Abstract
The aim of the present study was to determine and to compare through an indirect enzyme linked immunosorbent assay (ELISA) test, the presence of anti-Neospora caninum antibodies in city and farm dogs, as well as in farm cows, and the relationship among them. The correlation between anti-N. caninum antibodies in farm dogs and cattle was also assessed. The research was conducted in the dairy region of Tizayuca, Hidalgo, Mexico. The frequency of anti-N. caninum antibodies was significantly higher in farm dogs (n = 14) (51%) when compared to those from the city (n = 6) (20%) (P < 0.05), suggesting that farm dogs have a higher risk of exposure to the parasite. There was no significant difference in seropositivity between males (n = 11) (39%) and females (n = 9) (33%) (P > 0.05). The frequency of anti-N. caninum antibodies in farm cattle was significantly higher in farms with dogs (n = 158) (58%) when compared to those with no dogs (n = 43) (35%) (P < 0.05). These results suggest the possible transmission of the parasite from dogs to cattle.
Neosporosis has been considered an important disease in cattle and dogs over the last years (1). Recently, dogs proved to be a definitive host of N. caninum (2). The oocysts are excreted through dog feces and then, after they are consumed by an intermediary host (ruminants, horses, and wild animals), the sporozoites are liberated and infect the small intestine. They divide rapidly by endodyogeny and are converted into tachyzoites, which can infect both skeletal and cardiac muscles, connective tissue and liver, where they multiply. With time, cysts containing bradyzoites are developed. These show affinity for the central nervous system, peripheral nerves, and retina (2,3,4).
The most frequently infected intermediary host is cattle, in which abortion is the most common clinical sign. Morales et al (5) conducted a seroepidemiologic study in dairy cattle, proving that N. caninum infection is widely distributed among Mexican dairy herds. Although there is limited information concerning prevalence of N. caninum infection in dogs, cases of neosporosis have been reported from most of the developed countries of the world; including, North America, Europe, South Africa, Japan, Australia, and Costa Rica (6,7,8).
In Mexico there have been no studies on the prevalence of neosporosis in dogs, or its association with infection in dairy cattle. Because the dog is a definitive host of N. caninum (2), it is plausible that cattle are infected by exposure to canine oocysts, although this infection route remains to be proven. Thus, it is important to assess the prevalence of neosporosis in dogs and its correlation with infection in cattle. The objectives of the present study were to determine and to compare the presence of anti-N. caninum antibodies between city and dairy farm dogs, as well as to determine their correlation through an analysis of the risk factors involved in the transmission of the disease. For this purpose, an indirect enzyme linked immunosorbent assay (ELISA) test was used and a questionnaire was applied only to those farms that had dogs.
The study was conducted in the dairy region of Tizayuca, located at the Industrial Agricultural Center of Tizayuca, Hidalgo, Mexico. Serum samples from 57 dogs were taken. Twenty-seven belonged to 9 different farms and the remaining samples were obtained from dogs living in Mexico City, chosen by quota, non-probabilistic sampling which describes general characteristics from the population to study (9). In order to be included in the study, the dogs should have an owner and no previous contact with cattle.
For the serologic evaluation of cattle, 390 serum samples from 13 different farms were analyzed. Dogs were present at 9 of these farms. From each farm, samples from 30 cows were obtained through quota, non-probabilistic sampling. These farms were selected on the basis of identified neosporosis in herds from previous studies through serology in the mothers, and histopathology and immunohistochemistry in the fetus (5).
Sera were obtained by collecting 2 mL of blood from each animal, using sterile tubes without anticoagulant. The samples of blood from the cattle were taken from the coccigeal vein, while blood obtained from the dogs was taken from the cephalic vein.
The detection of anti-N. caninum antibodies in cattle, was assessed by an indirect ELISA test using both reagents and the method described by Herdchek anti-Neospora (sensitivity 100% and specificity 98.9%; IDEXX Laboratories Inc., Westbrook, Maine USA), with a 0.50 cut point (CP).
For the detection of antibodies to N. caninum, an ELISA test that had been developed for bovine sera was used. When used on canine sera, the anti-bovine immunoglobulin conjugate was replaced by an anti-canine IgG conjugate (Kirkegaard & Perry Laboratories Inc., Gaithersburg, Maryland, USA). A CP of 0.47 was chosen, based on the frequency distribution of the extinction values of all 57 canine sera (10). All samples were used at a 1:100 dilution. Although N. caninum is closely related to Toxoplasma gondii, Sarcocystis sp., and other apicomplexans, serologic cross-reactivity has not been a major issue in animals (11,12).
An identification of the risk factors in cattle was carried out using an analysis of the following variables: the dog's access to the food, water, or both destined to be consumed by cattle; the presence of dogs on the premises during the last 5 y; the dog's access to placentas, aborted fetuses, or both; and the presence of other animals species on the farms.
The frequency of sera seropositive to anti-N. caninum antibodies from both cattle and dogs was determined. In the former, seropositivity was evaluated taking into account the presence or absence of dogs in the farms. In the latter, positive cases were compared by their origin and sex. For dogs, positivity by Chi-squared test, the probability index (Odds ratio = OR), and their confidence intervals were assessed (13,14). The data obtained from the questionnaire of the farms for each of the different variables was coded into categories. These were evaluated using logistic regression, where the dependent variable was seropositivity for N. caninum (15). The variable selection was carried out by means of forward selection method, in which the only variables admitted were those with the likelihood ratio X2 of P ≤ 0.10 to enter and a P ≤ 0.05 to stay in the model.
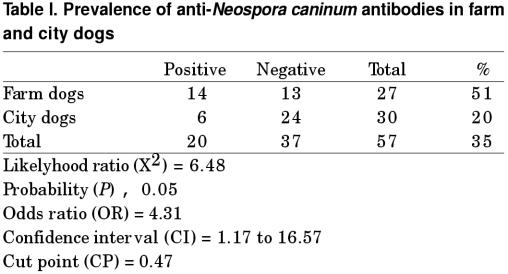
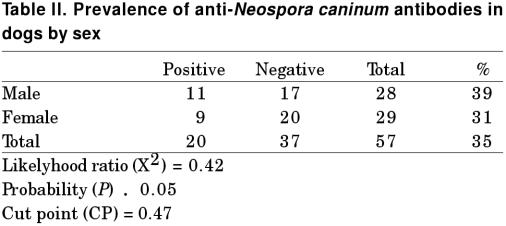
Based on the CP used (OD = 0.47), 14 out of 27 farm dogs (51%) and 6 out of 30 city dogs (20%) were positive to antiN. caninum antibodies. The difference between the positivity of farm and city dogs was statistically significant (P < 0.05), with an OR = 4.31 and a 1.17 to 16.57 confidence interval (Table I). When comparing the positivity between male (n = 11) (39%) and female (n = 9) (33%) dogs, no significant difference was found (P > 0.05) (Table II).
Table I.
Table II.
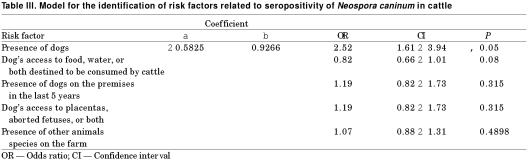
Even though the logistic regression model included the 2 variables; presence of dogs and dog's access to the food, water, or both destined to be consumed by cattle, only the first variable remained in the model (Table III). The seroprevalence in the seropositive cows was significantly higher (P < 0.05), when compared to the positive cows from farms with no dogs, OR = 2.52 and a confidence interval of 1.61 to 3.94.
Table III.
The prevalence of anti-N. caninum antibodies shown in the present study was significantly higher in farm dogs when compared with that from city dogs. This agrees with research conducted by Sawada et al (16) in Japan, with a 31% prevalence for farm dogs and 7% for city dogs. Also, Wouda et al (10) found similar results in Holland, 23.6% of farm dogs were positive, but only 5% of the sera from city dogs were positive. The high frequency of anti-N. caninum antibodies in farm dogs could be attributed to the consumption of fetuses or placentas infected with tachyzoites or cysts of N. caninum. Also, dogs could go from one farm to another and increase the probability of infection. Thus, some authors recommend not to feed dogs fetuses, fetal membranes, or dead calves, which could contain N. caninum cysts.
Another possible explanation for the high prevalence of anti-N. caninum antibodies in farm dogs, is contact with wild animals (coyotes and foxes) and other domestic species (horses, sheep, and goats). Evidence of infection by N. caninum in these species has been previously stated (17,18,19,20).
The presence of anti-N. caninum antibodies in city dogs could be associated with subclinical transplacental transmission through several generations. To a lesser extent, dogs could become infected by the horizontal route if they consume sporulated oocysts of N. caninum. Nevertheless, both modes of transmission could also be applicable to farm dogs (1,2).
The lack of a significant difference between male and female dogs to anti-N. caninum antibodies agrees with the studies conducted by Sawada et al and Barber et al (16,21), but differs from the findings of Wouda et al (10), where a higher frequency in females was found. This implies the need to perform more studies with larger sample sizes in order to establish a real sex predisposition.
A higher seroprevalence of anti-N. caninum antibodies was found in cattle from farms with dogs in comparison with those with no dogs. This is similar to that observed by Paré et al (22) in Québec, where a 22% seropositivity was observed in cattle from herds with dogs and a 7.5% seropositivity in cattle from herds with no dog. Moreover, Wouda et al (10) also states that the seroprevalence of anti-N. caninum antibodies in cattle was significantly less (P = 0.05) in those farms with no dogs. These results suggest a strong association between the seroprevalence to N. caninum in cattle and the presence of dogs, suggesting that dogs may be involved in the transmission of N. caninum to cattle as a definitive host (1). The elimination of oocysts of N. caninum through dog feces could contaminate the food and water that are then consumed by cattle, thus infecting them (3).
Although the seropositivity was lower in farms with no dogs, finding a seroprevalence of up to 46% could be associated with transient dogs. Another possibility is that seropositive cows in these farms were already infected when they were purchased. Many animals from countries with a high seroprevalence of bovine neosporosis are imported into the dairy region of Tizayuca, Hidalgo.
Vertical transmission is an important mode of infection which contributes significantly to the persistence of the infection within a herd. Several studies indicate that 80% or more seropositive cows may transmit the infection to their offspring. However horizontal transmission has also been identified (23,24,25).
The present study brings to light information concerning the relevance of banning dogs on dairy farms if neosporosis needs to be controlled or eradicated. If dogs are to be present, specific areas for their housing should be established, thus diminishing the presence of dog feces in the cattle's food that could contain N. caninum oocysts.
Footnotes
Address all correspondence and reprint requests to Dr. S.E. Morales; e-mail: morales@servidor.unam.mx
Received December 10, 2001. Accepted May 6, 2002.
References
- 1.Dubey JP. Vet Med Today: Clinical Update: Neosporosis in cattle: biology and economic impact. J Am Anim Hosp Assoc 1999;214:1160–1163. [PubMed]
- 2.McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, MacGuire AM. Dogs are definitive hosts of Neospora caninum. J Parasitol 1998;28:1473–1478. [PubMed]
- 3.Dubey JP.Vet Med Today: Clinical Update: Neosporosis in cattle: biology and economic impact. J Am Anim Hosp Assoc 1999;214:1160–1163. [PubMed]
- 4.Dubey JP, Koestner A, Piper RC. Repeated transplacental transmission of Neospora caninum in dogs. J Am Vet Med Assoc 1990;197:857–860. [PubMed]
- 5.Morales E, Trigo FJ, Ibarra F, Puente CE, Santacruz M. Seroprevalence study of bovine neosporosis in México. J Vet Diag Invest 2001;13:413–415. [DOI] [PubMed]
- 6.Barber JS, Gasser RB, Reichel MP, McMillan D, Trees AJ. Prevalence of antibodies to Neospora caninum in different canid populations. J Parasitol 1997;83:1056–1058. [PubMed]
- 7.Dubey JP, Lyndsay DS. Neosporosis. Parasitol today 1993;9:452–458. [DOI] [PubMed]
- 8.Morales JA, Dubey JP, Rodriguez E, Esquivel RL, Fritz D. Neosporosis and Toxoplasmosis associated paralysis in dogs in Costa Rica. Appl Parasitol 1995;36:179–184. [PubMed]
- 9.Kageyama Ma, SanÍn L, Romieu I, Manual de Muestreo Poblacional. Centro Panamericano de EcologÍa Humana y Salud. Organización Panamerica de la Salud. OMS 1997.
- 10.Wouda W, Dijkstra T, Kramer AM, Maanen C. Seroepidemiological evidence for a relationship between Neospora caninum infections in dogs and cattle. Int J Parasitol 1999;29:1677–1682. [DOI] [PubMed]
- 11.Dubey JP, Lindsay DS, Adams DS, et al. Serologic responses of cattle and other animals infected with Neospora caninum. Am J Vet Res 1996;57:329–336. [PubMed]
- 12.Wouda W, Brinkhof J, Van Maanen C, de Gee ALW, Moen AR. Serodiagnosis of neosporosis in individual cows and dairy herds: a comparative study of three enzyme-linked immunosorbent assays. Clin Diag Lab Immunol 1998;5:711–716. [DOI] [PMC free article] [PubMed]
- 13.Thurmond MC. Strategies to control Neospora infection in cattle. Bovine Prac 1995;29:60–63.
- 14.Bland M. An introduction to medical statistics 2nd ed. Oxford University Press Inc. New York. 1995.
- 15.Hosmer DW, Lemeslow S. Applied Logistic Regresion. Wiley. New York 1989.
- 16.Sawada M, Park CH, Kondo. Serological survey of antibody to Neospora caninum in Japanese dogs. J Vet Med Sci 1998;60:853–854. [DOI] [PubMed]
- 17.Lindsay DS, Kelly EJ, Mckown R, Stein FJ, Plozer J. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in coyotes (Canis latrans) and experimental infections of coyotes with Neospora caninum. J Parasitol 1996;82:657–659. [PubMed]
- 18.Buxton D, Maley SW, Pastoret PP, Bronchier B, Innes EA. Examination of red foxes (Vulpes vulpes) from Belgium for antibody to Neospora caninum and Toxoplasma gondii. Vet Rec 1997;141:308–309. [DOI] [PubMed]
- 19.Dubey JP, Porterfield ML. Neospora caninum (Apicomplexa) in an aborted equine fetus. J Parasitol 1990;76:732–734. [PubMed]
- 20.Dubey JP, Lindsay DS. Neospora caninum induced abortion in sheep. J Vet Diagn Invest 1990;2:230–233. [DOI] [PubMed]
- 21.Barber JS, Vam Hall L, Polis I, Trees AJ. Seroprevalence of antibodies to Neospora caninum in Belgian dogs. J Small Anim Pract 1997;38:15–16. [DOI] [PubMed]
- 22.Paré J, Fecteu G, Fortin M, Marsolais G. Seroepidemiologic study of Neospora caninum in dairy herds. J Am Vet Med Assoc 1998;213:1595–1598. [PubMed]
- 23.Paré J, Thurmond MC, Hietalo SK. Congenital Neospora caninum infection in dairy cattle and associated calfhood mortality. Can J Vet Res 1996;60:133–139. [PMC free article] [PubMed]
- 24.Bergeron N, Fecteau G, Paré I, Martineau R, Villeneuve A. Vertical and horizontal transmission of Neospora caninum in dairy herds in Québec. Can Vet J 2000;41:464–469. [PMC free article] [PubMed]
- 25.Wouda W, Moen Ar, Schukken YH. Abortion risk in progeny of cows that experienced a Neospora caninum epidemic. Theriogenology 1998;49:1311–1316. [DOI] [PubMed]