Abstract
Different doses of dexamethasone were evaluated for the treatment of cerebral trauma using a rat model of cerebral hematoma induced by intracerebral (IC) stereotaxic injections of collagenase. Control animals received an intracerebral collagenase injection followed by intraperitoneal (IP) saline injection. Sham operated animals received saline only (IC, IP). Forty-eight hours following the surgeries, the brains were removed from the euthanized animals. Cerebral hemispheres were separated and the 4 coronal sections (antero-posterior plane) were weighed. Each slice was dried for 24 h (100°C) and weighed again to establish brain water content. In hematoma-induced saline treated rats, significant differences in brain water content were observed when compared to sham operated animals. Rats treated with 1 mg/kg dexamethasone had a significant brain water content decrease; however, no significant differences were observed with higher doses of dexamethasone. In conclusion, low doses of dexamethasone seem to be beneficial for the treatment of cerebral trauma.
Low doses of dexamethasone have been used to treat cerebral edema associated with trauma and tumors in small animals (1). However, controversy still remains in the literature regarding the treatment of brain edema with dexamethasone after a severe head injury. Some authors (2) have suggested that high doses of dexamethasone may be beneficial to decrease edema, while others suggest that it does not improve outcome and may even have deleterious effects (3). High concentrations of potassium at the site of hematoma formation are associated with high doses of dexamethasone which may lead to neuronal degeneration (3).
Recent publications have shown that the collagenase-induced intracerebral hemorrhage in rats is a reproducible model that may be used to study the pathophysiolgogy of intracranial hematoma and associated cerebral edema (4,5,6). It has been suggested that the basal lamina, of blood vessels in the brain containing collagen (7,8,9), are affected by the release of collagenase secreted by mononuclear cells during inflammation and by metastatic tumors (10,11,12,13). Therefore, collagenase may be an intrinsic molecule responsible, at least in part, for the edema observed with cerebral trauma and tumors.
The objective of the present study was to evaluate different doses of dexamethasone (1, 5, and 30 mg/kg) for the treatment of cerebral hematoma induced by the intracerebral injection of collagenase at a dose sufficient to produce a significant intracerebral hematoma. Anti-inflammatory effects of dexamethasone are known to be mediated by mechanisms, such as blocking edema formation and extravasation of cells, at the inflammatory site. Since we did not evaluate histological changes, it is not possible to differentiate between the effects of dexamethasone on edema and hematoma formation independently.
Forty male Sprague-Dawley rats (5 groups of 8 animals), approximately 250 to 300 g body weight (BW) (Charles River, St-Constant, Québec) were used in this study. These rats were specific pathogen-free, originating from a barrier facility. Following their arrival, they were kept in a standard laboratory animal environment (fresh filtered air: 15 changes/h; temperature: 21 ± 3°C; humidity: 40–60%; and light-dark cycle: 12:12 h). They were housed in pairs in polycarbonate cages (Ancare, Bellmore, New York, USA) on hardwood bedding (Beta chip; Northeastern Products Company, Warrenburg, NewYork, USA) and acclimated for at least 3 d prior to the initiation of the study. Rats received reverse osmosis ultra violet (UV) treated water and a certified laboratory diet (Harlan Teklad Mouse/Rat diet, Bartonville, Illinois, USA) ad libitum. The experimental protocol was approved by the Institutional Animal Care and Use Committee prior to animal use and is in accordance with the guidelines of the Canadian Council on Animal Care (13).
Following anesthesia (pentobarbital 50 mg/kg IP; MTC Pharmaceuticals, Cambridge, Ontario), rats were placed in a stereotaxic instrument (David Kopf Instruments, Tujunga, California, USA). Rectal temperature was monitored during surgeries (Thermalert TH-8; Physitemp, Clifton, New Jersey, USA) and remained within normal limits (35.5–37.0°C) throughout the surgery for all animals. The skin above the skull was incised and a burr hole was made at the following stereotaxic coordinates (5): antero-posterior 0.0 mm and lateral 3.0 mm. Animals were injected via the burr hole 5 mm below the dura in the right cerebral hemisphere using a 5 μL syringe (Hamilton syringe; Reno, Nevada, USA). Hematoma-induced animals and negative controls received the collagenase solution and saline (2 μL) respectively. The collagenase solution was composed of 0.5 U collagenase Type VII (Sigma-Aldrich, Oakville, Ontario) in 2 μL saline (Abbott Laboratories, St-Laurent, Québec). The total injection time was 10 min. Following the injection, the needle was removed after 5 min to prevent reflux and the skin was sutured (silk 4.0). The animals were maintained under a heating lamp until recovery. Stereotaxic coordinates were chosen so that injections were localized in the caudate nucleus (14).
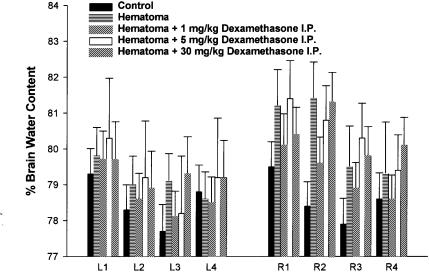
Negative controls (controls; Figure 1) were sham operated animals receiving an intracerebral injection of saline followed intraperitoneal saline at a dose volume of 5 mL/kg at 0.5 and 24 h. Positive controls (hematoma, Figure 1) received an intracerebral injection of the collagenase solution followed by intraperitoneal saline at a dose volume of 5 mL/kg at 0.5 and 24 h. Three hematoma-induced groups received 1, 5, and 30 mg/kg of dexamethasone (5 mg/mL; Vétoquinol N.A. Inc. Lavaltrie, Québec), respectively, at 0.5 and 24 h following the intracerebral injection of the collagenase solution.
Figure 1. Bar graph of brain water content (%) ± s from negative controls (sham-operated animals indicated as control), positive controls (hematoma-induced animals without dexamethasone treatment indicated as hematoma), and collagenase-induced hematoma dexamethasone treated animals. Water content was determined in 4 coronal antero-posterior brain sections (1, 2, 3, and 4), of approximately equal thickness taken from the right (R) and left (L) cerebral hemispheres.
Forty-eight hours following the intracerebral injections, animals were injected with pentobarbital (60 mg/kg, IP) and brains were quickly removed. Hemispheres were separated and cut in 4 coronal sections of approximately equal thickness in the antero-posterior direction identified as slice 1, 2, 3, and 4 of the right (R) and left (L) cerebral hemispheres. Each slice placed in a glass tube was weighed before and after drying for 24 h in an oven at 100°C. Brain water content was expressed as the percent change between wet weight (WW) and dry weight (DW) using the formula: WW-DW/WW × 100.
Unpaired t-tests were performed to assess differences between brain areas of selected groups. Statistical significance level was set a priori, at P < 0.05 adjusted with a Bonferroni correction. Statistical analyses were performed using software (Statistica, version 4.3; Statsoft, 1993; Tulsa, Oklahoma, USA).
Results from all experimental groups are presented in Figure 1. In hematoma controls, significant differences are observed between R2 sections (P < 0.0001) as well as R1 and R3 sections (P < 0.001). The increase in water content was, therefore, most significant in the slice (R2) corresponding to the collagenase injection site and less in the surrounding tissue (R1 and R3). Although the injection of collagenase was in the right cerebral hemisphere, the left hemisphere appeared affected, but to a much lesser extent.
Hematoma-induced animals that received low intraperitoneal doses of dexamethasone (1 mg/kg), showed a significant decrease in water content in R2 only (P < 0.001). These results suggest that low doses of dexamethasone decrease brain edema and, possibly, hematoma, following intracerebral collagenase injections mainly in the section (R2) where the hematoma is localized, although a non–significant decrease in brain water content also occurred in surrounding tissues.
No significant differences in brain water content were observed in animals that received an intracerebral injection of collagenase and intraperitoneal injections of dexamethasone at 5 and 30 mg/kg, respectively, when compared to hematoma controls.
Our results, using a collagenase-induced cerebral hematoma rat model, suggest that low doses of dexamethasone reduce cerebral brain water content, whereas high doses have no beneficial or detrimental effects on this parameter. This model has been shown to be highly reproducible and the histopathological changes are consistent with those seen in intracerebral hemorrhage (6). Locomotor deficits have been shown to correlate with the size of the lesion evaluated histologically (4), and nuclear magnetic imaging has been shown to correlate with behavioral and histological changes (5). Therefore, this seems to be a reliable model to study intracerebral hemorrhage, and alternatives methods can be used to assess therapeutic approaches for the restoration of neurological deficits following cerebral hemorrhage.
Previous investigations have found that high doses of corticosteroids were not beneficial in cases of brain trauma (16,17) and low doses of dexamethasone reduce inflammation and edema (18). Our findings are in accordance with these observations. In the collagenase-induced hematoma model (6), intracerebral bleeding was seen 10 min after the collagenase infusion. By 45 min intact erythrocytes were seen to dissect between normal brain tissue and by 4 h there was extensive bleeding without evidence of tissue necrosis. By 24 h, the lesion is composed of a central zone, consisting in necrotic tissue and erythrocytes, and a peripheral zone with necrotic glial and neuronal cells, as well as a few polymorphonuclear leukocytes. Therefore, the first 24 h are important in the formation of the hematoma. Our decision to treat at 0.5 h and 24 h following the injection, made in order to reproduce the clinical setting where, following head trauma, an animal would be treated in order to reduce edema during the critical post-traumatic phase. Contusions are associated with delayed edema, ischemia, and neurological deficits. Following a brain contusion, the maximal edematous and inflammatory responses have been shown to appear 4 to 6 d after the trauma (19,20). In rats, daily treatments for 5 d with low doses of dexamethasone (1 mg/kg), decreased edema following brain contusion (18). Although the hematoma produced in the contusion model appeared much less important in size, these results suggest that daily treatments with low doses of dexamethasone will also be beneficial in a clinical setting.
In conclusion, low doses of dexamethasone seem to be more beneficial for the treatment of cerebral trauma, whereas high dose have no beneficial effect. Further studies using the collagenase-induced model of the cerebral model are required to evaluate the histopathological and behavioral changes that occur with treatment, suggested by the results of this study.
Footnotes
Address all correspondence and reprint requests to Dr. Vachon; telephone: (450) 773-8521; fax: (450) 778-8109; e-mail: pascal.vachon@umontreal.ca
This work was supported by the MDS Pharma Services R&D Research Fund.
Received February 27, 2002. Accepted August 22, 2002.
References
- 1.Oliver JE, Lorenz MD. Handbook of veterinary neurology. WB Saunders, 1993.
- 2.Hall ED. High-dose glucocorticoid treatment improves neurological recovery in head-injured mice. J Neurosurg 1985;62:882–887. [DOI] [PubMed]
- 3.Mainig G, Deisenroth K. Dose-response relation for dexamethasone in cold lesion-induced brain edema in rats. Adv Neurol 1990;52:295–300. [PubMed]
- 4.Chesney JA, Kondoh T, Conrad JA, Low WC. Collagenase-induced intrastriatal hemorrhage in rats results in long-term locomotor deficits. Stroke 1995;26:312–316. [DOI] [PubMed]
- 5.Del Bigio MR, Yan HJ, Buist R, Peeling J. Experimental intracerebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates. Stroke 1996;27:2312–2319. [DOI] [PubMed]
- 6.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced cerebral hemorrhage in rats. Stroke 1990;21:801–807. [DOI] [PubMed]
- 7.McArdle JP, Muller HK, Roff BT, Murphy WH. Basal laminal redevelopment in tumours metastatic to brain: An immuno-peroxidase study using an antibody to Type IV collagen. Int J Cancer 1984;34:633–638. [DOI] [PubMed]
- 8.Sapsford I, Buontempo J, Weller RO. Basement membrane surfaces and perivascular compartments in normal human and glial tumors: A scanning electron microscope study. Neuropath Appl Neurobiol 1983;9:181–194. [DOI] [PubMed]
- 9.Alcolado R, Weller Ro, Parrish EP, Garrod D. The cranial arachnoid and pia matter in man: Anatomical and ultrastuctural observations. Neuropath Appl Neurobiol 1988;14:1–17. [DOI] [PubMed]
- 10.Janoff A, Zeligs JD. Vascular injury and lysis of basement membrane in vitro by neutral protease of human leukocytes. Science 1968;161:702–704. [DOI] [PubMed]
- 11.Harris ED Jr, Krane SM. Collagenases. N Engl J Med 1974;291:652–661. [DOI] [PubMed]
- 12.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 1989;320:365–376. [DOI] [PubMed]
- 13.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980;284:67–68. [DOI] [PubMed]
- 14.Guide to the Care and Use of Experimental Animals. Canadian Council on Animal Care, Ottawa, Ontario, Canada, 1993.
- 15.Paxinos GT, Watson C. The rat brain in stereotaxic coordinates, 4th ed, San Diego, Academic Press Inc., 1998.
- 16.Braakman R, Schouten HJ, Blaauw-van Dishoeck M, Minderhoud JM. Megadose steroids in severe head injury. Results of a prospective double-blind clinical trial. J Neurosurg 1983;58:326–330. [DOI] [PubMed]
- 17.Dearden NM, Gibson JS, McDowall DG, Gibson RM, Cameron MM. Effect of high-dose dexamethasone on outcome from severe head injury. J Neurosurg 1986;64:81–88. [DOI] [PubMed]
- 18.Holmin S, Mathiesen T. Dexamethasone and colchicine reduce inflammation and delayed oedema following experimental brain contusion. Acta Neurolchir 1996;138:418–424. [DOI] [PubMed]
- 19.Holmin S, Mathiesen T. Biphasic edema development after experimental brain contusion in rat. Neurosc Lett 1995;194:97–100. [DOI] [PubMed]
- 20.Holmin S, Mathiesen T, Shetye J, Biberfeld P. Intracerebral inflammatory response to experimental brain contusion. Acta Neurochir 1995;132:110–119. [DOI] [PubMed]