Abstract
AIMS: The aim of this study was to determine whether first-degree relatives (FDR) of patients with type 2 diabetes mellitus (T2DM) are at higher risk of non-alcoholic fatty liver disease (NAFLD) than healthy controls. METHODS: A total of 222 FDR of consecutive patients with T2DM aged between 35 and 55 years and 202 healthy individuals with no family history of diabetes were investigated for NAFLD. Fatty liver was diagnosed by ultrasonography using standard criteria. Height, weight, fasting glucose, alanine aminotransferase (ALT), total cholesterol and triglyceride were determined by routine laboratory methods. RESULTS: Compared to subjects with no family history of diabetes, the age and sex adjusted odds ratio (OR) of NAFLD was 1.83 (95% CI: 1.11-3.03) for FDR of patients with T2DM. After further adjusting for BMI, fasting glucose, ALT, asparate aminotransferase (AST), triglyceride and cholesterol, the multivariate OR of prevalent NAFLD in FDR of patients with T2DM compared with individuals with no family history of diabetes was 1.56 (95% CI: 0.85-2.86). CONCLUSIONS: The present study suggests that the relation between FDR of patients with T2DM and NAFLD is affected by the other covariates, in particular obesity, which points to a more complex relationship between the diseases. It appears that obesity and diabetes may independently predispose to NAFLD.
Keywords: type 2 diabetes, non-alcoholic fatty liver, obesity, first-degree relatives of T2DM patients, risk factors, ultrasonography
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in developed nations. It is associated with surrogate markers of cardiovascular morbidity [1, 2] and may progress to liver cirrhosis and hepatocellular carcinoma [3, 4]. NAFLD is reported to be related to obesity, diabetes mellitus, hypertension or hyperlipidemia [5-10]. These clinical features are also characteristics of the metabolic syndrome. However, NAFLD may occur in lean persons who appear otherwise healthy and do not seek medical help for their symptoms [9, 11].
NAFLD affects approximately 15-30% of the general population and its prevalence increases steadily to 70-90% in people with obesity or type 2 diabetes mellitus (T2DM) [12-15]. Patients with T2DM and NAFLD have significantly higher prevalence rates of coronary, cerebrovascular and peripheral vascular diseases than their counterparts without NAFLD [16]. There is familial clustering of obesity [17-22], diabetes [23-25], and NAFLD [18, 26]. The inheritance pattern is, however, unclear. Familial clustering of diabetes may support a genetic predisposition to NAFLD.
Environmental and genetic factors are likely to play a role in the pathogenesis of NAFLD. Observational studies of familial clustering of NAFLD [18] have prompted a search for genetic abnormalities that may predispose susceptible individuals to NAFLD. Some of the genes of interest include those influencing the development of T2DM; however, the risk of NAFLD in FDR of patients with T2DM remains unknown.
With the increasing prevalence of obesity and DM worldwide and the associated risk of NAFLD in such individuals, it becomes increasingly important to identify risk factors associated with susceptibility to obesity-related chronic liver disease.
The objective of this study was to examine the risk of NAFLD in FDR of patients with T2DM.
Subjects and methods
A total of 222 (44 men and 178 women) nondiabetic FDR of consecutive patients with T2DM aged 35-55, who sought treatment for diabetes at our clinic in the Endocrine and Metabolism Research Center affiliated to the Isfahan University of Medical Sciences, Iran, between March 2006 and March 2007, were evaluated. Diabetes mellitus was defined according to the American Diabetes Association criteria [27]. The group of FDR of T2DM was compared with a control group of 202 (108 men and 94 women) healthy adults with no family history of diabetes. Healthy controls were volunteers employed at the Isfahan University of Medical Sciences. The tenets of the Declaration of Helsinki were followed, institutional ethical committee approval was granted and an informed consent was signed by each participant.
Ultrasonography (US) of the abdomen was performed in all individuals for evidence of fatty liver. All ultrasound scans were performed by an expert sonographer (Sanaz Shayganfar) using a 3.5 MHz transducer (Honda, Electronics Co., Japan). The sonographer was unaware of the aims of the study and blinded to laboratory values. The diagnosis of fatty liver was made in the presence of diffusely increased liver echogenicity with evidence of contrast between the liver and kidney, diffusely increased liver echogenicity with blurring of the intrahepatic vessels and diaphragm, or bright hepatic echogenicity with poor penetration of the posterior hepatic segments and intrahepatic vessels or when the diaphragm was invisible [28]. NAFLD was assessed semi-quantitatively on a scale of 0 to 3: 0 = absent; 1 = mild; 2 = moderate and 3 = severe. We acknowledge that the interpretation of fatty liver using US is subjective and it is reported that US cannot reliably identify mild steatosis affecting less than 33% of the liver [29, 30]. Nonetheless, most investigators define grade 1 steatosis as affecting less than 33% of hepatocytes for a diagnosis of NAFLD [31, 32]. NAFLD was defined as the presence of fatty liver on US, without the following conditions: excessive alcohol consumption (women: ≥20 g/wk, men: ≥30 g/wk), positive hepatitis B surface antigen (HBsAg) or anti-hepatitis C virus antibody (anti-HCV), pregnancy, total parental nutrition, jejuneal bypass or extensive small bowel resection, or other known liver diseases like hepatoma, as determined by history, physical examination and screening blood tests. Subjects also were excluded from the diagnosis of NAFLD when they had ingested drugs known to produce fatty liver disease, such as steroids, estrogens, amiodarone, tamoxifen, or other chemotherapeutic agents within the previous 6 months.
The FDR of patients with T2DM included siblings or children. Half-siblings were excluded. Overnight fasting blood samples were taken and plasma was separated and analyzed on the same day. Alanine aninotransferase (ALT), asparate aminotransferase (AST), total cholesterol, triglyceride, and fasting blood glucose were assessed using standardized procedures. Body mass index (BMI in kg/m2) is recognized as the measure of overall obesity. Normal weight was defined as BMI < 25, overweight as BMI 25.0-29.9, and obesity as BMI ≥ 30. A glucose tolerance test, which involved ingesting 75 g of glucose in a volume of 300 ml, was performed in FDR of T2DM, who were classified as having normal glucose tolerance, impaired glucose tolerance or diabetes mellitus according to the American Diabetes Association criteria [27]. An interview was conducted at the time of the participant’s visit. The contents of the interview included demographic data, alcohol intake, history of viral hepatitis or another liver disease and medication history. The definition of high cholesterol and triglyceride levels was based on the National Cholesterol Education program (ATP III) criteria [33]. The definition of elevated AST and ALT was based on those of the kit manufacturers (Pars-Azmon, Iran).
Height and weight were measured with subjects in light clothes and without shoes, using standard apparatus. Weight was measured to the nearest 0.1 kg on a calibrated beam scale. Height was measured to the nearest 0.5 cm using a measuring tape.
Statistical analysis
To describe the association between FDR of patients with T2DM and risk of NAFLD we used two types of statistical analyses: age and sex-adjusted odds ratios and multivariate-adjusted odds ratios from multivariate logistic regression using the SPSS for Windows computer package (SPSS Inc., Chicago, IL, USA). We considered the following covariates in the multivariate-adjusted analyses: age, sex, BMI, fasting glucose, triglyceride, ALT, AST, and total cholesterol. To clarify the role of BMI further, we also conducted an analysis including the interaction terms of BMI as a continuous variable with family history of T2DM in the "full" model. All tests for statistical significance were two-tailed and performed at α < 0.05.
Results
Differences in distribution of several age and sex-adjusted characteristics among 222 FDR of patients with T2DM and 202 with no family history of diabetes are shown in Table 1. FDR of patients with T2DM were older, more overweight or obese, had higher age and sex-adjusted BMI, were less likely to be men and had lower ALT, AST and triglyceride than those with no family history of diabetes. The mean (SEM) age of FDR of patients with T2DM was 44.2 (0.5) years and 40.1 (0.5) for persons with no family history of diabetes.
Table 1. Age and sex-adjusted characteristics of first-degree relatives of T2DM patients and control subjects with no family history of diabetes.
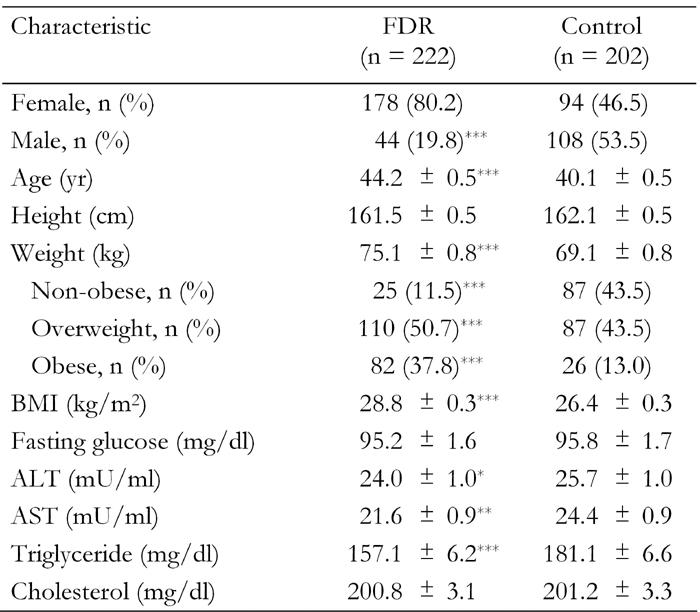
Data are age and sex-adjusted means ± SEM. Age and sex-adjusted means were calculated using general linear models. FDR: first-degree relatives of T2DM patients. Control: healthy persons without family history of T2DM. Weight categories: non-obese (BMI < 25), overweight (BMI 25.0-29.9), obese (BMI ≥ 30). ALT: alanine aminotransferase. AST: asparate aminotransferase. *p < 0.05, **p < 0.01, ***p < 0.001.
In this study 120 (28.3%) subjects with NAFLD were detected by US. 17.9% had mild/moderate and 10.1% severe NAFLD. Of the persons with no family history of diabetes, 25.7% had NAFLD (95% CI, 19.7-31.8). The overall prevalence rate of NAFLD among FDR of T2DM was 30.6% (95% CI, 24.6-36.7).
Those with NAFLD had higher BMI, weight, fasting glucose, ALT, triglyceride and cholesterol and were older (Table 2).
Table 2. Characteristics of subjects with and without non-alcoholic fatty liver disease (NAFLD).
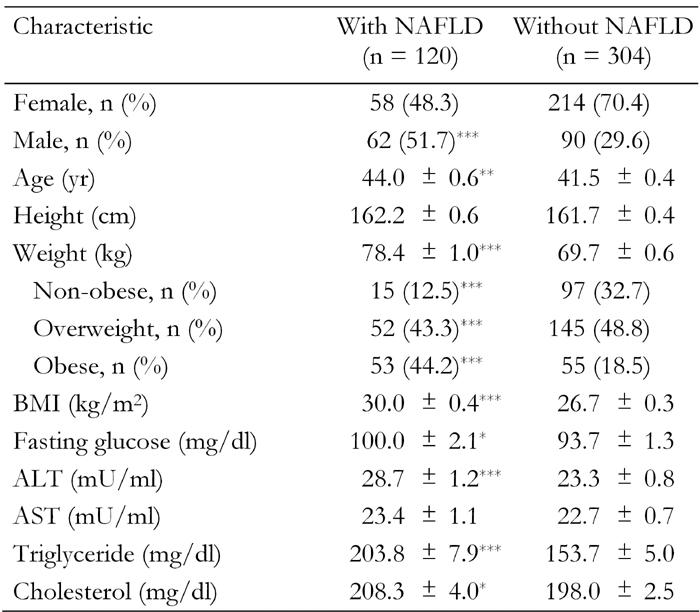
Data are age and sex-adjusted means ± SEM. Age and sex-adjusted means were calculated using general linear models. Weight categories: non-obese (BMI < 25), overweight (BMI 25.0-29.9), obese (BMI ≥ 30). ALT: alanine aminotransferase. AST: asparate aminotransferase. *p < 0.05, **p < 0.01, ***p < 0.001.
Compared with persons with no family history of diabetes, the age and sex adjusted risk of NAFLD was 83% higher in FDR of patients with T2DM (odds ratio (OR) 1.83, 95% CI, 1.11-3.03) in age and sex-adjusted models. Controlling for age, sex and BMI reduced the relationship between FDR of patients with T2DM and NAFLD (OR 1.22, 95% CI, 0.70-2.10) compared to the model adjusted for age and sex. In a multivariate model, the additional adjustment for other covariates increased the relationship between FDR of patients with T2DM and NAFLD risk compared to the model adjusted for age, sex and BMI (OR 1.56, 95% CI, 0.85-2.86) (Table 3).
Table 3. Prevalence rates and odds ratios of non-alcoholic fatty liver disease.
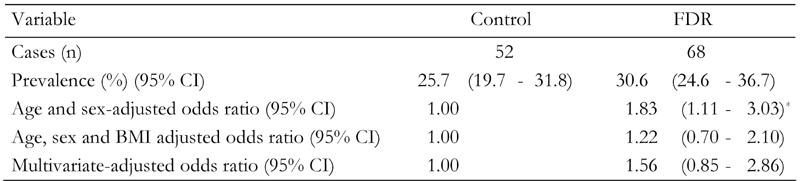
Data are odds ratios (95% CI in parentheses) calculated by binary logistic regression analysis. Calculation adjusted for age, sex, body mass index, fasting blood glucose, ALT, AST, triglyceride and cholesterol. FDR: first-degree relatives of T2DM patients. Control: healthy persons without family history of T2DM. BMI = body mass index. *p < 0.001.
The higher risk of NAFLD among FDR of patients with type 2 diabetes could be related to obesity. Analyses stratified by obesity status are shown in Table 4. A similar association was observed among overweight (BMI ≥ 25) (OR 1.19, 95% CI, 0.50-2.85), obese (BMI ≥ 30) (OR 1.84, 95% CI, 0.49-6.99) or non-obese (OR 1.58, 95% CI, 0.24-10.36) individuals after multivariate adjustment. This association was not statistically significant, possibly because of the small number of NAFLD.
Table 4. Prevalence rates and odds ratios of non-alcoholic fatty liver disease by obesity status.
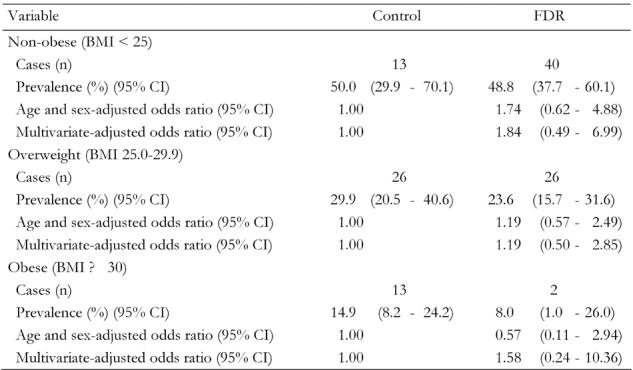
Data are odds ratios (95% CI in parentheses) calculated by binary logistic regression analysis. Calculation adjusted for age, sex, body mass index, fasting blood glucose, ALT, AST, triglyceride and cholesterol. FDR: first-degree relatives of T2DM patients. Control: healthy persons without family history of T2DM. BMI = body mass index.
Discussion
This study did not confirm that NAFLD is significantly associated with FDR of T2DM patients. However, the relation between FDR of T2DM patients and NAFLD seems to be affected by other covariates, including BMI, indicating a more complex relationship. To the best of our knowledge, no other study with similar results is available. The non-significant risk of NAFLD for FDR of patients with T2DM was amplified in the presence of overweight and obesity. In the light of the existing literature and our new results, overweight/obesity and diabetes may independently predispose to NAFLD. However, we were unable to examine the relation between FDR of T2DM patients and risk of NAFLD in obesity subgroups because of the small number of cases. This question remains to be clarified in subsequent studies.
NAFLD is significantly associated with several conditions such as obesity, diabetes mellitus, hypertension, hyperlipidemia and the metabolic syndrome [5-11], but the causality is unclear. These conditions may play an important role as confounding factors. Therefore, major susceptibility genes may account for at least some of these associations [19].
Obesity is associated with type 2 diabetes and with NAFLD. Our findings confirm those obtained in other studies [5-11], in which obesity increased the risk for NAFLD. Overweight FDR of T2DM patients were at higher risk of NAFLD than non-obese FDR. Our finding that the risk of NAFLD was non-significantly associated with increasing obesity among FDR of T2DM was based on only 2 cases among non-obese FDR of patients with T2DM. Although there was no statistically significant risk for FDR of T2DM patients, our results could not exclude a 20-80% increased NAFLD. Several studies have shown that measures of obesity show strong heritability [34]. In Iran, a recent nationwide study revealed that 43.0% of men and 57.0% of women were overweight or obese and 11.0% of men and 25.0% of women were obese. The prevalence of abdominal obesity was 11.4% in men and 57.5% in women [35]. NAFLD share many common features with T2DM and obesity: all seem to be familial. This suggests that genetic factors beside lifestyle, obesity, diabetes mellitus, and dyslipidemia may be part of the risk factors for NAFLD. However, it is also possible that these two diseases are two distinct entities, pathologic phenomena closely related to obesity and overweight which share the same process of pathogenesis.
Our study has several limitations. Firstly, the number of subjects is relatively small. Secondly, the control group in this study was recruited from employees of the Isfahan University of Medical Sciences and may not be representative of healthy individuals from the community. In particular, the control group was not matched for BMI with FDR of T2DM, although we adjusted for BMI in multivariate analysis and analyzed separately for overweight and obese and normal subjects. After adjustment, however, the differences were not statistically significant.
In the present study, the diagnosis of NAFLD was based on the exclusion of the known etiologic factors responsible for liver disease and ultrasound examination results, but the diagnosis was not confirmed by liver biopsy results. A liver biopsy is the gold standard for ascertaining fatty liver disease, but it is invasive and can cause complications. Ultrasound, however, with sensitivity of 80% to 95% and specificity of 90% to 95%, is widely available and relatively accurate for the diagnosis of fatty liver disease [28, 36-39]. Although US has some limitations in distinguishing a fatty liver from other liver diseases, the present study used US as a non-invasive method of examining subjects in sufficient numbers.
In summary, the findings of this study illustrate for the first time the NAFLD in FDR of patients with T2DM. Our study indicates that the relationship between FDR and NAFLD is affected by the other covariates, indicating a somewhat complex relationship. The risk of NAFLD is also associated with overweight and obesity. Our findings highlight the need for further studies to provide a more complete picture of the situation and to identify gaps or deficiencies that may need to be addressed. Our results also emphasize the importance of controlling all known diabetes risk factors, especially overweight and obesity, in FDR of patients with T2DM. The results of this study will be evaluated in large epidemiologic studies that interface with the ability to conduct familial genetic assessment of candidate genes for NAFLD in FDR of patients with T2DM.
Acknowledgments
We are grateful to Mr. Majid Abyar for computer technical assistance and two anonymous reviewers for their valuable comments.
References
- 1.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 2.Targher G, Bertolini L, Padovani R, Zenari L, Zoppini G, Falezza G. Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men: role of visceral fat accumulation. Diabetes Care. 2004;27:2498–2500. doi: 10.2337/diacare.27.10.2498. [DOI] [PubMed] [Google Scholar]
- 3.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, Rizzetto M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 4.Zen Y, Katayanagi K, Tsuneyama K, Harada K, Araki I, Nakanuma Y. Hepatocellular carcinoma arising in non-alcoholic steatohepatitis. Pathol Int. 2001;51:127–131. doi: 10.1046/j.1440-1827.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 5.Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 8.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 9.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 10.Friis-Liby I, Aldenborg F, Jerlstad P, Rundstrom K, Bjornsson E. High prevalence of metabolic complications in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2004;9:865–869. doi: 10.1080/00365520410006431. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–2175. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 12.Adams LA, Angulo P. Recent concepts in non-alcoholic fatty liver disease. Diabet Med. 2005;22:1129–1133. doi: 10.1111/j.1464-5491.2005.01748.x. [DOI] [PubMed] [Google Scholar]
- 13.Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005;16:421–427. doi: 10.1097/01.mol.0000174153.53683.f2. [DOI] [PubMed] [Google Scholar]
- 14.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroentrol. 2006;40(3 Suppl 1):S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 15.Neuschwander-Tetri BA. Nonalcoholic steatohepatitis and the metabolic syndrome. Am J Med Sci. 2005;330(6):326–335. doi: 10.1097/00000441-200512000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Targher G, Bertolini L, Padovani R, Poli F, Scala L, Tessari R, Zenari L, Falezza G. Increased prevalence of cardiovascular disease in type 2 diabetes patients with non-alcoholic fatty liver disease. Diabet Med. 2006;23:403–409. doi: 10.1111/j.1464-5491.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 17.Tripathy D, Lindholm E, Isomaa B, Saloranta C, Tuomi T, Groop L. Familiality of metabolic abnormalities is dependent on age at onset and phenotype of the type 2 diabetic proband. Am J Physiol Endocrinol Metab. 2003;285(6):E1297–E1303. doi: 10.1152/ajpendo.00113.2003. [DOI] [PubMed] [Google Scholar]
- 18.Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957–2961. doi: 10.1111/j.1572-0241.2001.04667.x. [DOI] [PubMed] [Google Scholar]
- 19.Weijnen CF, Rich SS, Meigs JB, Krolewski AS, Warram JH. Risk of diabetes in siblings of index cases with Type 2 diabetes: implications for genetic studies. Diabet Med. 2002;19:41–50. doi: 10.1046/j.1464-5491.2002.00624.x. [DOI] [PubMed] [Google Scholar]
- 20.Rice T, Despres JP, Daw EW, Gagnon J, Borecki IB, Perusse L, Leon AS, Skinner JS, Wilmore JH, Rao DC et al. Familial resemblance for abdominal visceral fat: the HERITAGE family study. Int J Obes Relat Metab Disord. 1997;21:1024–1031. doi: 10.1038/sj.ijo.0800511. [DOI] [PubMed] [Google Scholar]
- 21.Lemiux S. Genetic susceptibility to visceral obesity and related clinical implications. Int J Obes Relat Metab Disord. 1997;21:831–838. doi: 10.1038/sj.ijo.0800488. [DOI] [PubMed] [Google Scholar]
- 22.Park HS, Yim KS, Cho SI. Gender differences in familial aggregation of obesity-related phenotypes and dietary intake pattern in Korean families. Ann Epidemiol. 2004;14:486–491. doi: 10.1016/j.annepidem.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Li JK, Ng MC, So WY, Chiu CK, Ozaki R, Tong PC, Cockram CS, Chan JC. Phenotypic and genetic clustering of diabetes and metabolic syndrome in Chinese families with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2006;22(1):46–52. doi: 10.1002/dmrr.577. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Co-segregation of obesity with familial aggregation of type 2 diabetes mellitus. Diabetes Obes Metab. 2000;2:149–154. doi: 10.1046/j.1463-1326.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 25.Elbein SC. The genetics of human noninsulin-dependent (type 2) diabetes mellitus. J Nutr. 1997;127(9):1891S–1896S. doi: 10.1093/jn/127.9.1891S. [DOI] [PubMed] [Google Scholar]
- 26.Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Ann J Med. 2000;108(1):9–13. doi: 10.1016/s0002-9343(99)00315-0. [DOI] [PubMed] [Google Scholar]
- 27.Committee on the Diagnosis and Classification of Diabetes. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 28.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. BMJ. 1986;292:13–15. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 30.Scatarige JC, Scott WW, Donovan PJ, Siegelman SS, Sanders RC. Fatty infiltration of the liver: ultrasonographic and computed tomographic correlation. J Ultrasound Med. 1984;3:9–14. doi: 10.7863/jum.1984.3.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Choudhury J, Sanyal AJ. Clinical aspects of fatty liver disease. Semin Liver Dis. 2004;24:349–362. doi: 10.1055/s-2004-860864. [DOI] [PubMed] [Google Scholar]
- 32.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 33.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 34.Selby JV, Newman B, Quesenberry CP Jr, Fabsitz RR, Carmelli D, Meaney FJ, Slemenda C. Genetic and behavior on body fat distribution. Int J Obes. 1990;14:593–602. [PubMed] [Google Scholar]
- 35.Janghorbani M, Amini M, Willett WC, Gouya MM, Delavari AR, Alikhani S, Mahdavi AR. First national survey of prevalence of overweight, underweight, and abdominal obesity in Iranian adults. Obesity. 2007;15:2797–2808. doi: 10.1038/oby.2007.332. [DOI] [PubMed] [Google Scholar]
- 36.Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol. 1991;43:26–31. doi: 10.1016/s0009-9260(05)80350-2. [DOI] [PubMed] [Google Scholar]
- 37.Mathiesen UL, Franzen LE, Aselius H, Resjo M, Jacobsson L, Foberg U, Fryden A, Bodemar G. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis. 2002;34:516–522. doi: 10.1016/s1590-8658(02)80111-6. [DOI] [PubMed] [Google Scholar]
- 38.Hultcrantz R, Gabrielsson N. Patients with persistent elevation of aminotransferases: investigation with ultrasonography, radionuclide imaging and liver biopsy. J Intern Med. 1993;233:7–12. doi: 10.1111/j.1365-2796.1993.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 39.Layer G, Zuna I, Lorenz A, Haberkorn U, Bannasch P, van Kaick G, Rath U. Computerized ultrasound B-scan texture analysis of experimental diffuse parenchymal liver disease: correlation with histopathology and tissue composition. J Clin Ultrasound. 1999;19:193–201. doi: 10.1002/jcu.1870190402. [DOI] [PubMed] [Google Scholar]


