety of different endothelial cell types, including human umbilical vein endothelial cells (HUVEC), aortic and microvascular endothelial cells, and in response to stimuli ranging from serum-deprivation, hyperosmolarity and tumour necrosis factor-α (TNFα)-treatment to irradiation 36-41. Ang1 appears to inhibit apoptosis in some non-endothelial cells, including mouse cortical neurons 42, and skeletal and cardiac myocytes 10. Tie2 is expressed in the neurons but not in the myocytes, leaving the receptor for Ang1 unknown in the latter.
The anti-apoptotic effects of Ang1 involve the phosphatidylinositol 3-kinase (PI3K)/Akt pathway 21,41, which is activated in response to Ang1 through recruitment of the regulatory p85 subunit of PI3K to phosphorylated tyrosine residue 1102 in the intracellular domain of Tie2 43,44. Stimulation of Akt via Ang1 activated PI3K results in phosphorylation and inhibition of the forkhead transcription factor FKHR in endothelial cells 45. FKHR induces endothelial apoptosis and regulates expression of several genes in endothelium involved in vascular destabilization and remodelling, including Ang2 45. This inhibitory effect of Ang1 on FKHR is consistent with its role in promoting vessel quiescence. In addition to cell survival, PI3K plays critical roles in a number of other cellular actions of Ang1, including modulation of gene expression, activation of endothelial migration and suppression of inflammatory phenotype. The main signalling pathways known to be activated by Ang1 are summarized in Figure 1.
Figure 1. Angiopoietin-1 signalling.
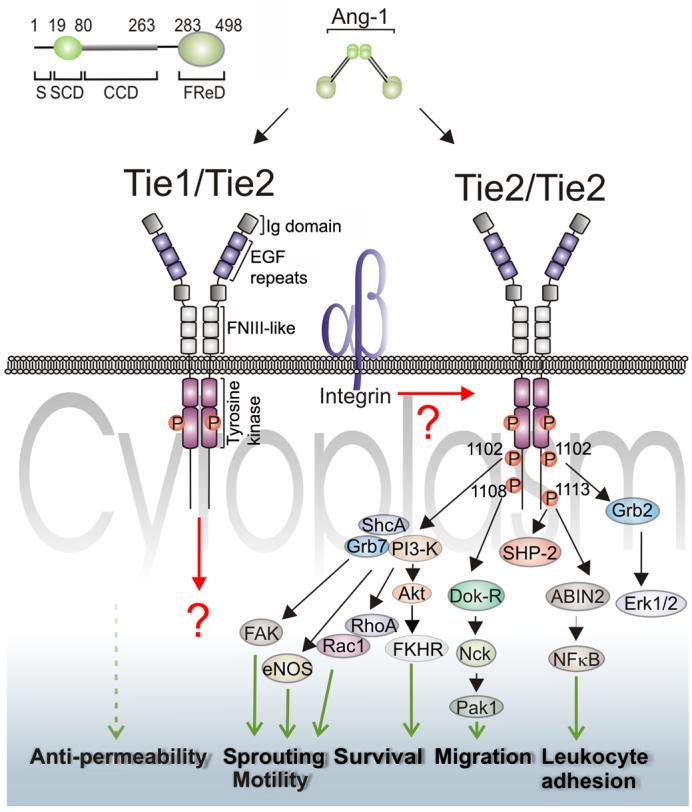
Schematic representation of established and potential signalling pathways activated by Ang1 in endothelial cells. Key phosphotyrosines are shown for the carboxy-terminus of Tie2 and cellular functions regulated by Ang1 are indicated towards the bottom of the figure. The domain structure of Tie2 is very similar to the related Tie1 receptor tyrosine kinase. Tie2 is the well established transducer of Ang1 signals, but recent data indicates that Tie1 is also activated by Ang1. Furthermore, heteromeric complexes between Tie1 and Tie2 were detected in cells expressing both receptors. However, possible signalling emanating from these heteromeric receptor complexes remains to be defined. Integrins have been found to interact with both Ang1 and Tie2, but the importance of these interactions on Tie2 signalling are largely unknown. Other details of the receptors, signalling intermediates and functions are given in the text. The top left corner of the figure depicts the structural features of human Ang1 together with approximate amino acid residue number flanking each domain. Following the secretory leader sequence (S) is the superclustering domain (SCD), coiled-coil domain (CCD), hinge region (H) and fibrinogen-related domain (FReD).
Migration
Ang1 stimulates endothelial cell migration 46-49 and this requires Tie2, as shown by experiments with endothelial cells from Tie2-deficient mice 50. The signalling pathways by which Ang1 stimulates endothelial migration involve both PI3K and the adaptor protein Dok-R. Following Tie2 activation Dok-R is recruited to the receptor and becomes phosphorylated creating interaction sites for Nck and the serine kinase p21 activating kinase (PAK1) 51. Recruitment of Dok-R to activated Tie2 is essential for Tie2-mediated migration 51. This binding to Tie2 requires both PTB and PH domains of the adaptor, with the PTB domain interacting with phosphorylated Y1108 on Tie2 and the PH domain being involved in PI3K-dependent membrane localization 50. The adaptor protein, ShcA, is also recruited to Tie2 in endothelial cells following Ang1 stimulation and appears to have a role in migration and organization 52. Increased endothelial motility in response to Ang1 also involves the GTPases RhoA and Rac1 as indicated by their activation in response to Ang1 and the ability of dominant-negative forms of the GTPases to suppress Ang1-induced endothelial motility 48,53.
Outgrowth of neurons from rat dorsal root ganglia, and migration of rat smooth muscle cells have also been found to be activated by Ang1 and both of these non-endothelial cell types express Tie2 54-56. Interestingly, Tie2-negative fibroblasts show increased movement in response to Ang1, suggesting that, in these cells at least, the ligand can increase motility via receptors other than Tie2 11.
Reorganization
A cellular effect of Ang1 related to migration and reflecting the remodelling effects of Ang1 in vivo is the ability of the ligand to induce reorganization of cultured endothelial cells into tubule-like structures invading into three-dimensional matrices 49,52,57,58. Ang1-stimulated reorganization of endothelial tubules and invasion into matrices is not seen in endothelial cells lacking Tie2 57 but can be stimulated by a Tie2-activating antibody in the rat aortic ring assay 59. Consistent with such remodelling effects, Ang1 stimulates production of proteases including plasmin and matrix metalloproteases, as well as suppressing secretion of tissue inhibitor of metalloprotease-2 58. Use of inhibitors and dominant-negative constructs has indicated a number of signalling intermediates involved in Ang1-induced re-organization of endothelium and motility, including PI3K, the adaptor protein ShcA, focal adhesion kinase and endothelial nitric oxide synthase 49,52,58,60. Thus, Ang1 activation of nitric oxide synthase (eNOS), has been reported in HUVEC and porcine coronary artery endothelial cells 49,60, and the ligand stimulates tyrosine phosphorylation of focal adhesion kinase (FAK) in HUVEC 58. Ang1 also stimulates Erk1/2 in endothelial cells 11,45,47,61,62 and this probably involves the upstream adaptor Grb2 that gets recruited to Y1102 in activated Tie2 44. The tyrosine phosphatase and adaptor protein SHPTP2 is also recruited to activated Tie2 59,63 and could also participate in Erk1/2 stimulation via its ability to recruit Grb2 64.
Proliferation
Ang1 has been reported to have no 46,47, mild 15,57 or significant 65 mitogenic activity in endothelial cells. Additional growth factors may be necessary for Ang1 to induce endothelial proliferation and more work is required to define conclusively the conditions under which it may act to stimulate proliferation.
Inflammatory gene expression
Ang1 suppresses adhesion of leukocytes to VEGF-stimulated HUVEC 66 by inhibiting the expression of a number of inflammation-associated adhesion molecules. Specifically, E-selectin, intercellular adhesion molecule-1 (ICAM1) and vascular cell adhesion molecule-1 (VCAM1) expression is decreased by Ang1 in VEGF-activated HUVEC 66,67. Ang1 induces a transient small increase in P-selectin expression at the surface of HUVEC, with a corresponding increased neutrophil adhesion, within the first few minutes of treatment with Ang1 68. In addition to effects on adhesion molecule expression, Ang1 blocks TNFα and VEGF-induced tissue factor expression in HUVEC 69. The signalling mechanism for these anti-inflammatory actions remains to be defined, although inhibitor and dominant-negative experiments show PI3K and Akt are required 66,69. In addition, Ang1 stimulation of endothelial cells promotes recruitment of A20 binding inhibitor of NFκB-2 (ABIN2) to Tie2 and this has been implicated in Ang1 suppression of NFκB 70, a transcription factor critical for regulation of inflammatory gene expression.
Permeability
An important cellular effect of Ang1 is its ability to improve integrity of endothelial monolayers. Permeability of monolayers of cultured HUVEC, human umbilical artery endothelial cells and glomerular endothelial cells is suppressed by Ang1 67,71. The ligand augments integrity of unstimulated monolayers and counteracts the increased permeability following activation with thrombin, VEGF or TNFα 67,71. Again, the signalling mechanisms mediating these effects have yet to be delineated. Tyrosine phosphorylation of vascular endothelial cadherin (VE-Cad) and platelet endothelial cell adhesion molecule-1 (PECAM1) is decreased by Ang1 in endothelial cells 67. The ligand also suppresses dissociation of VE-Cad from β-catenin and these effects could be involved in suppression of inter-endothelial leakage 67,72.
Haematopoiesis
Both Tie1 and Tie2 were cloned from leukaemia cell lines 27,73. They are expressed in haematopoietic progenitor cells 74,75, and consistent with a role in haematopoiesis, the Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche 76.
Putative signalling from Tie1 and integrins
The vast majority of studies on cellular actions and signalling by Ang1 have focused on Tie2. With the recent findings, that Ang1 may signal through Tie1 and possibly, to some extent via integrins, it will be important to define the signalling pathways and cellular functions that these additional receptors regulate. Studies with Tie1 gene targeted mice have shown that in vivo, Tie1 is required cell autonomously for endothelial cell survival during late embryogenesis 3,77. Thus it is likely that Tie1 has a role in suppression of endothelial apoptosis, possibly via the PI3K pathway. Supporting this it has been recently shown that the Tie1 intracellular domain can recruit the p85 subunit of PI3K and, using a chimeric receptor approach, that this is involved in Akt activation and suppression of UV-induced apoptosis in transfected fibroblasts 78. The tyrosine in Tie1 involved in interaction with the p85 subunit of PI3K is present in the motif Y1117VNM. This motif is more similar to the preferred binding motif of p85 79 than the equivalent Y1102VNT sequence in Tie2, suggesting that activated Tie1 could be a more potent activator of PI3K than Tie2. Another important difference between signalling motifs in Tie2 and Tie1 is that a key tyrosine residue in Tie2 involved in regulating endothelial migration, Y1108, does not have an equivalent in Tie1, where phenylalanine replaces it. As discussed above Y1108 in Tie2 is responsible for recruiting Dok-R/Nck/PAK1, which is essential for Tie2-mediated endothelial migration 50,51. It may be, therefore, that although Tie1 could be a strong activator of PI3K and endothelial survival it would not stimulate endothelial migration. Indeed, as PI3K has also been implicated in the anti-inflammatory activity of Ang1, it is an intriguing possibility that Tie1 could promote protective actions of endothelial survival and anti-inflammatory activities without promoting endothelial migration associated with vessel remodelling.
The idea that Ang1 signals through integrins is supported by the finding that in myocytes, that lack Tie receptors, Ang1 activation of Akt and Erk1/2, as well as survival, is inhibited by RGD peptides and integrin-blocking antibodies 10. Furthermore, a recent study found that the isolated Ang1 fibrinogen domain is able to bind α5β1 and suppress permeability of an endothelial monolayer without stimulating Tie2 34. Thus, integrins may signal from Ang1 independently of Tie2. However, the integrin α5β1 also contributes to Tie2 signalling; it associates constitutively with Tie2 and increases the sensitivity of the receptor tyrosine kinase to Ang1, with the receptor and downstream substrate p85 becoming phosphorylated at lower concentrations of Ang1 when the integrin is engaged 35. Ang1-activated endothelial motility is also enhanced in endothelial cells adhering to the α5β1 ligand, fibronectin 35.
The specific signalling pathways and downstream cellular and in vivo functions regulated by Ang1 need defining. The interactions between Tie2 and the putative new Ang1 receptors require investigation, do any of the receptors act independently to mediate discrete actions of Ang1 or do they co-operate in overlapping functions? For example, there is strong evidence that Ang1 can act through Tie2 in cultured endothelial cells to inhibit apoptosis and stimulate migration, and activation of Tie2 does promote remodelling of endothelial tubes. However, the contribution of other Ang1 receptors to each of these cellular actions is yet to be defined. Furthermore, the involvement of Tie1 and integrins in the effects of the ligand on monolayer integrity or inflammatory gene expression require definition.
VASCULAR PROTECTIVE EFFECTS OF ANGIOPOIETIN-1 IN VIVO
Some of the cellular effects of Ang1 suggest that the ligand would be vascular protective in vivo and Table 2 summarizes the main cellular and in vivo effects of Ang1. Certainly, Ang1 has been found to promote blood vessel survival, inhibit vascular leakage and suppress vessel inflammation.
Table 2.
Principal vascular cellular and in vivo actions of Ang1
| Cellular Effects | Effects in vivo |
|---|---|
| Inhibits apoptosis 21,36-41,47,115-117 | Stimulates vessel survival, inhibits regression 80,81,90 |
| Stimulates migration 11,44,47-49,52 | Stimulates remodelling 90-92 |
| Stimulates tube formation/invasion 49,52,57,58,60,118 | Stimulates angiogenesis, alone or with VEGF/TNFα 40,60,84,93-95 |
| Stimulates protease production 58 | Stimulates lymphatic proliferation & sprouting in mice 96,97 |
| Stimulates proliferation 15,65 | |
| Inhibits leukocyte adhesion 66,67 | Inhibits leukocyte adhesion and infiltration 82,86,88 |
| Inhibits adhesion molecule expression 66,67 | Inhibits allograft fibrosis 88 |
| Inhibits Tissue Factor expression 69 | Inhibits endotoxaemia 87 |
| Inhibits NFκB 70 | |
| Promotes monolayer integrity 53,67,71,72 | Inhibits vascular leakage 82-85 |
Vessel survival
Evidence for increased vessel survival in response to Ang1 in vivo is provided in studies of radiation-exposed mice, where endothelial survival in microvessels of the intestinal villi is increased by the ligand 80. The protective effects of Ang1 in this model differed between different vascular beds, with little protection afforded to liver microvessels but inhibition of apoptosis in lung and intestinal endothelial cells following intravenous administration of the ligand. The ligand also inhibits apoptosis of microvessel endothelium in rats treated with monocrotaline to induce pulmonary injury and hypertension 81.
Permeability
Plasma leakage from skin vessels following treatment with inflammatory stimuli or VEGF is decreased by Ang1 82,83. This effect occurs with both acute and chronic Ang1 administration. VEGF-induced retinal vessel permeability is also decreased by Ang1 and the ligand reduces vascular leakage in the brain following experimental embolic cerebral occlusion in mice 84,85.
Inflammation
Ang1 has anti-inflammatory effects on the vasculature. This is seen in skin of mice transgenically overexpressing VEGF, where leukocyte adhesion is decreased by co-expression of Ang1 82. Decreased adhesion of leukocytes, as well as depressed expression of ICAM1, is also seen in retinal vessels of diabetic rats following Ang1 administration 86. Similarly, expression of the leukocyte adhesion molecules Eselectin, ICAM1 and VCAM1 is suppressed by Ang1 in pulmonary vessels of lipopolysacharide-treated mice 87. Further evidence for in vivo anti-inflammatory effects of Ang1 is provided by the decrease in leukocyte infiltration and fibrosis seen in cardiac allografts of rats overexpressing the ligand 88. Importantly, Ang2 has recently been shown to be a key regulator of vascular inflammation 89. Ang2 appears to be required for TNFα-induced monocyte adhesion to cultured HUVEC and expression of ICAM1 and VCAM1 89. Mice deficient in Ang2 have a markedly attenuated inflammatory response to Staphylococcus aureus and other stimuli and administration of Ang2 reverses this 89. These data are consistent with maintenance of endothelial quiescence via the anti-inflammatory actions of Ang1 and reversal of this by Ang2 antagonistic activity.
Remodelling
In addition to vessel protection, Ang1 has been implicated in vessel remodelling. In developing mice treatment with Ang1 results in enlargement of vessels, particularly on the venous side of the microvasculature 90-92. Localized overexpression of Ang1 in the liver of developing mice induces vascular remodelling effects including hepatic arterial sprouting and enlargement, as well as portal vein dilation 91. The effects of Ang1 on vessel enlargement are more restricted in adult mice with reports of moderate and reversible increases in vessel size in liver, and enlargement of tracheal vessels 90-92. Stimulation of remodelling by Ang1 manifested by apparent angiogenesis has been reported in some studies. A potent Ang1 variant, COMP-Ang1, was found to stimulate angiogenesis in the mouse corneal micropocket assay 93. Others have found Ang1 to be angiogenic in the presence of co-activators such as TNFα or VEGF 40,94, or even to suppress angiogenesis 84,95. Some of the variation in effects observed in these studies may be due to dissimilarities in potency and Ang1 administration route, along with possible differences in sensitivities between vascular beds.
Lymphatic growth
As well as effects on blood vessels, Ang1 stimulates growth of lymphatics, with increased lymphangiogenesis and lymphatic sprouting and hyperplasia observed in mice in response to Ang1 96,97.
Pulmonary hypertension
Ang1 has been implicated in pulmonary hypertension (PH), a disease in which pulmonary arterial pressure is increased and which is associated with increased smooth muscle coverage of pulmonary arterioles 98. However, the precise role of the ligand in PH pathogenesis is yet to be resolved. Increased expression of Ang1 at the mRNA and protein level has been reported in patients with PH, as well as increased levels of activated (phosphorylated) Tie2 99. These changes have been associated with changes in expression of regulators of smooth muscle cell proliferation, specifically suppression of bone morphogenic receptor type 1A (BMPR1A) and increase expression of serotonin 99,100. It has been suggested that these events lead to elevated proliferation of smooth muscle cells around pulmonary arterioles resulting in the increased muscularization of the vessels 99,100. In support of a causative role for Ang1, transgenic overexpression of Ang1 in rat lung can result in a PH-like phenotype and expression of soluble Tie2 ectodomain, to sequester Ang1, suppresses the pathology in monocrotaline- and Ang1 induced models of PH in rats 100,101. However, some anomalies in this model of PH pathogenesis need resolving. For example, it is not clear why Tie2 appears un-activated in normal lung vessels when others have reported the receptor to be stimulated in quiescent lung vasculature 102; furthermore, Ang1 has not previously been reported to activate proliferation of smooth muscle cells surrounding vessels. In contrast to a causative role, Ang1 has also reported to protect against development of PH in the rat monocrotaline model 81. In this case, it is hypothesized that endothelial apoptosis could lead to decreased vessel numbers in the pulmonary bed causing increased resistance and that Ang1 may protect against such endothelial loss 81. Multiple factors contribute to development of PH in humans 103 and additional work will be required to define the importance of Ang1, as well as the potential risks of elevating Ang1, in development of PH. In particular, the apparently contradictory findings of elevated Ang1 in PH and the protective effects of the ligand require further clarification. One possibility is that the reported increased Ang1 is a compensatory response rather than an initiating factor 104.
POTENTIAL CLINICAL APPLICATIONS OF ANGIOPOIETIN-1
Clearly, the effects of Ang1 in vivo suggest that manipulation of this ligand could have therapeutic potential. Such manipulation could include direct administration of Ang1 or potent Ang1 mimetics as well as functionally increasing Ang1 by decreasing or blocking competitive antagonists such as Ang2.
Microvascular regression contributes to a number of diseases including diabetic retinopathy, allograft vasculopathy and sepsis 105 and the anti-apoptotic effects of Ang1 would have possible therapeutic applications in counteracting such regression. Promotion of endothelial survival would also be beneficial in attempts to induce rapid vascularization of transplanted tissues and in limiting radiation damage associated with radiotherapy. Preclinical studies support the concept of therapeutic use of Ang1 to limit endothelial death. These include reports of the pro-survival effects of Ang1 on microvessels of the intestine in a radiation therapy mouse model, discussed above, 80 and increased survival of transplanted skin flaps in a rat model of reconstructive surgery 106.
Vascular leakage is problematic in inflammatory conditions, such as asthma, as well as diabetic retinopathy, stroke and a variety of other pathologies 107-109. The potential therapeutic use of Ang1 to limit oedema is clearly indicated by its ability to inhibit VEGF- and inflammation-induced leakage from vessels of the skin in animal models 82,83. Ang1 suppression of VEGF-induced permeability of retinal vessels in mice provides a further pre-clinical demonstration of its therapeutic potential 84. In a mouse model of stroke, Ang1 inhibited leakage from cerebral vessels and decreased lesion size 85. In addition to decreasing vessel leakage, Ang1 could contribute to resolution of oedema via its actions on lymphatic vessel growth 97.
The suppression of inflammatory gene expression in endothelial cells by Ang1 suggests that the ligand may be useful in suppressing vascular inflammation. This is supported by the pre-clinical studies that show inhibitory effects of Ang1 on leukocyte adhesion and extravasation in animal models of diabetic retinopathy, allograft arteriosclerosis and sepsis 86-88.
Ang1 has significant therapeutic potential. However, its actions to promote vessel remodelling, angiogenesis, and its possible involvement in PH could limit its usefulness. Ways to maximize the vascular protective effects of the ligand and minimize any potential for deleterious actions will need to be sought. A better understanding of the mechanisms of action of Ang1 will be required. This could then allow manipulation at the level of ligand, receptor(s) or intracellular signals to promote the desired therapeutic actions.
CONCLUSIONS
The effects of Ang1 on endothelial cells and blood vessels broadly fall into two categories; those associated with promotion of vessel protection, and those related to vessel remodelling and angiogenesis. Pre-clinical studies support possible therapeutic manipulation of Ang1 for promotion of vessel survival in transplanted tissue, suppression of vascular leakage in stroke as well as protection from endotoxaemia, diabetic retinopathy and allograft arteriosclerosis.
Ang1 mediates its effects through the Tie2 receptor tyrosine kinase. Recent results suggest that the situation may be more complex, as it was shown that the related receptor Tie1 can be activated by Ang1 and thus may mediate Ang1 induced signalling. In addition, integrins appear to interact with both the ligand and the Tie2 receptor. Differences in signalling motifs between these three receptors, such as the possible lack of a Dok-R recruitment site in Tie1, suggest that they could have some distinct signalling and cellular activities. It will be important to define the specific functions of Ang1 that are mediated by each receptor in the cellular and in vivo context, the signalling pathways involved, as well as the significance of interactions and cross-talk between the receptors. It is certainly possible that a better understanding of Ang1 and its receptors will lead to identification of novel cellular, vascular and possibly non-vascular activities controlled by Ang1 via these receptors. The different cellular and signalling effects of the Ang receptors could provide new opportunities for therapeutic manipulation of the vasculature. Thus specific activation of one or a combination of Ang1 receptors, and associated signalling pathways, may promote optimum vessel protection whilst minimizing the possibility of inducing inappropriate vascular remodelling, for example. Correspondingly, other receptors or receptor combinations could be inhibited where it would be beneficial to induce vessel regression.
Irrespective of these possibilities, the unique vascular protective effects of Ang1 make this ligand very attractive for exploitation to provide novel therapeutic options applicable to a wide range of pathologies involving the vasculature.
Acknowledgements
Studies in the authors laboratories have been supported by NIH grant 5 R01 HL075183-02 (KA) and the British Heart Foundation (NB).
Footnotes
Conflict of Interest: None.
References
- 1.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 2.Dumont D, Gradwohl G, Fong G, Puri M, Gertsenstein M, Auerbach A, Breitman M. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 3.Sato T, Tozawa Y, Deutsch U, Wolburg-Bucholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 4.Patan S. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc Res. 1998;56:1–21. doi: 10.1006/mvre.1998.2081. [DOI] [PubMed] [Google Scholar]
- 5.Puri MC, Partanen J, Rossant J, Bernstein A. Interaction of the TEK and TIE receptor tyrosine kinases during cardiovascular development. Development. 1999;126:4569–80. doi: 10.1242/dev.126.20.4569. [DOI] [PubMed] [Google Scholar]
- 6.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 7.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand Angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 9.Saharinen P, Kerkela K, Ekman N, Marron M, Brindle N, Lee GM, Augustin H, Koh GY, Alitalo K. Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J Cell Biol. 2005;169:239–243. doi: 10.1083/jcb.200411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 Promotes Cardiac and Skeletal Myocyte Survival Through Integrins. Circ Res. 2005;96:e8–24. doi: 10.1161/01.RES.0000158285.57191.60. [DOI] [PubMed] [Google Scholar]
- 11.Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct Cell Adhesion to the Angiopoietins Mediated by Integrins. J Biol Chem. 2001;276:26516–26525. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- 12.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 13.Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, McClain J, Copeland NG, Gilbert DJ, Jenkins NA, Huang T, Papadopoulos N, Maisonpierre PC, Davis S, Yancopoulos GD. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci USA. 1999;96:1904–9. doi: 10.1073/pnas.96.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim I, Kim J-H, Moon S-O, Kwak HJ, Kim N-G, Koh G-Y. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000;19:4549–4552. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]
- 15.Teichert-Kuliszewska K, Maisonpierre PC, Jones N, Campbell AI, Master Z, Bendeck MP, Alitalo K, Dumont DJ, Yancopoulos GD, Stewart DJ. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001;49:659–70. doi: 10.1016/s0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee HJ, Cho C-H, Hwang S-J, Choi H-H, Kim K-T, Ahn SY, Kim J-H, Oh JL, Lee GM, Koh GY. Biological characterization of angiopoietin-3 and angiopoietin-4. FASEB J. 2004;18:1200–1208. doi: 10.1096/fj.03-1466com. [DOI] [PubMed] [Google Scholar]
- 17.Procopio WN, Pelavin PI, Lee WM, Yeilding NM. Angiopoietin-1 and -2 coiled coil domains mediate distinct homo-oligomerization patterns, but fibrinogen-like domains mediate ligand activity. J Biol Chem. 1999;274:30196–201. doi: 10.1074/jbc.274.42.30196. [DOI] [PubMed] [Google Scholar]
- 18.Barton WA, Tzvetkova D, Nikolov DB. Structure of the angiopoietin-2 receptor binding domain and identification of surfaces involved in Tie2 recognition. Structure (Camb) 2005;13:825–32. doi: 10.1016/j.str.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Davis S, Papadopoulos N, Aldrich TH, Maisonpierre PC, Huang T, Kovac L, Xu A, Leidich R, Radziejewska E, Rafique A, Goldberg J, Jain V, Bailey K, Karow M, Fandl J, Samuelsson SJ, Ioffe E, Rudge JS, Daly TJ, Radziejewski C, Yancopoulos GD. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat Struct Biol. 2003;10:38–44. doi: 10.1038/nsb880. [DOI] [PubMed] [Google Scholar]
- 20.Kim K-T, Choi H-H, Steinmetz MO, Maco B, Kammerer RA, Ahn SY, Kim H-Z, Lee GM, Koh GY. Oligomerization and multimerization is critical for angiopoietin-1 to bind and phosphorylate tie2. J Biol Chem. 2005;280:20126–20131. doi: 10.1074/jbc.M500292200. [DOI] [PubMed] [Google Scholar]
- 21.Kim I, Kim HG, So J-S, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 22.Stratmann A, Risau W, Plate KH. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol. 1998;153:1459–66. doi: 10.1016/S0002-9440(10)65733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand Angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 24.Jones PF. Not just angiogenesis--wider roles for the angiopoietins. J Pathol. 2003;201:515–27. doi: 10.1002/path.1452. [DOI] [PubMed] [Google Scholar]
- 25.Gerritsen ME, Tomlinson JE, Zlot C, Ziman M, Hwang S. Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br J Pharmacol. 2003;140:595–610. doi: 10.1038/sj.bjp.0705494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. Hypoxia-Inducible Factor-1 Mediates Activation of Cultured Vascular Endothelial Cells by Inducing Multiple Angiogenic Factors. Circ Res. 2003;93:664–673. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- 27.Partanen J, Armstrong E, Makela TP, Korhonen J, Sandberg M, Renkonen R, Knuutila S, Huebner K, Alitalo K. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992;12:1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene. 1992;7:71–80. [PubMed] [Google Scholar]
- 29.Runting AS, Stacker SA, Wilks AF. tie2, a putative protein tyrosine kinase from a new class of cell surface receptor. Growth Factors. 1993;9:99–105. [PubMed] [Google Scholar]
- 30.Fiedler U, Krissl T, Koidl S, Weiss C, Koblizek T, Deutsch U, Martiny-Baron G, Marme D, Augustin HG. Angiopoietin-1 and Angiopoietin-2 Share the Same Binding Domains in the Tie-2 Receptor Involving the First Ig-like Loop and the Epidermal Growth Factor-like Repeats. J Biol Chem. 2003;278:1721–1727. doi: 10.1074/jbc.M208550200. [DOI] [PubMed] [Google Scholar]
- 31.Marron MB, Hughes DP, Edge MD, Forder CL, Brindle NPJ. Evidence for heterotypic interaction between the receptor tyrosine kinases TIE-1 and TIE-2. J Biol Chem. 2000;275:39741–39746. doi: 10.1074/jbc.M007189200. [DOI] [PubMed] [Google Scholar]
- 32.Tsiamis AC, Morris PN, Marron MB, Brindle NPJ. Vascular endothelial growth factor modulates the Tie-2:Tie-1 receptor complex. Microvasc Res. 2002;63:149–158. doi: 10.1006/mvre.2001.2377. [DOI] [PubMed] [Google Scholar]
- 33.Chen-Konak L, Guetta-Shubin Y, Yahav H, Shay-Salit A, Zilberman M, Binah O, Resnick N. Transcriptional and post-translation regulation of the Tie1 receptor by fluid shear stress changes in vascular endothelial cells. FASEB J. 2003;17:2121–2123. doi: 10.1096/fj.02-1151fje. [DOI] [PubMed] [Google Scholar]
- 34.Weber CC, Cai H, Ehrbar M, Kubota H, Martiny-Baron G, Weber W, Djonov V, Weber E, Mallik AS, Fussenegger M, Frei K, Hubbell JA, Zisch AH. Effects of protein and gene transfer of the Ang1 fibrinogen-like receptor-binding domain for endothelial and vessel organization. J Biol Chem. 2005;280:22445–22453. doi: 10.1074/jbc.M410367200. [DOI] [PubMed] [Google Scholar]
- 35.Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between {alpha}5{beta}1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999;448:249–53. doi: 10.1016/s0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- 37.Kwak HJ, Lee SJ, Lee YH, Ryu CH, Koh KN, Choi HY, Koh GY. Angiopoietin-1 Inhibits Irradiation- and Mannitol-Induced Apoptosis in Endothelial Cells. Circulation. 2000;101:2317–3224. doi: 10.1161/01.cir.101.19.2317. [DOI] [PubMed] [Google Scholar]
- 38.Papapetropoulos A, García-Cardeña G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79:213–23. [PubMed] [Google Scholar]
- 39.Kim I, Moon S, Han C, Pak YK, Moon SK, Kim JJ, Koh GY. The angiopoietin-tie2 system in coronary artery endothelium prevents oxidized low-density lipoprotein-induced apoptosis. Cardiovasc Res. 2001;49:872–881. doi: 10.1016/s0008-6363(00)00295-9. [DOI] [PubMed] [Google Scholar]
- 40.Chen J-X, Chen Y, DeBusk L, Lin W, Lin PC. Dual functional roles of Tie-2/angiopoietin in TNF-{alpha}-mediated angiogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H187–195. doi: 10.1152/ajpheart.01058.2003. [DOI] [PubMed] [Google Scholar]
- 41.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 42.Valable S, Bellail A, Lesne S, Liot G, MacKenzie ET, Vivien D, Bernaudin M, Petit E. Angiopoietin-1-induced phosphatidyl-inositol 3-kinase activation prevents neuronal apoptosis. FASEB J. 2003;17:443–445. doi: 10.1096/fj.02-0372fje. [DOI] [PubMed] [Google Scholar]
- 43.Kontos CD, Stauffer TP, Yang WP, York JD, Huang L, Blanar MA, Meyer T, Peters KG. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol. 1998;18:4131–40. doi: 10.1128/mcb.18.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont DJ. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem. 1999;274:30896–905. doi: 10.1074/jbc.274.43.30896. [DOI] [PubMed] [Google Scholar]
- 45.Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai K-M, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18:1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem. 1998;273:18514–21. doi: 10.1074/jbc.273.29.18514. [DOI] [PubMed] [Google Scholar]
- 47.Fujikawa K, de Aos Scherpenseel I, Jain SK, Presman E, Christensen RA, Varticovski L. Role of PI 3-kinase in angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells. Exp Cell Res. 1999;253:663–72. doi: 10.1006/excr.1999.4693. [DOI] [PubMed] [Google Scholar]
- 48.Cascone I, Audero E, Giraudo E, Napione L, Maniero F, Philips MR, Collard JG, Serini G, Bussolino F. Tie-2 - dependent activation of RhoA and Rac1 participates in endothelial cell motility triggered by angiopoietin-1. Blood. 2003;102:2482–2490. doi: 10.1182/blood-2003-03-0670. [DOI] [PubMed] [Google Scholar]
- 49.Chen JX, Lawrence ML, Cunningham G, Christman BW, Meyrick B. HSP90 and Akt modulate Ang-1-induced angiogenesis via NO in coronary artery endothelium. J Appl Physiol. 2004;96:612–20. doi: 10.1152/japplphysiol.00728.2003. [DOI] [PubMed] [Google Scholar]
- 50.Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, Dumont DJ. A Unique Autophosphorylation Site on Tie2/Tek Mediates Dok-R Phosphotyrosine Binding Domain Binding and Function. Mol Cell Biol. 2003;23:2658–2668. doi: 10.1128/MCB.23.8.2658-2668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Master Z, Jones N, Tran J, Jones J, Kerbel RS, Dumont DJ. Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak. EMBO J. 2001;20:5919–5928. doi: 10.1093/emboj/20.21.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Audero E, Cascone I, Maniero F, Napione L, Arese M, Lanfrancone L, Bussolino F. Adaptor ShcA Protein Binds Tyrosine Kinase Tie2 Receptor and Regulates Migration and Sprouting but Not Survival of Endothelial Cells. J Biol Chem. 2004;279:13224–13233. doi: 10.1074/jbc.M307456200. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Hahn CN, Parsons M, Drew J, Vadas MA, Gamble JR. Role of protein kinase C{zeta} in thrombin-induced endothelial permeability changes: inhibition by angiopoietin-1. Blood. 2004;104:1716–1724. doi: 10.1182/blood-2003-11-3744. [DOI] [PubMed] [Google Scholar]
- 54.Kosacka J, Figiel M, Engele J, Hilbig H, Majewski M, Spanel-Borowski K. Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell Tissue Res. 2005;320:11–19. doi: 10.1007/s00441-004-1068-2. [DOI] [PubMed] [Google Scholar]
- 55.Iurlaro M, Scatena M, Zhu W-H, Fogel E, Wieting SL, Nicosia RF. Rat aorta-derived mural precursor cells express the Tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–3643. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- 56.Metheny-Barlow LJ, Tian S, Hayes AJ, Li LY. Direct chemotactic action of angiopoietin-1 on mesenchymal cells in the presence of VEGF. Microvasc Res. 2004;68:221–30. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–32. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- 58.Kim I, Kim HG, Moon S-O, Chae SW, So J-N, Koh KN, Ahn BC, Koh GY. Angiopoietin-1 Induces Endothelial Cell Sprouting Through the Activation of Focal Adhesion Kinase and Plasmin Secretion. Circ Res. 2000;86:952–959. doi: 10.1161/01.res.86.9.952. [DOI] [PubMed] [Google Scholar]
- 59.Hansbury MJ, Nicosia RF, Zhu WH, Holmes SJ, Winkler JD. Production and characterization of a Tie2 agonist monoclonal antibody. Angiogenesis. 2001;4:29–36. doi: 10.1023/a:1016678828930. [DOI] [PubMed] [Google Scholar]
- 60.Babaei S, Teichert-Kuliszewska K, Zhang Q, Jones N, Dumont DJ, Stewart DJ. Angiogenic Actions of Angiopoietin-1 Require Endothelium-Derived Nitric Oxide. Am J Pathol. 2003;162:1927–1936. doi: 10.1016/S0002-9440(10)64326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim I, Ryu YS, Kwak HJ, Ahn SY, Oh JL, Yancopoulos GD, Gale NW, Koh GY. EphB ligand, ephrinB2, suppresses the VEGF- and angiopoietin 1-induced Ras/mitogen-activated protein kinase pathway in venous endothelial cells. FASEB J. 2002;16:1126–8. doi: 10.1096/fj.01-0805fje. [DOI] [PubMed] [Google Scholar]
- 62.Harfouche R, Gratton JP, Yancopoulos GD, Noseda M, Karsan A, Hussain SN. Angiopoietin-1 activates both anti- and proapoptotic mitogen-activated protein kinases. FASEB J. 2003;17:17. doi: 10.1096/fj.02-0698fje. [DOI] [PubMed] [Google Scholar]
- 63.Huang L, Turck C, Rao P, Peters K. GRB2 and SH-PTP2:potentially important endothelial signaling molecules downstream of the TEK/TIE2 receptor tyrosine kinase. Oncogene. 1995;11:2097–2103. [PubMed] [Google Scholar]
- 64.Li W, Nishimura R, Kashishian A, Batzer AG, Kim WJH, Cooper JA, Schlessinger J. A new function for a phosphotyrosine phosphatase: Linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanda S, Miyata Y, Mochizuki Y, Matsuyama T, Kanetake H. Angiopoietin 1 Is Mitogenic for Cultured Endothelial Cells. Cancer Res. 2005;65:6820–6827. doi: 10.1158/0008-5472.CAN-05-0522. [DOI] [PubMed] [Google Scholar]
- 66.Kim I, Moon S-O, Park SK, Chae SW, Koh GY. Angiopoietin-1 Reduces VEGF-Stimulated Leukocyte Adhesion to Endothelial Cells by Reducing ICAM-1, VCAM-1, and E-Selectin Expression. Circ Res. 2001;89:477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 67.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-Inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603–607. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 68.Lemieux C, Maliba R, Favier J, Theoret J-F, Merhi Y, Sirois MG. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105:1523–1530. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 69.Kim I, Oh JL, Ryu YS, So JN, Sessa WC, Walsh K, Koh GY. Angiopoietin-1 negatively regulates expression and activity of tissue factor in endothelial cells. FASEB J. 2002;16:126–8. doi: 10.1096/fj.01-0556fje. [DOI] [PubMed] [Google Scholar]
- 70.Hughes DP, Marron MB, Brindle NPJ. The Antiinflammatory Endothelial Tyrosine Kinase Tie2 Interacts With a Novel Nuclear Factor-{kappa}B Inhibitor ABIN-2. Circ Res. 2003;92:630–636. doi: 10.1161/01.RES.0000063422.38690.DC. [DOI] [PubMed] [Google Scholar]
- 71.Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004;15:566–74. doi: 10.1097/01.asn.0000115397.22519.03. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Pampou S, Fujikawa K, Varticovski L. Opposing effect of angiopoietin-1 on VEGF-mediated disruption of endothelial cell-cell interactions requires activation of PKCbeta. J Cell Physiol. 2004;198:53–61. doi: 10.1002/jcp.10386. [DOI] [PubMed] [Google Scholar]
- 73.Iwama A, Hamaguchi I, Hashiyama M, Murayama Y, Yasunaga K, Suda T. Molecular cloning and characterization of mouse TIE and TEK receptor tyrosine kinase genes and their expression in hematopoietic stem cells. Biochem Biophys Res Comm. 1993;195:301–309. doi: 10.1006/bbrc.1993.2045. [DOI] [PubMed] [Google Scholar]
- 74.Batard P, Sansilvestri P, Seheinecker C, Knapp W, Debili N, Vainchenker W, Buhring H, Monier M, Kukk E, Partanen J, Matikainen M, Alitalo R, Hatzfeld J, Alitalo K. The tie receptor tyrosine kinase is expressed by human hematopoietic progenitor cells and by a subset of megakaryocytic cells. Blood. 1996;87:2212–2220. [PubMed] [Google Scholar]
- 75.Hashiyama M, Iwama A, Ohshiro K, Kurozumi K, Yasunaga K, Shimizu Y, Masuho Y, Matsuda I, Yamaguchi N, Suda T. Predominant expression of a receptor tyrosine kinase, TIE, in hematopoietic stem cells and B cells. Blood. 1996;87:93–101. [PubMed] [Google Scholar]
- 76.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 77.Puri M, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14:5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kontos CD, Cha EH, York JD, Peters KG. The endothelial receptor tyrosine kinase Tie1 activates phosphatidylinositol 3-kinase and Akt to inhibit apoptosis. Mol Cell Biol. 2002;22:1704–13. doi: 10.1128/MCB.22.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–78. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 80.Cho C-H, Kammerer RA, Lee HJ, Yasunaga K, Kim K-T, Choi H-H, Kim W, Kim SH, Park SK, Lee GM, Koh GY. Designed angiopoietin-1 variant, COMP-Ang1, protects against radiation-induced endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101:5553–5558. doi: 10.1073/pnas.0307575101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao YD, Campbell AIM, Robb M, Ng D, Stewart DJ. Protective Role of Angiopoietin-1 in Experimental Pulmonary Hypertension. Circ Res. 2003;92:984–991. doi: 10.1161/01.RES.0000070587.79937.F0. [DOI] [PubMed] [Google Scholar]
- 82.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 83.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nature Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 84.Nambu H, Nambu R, Oshima Y, Hackett SF, Okoye G, Wiegand S, Yancopoulos G, Zack DJ, Campochiaro PA. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004;11:865–73. doi: 10.1038/sj.gt.3302230. [DOI] [PubMed] [Google Scholar]
- 85.Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683–7. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 86.Joussen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q, Moromizato Y, Bursell SE, Wiegand SJ, Rudge J, Ioffe E, Yancopoulos GD, Adamis AP. Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol. 2002;160:1683–93. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Witzenbichler B, Westermann D, Knueppel S, Schultheiss H-P, Tschope C. Protective Role of Angiopoietin-1 in Endotoxic Shock. Circulation. 2005;111:97–105. doi: 10.1161/01.CIR.0000151287.08202.8E. [DOI] [PubMed] [Google Scholar]
- 88.Nykanen AI, Krebs R, Saaristo A, Turunen P, Alitalo K, Yla-Herttuala S, Koskinen PK, Lemstrom KB. Angiopoietin-1 Protects Against the Development of Cardiac Allograft Arteriosclerosis. Circulation. 2003;107:1308–1314. doi: 10.1161/01.cir.0000054623.35669.3f. [DOI] [PubMed] [Google Scholar]
- 89.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Thurston G, Gale NW, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nature Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 90.Baffert F, Thurston G, Rochon-Duck M, Le T, Brekken R, McDonald DM. Age-Related Changes in Vascular Endothelial Growth Factor Dependency and Angiopoietin-1-Induced Plasticity of Adult Blood Vessels. Circ Res. 2004;94:984–992. doi: 10.1161/01.RES.0000125295.43813.1F. [DOI] [PubMed] [Google Scholar]
- 91.Ward NL, Haninec AL, Van Slyke P, Sled JG, Sturk C, Henkelman RM, Wanless IR, Dumont DJ. Angiopoietin-1 Causes Reversible Degradation of the Portal Microcirculation in Mice: Implications for Treatment of Liver Disease. Am J Pathol. 2004;165:889–899. doi: 10.1016/S0002-9440(10)63351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thurston G, Wang Q, Baffert F, Rudge J, Papadopoulos N, Jean-Guillaume D, Wiegand S, Yancopoulos GD, McDonald DM. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development. 2005;132:3317–3326. doi: 10.1242/dev.01888. [DOI] [PubMed] [Google Scholar]
- 93.Cho C-H, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim K-T, Kim I, Choi H-H, Kim W, Kim SH, Park SK, Lee GM, Koh GY. COMP-Ang1: A designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–40. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 95.Nambu H, Umeda N, Kachi S, Oshima Y, Akiyama H, Nambu R, Campochiaro PA. Angiopoietin 1 prevents retinal detachment in an aggressive model of proliferative retinopathy, but has no effect on established neovascularization. J Cell Physiol. 2005;204:227–235. doi: 10.1002/jcp.20292. [DOI] [PubMed] [Google Scholar]
- 96.Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, Maekawa H, Kimura Y, Ohmura M, Miyamoto T, Nozawa S, Koh GY, Alitalo K, Suda T. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 97.Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4658. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 98.Perros F, Dorfmuller P, Humbert M. Current insights on the pathogenesis of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2005;26:355–64. doi: 10.1055/s-2005-916149. [DOI] [PubMed] [Google Scholar]
- 99.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, Yuan JX, Deutsch R, Jamieson SW, Thistlethwaite PA. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med. 2003;348:500–9. doi: 10.1056/NEJMoa021650. [DOI] [PubMed] [Google Scholar]
- 100.Sullivan CC, Du L, Chu D, Cho AJ, Kido M, Wolf PL, Jamieson SW, Thistlethwaite PA. Induction of pulmonary hypertension by an angiopoietin 1/TIE2/serotonin pathway. Proc Natl Acad Sci USA. 2003;100:12331–12336. doi: 10.1073/pnas.1933740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kido M, Du L, Sullivan CC, Deutsch R, Jamieson SW, Thistlethwaite PA. Gene transfer of a TIE2 receptor antagonist prevents pulmonary hypertension in rodents. J Thorac Cardiovasc Surg. 2005;129:268–76. doi: 10.1016/j.jtcvs.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 102.Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 1997;81:567–574. doi: 10.1161/01.res.81.4.567. [DOI] [PubMed] [Google Scholar]
- 103.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation. 2005;111:534–8. doi: 10.1161/01.CIR.0000156326.48823.55. [DOI] [PubMed] [Google Scholar]
- 104.Rudge JS, Thurston G, Yancopoulos GD. Angiopoietin-1 and Pulmonary Hypertension: Cause or Cure? Circ Res. 2003;92:947–949. doi: 10.1161/01.RES.0000074031.94558.99. [DOI] [PubMed] [Google Scholar]
- 105.Stefanec T. Endothelial Apoptosis: Could It Have a Role in the Pathogenesis and Treatment of Disease? Chest. 2000;117:841–854. doi: 10.1378/chest.117.3.841. [DOI] [PubMed] [Google Scholar]
- 106.Jung H, Gurunluoglu R, Scharpf J, Siemionow M. Adenovirus-mediated angiopoietin-1 gene therapy enhances skin flap survival. Microsurgery. 2003;23:374–80. doi: 10.1002/micr.10140. [DOI] [PubMed] [Google Scholar]
- 107.Persson CG. Role of plasma exudation in asthmatic airways. Lancet. 1986;2:1126–9. doi: 10.1016/s0140-6736(86)90533-7. [DOI] [PubMed] [Google Scholar]
- 108.Cunha-Vaz J. Diabetic macular edema. Eur J Ophthalmol. 1998;8:127–30. doi: 10.1177/112067219800800301. [DOI] [PubMed] [Google Scholar]
- 109.Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for Vascular Protection After Acute Ischemic Stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 110.Partanen J, Puri M, Schwartz L, Fischer K-D, Bernstein A, Rossant J. Cell autonomous functions of the receptor tyrosine kinase TIE in a late phase of angiogenic capillary growth and endothelial cell survival during murine development. Development. 1996;122:3013–3021. doi: 10.1242/dev.122.10.3013. [DOI] [PubMed] [Google Scholar]
- 111.Puri MC, Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci USA. 2003;100:12753–12758. doi: 10.1073/pnas.2133552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–71. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 113.Gale N, Thurston G, Hackett S, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte M, Jackson D, Suri C, Campochiaro P, Wiegand S, Yancopoulos G. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 114.Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–7. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 115.Harfouche R, Hassessian HM, Guo Y, Faivre V, Srikant CB, Yancopoulos GD, Hussain SN. Mechanisms which mediate the antiapoptotic effects of angiopoietin-1 on endothelial cells. Microvasc Res. 2002;64:135–47. doi: 10.1006/mvre.2002.2421. [DOI] [PubMed] [Google Scholar]
- 116.Tadros A, Hughes DP, Dunmore BJ, Brindle NPJ. ABIN-2 protects endothelial cells from death and has a role in the antiapoptotic effect of angiopoietin-1. Blood. 2003;102:4407–4409. doi: 10.1182/blood-2003-05-1602. [DOI] [PubMed] [Google Scholar]
- 117.DeBusk LM, Hallahan DE, Lin PC. Akt is a major angiogenic mediator downstream of the Ang1/Tie2 signaling pathway. Exp Cell Res. 2004;298:167–77. doi: 10.1016/j.yexcr.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 118.Hayes AJ, Huang WQ, Mallah J, Yang D, Lippman ME, Li LY. Angiopoietin-1 and Its Receptor Tie-2 Participate in the Regulation of Capillary-like Tubule Formation and Survival of Endothelial Cells. Microvasc Res. 1999;58:224–237. doi: 10.1006/mvre.1999.2179. [DOI] [PubMed] [Google Scholar]


