Abstract
The use of naturally-farrowed, artificially-reared piglets as an alternative model to study Haemophilus parasuis infections was evaluated. Two trials were performed in order to evaluate the proposed model. In trial 1, animals were vaccinated and challenged with H. parasuis. Results showed that the proposed model was effectively used to evaluate protective immunity against this organism. In trial 2, animals were challenged with different doses of H. parasuis. Results showed that the severity of clinical signs and lesions tended to increase with higher doses. The reproduction of clinical signs and lesions characteristic of H. parasuis systemic infection was successful in both trials, proving that this model is a viable alternative to specific-pathogen free and cesarean-derived, colostrum-deprived pigs.
Haemophilus parasuis is an early colonizer of the upper respiratory tract of swine. This comensal organism can be found in the nasal cavity (1,2), tonsillar area (3), and trachea (4) of healthy animals. Factors involved in systemic invasion or development of protective immunity by H. parasuis are largely unknown. Reproduction of systemic infection using naturally colonized animals is frequently unsuccessful, since these animals appear to develop a cross-protective immunity against the strains used for challenge. Another factor that may influence successful experimental infection of naturally colonized animals with H. parasuis, is the presence of maternal antibodies. It is known that maternal antibodies generally start to decrease as animals enter the nursery (5). By this time, colonized animals start to develop their own immunity against H. parasuis, which can prevent systemic infection following experimental challenge. Alternative animal models, such as mice and guinea pigs, have been proposed to study the pathogenesis of H. parasuis (6,7). Although no lesions were observed in most inoculated mice, guinea pigs inoculated intramuscularly or in the lung, developed serositis (6). A greater success in reproducing systemic infection by H. parasuis has been achieved by using specific-pathogen free (SPF) pigs (8,9,10,11). However, it is extremely difficult to find swine herds that are not colonized by H. parasuis.
Cesarean-derived, colostrum-deprived (CDCD) pigs have been used successfully to study disease caused by H. parasuis (2,12,13). The major features that make CDCD pigs an ideal model to study H. parasuis infections are the lack of natural colonization by H. parasuis and maternal immunity. However, the high costs and workload involved in obtaining CDCD animals make this model unsuitable for most studies. The development of an alternative pig model, that would maintain the immunological and exposure status of CDCD pigs combined with broad accessibility, would be very useful when studying H. parasuis infections. In this report, the use of naturally-farrowed, artificially-reared pigs was evaluated for experimental reproduction of systemic infection by H. parasuis.
Two trials were performed in order to evaluate the feasibility of using naturally-farrowed, artificially-reared piglets to study H. parasuis infections. Naturally-farrowed piglets were obtained and maintained as described previously (14), with some modifications. In the 1st trial, 10 piglets were obtained from a Hampshire sow herd positive for H. parasuis. The sow was closely monitored during the days preceding the expected farrowing date (114 to 115 d) in order to avoid contact between the newly born piglets and the floor or sow. After the production of milk by the sow was first observed, the sow was washed using a 2% iodine solution (Land O'Lakes, Minneapolis, Minnesota, USA). Piglets were collected as they were born and disinfected using a 70% alcohol solution. The umbilical cord was disinfected using a 2% iodine solution and clamped using a navel cord clamp. Each piglet received 10 mg/kg of gentamicin (Schering-Plough, Kenilworth, New Jersey, USA) by the oral route in order to prevent Escherichia coli infections. Piglets were all transferred into a clean and disinfected plastic box and maintained in the farrowing room until farrowing was completed. A heat lamp was used to maintain the ideal temperature for the newborns (between 28 and 32°C). After farrowing was completed, animals were transferred to a previously cleaned and disinfected isolation unit. The floor of the room where piglets were allocated was covered with autoclaved straw and heat lamps were brought in to provide the ideal environmental conditions for the newborns. Piglets were immediately fed untreated bovine colostrum upon entering the isolation unit. The bovine colostrum was warmed to 37°C and delivered to the piglets using a nurser bottle. The 1st day, piglets were fed ad libitum at 2-hour intervals from 8:00 am until 10:00 pm. Clean water was available at all times. On days 2 and 3 after birth, piglets were fed a range of 40 to 50 mL of bovine colostrum per pig, at 2-hour intervals, and received an additional gentamicin treatment (10 mg/kg, once a day, orally). On days 4 and 5, piglets were fed a mixture containing 50% bovine colostrum and 50% milk replacer for puppies (Esbilac; PetAg Inc. Hampshire, Illinois, USA). The interval between feedings was increased to 4 h, and piglets started to drink off plates. Straw was removed from the room on day 5 after birth. On days 6 and 7, piglets were fed a mixture containing 50% milk replacement (Esbilac; PetAg Inc.) and 50% non-medicated dry food (Theis Research Pig Starter; Mankato, Minnesota, USA) off plates. After 1 wk, piglets were fed only dry food, which was initially moistened using tap water and gradually fed dry. Animals were observed daily for clinical signs. Treatment with gentamicin was repeated when severe diarrhea was observed. All antibiotic treatments were interrupted at least 1 wk prior to challenge. If the animals presented severe diarrhea, the floor of the room was disinfected with 2% iodine solution (Land O'Lakes) at least once a day to avoid spread of infection.
In order to evaluate the use of naturally-farrowed, artificially-reared piglets in a vaccine trial, 6 piglets were vaccinated with a killed vaccine (H. parasuis strain 29775, 1 × 109 CFU/mL, 3 mL dose by the intramuscular route, using an oil-in-water adjuvant) on weeks 2 and 4 after birth. Four pigs in the control group were not vaccinated. Haemophilus parasuis isolation was attempted from nasal swabs collected from all animals before challenge. The homologous challenge was performed 2 wk after the 2nd vaccination, at 6 wk of age. All animals were inoculated with 3 mL of a live H. parasuis culture (strain 29775) containing 1 × 109 CFU/mL, by the intratracheal route. Necropsy was performed on days 3 and 7 after challenge. During the necropsy, swabs from the thoracic and abdominal cavities, brain, and joints were collected for detection of H. parasuis by isolation and polymerase chain reaction (PCR) (15). Samples from the brain, lung, pericardium, liver, and spleen were collected for histology.
The 2nd trial was performed to evaluate how naturally-farrowed, artificially-reared piglets would respond to different vaccination challenge doses. The same protocol was repeated, with some modifications. Twenty-six piglets were obtained from randomly selected sows from a 5000-sow H. parasuis positive herd, which were farrowed on the same date. Piglets were processed as described before. However, disinfection was performed with a biocide (Virkon; Vétoquinol, Princeville, Quebec), instead of a 70% alcohol solution. After processing, the piglets were transferred to an isolation unit previously cleaned and disinfected, at a high security laboratory (Centro de Investigación en Sanidad Animal (CISA), Spain). Animals were maintained in elevated pens, with heated plastic flooring pads as well as heat lamps. Animals were fed and treated as described previously. Electrolytes (BioDiet 50; ) were placed in a plate between feedings and overnight. As bovine colostrum was terminated on day 3, the diet was gradually changed to milk replacement (Esbilac; Petag Inc.) and dry food (SCA- Startrite 100). Animals were treated with 10 mg/kg of gentamicin during 3 consecutive days when diarrhea was observed, and with 3 mg/kg of ceftiofur (Pharmacia Animal Health, Kalamazoo, Michigan, USA) when swollen joints were observed. All antibiotic treatments were interrupted 1 wk before challenge. The room was cleaned with biocide (Virkon; Vétoquinol) once a day. Animals were randomly divided into 5 groups and inoculated at 3 wk of age with 104, 106, 108, and 109 CFU/mL by the intratracheal route, using H. parasuis strain 29775. The control group was inoculated with PBS. Animals were observed daily for clinical signs after the vaccination challenge. Necropsies were performed on days 2 and 3 postinfection (PI). During necropsy, swabs from the thoracic and abdominal cavities, brain, and joints were collected for H. parasuis isolation and PCR (15). This experiment was approved by the Institutional Animal Care and Use Committee (IACUC), University of Minnesota, Minneapolis, Minnesota, USA.
In trial 1, from the 10 animals initially obtained, 5 remained alive when the vaccination challenge was performed. One animal, which did not adapt with the feeding on plates, continued to be fed 50% milk replacement (Esbilac; Petag Inc.) and 50% dry food using a nurser bottle. Despite the efforts to feed this animal, it died during the 1st wk. The remaining 4 animals died in weeks 2 and 3 after developing vomiting and diarrhea. Escherichia coli was isolated in pure culture from the peritoneal and thoracic cavities of these animals. Haemophilus parasuis was not isolated from the nasal cavities of vaccinated and non-vaccinated animals before challenge. In week 6, 4 vaccinated animals and 1 non-vaccinated animal were challenged with H. parasuis. The non-vaccinated control developed high fever (105°C) and arthritis of the left carpal joint on day 1 postinoculation (PI). On day 2 PI, this animal developed severe generalized arthritis and was reluctant to walk. On day 3 PI, the control pig showed severe central nervous system (CNS) signs, characterized by prostration and trembling. Vaccinated animals showed no clinical signs after challenge. The control pig and 2 vaccinated pigs were necropsied on day 3 PI. At necropsy, severe fibrinous polyserositis, arthritis, and meningitis were observed in the control pig. These lesions were further evaluated and confirmed by histopathology. Haemophilus parasuis was consistently isolated in pure culture from collected samples and all swabs were PCR positive for H. parasuis. Vaccinated animals showed no lesions at necropsy and were PCR and culture negative for H. parasuis. One of the remaining vaccinated animals developed a swollen joint on day 6 PI. The 2 remaining animals were necropsied on day 7 PI. At necropsy, fibrinous arthritis was observed in the animal that previously showed clinical signs of arthritis. Escherichia coli was isolated in pure culture from the affected joint. The 2nd animal showed no macroscopic lesions. Samples from both animals were PCR and culture negative for H. parasuis.
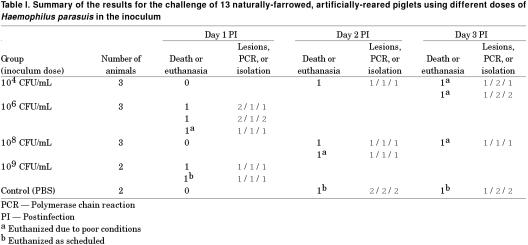
In trial 2, from the 26 animals initially obtained, 13 survived for the vaccination challenge on week 3. On day 6 after birth, 2 piglets developed pustular dermatitis. By day 7, nearly all pigs had skin lesions characteristic of Staphylococcus infection. On day 8, 1 piglet that was showing severe dermatitis died and Staphylococcus spp. was isolated in pure culture from cardiac blood. Another piglet showing severe pustular dermatitis was euthanized on day 8, 2 piglets died on day 9, and 1 more piglet died on day 10. On day 10, blood samples from the remaining piglets were cultured in order to assess the prevalence of systemic infection. Staphylococcus spp. was isolated in pure culture from 2 of the 21 remaining piglets. On day 14 after birth, 2 piglets died and 1 piglet was euthanized. At necropsy, these piglets had dry pustules on the skin and severe subcutaneous edema and colon edema, suggestive of toxemia by E. coli. No organisms were isolated from peritoneal and thoracic swabs. On day 15, 3 other animals showed severe subcutaneous edema and were euthanized and 2 more animals with similar symptoms were euthanized on day 16. Escherichia coli and Staphylococcus spp. were not isolated from these animals. The experimental design for evaluation of different challenge doses and results for macroscopic lesions, isolation and PCR are summarized in Table I. Nine of the 11 inoculated animals developed lesions characteristic of H. parasuis infection. Haemophilus parasuis was isolated from 9 animals and all inoculated animals were PCR positive. The control animals did not develop any clinical signs. One of the control animals showed a small adhesion in the pericardium. However, both control animals were negative for H. parasuis using isolation and PCR methods of detection.
Table I.
The use of naturally-farrowed, artificially-reared pigs as an alternative model to study H. parasuis infections was evaluated. Results from trial 1 showed that the non-vaccinated pig inoculated with H. parasuis strain 29775 developed systemic disease after challenge, which was confirmed by the presence of lesions, PCR, or isolation. Results from trial 2 showed that all challenged pigs were systemically infected, based on clinical signs, lesions, PCR and H. parasuis isolation. Performance of the 2 described trials demonstrated the feasibility of using the proposed model in vaccine and pathogenesis studies.
The success of obtaining naturally-farrowed, artificially-reared piglets was dependent on several critical factors. The intensive feeding regimen that was adopted prevented dehydration, caused on occasion by the transport of piglets from the farm to the isolation units, and reduced mortality of piglets reluctant to eat off plates. Strict hygiene reduced cross-contamination of piglets during episodes of diarrhea. The constant evaluation of clinical signs and immediate treatment also helped to improve the survival rate. We have previously attempted to raise piglets using only a milk replacer (Esbilac; Petag Inc.). The mortality rates reached 80 to 90% during the 1st wk. Piglets rapidly succumbed to severe vomiting and diarrhea 4 d after birth. The use of bovine colostrum improved the survival rate of artificially-reared piglets considerably and significantly reduced the onset of vomiting and diarrhea. These results are in accordance with previous reports (14,16,17).
Considering the adopted protocols and described outcomes, it can be concluded that different groups of pigs raised in different conditions may be more susceptible to different sets of agents. The major cause of death in trial 1 was diarrhea caused by E. coli. Preventative treatment of animals with gentamicin might have reduced, but not prevented, colonization by E. coli. One vaccinated animal, which had suffered previous episodes of diarrhea, developed arthritis due to an E. coli infection after the vaccination challenge with H. parasuis. Although this animal did not develop systemic infection by H. parasuis, the stress of challenge may have precipitated the E. coli infection.
Some diarrhea was also observed in trial 2. However, the major causes of death in this group were severe dermatitis followed by systemic infection by Staphylococcus spp. and toxemia by E. coli, which was characterized by severe subcutaneous and colon edema. The excessive cleaning of piglets due to their diarrhea may have resulted in an imbalance of the normal flora of the skin, which facilitated the observed dermatitis. Young animals are particularly susceptible to dermatitis caused by Staphylococcus spp. However, systemic infections by this agent are extremely rare (18). The naïve status of the artificially-reared piglets combined with the lack of maternal immunity makes these animals highly susceptible to systemic infections. Edema were observed when the diet offered to the animals was changed to completely dry food. The high consumption, together with the high protein content in the dry food could have facilitated the development of toxemia by E. coli.
All of the animals developed systemic disease. Individual differences were observed regarding the development of clinical signs and lesions due to H. parasuis infection, within and between groups. Two out of 3 animals inoculated with 106 CFU/mL and 1 out of 2 animals inoculated with 109 CFU/mL died on day 1 PI. One of the animals inoculated with 109 CFU/mL did not develop a fever or clinical signs after challenge. Animals inoculated with 108 CFU/mL developed delayed systemic infections compared with animals inoculated with 106 CFU/mL. Similarly, Amano et al (11) did not find any major differences when SPF pigs were experimentally inoculated with 105, 106, and 107 CFU/mL. The ideal dose used for reproduction of H. parasuis infections depends on the characteristics of the study of interest. However, one should be aware of the possibility of different responses among animals inoculated with similar doses. One of the factors that should be considered regarding these differences is the use of antibiotics. Although animals received antibiotics before challenge, all treatments were terminated 1 wk before challenge, as instructed by the manufacturer. One week before challenge, animals that still required further treatment were euthanized.
The naturally-farrowed, artificially-reared pig model can be used to produce H. parasuis-free piglets even when obtained from H. parasuis-positive herds. Although the model proved suitable for H. parasuis studies, it requires further refinements in order to minimize the excessive prechallenge death losses that were experienced in these trials. This model is currently being used in the CISA to study the pathogenesis of H. parasuis systemic infection. The survival rate of naturally-farrowed, artificially-reared pigs has now been improved to 80%.
Footnotes
Acknowledgments
The authors thank PIC International Group and the PathoCHIP team for partially funding this project and the Centro de Investigación en Sanidad Animal (CISA, Spain) for providing the facilities and personal for the experiment. The authors also thank Carmen Cia (PIC Spain), Carmen Sanchez (CISA), and Miguel Sanchez (CISA) for their valuable help during the development of the 2nd trial.
Address all correspondence and reprint requests to Dr. C. Pijoan; telephone: (612) 625-1233; fax: (612) 625-1220; e-mail: pijoa001@tc.umn.edu
Received March 11, 2002. Accepted July 11, 2002.
References
- 1.Amano H, Shibata M, Kajio N, Morozumi T. Pathologic observations of pigs intranasally inoculated with serovar 1, 4 and 5 of Haemophilus parasuis using immunoperoxidase method. J Vet Med Sci 1994;56:639–644. [DOI] [PubMed]
- 2.Vahle JL, Haynes JS, Andrews JJ. Interaction of Haemophilus parasuis with nasal and tracheal mucosa following intranasal inoculation of cesarean-derived colostrum-deprived (CDCD) swine. Can J Vet Res 1997;61:200–206. [PMC free article] [PubMed]
- 3.Oliveira S, Batista L, Torremorell M, Pijoan C. Experimental colonization of piglets and gilts with systemic strains of Haemophilus parasuis and Streptococcus suis to prevent disease. Can J Vet Res 2001;65:161–167. [PMC free article] [PubMed]
- 4.Segales J, Domingo M, Solano GI, Pijoan C. Immunohistochemical detection of Haemophilus parasuis serovar 5 in formalin-fixed, paraffin-embedded tissues of experimentally infected swine. J Vet Diagn Invest 1997;9:237–243. [DOI] [PubMed]
- 5.Solano-Aguilar GI, Pijoan C, Rapp-Gabrielson V, Collins J, Carvalho LF, Winkelman N. Protective role of maternal antibodies against Haemophilus parasuis infection. Am J Vet Res 1999;60:81–87. [PubMed]
- 6.Morozumi T, Hiramune T, Kobayashi K. Experimental infections of mice and guinea pigs with Haemophilus parasuis. Natl Inst Anim Health Q (Tokyo) 1982;22:23–31. [PubMed]
- 7.Rapp-Gabrielson VJ, Gabrielson DA, Schamber GJ. Comparative virulence of Haemophilus parasuis serovars 1 to 7 in guinea pigs. Am J Vet Res 1992;53:987–994. [PubMed]
- 8.Rosendal S, Boyd DA, Gilbride KA. Comparative virulence of porcine Haemophilus bacteria. Can J Comp Med 1985;49:68–74. [PMC free article] [PubMed]
- 9.Nielsen R. Pathogenicity and immunity studies of Haemophilus parasuis serotypes. Acta Vet Scand 1993;34:193–198. [DOI] [PMC free article] [PubMed]
- 10.Amano H, Shibata M, Kajio N, Morozumi T. Pathogenicity of Haemophilus parasuis serovars 4 and 5 in contact-exposed pigs. J Vet Med Sci 1996;58:559–561. [DOI] [PubMed]
- 11.Amano H, Shibata M, Takahashi K, Sasaki Y. Effects on endotoxin pathogenicity in pigs with acute septicemia of Haemophilus parasuis infection. J Vet Med Sci 1997;59:451–455. [DOI] [PubMed]
- 12.Rapp-Gabrielson V, Kocur GJ, Claark JT, Stephen KM. Haemophilus parasuis: immunity in swine after vaccination. Vet Med 1997;92:83–90.
- 13.Vahle JL, Haynes JS, Andrews JJ. Experimental reproduction of Haemophilus parasuis infection in swine: clinical, bacteriological, and morphologic findings. J Vet Diagn Invest 1995;7:476–480. [DOI] [PubMed]
- 14.Gomez GG. The colostrum-deprived, artificially-reared, neonatal pig as a model animal for studying rotavirus gastroenteritis. Front Biosci 1997;2:471–481. [DOI] [PubMed]
- 15.Oliveira S, Galina L, Pijoan C. Development of a PCR test to diagnose Haemophilus parasuis infections. J Vet Diagn Invest 2001;13:495–501. [DOI] [PubMed]
- 16.McCallum MI, Elliot JI, Owen BD. Survival of colostrum-deprived neonatal piglets fed gamma-globulins. Can J Anim Sci 1977;57:151–158.
- 17.Drew MD, Owen BD. The provision of passive immunity to colostrum-deprived piglets by bovine or porcine serum immunoglobulins. Can J Anim Sci 1988;68:1277–1284.
- 18.Wegener HC, Skov-Jensen EW. Exsudative epidermitis. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa State University Press, 1999:469–474.