Abstract
Background
Glyceroneogenesis, i.e. formation of triglyceride glycerol from pyruvate, is a critical component of triglyceride fatty acid cycling in-vivo. The quantitative contribution of glyceroneogenesis to triglyceride glycerol and its hormonal regulation have not been examined in humans.
Methods
We have quantified the contribution of pyruvate to VLDL triglycerides in subjects with type II diabetes mellitus using the deuterium labeling of body water technique. Subjects with type II diabetes were studied before and after a six-month behavioral intervention therapy, during fasting and during a hyperinsulinemic normoglycemic clamp. Response to glucagon infusion was examined in five healthy subjects after an overnight fast.
Results
Glyceroneogenesis contributed ∼54% to VLDL triglyceride glycerol in type II diabetes as compared with ∼12% contribution of plasma glucose. There was no effect of insulin plus glucose during hyperinsulinemic clamp on glyceroneogenesis, even following clinical interventions, when insulin sensitivity had improved. In healthy subjects, the contribution of triosephosphates to plasma VLDL triglycerides was ∼45%.
Conclusion
Glyceroneogenesis, in contrast to glycolysis, is the predominant source of triglyceride-glycerol carbon for VLDL triglycerides in subjects with type II diabetes. The contribution of glyceroneogenesis to triglyceride-glycerol is not affected by short (4h) infusion of insulin in type 2 diabetes.
BACKGROUND
The failure to regulate the concentration of free fatty acids (FFA) in the blood is a critical factor in the genesis of insulin resistance in humans [1]. An important element in the control of blood FFA levels is their rate of re-esterification in the liver; as much as 65% of the FFA released from the adipose tissue during fasting are re-esterified and re-deposited [2]. This triglyceride/fatty acid cycling insures that fatty acids are available to support energy requirements. A central feature of the cycle is glyceroneogenesis, an abbreviated version of gluconeogenesis, in which carbon from sources other than glucose or glycerol contribute to the formation of the glycerol-3-phosphate, which is converted to the glyceride-glycerol of triglycerides [2]. The key enzyme in this pathway, the cytosolic form of phosphoenolpyruvate carboxykinase (PEPCK-C), is present in varying activities in tissues that synthesize triglycerides, such as liver, white and brown adipose tissue and muscle [3]. Alterations in the expression of PEPCK-C in various tissues of transgenic mice have been shown to be associated with changes in triglyceride accumulation or depletion [4-7].
Despite its potential physiological and clinical relevance, there is little information available concerning the rate of glyceroneogenesis and its regulation in humans. The extent of glyceroneogenesis in vivo has been quantified in adipose tissue and liver of rats [8-10] and in the livers of humans [11] using various isotopic tracer techniques. The study with humans demonstrated that hepatic glyceroneogenesis accounted for 30-60% of the plasma triglyceride-glycerol following an overnight fast. Plasma glycerol provided a low 5% of triglyceride-glycerol. There are no data, however, on the relative contribution of glyceroneogenesis vs. glycolysis for glycerol-3-phosphate and their hormonal regulation in humans. In the present study, we have examined the quantitative contribution of glyceroneogenesis to plasma triglyceride-glycerol in subjects with type II diabetes. The response to intravenous glucose and insulin infusion was examined in subjects with diabetes before and after improvement in insulin sensitivity as a result of diet and behavior modification. Since glyceroneogenesis and gluconeogenesis have a common pathway, the impact of stimulation of gluconeogenesis by glucagon was examined in healthy controls. The data underscore the role of glyceroneogenesis in the reesterification of fatty acids in the liver, and suggest that the regulation of glucose synthesis in the liver is not linked to the regulation of glyceroneogenesis.
METHODS
Hepatic glyceroneogenesis was quantified using the deuterium labeling of the body water [12] in subjects with type II diabetes and in healthy controls. The response to a hyperinsulinemic normoglycemic clamp was determined in type II diabetic subjects. In addition, the response to intravenous glucagon was examined in healthy subjects.
The contribution of glucose and pyruvate carbon to glyceride-glycerol in plasma VLDL triglycerides was quantified in ten type II diabetic subjects before and after six months of treatment including behavioral intervention alone (n=8) and combined (n=2) with intestinal lipase inhibitor, Orlistat® [13,14]. The data of the two subjects on Orlistat® were not different from those on the placebo; therefore the entire group of ten is reported together. The data of weight loss, insulin resistance, regional adiposity and fatty acids have been reported previously [13,14]. The clinical and metabolic data of study subjects are displayed in Table 1. After enrollment, the volunteers with type II diabetes mellitus were asked to withdraw from prior diabetes medication for a four-week baseline period. A nutritional plan for weight maintenance was provided during this period. Following a standardized meal the evening before the study, the subjects fasted overnight. The next morning, an oral dose of deuterated water (∼125 g) was administered in order to achieve a total body water [2H] enrichment ∼0.4%. The rate of glucose production was quantified by infusing a prime constant rate infusion of [6,6-2H2]glucose intravenously. The priming dose of the tracer was adjusted in proportion to the magnitude of fasting hyperglycemia. Following 4h of tracer infusion for a baseline determination, a hyperinsulinemic normoglycemic clamp was instituted to quantify insulin sensitivity and to examine the effect of glucose and insulin infusion on the incorporation of glucose and pyruvate into triglyceride. A 4h continuous infusion of insulin was given at 40 μU/m2.min. Plasma glucose was measured every five minutes and was allowed to decrease until normoglycemia (100 mg.dl-1) was achieved, which was then maintained with an adjustable infusion of 20% glucose. Blood samples for the enrichment of [6,6-2H2]glucose and for the 2H enrichment of VLDL triglyceride glycerol were obtained at periodic intervals. The dextrose infusion was pre-labeled with [6,6-2H2]glucose to maintain stable enrichment of plasma glucose [15].
Table 1.
Clinical and Metabolic Data on Subjects with Type II Diabetes
| Age yrs | Weight kg | BMI kg/m2 | Glucose mg/dl | Insulin μU/ml | HOMA-IR§ | HBA1c % | FFA μmol/L | TRIG mg/dl | |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 55.9 (6.2) | 95.9 (18.00) | 33.3 (6.3) | 162.4 (38.0) | 17.8 (9.4) | 6.6 (3.2) | 7.8 (1.3) | 650.0 (141.0) | 153.3 (60.2) |
| 6 months | 56.4 (6.2) | 87.1* (14.3) | 30.3* (5.1) | 121.9+ (46.5) | 12.6+ (7.7) | 3.6* (1.9) | 6.8 (2.2) | 616.1 (90.9) | 104.1+ (37.3) |
Data are mean (± SD). Ten type II diabetic subjects were studied at baseline and six months after an intervention consisting of diet and behavior modification as described in the Methods. Blood samples were obtained after an overnight fast.
BMI: Body mass index
HOMAR-IR: homeostasis model of assessment - insulin resistance (fasting plasma glucose x fasting plasma insulin)/22.5; FFA - plasma free fatty acid; TRIG - plasma triglycerides
Significantly different from the baseline, paired t
p<0.003
p<0.01
Five healthy subjects, age 40 ± 7.6 years, body weight 78.6 ± 13.0 kg, BMI 26.1 ± 1.5 kg/m2 (mean ± SD) were studied after an overnight fast. They were also given oral [2H2]O, 4 g.kg-1 body weight divided into two doses given at 4:00 a.m. and 6:00 a.m. and [6,6-2H2]glucose tracer as described above. After a basal period of 3 h, the response to an unopposed effect of glucagon was examined by infusing octreotide (50 μg.h-1) along with glucagon (∼3 ng.kg-1.min-1) for 3 h. Blood samples were obtained at frequent intervals though an indwelling cannula.
Analytical Methods
The deuterium enrichment of hydrogens on C-6 of glucose was measured by periodate oxidation followed by conversion of the formaldehyde formed into hexamethylenetetramine (HMT), as described previously [16]. Enrichment of mono-labeled m1 and di-labeled m2 species of HMT was quantified using GC-mass spectrometry. VLDL triglycerides were separated from the plasma by ultracentrifugation [17]. The triglycerides were further extracted using chloroform:methanol (2:1). The isolated triglycerides were hydrolyzed using KOH/ethanol; glycerol was isolated by sequential ion exchange chromatography followed by liquid chromatography. The aqueous layer was passed through an ion exchange column, diameter 0.5 cm, consisting of AG 1×8 formate and AG 50W × 8 resins. The neutral fraction, eluted with 5 ml water, was dried, reconstituted and further purified using an Agilent HPLC system (Agilent Technologies; Wilmington, DW). Aminex HPX-87 column 300 cm × 7.8 mm was used (Bio-Rad Laboratories, Inc.; Hercules, CA). The column temperature was maintained at 80°C, and glycerol was eluted using HPLC grade water at 0.6 ml.min-1. The glycerol peak was evaporated to dryness. An aliquot of glycerol was treated with periodic acid, resulting in the cleavage of both C-1 and C-3 as formaldehyde, which was then converted to hexamethylenetetramine as described above. Another aliquot of glycerol was enzymatically converted to glycerol-3-phosphate by incubating for one h at 37°C with 5 mM ATP and 2 units glycerokinase in 0.1M phosphate buffer, pH 7.4. Glycerol-3-phosphate was isolated by ion exchange chromatography using AG 1 × 8 resin in 1N formic acid. The column was sequentially eluted with water, 1N formic acid and 4N formic acid. Glycerol-3-phosphate appeared in the 4 N formic acid fraction. The recovery was ∼80%. The isolated glycerol-3-phosphate was treated with periodic acid, resulting in the cleavage of only C-1 of glycerol as formaldehyde, which was then converted to hexamethylenetetramine [16]. The deuterium enrichments of hydrogens on C-1 of glycerol were then subtracted from the sum of C-1 and C-3 in order to estimate the enrichments on hydrogens on C-3.
Calculation
The fractional contribution of gluconeogenesis to glucose was calculated as follows:
% Gluconeogenesis from pyruvate = 100 x (0.5 x 2H enrichment of glucose C-6 / 2H enrichment in body water)
The 2H enrichment of glucose C-6 is halved because 2 hydrogens bound to C-6 glucose are enriched. Because of the extensive exchange between the hydrogens of C-3 of pyruvate and the hydrogens in body water (at alanine aminotransferase and during the oxaloacetate, malate, and fumarate exchange in the TCA cycle), the [2H] enrichment of body water and that of H on C-3 of pyruvate is expected to be the same. Therefore the 2H enrichment of body water has been used as a measure of 2H enrichment of C-3 of pyruvate - the precursor for both gluconeogenesis and glyceroneogenesis [12,19].
The fractional contribution of pyruvate to VLDL triglyceride was calculated similarly.
% Contribution of pyruvate to triglyceride glycerol = 100 x (0.5 x 2H enrichment of C-3 glycerol / 2H enrichment in body water). The 2H enrichment of C-3 glycerol is halved because of 2 hydrogens bound to C-3 of glycerol.
Since the hydrogens on C-1 of glycerol can also be labeled from body water during isomerization of glyceraldehydes-3-phosphate, estimates of glyceroneogenesis using labeling on C-1 should be designated as the contribution of triosephosphate rather than that of pyruvate.
Contribution of glucose
The fraction of triglyceride glycerol derived from glucose was estimated by comparing the m2 enrichment on carbon 3 of glycerol (derived from two deuteriums on C-6 of [6,6-2H2]glucose) with that on C-6 of glucose. Carbon 6 of glucose forms carbon 3 of triglyceride glycerol. Therefore, [2H2] tracer from C-6 of glucose will not be expected to appear on C-1 of glycerol. Such was the case both during the basal state and during the hyperinsulinemic clamp in the diabetic subjects (Table 3).
Table 3.
M2 Enrichment (%) of Plasma Glucose, VLDL Triglyceride Glycerol and the Contribution of Plasma Glucose to VLDL Triglyceride
| m2 Enrichment of Glucose |
m2 Enrichment of Glycerol |
% TG from Glucose |
||||||
|---|---|---|---|---|---|---|---|---|
| C-6 | C-1 | C-3 | ||||||
| Basal | Clamp | Basal | Clamp | Basal | Clamp | Basal | Clamp | |
| Baseline | 1.86 ± 0.31 | 2.39 ± 0.25 | 0.018 ± 0.03 | 0.016 ± 0.03 | 0.144 ± 0.11 | 0.146 ± 0.07 | 15.7 ± 11.9 | 12.3 ± 6.3 |
| 6 Months | 2.03 ± 0.30 | 2.45 ± 0.29 | 0.014 ± 0.01 | 0.014 ± 0.02 | 0.126 ± 0.07 | 0.150 ± 0.07 | 12.7 ± 7.9 | 11.8 ± 4.9 |
Data are mean ± SD; TG = triglyceride glycerol
Ten type II subjects were studied at baseline and following 6 months of intervention consisting of diet and behavior (please see methods). Following an overnight fast, [6,6-2H2]glucose was administered as a prime constant rate infusion. After a basal observation of 4 h, a hyperinsulinemic euglycemic clamp was performed for 4 h. [2H2] enrichment (m2) of C-6 glucose and of C-1 and C-3 of glycerol were quantified.
Triglyceride glycerol from glucose (%) = 100 x (2 x m2 enrichment of C-3 glycerol / m2 enrichment of C-6 glucose)
The results are multiplied by 2 since each molecule of glucose forms two molecules of glycerol.
All data are presented as mean ± SD. Changes within the group were evaluated by paired t-test. Linear regression was calculated by the method of least squares.
RESULTS
The clinical and metabolic data in the subjects with type II diabetes are displayed in Table 1 The subjects were obese with high body mass index, had fasting hyperglycemia and hyperinsulinemia, and had high plasma free fatty acid and triglyceride levels. In addition, they showed evidence for insulin resistance (HOMA: homeostatic model of assessment) [18]. Six months of intervention resulted in loss of body weight and significant improvement in all clinical and metabolic parameters. Of note, there was a significant reduction in plasma insulin levels, the insulin resistance index (HOMA) and a decrease in plasma triglyceride levels.
Glucose kinetics and gluconeogenesis from pyruvate
The deuterium enrichment of hydrogens on C-6 of glucose is displayed in Table 2 along with the 2H enrichment of body water. A steady state enrichment was achieved both for m1, [2H from body water], representing gluconeogenesis from pyruvate and for m2 representing the dilution of infused [6,62H2]glucose. The rate appearance (Ra) of glucose calculated from the dilution of infused tracer was 169.5 ± 32.2 mg.min-1 prior to intervention and 150.7 ± 27.5 mg.min-1 (p<0.02) following 6 months of intervention. Since all subjects had a significant loss in body weight (p<0.003), the glucose Ra, when normalized for body weight (mg.kg-1.min-1), was not significantly different. Intervention and weight loss had no impact on the fractional contribution of gluconeogenesis from pyruvate during the basal state (Baseline: 60.0 ± 9.4%; 6 Months: 60.9 ± 7.8%).
Table 2.
2H Enrichment (%), m1 and m2, of C-6 of Plasma Glucose During the Basal State and During Hyperinsulinemic Normoglycemic Clamp
| Basal |
Hyperinsulinemic Clamp |
|||||||
|---|---|---|---|---|---|---|---|---|
| Min | -30 | -15 | 0 | +90 | +210 | +240 | Body Water | |
| Baseline (10) | m1 | 0.37 ± 0.07 | 0.39 ± 0.05 | 0.40 ± 0.06 | 0.44 ± 0.08 | 0.35 ± 0.13 | 0.35 ± 0.13 | 0.34 ± 0.02 |
| m2 | 1.82 ± 0.34 | 1.93 ± 0.30 | 1.97 ± 0.27 | 2.29 ± 0.37 | 2.46 ± 0.30 | 2.41 ± 0.27 | ||
| 6 Months (10) | m1 | 0.40 ± 0.08 | 0.39 ± 0.07 | 0.40 ± 0.07 | 0.38 ± 0.09 | 0.26 ± 0.11 | 0.23 ± 0.10 | 0.33 ± 0.03 |
| m2 | 2.03 ± 0.33 | 2.03 ± 0.31 | 2.05 ± 0.29 | 2.35 ± 0.36 | 2.51 ± 0.29 | 2.47 ± 0.29 | ||
Data are mean ± SD
Type II diabetic subjects were studied at baseline and following 6 months of intervention consisting of diet and behavior modification (please see methods). Following an overnight fast, [6,6-2H2]glucose was administered as a prime constant rate infusion. The subjects were also given a dose of [2H2]O in order to label the body water pool. After a basal period of 4 h, a hyperinsulinemic normoglycemic clamp was performed. The labeled glucose was also infused during the clamp.
Intervention resulted in an improvement in insulin sensitivity as evidence by higher glucose uptake during the clamp (Baseline: 246 ± 122 mg.min-1; 6 Months: 335 ± 153 mg.min-1; p=0.07). There was a significant dilution of the m1 enrichment on carbon-6 of glucose during normoglycemic hyperinsulinemia (clamp) following clinical intervention, from 0.40 ± 0.7% to 0.23 ± 0.1% (Table 2). However, the precise contribution of gluconeogenesis during the hyperinsulinemic clamp could not be calculated due to the large contribution of m1 from the infused glucose and because of a lack of isotopic tracer equilibrium during the infusion of glucose and insulin.
Source of VLDL triglyceride glycerol
Contribution of glucose: As shown in Table 3, m2 enrichment of C-1 of glycerol was minimal or insignificant, and the m2 enrichment of glycerol as a result of [6,6-2H2]glucose infusion was only evident on C-3 of glycerol. Approximately 16% of triglyceride glycerol was derived from glucose. The contribution of glucose to triglyceride glycerol did not change as a result of six months of clinical intervention or acutely during insulin plus glucose infusion (hyperinsulinemic normoglycemic clamp).
Contribution of pyruvate (glyceroneogenesis)
The contribution of pyruvate to VLDL triglyceride glycerol was estimated from the [2H] enrichment of carbon-3 (m1) of glycerol. The m1 enrichment on C-1 was significantly less than that on C-3 in the baseline study, both during the basal state and during the hyperinsulinemic clamp (Table 4). These differences were present subsequent to interventions in nine of ten subjects. The estimated contribution of glyceroneogenesis (pyruvate) to triglyceride glycerol was ∼53% in the basal state and remained unchanged during the hyperinsulinemic normoglycemic clamp. In addition, dietary and physical activity intervention and associated improvement in insulin sensitivity had no effect on the contribution of pyruvate to triglyceride glycerol (Table 5).
Table 4.
Hepatic Glyceroneogenesis in vivo
| Deuterium enrichment (m1) in body water |
Deuterium enrichment (m1) on Triglyceride Glycerol |
TG from Pyruvate |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| % | C-1 + C-3 | C-1 | C-3 | % | |||||
| Basal | Clamp | Basal | Clamp | Basal | Clamp | Basal | Clamp | ||
| Baseline | 0.34 (0.02) | 0.32 (0.07) | 0.35 (0.06) | 0.29* (0.06) | 0.30§ (0.05) | 0.36* (0.09) | 0.36§ (0.08) | 53 (11.6) | 53.4 (9.6) |
| 6 Months | 0.33 (0.03) | 0.33 (0.07) | 0.34 (0.07) | 0.31 (0.06) | 0.29 (0.08) | 0.36 (0.13) | 0.37 (0.12) | 53.9 (18.0) | 56.3 (16.9) |
Data are mean (± SD); TG = triglyceride glycerol
Significantly different, paired t-test
p<0.015
p<0.01.
Ten type II subjects were studied before and following 6 months of diet and behavior modification. After an overnight fast, [2H2] enrichment (m1) of body water, and that of hydrogens on C-1 and C-3 of triglyceride were determined.
In the healthy control subjects, the contribution of pyruvate to glucose was 38.4 ± 10.8%. The contribution of triosephosphate to plasma triglyceride glycerol, measured by 2H enrichment of C-1 of triglyceride glycerol, was 44.9 ± 9.3%. Intravenous glucagon for 3 hours did not affect gluconeogenesis, while it caused a small but significant increase (p<0.01) in the contribution of trioses to plasma triglyceride glycerol.
Correlations
A positive correlation was observed between gluconeogenesis and glyceroneogenesis in type II diabetic subjects after interventions when their insulin sensitivity had improved (r2 = 0.564).
DISCUSSION
In the present study, we have examined the relative contribution of glucose and pyruvate to triglyceride glycerol and the impact of insulin in type II diabetes. The data show that pyruvate via triosephosphates, i.e. glyceroneogenesis and not glucose via glycolysis, is the major carbon source for the VLDL triglyceride glycerol in humans. The contribution of glucose to triglyceride was low compared with that of pyruvate. Acute infusion of insulin and glucose, during a hyperinsulinemic clamp, had no impact on the contribution of glucose or pyruvate to triglyceride glycerol in type II diabetic subjects before or after improvement in insulin sensitivity. An acute infusion of glucagon caused an increase in the triglyceride glycerol from triosephosphate in healthy controls. These data suggest that the primary source for VLDL triglyceride glycerol carbon in humans is pyruvate and that plasma glucose makes only a small contribution to triglyceride glycerol, even when glucose plus insulin are administered acutely.
We used the total body water labeling method, using [2H2]O to quantify the contribution of pyruvate to triglyceride glycerol. As discussed previously [12,19], the methyl hydrogens (C3) of pyruvate which form C6 of glucose and C3 of glyceraldehyde-3-P, exchange with hydrogens in the body water so that [2H] enrichment of the hydrogens bound to the C3 of pyruvate or phosphoenolpyruvate is similar to that of the water. One hydrogen on the C1 of dihydroxyacetone phosphate (DHAP), the immediate precursor of glycerol-3-phosphate, is obtained from body water during the conversion of phosphoenolpyruvate to glyceraldehyde-3-P. The second hydrogen is also obtained from body water during the isomerization of DHAP and glyceraldehydes-3-P, so that the [2H] enrichment of both the hydrogens on C1 of glycerol-3-phosphate is the same as that of body water. Thus, the [2H] enrichment of hydrogens on the C1 and C3 of triglyceride glycerol formed from pyruvate will be the same as that of body water. The hydrogens on C3 truly represent the contribution of pyruvate, while those on C1 represent the contribution of triosephosphate. As shown in Table 4, the 2H enrichment of C1 and C3 of triglyceride glycerol in type II diabetic subjects was the same after the intervention study, both during the basal state and during insulin-glucose infusion. The 2H enrichment on C-1 was significantly less than that on C-3 during the baseline study, when the magnitude of resistance to insulin was high. The reason(s) for the difference is not readily apparent.
Sources of carbon for triglyceride
As shown in tables 3 and 4, pyruvate was the predominant sources of triglyceride glycerol carbon, and that glucose C was a minor contributor. The latter is of particular interest since glucose, insulin infusion during hyperinsulinemic clamp, had no effect on the contribution of glucose in the type II diabetic subjects. The insulin clamp study was performed for four hours, when glucose uptake by the liver should have increased, particularly in the studies performed following the intervention, when insulin sensitivity had improved. Whether the observed contribution of glucose to triglyceride is a net contribution or simply represents an appearance of label due to futile cycling cannot be ascertained from the present data.
Glucose carbon recycling via pyruvate, either through the periphery (muscle) or as a result of hepatic zonation, cannot be estimated from these studies since hydrogens on C-3 of pyruvate and C-1 trioses, irrespective of the source of carbon and labeling pattern of hydrogen, will rapidly equilibrate with body water and become enriched to the same magnitude as body water.
Our data show that glyceroneogenesis and glucose contributed ∼50% and ∼15% to glyceride-glycerol, respectively. Our previous data have shown that the contribution of plasma glycerol was ∼5% to triglyceride glycerol (11). The discrepant ∼30% represents the unlabeled triglyceride pool in the circulation because of the slow turnover rate of this pool. We speculate that had we continued the studies for a prolonged period, the relative contribution of glyceroneogenesis, glucose and glycerol, although unchanged, would have been ∼70%, 23% and 7% (total = 100%), respectively.
Effect of insulin administration
Normoglycemic hyperinsulinemic clamp studies showed that insulin sensitivity had significantly improved in the type II diabetic subjects following six months of dietary and behavioral intervention. However, it had no impact on the contribution of glucose or pyruvate to triglyceride glycerol (Tables 3 and 4). Although improved sensitivity to insulin appeared to result in lower contribution of gluconeogenesis from pyruvate to glucose, accurate estimates of gluconeogenesis during the hyperinsulinemic clamp could not be made due to lack of equilibrium in the glucose pools. Data from several studies suggest that the acute effect of insulin on glucose production is primarily via suppression of glycogenolysis and not gluconeogenesis [20-23].
The lack of any effect of insulin on contribution of glucose to triglyceride glycerol is of interest. During normoglycemic hyperinsulinemic clamp, the hepatic glucose output decreased. A lack of change in the contribution of glucose to triglyceride glycerol suggests that hyperinsulinemia during the clamp did not increase hepatic glucose uptake, nor did it effect the intrahepatic regulation of the synthesis of triglyceride glycerol from glucose. In addition, insulin infusion did not impact the conversion of pyruvate to triglyceride glycerol before and after clinical intervention. These data are consistent with the observation on the acute effects of insulin on gluconeogenesis, which show a minimal effect both in humans and in animals [20-23]. Acute administration of insulin in healthy subjects has been shown to suppress VLDL secretion [24,25]. Such an effect of insulin on VLDL secretion would not impact the fractional contributions of pyruvate (or triosephosphate) to VLDL-triglyceride, as seen in the present study, although the total quantitative contribution (fraction × VLDL secretion rate) would decrease.
These data suggest that the major effect of acute infusion of insulin on gluconeogenesis is at a site in the pathway that is above the triosephosphate branch point between gluconeogenesis and glyceroneogenesis, most likely at fructose-1,6-biphosphatase (FBPase). This enzyme is highly regulated and controls the potential “futile cycling” of carbon between fructose-6-phosphate and fructose-1,6-biphosphate [26,27]. The relative activity of both FBPase and phosphofructokinase is controlled by change in fructose-2,6-biphosphate and AMP; the levels of both of these compounds would be expected to be altered by the metabolic events initiated by infusion of insulin. In addition, glyceroneogenesis and gluconeogenesis will not be affected acutely by insulin infusion, since PEPCK-C is not allosterically controlled and the flux over this enzyme would not be altered by a 4h infusion of insulin [3]. Insulin effects PEPCK-C by inhibiting the transcription of the gene, and hence the effects of insulin are not apparent acutely, as in the present study.
Correlation between gluconeogenesis and glyceroneogenesis
The positive correlation between gluconeogenesis and glyceroneogenesis in type II diabetic subjects following the improvement in insulin sensitivity suggests substrate regulation of gluconeogenesis and glyceroneogenesis, i.e. the carbon flow from pyruvate to glucose and to glycerol-3-phosphate are parallel and are determined by the uptake and delivery of pyruvate carbon to the liver.
In response to glucagon, there was a minimal effect on the fractional contribution of pyruvate to glucose in healthy controls, and a small increase in the contribution of triosephosphate to VLDL triglyceride glycerol. These data are consistent with the observation by others [28-30] that the acute effect of glucagon on glucose Ra is by increasing glycogenolysis rather than gluconeogenesis. We assume that the increase in the contribution of triosephosphate to triglyceride glycerol was the consequence of an increase in cAMP, which inhibits futile cycling at pyruvate kinase, resulting in increased flux of pyruvate carbon to glyceraldehydes-3-phosphate [31,32]. Whether glucagon has any effect on VLDL triglyceride transport is not known.
Data from type II diabetics show that pyruvate remained the predominant source of triglyceride glycerol. The infusion of insulin for 4 h had no effect on the fractional contribution in type II diabetic subjects before or after the dietary and behavioral intervention. These data suggest that the primary regulator of the quantitative appearance of trioses in the plasma VLDL triglyceride pool may be the rate of release of VLDL triglycerides.
Glyceroneogenesis and gluconeogenesis share a common pathway
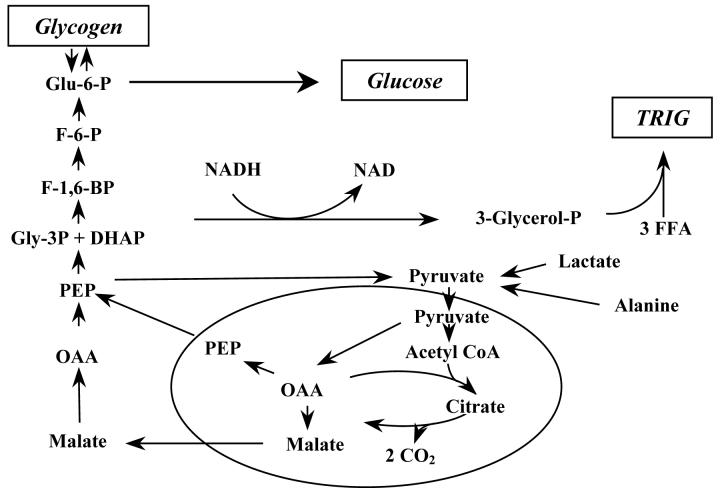
The metabolic pathway for the synthesis of triglyceride glycerol and glucose share a common set of reactions. Substrates such as lactate or alanine provide carbon for both pathways; the branch point is at the conversion of dihydroxyacetone phosphate (DHAP) to glycerol-3-phosphate via glycerol-3-phosphate dehydrogenase (Fig. 1); the latter compound is the precursor of triglyceride glycerol. Glycerol-3-phosphate dehydrogenase is a redox sensitive reaction, in which NADH is oxidized to NAD. The rate of flux of triose phosphate to glucose via this pathway is rapid and the diversion of DHAP to glycerol-3-phosphate from this pool is dependent on the redox state of the liver. In addition, the equilibrium of glycerol-3-phosphate dehydrogenase strongly favors the formation of glycerol-3-phosphate. During periods of glyceroneogenesis/gluconeogenesis, the oxidation of both fatty acids and lactate would provide the reducing equivalents needed to drive the synthesis of glycerol-3-phosphate. The quantitatively higher flux of pyruvate to triose phosphate to glucose (compared with pyruvate to glycerol-3-phosphate) explains our observation that glycerol accounts for only 6% of the triglyceride found in the VLDL triglyceride of overnight-fasted humans [11]; the glycerol-3-phosphate formed from glycerol via glycerol kinase is diluted by the greater flow of triose phosphate from gluconeogenic precursors, such as lactate and alanine, through the triose phosphate pool. The important role of cytosolic redox state in the regulation of the diversion of triose phosphate from gluconeogenesis to glyceroneogenesis is supported by the studies of Siler et al [33], which demonstrated that ethanol greatly enhanced the rate of synthesis of triglyceride glycerol via glyceroneogenesis in the perfused rat liver. This was attributed to a shift in the NAD/NADH ratio induced by the metabolism of ethanol.
Figure 1. The common pathway of glyceroneogenesis and gluconeogenesis in the mammalian liver.
The pathway for the conversion of precursors such as alanine, lactate and pyruvate to either triglyceride or glucose is shown. The major branch point in the pathway is the reduction of DHAP to 3- glycerol phosphate by 3-glycerol phosphate dehydrogenase. This reaction is redox sensitive and its equilibrium favors the formation of 3-glycerol phosphate. Our data demonstrate that insulin controls glyceroneogenesis independently of gluconeogenesis, perhaps by an alteration in flux through the FBPase reaction.
Acknowledgment
The authors thank Ed Burkett for technical support and Joyce Nolan for secretarial assistance.
This work was supported by NIH grants PO1 HD11089, RO1 HD042154, RO1 DK58620 (RWH), and University of Pittsburgh Obesity and Nutrition Research Center (P30 DK046204).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:10S–16S. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reshef L, Olswang Y, Cassuto H, et al. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278:30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 3.Hanson RW, Reshef L. Glyceroneogenesis revisited. Biochimie. 2003;85:1199–1205. doi: 10.1016/j.biochi.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Franckhauser S, Muñoz S, Pujol A, et al. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes. 2002;51:624–630. doi: 10.2337/diabetes.51.3.624. [DOI] [PubMed] [Google Scholar]
- 5.Franckhauser S, Muñoz S, Elias I, Ferre T, Bosch F. Adipose overexpression of phosphoenolpyruvate carboxykinase leads to high susceptibility to diet-induced insulin resistance and obesity. Diabetes. 2006;55:273–280. doi: 10.2337/diabetes.55.02.06.db05-0482. [DOI] [PubMed] [Google Scholar]
- 6.Olswang Y, Cohen H, Papo O, et al. A mutation in the peroxisome proliferators-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc Natl Acad Sci USA. 2002;99:625–630. doi: 10.1073/pnas.022616299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess SC, Hausler N, Merritt M, et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem. 2004;279:48941–48949. doi: 10.1074/jbc.M407120200. [DOI] [PubMed] [Google Scholar]
- 8.Botion LM, Brito MS, Brito NA, Brito SR, Kettelhut IC, Migliorini RH. Glucose contribution to in vivo synthesis of triglyceride and fatty acids in rats adapted to a high-protein, carbohydrate-free diet. Metabolism. 1998;47:1217–1221. doi: 10.1016/s0026-0495(98)90326-2. [DOI] [PubMed] [Google Scholar]
- 9.Brito SC, Festuccia WL, Kawashita NH, et al. Increased glyceroneogenesis in adipose tissue from rats adapted to a high-protein, carbohydrate-free diet: role of dietary fatty acids. Metab Clin Exper. 2006;55:84–89. doi: 10.1016/j.metabol.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Chen JL, Peacock E, Samady W, et al. Physiologic and pharmacologic factors influencing glyceroneogenic contribution to triglyceride measured by mass isotopomer distribution analysis. J Biol Chem. 2005;280:25396–25402. doi: 10.1074/jbc.M413948200. [DOI] [PubMed] [Google Scholar]
- 11.Kalhan SC, Mahajan S, Burkett E, Reshef L, Hanson RW. Glyceroneogenesis and the source of glycerol for hepatic triacylglycerol synthesis in humans. J Biol Chem. 2001;276:12928–12931. doi: 10.1074/jbc.M006186200. [DOI] [PubMed] [Google Scholar]
- 12.Chandramouli V, Ekberg K, Schumann WC, Kalhan SC, Wahren J, Landau BR. Quantifying gluconeogenesis during fasting. Am J Physiol. 1997;273:E1209–E1215. doi: 10.1152/ajpendo.1997.273.6.E1209. [DOI] [PubMed] [Google Scholar]
- 13.Kelley DE, Harper P, Kuller LH, Mancino J, McKolanis TM, Kalhan S. Effects of moderate weight loss and orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes. Diabetes Care. 2004;27:33–40. doi: 10.2337/diacare.27.1.33. [DOI] [PubMed] [Google Scholar]
- 14.Kelley DE, McKolanis TM, Hegazi RAF, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906–E916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 15.Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987;36:914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- 16.Kalhan SC, Trivedi R, Singh S, Chandramouli V, Schumann WC, Landau BR. A micromethod for the measurement of deuterium bound to carbon 6 of glucose to quantify gluconeogenesis in vivo. J Mass Spectrom. 1995;30:1588–1592. [Google Scholar]
- 17.Egusa G, Brady DW, Grundy SM, Howard BV. Isopropanol precipitation method for the determination of apolipoprotein B specific activity and plasma concentrations during metabolic studies of very low density lipoprotein and low density lipoprotein apolipoprotein. Br J Lipid Res. 1983;24:1261–1267. [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β cell function from fasting plasma insulin and glucose concentration in man. Diabetes. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Use of 2H2O for estimating rates of gluconeogenesis: application to the fasted state. J Clin Invest. 1995;95:172–178. doi: 10.1172/JCI117635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgerton DS, Cardin S, Emshwiller M, et al. Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes. 2001;50:1872–1882. doi: 10.2337/diabetes.50.8.1872. [DOI] [PubMed] [Google Scholar]
- 21.Hausler N, Browning J, Merritt M, et al. Effects of insulin and cytosolic redox state on glucose production pathways in the isolated perfused mouse liver measured by integrated 2H and 13C NOR. Biochem J. 2006;394:465–473. doi: 10.1042/BJ20051174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sindelar DK, Balcom JH, Chu CA, Neal DW, Cherrington AD. A comparison of the effects of selective increases in peripheral or portal insulin on hepatic glucose production in the conscious dog. Diabetes. 1996;45:594–1604. doi: 10.2337/diab.45.11.1594. [DOI] [PubMed] [Google Scholar]
- 23.Edgerton DS, Lautz M, Scott M, et al. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest. 2006;116:521–527. doi: 10.1172/JCI27073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis GF, Steiner G. Acute effects of insulin in the control of VLDL production in humans. Diabetes Care. 1996;19:390–393. doi: 10.2337/diacare.19.4.390. [DOI] [PubMed] [Google Scholar]
- 25.Mittendorfer B, Patterson BW, Klein S, Sidossis LS. VLDL-triglyceride kinetics during hyperglycemia-hyperinsulinemia: effects of sex and obesity. Am J Physiol Endocrinol Metab. 2003;294:E708–E715. doi: 10.1152/ajpendo.00411.2002. [DOI] [PubMed] [Google Scholar]
- 26.Pilkis SJ, Chrisman TD, El-Maghrabi MR, et al. The action of insulin on hepatic fructose 2,6-biphosphate metabolism. J Biol Chem. 1983;158:1495–1503. [PubMed] [Google Scholar]
- 27.Wu C, Khan S, Peng L-J, Li H, Carmella SS, Lange AJ. Perturbation of glucose flux in the liver by decreasing fructose-2,6-biphosphate levels causes hepatic insulin resistance and hyperglycemia. Am J Physiol Endocrinol Metab. 2006 Apr; doi: 10.1152/ajpendo.00126.2006. doi:10.1152/ajpendo.00126.2006. [DOI] [PubMed] [Google Scholar]
- 28.Magnusson I, Rothman DL, Gerard DP, Katz LD, Shulman GI. Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes. 1995;44:185–189. doi: 10.2337/diab.44.2.185. [DOI] [PubMed] [Google Scholar]
- 29.Hendrick GK, Frizzell RT, Williams PE, Cherrington AD. Effect of hyperglucagonemia on hepatic glycogenolysis and gluconeogenesis after a prolonged fast. Am J Physiol. 1990;258:E841–E849. doi: 10.1152/ajpendo.1990.258.5.E841. [DOI] [PubMed] [Google Scholar]
- 30.Lecavalier L, Bolli G, Gerich J. Glucagon-cortisol interactions on glucose turnover and lactate gluconeogenesis in normal humans. Am J Physiol. 1990;258:E569–E575. doi: 10.1152/ajpendo.1990.258.4.E569. [DOI] [PubMed] [Google Scholar]
- 31.Pilkis SJ, Claus TH, el-Maghrabi MR. The role of cyclic AMP in rapid and long-term regulation of gluconeogenesis and glycolysis. Adv Second Messenger Phosphoprotein Res. 1988;22:175–191. [PubMed] [Google Scholar]
- 32.Pilkis SJ, el-Maghrabi MR, McGrane M, Pilkis J, Claus TH. Regulation by glucagon of hepatic pyruvate kinase, 6-phosphofructo 1-kinase, and fructose-1,6-biphosphatase. Fed Proc. 1982;41:2623–2628. [PubMed] [Google Scholar]
- 33.Siler SQ, Neese RA, Parks EJ, Hellerstein MK. VLDL-triglyceride production after alcohol ingestion, studied using [2-13C1]glycerol. J Lipid Res. 1998;39:2319–2328. [PubMed] [Google Scholar]