Abstract
Nursing sickness, the largest single cause of mortality in adult female mink (Mustela vison), is an example of a metabolic disorder, which develops when the demands for lactation require extensive mobilization of body energy reserves. The condition is characterized by progressive weight loss, emaciation, and dehydration with high concentrations of glucose and insulin in the blood. Morbidity due to nursing sickness can be as high as 15% with mortality around 8%, but the incidence is known to vary from year to year. Stress has been shown to trigger the onset of the disease and old females and females with large litters are most often affected. Increasing demand for gluconeogenesis from amino acids due to heavy milk production may be a predisposing factor. Glucose metabolism is inextricably linked to that of protein and fats. In obesity (or lipodystrophy), the ability of adipose tissue to buffer the daily influx of nutrients is overwhelmed (or absent), interfering with insulin-mediated glucose disposal and leading to insulin resistance. Polyunsaturated fatty acids of the n-3 family play an important role in modulating insulin signalling and glucose uptake by peripheral tissue. The increasing demand on these fatty acids for milk fat synthesis towards late lactation may result in deficiency in the lactating female, thus impairing glucose disposal. It is suggested that the underlying cause of mink nursing sickness is the development of acquired insulin resistance with 3 contributing key elements: obesity (or lipodystrophy), n-3 fatty acid deficiency, and high protein oxidation rate. It is recommended that mink breeder females be kept in moderate body condition during fall and winter to avoid fattening or emaciation. A dietary n-3 fatty acid supplement during the lactation period may be beneficial for improved glycemic control. Lowering of dietary protein reduces (oxidative) stress and improves water balance in the nursing females and may, therefore, prevent the development and help in the management of nursing sickness. It is also surmised that other, thus far unexplained, metabolic disorders seen in male and female mink may be related to acquired insulin resistance.
Introduction
Nursing sickness is the largest single cause of mortality in adult female mink (Mustela vison). It is a classic example of a metabolic disorder, which develops when the demands for production require extensive mobilization of body energy reserves. The female enters a strongly negative energy balance and death usually follows soon after the first clinical signs. The etiology of this disorder has eluded scientists for decades (1) and there appears to be several contributing constituents. Clausen et al (2), for example, characterize nursing sickness as an unknown complex of nutritional, metabolic, and environmental factors which influence the ability and capacity of the lactating female to meet the extreme demands for energy turnover and milk production. Any additional stress, such as sudden change in environmental conditions, inanition, and inadequate availability of feed and water, may be of clinical importance as precipitating factors initiating clinical signs and further development of nursing sickness (2). Another previously suggested model (3) for the pathogenesis of nursing sickness considers the following causal factors: fluid deficit, energy deficit, genetic susceptibility, and stress. When these factors coincide during a period of susceptibility in late lactation, nursing sickness will result (3). Although the ranch level epidemiology and the clinical pathology of nursing sickness are well documented, the cause of this metabolic disorder remains unclear. This review paper will examine the known contributing factors and will discuss a new hypothesis for the pathogenesis of nursing sickness in mink.
Ranch level and individual level epidemiology
Studies on ranch level epidemiology in Denmark (2) and Canada (4) indicate that the incidence of this metabolic disorder fluctuates from year to year with a morbidity of 2 to 15% and mortality as high as 8%. In Denmark it is estimated that about 30 000 to 150 000 females are lost annually due to this disease (5). The condition most often affects older females and females with larger litters, but is not associated with any particular colour type. Typical onset of nursing sickness is seen around 42 d post-partum and mortality around 46 d (4).
At the individual level, the condition is characterized by progressive weight loss, lethargy, loss of appetite, emaciation, and extreme dehydration (2,6). The female is often unwilling to leave the nest and may exhibit gastric ulcers and melena (2). There is also evidence of hepatic lipidosis with distinct vacuolization of hepatocytes and renal tubular epithelial cells, possibly indicating a progressive catabolic state (2), as well as adrenal cortical hyperplasia (7). The latter signs are also seen in healthy female mink during the lactation period and are, therefore, not per se indicative of nursing sickness (4,7).
The clinical and biochemical signs related to severe extracellular volume depletion, tissue catabolism, and emaciation include extremely low concentrations of sodium and chloride in the plasma and urine, abnormally high plasma concentrations of glucose, protein, and creatinine, as well as increased osmolality, blood urea nitrogen, phosphorus, and potassium (8). It is suggested (8) that the high glucose and insulin levels are observed in response to stress-induced release of regulatory hormones, such as epinephrine, cortisol, and glucagon. There may also be a lowered peripheral responsiveness to insulin, as well as impaired renal degradation of insulin due to the concentrating ability of the kidneys being compromised. However, there are no signs of lactic acidosis or ketosis in the sick dams (8). Wamberg et al (8) defined nursing sickness as “severe dehydration and emaciation due to heavy loss of energy, water, and body mass along with increased milk production” and characterize the condition by circulatory insufficiency and distinct metabolic disturbances including hormonal control mechanisms for protein degradation, glucose utilization, energy conservation, and water and electrolyte turnover. In the nursing female, coma and death are often imminent after the clinical signs have been observed. Early in the disease some individuals do respond well to oral, subcutaneous, or intraperitoneal electrolyte therapy (5,9).
Effect of age, litter size, and weight loss
The increasing age of the lactating dam, followed by litter size and female weight loss, have been indicated to be the major determinants for the development of nursing sickness (2). Schneider et al (4) have also singled out litter size as a factor consistently associated with nursing disease. According to Korhonen and others (10), mink females with average (4 to 6 kits) and large (7 to 9 kits) litters lose significantly more weight 3 wk after parturition compared to females with smaller litters. Moreover, the number of kits and their total weight were both shown to be important factors affecting the weight loss of the female (10). The study by Clausen et al (2) demonstrated that female mink affected by nursing sickness lose over 30% of their body weight during lactation, whereas apparently healthy dams lost, on average, only 14% (2). This was observed in both the standard black and pastel colour type females. The total number and weight of the kits raised by standard black females suffering from nursing sickness was significantly larger 43 d postpartum than that of healthy controls. However, in the pastel colour phase, the litter size and the total litter biomass did not differ between the sick and the healthy dams (2). No significant difference was observed in the incidence of nursing sickness between the 2 colour phases of mink (black 14.4%, pastel 14.6%) (2).
Role of dehydration and salt deficiency
Hansen et al (11) have been able to produce biochemical changes identical to those found in nursing sickness by using loop diuretics. Findings, including extracellular volume depletion, dehydration, aldosteronism, extremely low urinary sodium, and low urinary osmolality, were consistent with those observed in nursing sickness; however, no clinical illness was diagnosed in the lactating females (11). The authors concluded that the volume depletion and salt deficiency associated with nursing sickness are consequences of, rather than reasons for, the disease. According to Hansen et al (11) the real problem is the onset of inanition, which leads to salt deficiency.
Dietary salt supplement of 1.2 to 1.3% in the dry matter is often used in mink diets during the nursing period to avoid nursing sickness (12). It is also suggested that salt acts as an appetite stimulant (13). A salt level of 1.00 g NaCl MJ−1 in the feed has been shown to reduce the incidence of nursing sickness from 22% down to 7% in the non-supplemented control diet (0.53 g NaCl MJ−1) (13). Female weight loss was also reduced from 24% to 15% due to the increase in the dietary NaCl level. It is noteworthy, however, that the females fed the NaCl supplemented diet weaned fewer kits (5.6 born, 4.5 weaned), than the females fed the non-supplemented diet (5.7 born, 5.1 weaned) (13). Thus, it is unclear whether the reduced nursing sickness incidence is a result of the salt supplement or an outcome of the reduced nursing demand.
In a recent feed palatability study (14) the use of a 0.5% NaCl supplement in the diet did not increase dry matter feed consumption by female or male mink, whereas water intake and urine excretion were significantly elevated. This may have significant practical implications to the females' water balance during lactation resulting in reduced availability of water for milk synthesis. Furthermore, the higher salt content may increase the water requirement of the kits which are starting to consume solid feed around 25 to 26 d of age (15), further accentuating the nursing demand on the female as suggested based on practical observations (16). This is supported by a recent finding by Clausen et al (17), who demonstrated that the kits have a limited capacity to concentrate urine and excrete sodium, and recommended that the dietary salt content should not exceed 0.5 g of NaCl per 100 kcal. For the nursing female, a dietary salt supplement (0.42-0.50 g NaCl 100 kcal−1) may be considered beneficial (17). This is possibly due to the increased water intake and urine excretion (14), which keeps the kidneys perfused and functional, and is thus similar to the effects of rehydration therapy by oral or intraperitoneal electrolytes (5,9).
Stress as a contributing factor
It has been demonstrated that plasma cortisol levels are elevated in the female mink during the latter part of the nursing period (approximately 80–90 nmol L−1) and peak around the time of weaning (approximately 170 nmol L−1) (18). This stress response appears not to be influenced by the actual time of weaning, for example, whether it occurs at 42 versus 49 d of age (19). During weaning stress the females stop eating and lose about 6 to 8% of their body weight in 24 h (18). It is interesting to note that plasma cortisol levels were significantly lowered about 1 wk after weaning, implying reduced stress on the dams at this time point (18,19). These results indicate that weaning is the most vulnerable and stressful period to the mink dams irrespective of the actual timing of weaning (19). Handling of females during this time is suggested to be minimal as this may trigger the development of nursing sickness post-weaning (18). This is in accordance with Schneider et al (4,7) who reported postweaning cases of nursing sickness when the kits were weaned at 6 wk of age. The authors conclude that weaning may act as a stressor precipitating clinical disease in highly susceptible females. The ranch incidence rate, however, was not affected by the time of weaning (4). It appears that the metabolic syndrome of nursing sickness is perhaps transitory in nature as clinically ill females may show recovery if their level of stress is reduced and the demands of lactation no longer exist. It is thus necessary to emphasize the need for close monitoring of the females and prudent weaning practices during the latter part of the nursing period.
Glucose homeostasis and the requirements for lactation
Gluconeogenesis and glycolysis
As an obligate carnivore, the mink has higher enzyme activities for gluconeogenesis and amino acid catabolism than omnivore species (20). According to Berestov (21), carnivore fur-bearing animals have high serum glucose levels already during the first months of life, the values ranging from 7.38 to 10.55 mmol L−1. In the standard dark colour phase of mink, serum glucose values of 190 mg 100 mL−1 have been reported for males and 177 mg 100 mL−1 for females at 6 mo of age (22). The mink can maintain a stable blood glucose concentration when fed carbohydrate-free diets, except when there is a shortage of amino acids, which are percursors of gluconeogenesis. Petersen et al (23) stated that mink are able to maintain glucose homeostasis when glycogen stores are almost exhausted and have shown that glycolytic and gluconeogenetic enzymes are only of minor importance in regulating carbohydrate metabolism in the liver. The activities of glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK) activities have been shown to be very high in the mink (24), indicating high capacity for gluconeogenesis. Moreover, the activities of G6Pase and pyruvate kinase (PK) were significantly higher in females in comparison to males (24). The female mink can, therefore, be expected to exhibit more pronounced gluconeogenesis and glycolysis than the male. As a result, her metabolism may be more responsive to dietary protein and amino acid levels, as well as capable of more rapid mobilization of glucose reserves. Fink and Børsting (25) have recently demonstrated that the lactating mink has indeed a high ability to adapt to variations in the supply of dietary protein as well as carbohydrates.
The mink is able to regulate the intestinal brush-border sugar transporter activity in response to changing levels of carbohydrates in the diet (26). This is unlike another strict carnivore, the cat, which cannot increase sugar uptake from the gut lumen and develops diarrhea when fed large amounts of carbohydrates (27). The mink also has the ability to store excess glucose as glycogen when fed substantial amounts of carbohydrates (20).
Milk production
The mink produces milk with a high dry matter and energy content (28). The dry matter content increases from 19.5% day 3 post-partum to 25.3% at day 25 and up to 37.6% at day 39, whereas the fat content increases from 38.5% to 40.7% and 49.7%, respectively. During the same time frame the milk protein levels are 37.9%, 30.0%, and 25.8%, and lactose levels 11.3%, 4.0%, and 0.3% (28). During the 3rd to 4th wk of lactation the mink dam produces up to 190 g of milk per day (29), which equals nearly 20% of her own body weight, placing very heavy demands on energy metabolism during this time. The daily output of lactose and other sugars in the milk of the female mink nursing a litter of 8 kits is at least 9 g per day (20), which is within the same range as the total absorption of glucose (30). According to Børsting and Gade (20), gluconeogenesis from amino acids accounts for over 70% of the glucose requirements in lactating mink. Litter size has been shown to influence the rate of glucose synthesis. Børsting and Damgaard (30) observed higher glucose production in females nursing 8 kits in comparison to those nursing only 4, placing greater demands for gluconeogenesis by females with large litters. This increased demand for glucose may be considered a significant predisposing factor for the development of nursing sickness. Thus, the etiology appears to be closely tied with a disruption in glucose homeostasis, as suggested by Børsting and Gade (20).
It is interesting to note, however, that in a glucose tolerance test (31) the tolerance curves did not differ between female mink nursing small or large litters. On the other hand, the blood insulin concentration was found to be higher 2 h postprandially in the nursing female mink (30) than what was observed after an intravenous infusion of glucose in the tolerance test (31). This suggests a more pronounced regulation of insulin (and perhaps also glucagon) by plasma amino acid levels rather than by glucose, a mechanism similar to that observed in the cat (32). The lack of a directly glucose-dependent regulation of insulin may contribute to the disruption in glucose homeostasis during the nursing period, where gluconeogenesis from amino acids (high protein diets traditionally fed during nursing) and dietary carbohydrate both elevate blood glucose.
Energy nutrition during lactation
Fink and others (25,33) have recently shown that the lactating mink dam is capable of utilizing high dietary carbohydrate levels without critical elevation in blood glucose when fed diets with varying metabolizable energy (ME) ratios from protein (CP), fat (CF), and carbohydrates (CHO). Feeding of the lowest protein diet, with an ME ratio of 31:37:32 (CP:CF:CHO), resulted in the lowest heat production in the nursing females and the least amount of protein being oxidized for energy purposes (33). The females also produced the most milk and raised heavier kits (33). These results are in agreement with a study by Pölönen and coworkers (34) who demonstrated that a dietary corn syrup supplement, a readily available carbohydrate source, helped maintain female body condition during lactation. Lowering the dietary protein level and elevating the carbohydrate content during lactation may also have significant practical implications. There appears to be a possibility, by dietary manipulation, to reduce heat stress on the female by lowering heat production and consequently reducing the need for heat dissipation particularly during hot weather. Also, the amount of water voided in the urine on a low protein, high carbohydrate diet is less, due to a reduced requirement for the excretion of nitrogen from protein catabolism (33). This can be expected to result in lower stress levels in the dam as well as improve water balance and thus help prevent or manage nursing sickness.
Seasonal changes in mink body condition
Dietary fat is the main source of energy for mink, contributing about 35 to 55% of the animals' ME requirement. During the fall, from September to November, the mink lays down substantial subcutaneous, mesenteric, and inguinal fat depots to serve as energy reserves during winter (35,36). These labile stores are extensively mobilized and used for energy during fasting or food deprivation, and for the production of milk fat by the female. In the mink de novo synthesis of fatty acids in the mammary gland accounts for only 5% of milk fat production (37), the rest being derived from external sources, such as the diet and body fat deposits. The profiles of dietary marine, animal, and plant fat sources differ greatly, and result in respective changes in mink body (15,35) and milk fatty acid profiles (37). Calculations based on estimated milk production volumes (29) and the reported milk fat content (28) show that the female mink transfers about 20 g of milk fat a day during the 3rd to 4th wk of lactation. This demand is estimated to nearly double in late lactation as the fat content in the milk continues to increase (28). Strong dietary linkage to nursing sickness may be surmised as there is evidence that a diet based on poultry offal has resulted in extremely high incidence, with over 40% morbidity and over 30% mortality (38).
Body weight of the mink fluctuates markedly according to season being, the heaviest during the winter and the lowest during the summer months (39). Short-term variations in body weight can be observed particularly during winter due to weather conditions, cold weather inducing both reduced feed intake and significantly diminished locomotor activity (40). The total daily rate of activity from January to February (average ambient temperature between −8 and 0°C) is about 198 min, with no relationship between locomotor activity and feed consumption or locomotor activity and body weight. The smaller mink were, however, shown to eat more frequently (40). These data are for male mink only, but may be expected to be similar for the females, as well as indicating a relatively sedentary lifestyle. The winter months preceding the breeding season (in March) are the time when the mink possess the largest deposits of adipose tissue (35,36).
Tauson (41), as well as Korhonen and Niemelä (36), suggested that breeder mink should be put on a restricted feeding regime during the fall to keep them in moderate condition and to avoid excessive fattening. They also argue that the breeding success of obese mink is poor as a result of larger number of barren females and increased kit losses. Nutritional flushing, where the female mink is slimmed down by restricting feed intake and then brought up in body condition by ad libitum feeding prior to breeding, is a common management practice particularly regarding young females in order to increase reproductive success (41). Older females are often not slimmed down prior to breeding, as no reproductive advantage has been demonstrated. According to Tauson and Aldén (42) female mink aged 1 to 3 y tend to perform better on a high feeding intensity, whereas older mink give best results at a low feeding level. This close relationship between the plane of nutrition and reproductive longevity has also been demonstrated in the males (43). Restrictive feeding of the female mink during pregnancy, on the other hand, has been shown to reduce litter size, increase preweaning mortality, and result in reduced weight gain of the kits (44). It is evident that the mink is subject to fluctuations in body condition due to seasonal changes in plane of nutrition as well as hormonal status and that these changes influence body condition impacting both health and reproductive success.
A metabolic mystery or a familiar foe?
Rearranging the puzzle
The puzzle of nursing sickness still remains to be solved. The previously suggested model (3) for the pathogenesis considers 4 main factors: 1) fluid deficit, 2) energy deficit, 3) genetic susceptibility, and 4) stress. These all influence a period of susceptibility resulting in nursing sickness. The fluid deficit is affected by kits, temperature, and water source, and the energy deficit by feed composition, whereas the genetic susceptibility has been linked to colour type. The dam's stress level, on the other hand is, influenced by weaning, cage design, and environmental factors (3). This model is complex and detailed, yet very general, and it does not adequately explain the causative relationships between the contributing factors and nursing sickness. Many of the key components have been investigated to date as potential primary causes, but they appear to be consequences or symptoms rather than actual precipitating factors. Therefore, a thorough re-examination of the proposed model is warranted.
Glucose, lipid, and protein metabolism connected
As previously outlined, glucose homeostasis in the mink is directly dependent on protein and amino acid nutrition. However, the metabolism of both carbohydrates and protein is inextricably linked to that of lipids, which have received very little attention regarding the etiology of nursing sickness. The role of the adipose tissue is to buffer the daily influx of dietary nutrients and to maintain energy homeostasis in the body through multiple mechanisms (45). Increasing fat storage in adipose tissue, as seen in obesity, can lead to widespread changes in glucose and lipid metabolism, and other physiological systems (such as the cardiovascular system) (45). It is noteworthy that the normal buffering capacity of adipose tissue is also lost in lipodystrophy, the absence of adipose tissue (45). When the buffering capacity of adipose tissue is overwhelmed (or adipose tissue is not present), fat is accumulated in other tissues interfering with insulin-mediated glucose disposal. This leads to the development of acquired insulin resistance, also commonly known as type 2 diabetes or syndrome X (45). It appears that adipose tissue, as a major endocrine and secretory organ (46), is able to regulate lipid metabolism locally, as well as in the liver, muscle, and brain (47). From the standpoint of the female mink, the development of hyperglycemia and hyperinsulinemia and, potentially, insulin resistance is a possible result of being either obese or emaciated.
Recently, it has been shown in rats that dietary fish oil reduces blood glucose levels, improves glucose tolerance, and increases insulin-stimulated glucose transport and metabolism in fat cells (48). Compared to saturated fats and fats high in the n-6 fatty acids, fish oils high in polyunsaturated n-3 fatty acids also reduce the amount of white adipose tissue (48). It is plausible that the n-3 fatty acids may play a role in regulating glucose homeostasis in the nursing female mink. The increasing demand for milk fat synthesis towards late lactation (28), and the prominent levels of the n-3 fatty acids in mink milk (37), can be expected to result in increasing amounts of the n-3 fatty acids being secreted in the milk. Since the mink mammary gland does not possess chain elongation and desaturation enzyme systems (37), the n-3 fatty acids must originate either from the diet or from the labile stores of body fat. Based on the estimated milk volumes (29), milk fat percentages (28), and the dietary and milk fatty acid profiles (37) it can be extrapolated that a deficiency in n-3 fatty acids may develop in the nursing female mink during peak to late lactation. This in turn may result in reduced insulin signalling and the development of hyperglycemia. The implication of the role of n-3 fatty acids in the development of hyperglycemia, and subsequently nursing sickness, is consistent with the higher nursing (milk fat) demand posed by a large litter (2,4). Weaning and the cessation of lactation also eliminates the augmented demand for n-3 fatty acids. Interestingly, the lower levels of n-3 fatty acids present in poultry fat (15) may offer an explanation to the dramatically higher incidence of nursing sickness reported when fed a poultry-based diet (38).
It is conceivable that the metabolic syndrome known as nursing sickness in the mink is closely linked to fatty acid metabolism and the degree of obesity or lipodystrophy. Rearranging the suggested relationships between the previously identified contributing factors (3) and considering 2 additional parameters, namely hyperglycemia and insulin resistance, to be pre-existing conditions rather than just clinical symptoms of nursing sickness dramatically alters the proposed pathogenesis cascade for this metabolic disorder. I suggest the possibility that the underlying cause of nursing sickness in the mink may be acquired insulin resistance. The history of obesity (or lipodystrophy) and the development of n-3 fatty acid deficiency during lactation are suggested as the main causal factors.
In addition to obesity (or lipodystrophy) or n-3 deficiency, there may be yet another contributing element. Oxidative stress is suggested to play a key role in the etiology of degenerative diseases, such as cataractous lenses, atherosclerosis, rheumatoid arthritis, Alzheimer's disease, and diabetes (49). In diabetic patients, blood plasma protein fluorescence is increased and vitamin C concentration reduced, indicating increased protein oxidation (49). The high protein diet traditionally fed to mink during the breeding and lactation periods is likely to subject the breeder females to high oxidative stress. Increased protein oxidation rates with increasing levels of dietary protein has been documented in lactating mink (25,33). In poultry production, high dietary protein levels have been demonstrated to directly regulate lipid metabolism (50). A rapid decrease in the expression of genes implicated in lipogenesis was observed, such as fatty acid synthase, acetyl CoA carboxylase, and malic enzyme (50). In the same study (50), plasma thyroxine, uric acid, and non-esterified fatty acids (NEFA) were shown to be elevated by high protein feeding.
Evidence shows that feeding diets of high protein, subjects the lactating female mink to significant increases in metabolic rates (33), predisposing them to high oxidative stress (49). High protein diets may also directly alter lipid metabolism (50). This hypothesis may further explain the extremely high nursing sickness morbidity and mortality reported in the mink when they are fed poultry based diets (38). In a pathological study (38), the level of dietary protein was over 60% in dietary dry matter contributing around 60% of ME. It is possible that the level of dietary protein plays a role in the development of nursing sickness in mink. The mechanism by which this may happen is 2-fold, namely by causing increased oxidative stress resulting in depletion of protective antioxidants (49) and by elevating the levels of circulating NEFA (50), both leading to insulin resistance.
Acquired insulin resistance
The most typical clinical signs of acquired insulin resistance (type 2 diabetes) in other (carnivore) species include hyperglycemia (extracellular glucose excess), reduced uptake of glucose by cells (intracellular glucose deficit), insulin resistance by peripheral tissue, renal failure if uncontrolled, and significant muscle and adipose tissue breakdown. The condition is also aggravated by stress and is often associated with obesity and a sedentary lifestyle (51,52,53).
There are currently no reports in the literature that describe insulin resistance or diabetes mellitus in the mink. The condition is also poorly understood in a closely related member of the weasel family, the ferret (Mustela putorius) (54). A case report on a wild-caught, male, black-footed ferret (Mustela nigripes) describes weight loss, polyphagia, polydipsia, and polyuria. Pathological findings included hyperglycemia, elevated concentrations of aspartate amino transferase and lactate dehydrogenase, as well as glucosuria and ketonuria (54). Inadequate insulin release or peripheral resistance were suggested to be the causal factors. According to Fox and Marini (54) additional clinical experience and more detailed case reports are required to more fully characterize diabetes mellitus in the ferret. The similarities between the clinical symptoms of the metabolic syndrome associated with acquired insulin resistance and the nursing sickness observed in the mink are striking and ought to be further investigated.
New hypothesis for the pathogenesis of nursing sickness in the mink
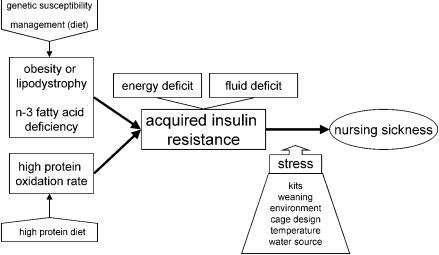
The proposed new hypothesis for the pathogenesis of nursing sickness is presented in Figure 1. There are 3 key components in this model: 1) history of obesity or lipodystrophy; 2) development of n-3 fatty acid deficiency; and 3) high protein oxidation rate, which may together or independently lead into the development of acquired insulin resistance. The animal's body condition and composition are affected by genetic susceptibility and by management (diet), whereas the high protein oxidation rate is induced by a high protein diet. The acquired insulin resistance has 2 significant associated factors, namely fluid and energy deficit. The precipitating factor in the development of nursing sickness is stress, the level of which is influenced by the number of kits; the process of weaning; and the environment of the mink, such as cage design, temperature, and water source. According to this model, the clinical manifestation of nursing sickness equals the terminal stage in type 2 diabetes, where the animal is “starving in the midst of plenty” (51) with increased tissue catabolism, has developed glucose-induced diuresis with very low urinary sodium and chloride concentrations, and is severely dehydrated. At this stage coma and death are often imminent.
Figure 1. Proposed hypothesis for the etiology of nursing sickness in mink.
Until a more complete understanding of the causative components of nursing sickness and an effective (preventive) treatment are available, it is recommended that female breeder mink be maintained in moderate body condition to avoid obesity and emaciation and the potential development of acquired insulin resistance. This is very important for the proper conditioning of females during the fall and winter in preparation for breeding, as well as during pregnancy and lactation. In order to alleviate nursing burden on the females, large litters may be fostered. Stress should be reduced during late lactation and around the time of weaning by providing a cool and quiet environment, palatable feed, ample water, and avoiding unnecessary handling. Early weaning may also be helpful in preventing the onset of the catabolic state. A dietary n-3 fatty acid supplement, especially during the lactation period, may be beneficial for improved glycemic control in the nursing female mink. Lowering of the dietary protein and elevating the level of carbohydrates may alleviate both oxidative stress and heat stress on the nursing females. This dietary change also reduces the amount of water voided in the urine due to reduced protein catabolism aiding in the prevention and management of this complex metabolic syndrome.
Conclusions
In summary, it is postulated that the underlying cause of mink nursing sickness is the development of acquired insulin resistance. There are 3 possible key elements leading to this development in the female mink: 1) obesity or lipodystrophy, 2) n-3 fatty acid deficiency, and 3) high protein oxidation rate. Given the widespread incidence of type 2 diabetes in carnivore companion animals it may be surmised that other, thus far unexplained, metabolic disorders seen in both female and male mink during other times of the production cycle may have their origins in the acquired insulin resistance syndrome. The mink may offer a new animal model for the study of diabetes in carnivores and may also provide a useful new research tool for human medicine.
Footnotes
Acknowledgments
The author thanks the Natural Sciences and Engineering Research Council, Agriculture and Agri-Food Canada, the Canada Mink Breeders' Association, and the Nova Scotia Fur Institute for supporting this research. I would also like to acknowledge Dr. Tom Hutchison and Dr. Gordon Finley, Veterinary Services of the Nova Scotia Department of Agriculture and Fisheries, for their feedback and encouragement. This hypothesis was first presented in the Nova Scotia Mink Breeders' Association Education Seminar in 2002. I wish to thank all of the mink producers who have offered valuable practical insight into this metabolic mystery.
Address all correspondence and reprint requests to Dr. Rouvinen-Watt; telephone: (902) 893-6646; fax: (902) 895-6734; e-mail: krouvinen@nsac.ns.ca
Received July 8, 2002. Accepted February 13, 2003.
References
- 1.Hartsough GR, Gorham J. Sanitation and health. In: The Blue Book of Fur Farming, Madison: Editorial Service Company, 1970:103–127.
- 2.Clausen TN, Olesen CR, Hansen O, Wamberg S. Nursing sickness in lactating mink (Mustela vison). I. Epidemiological and pathological observations. Can J Vet Res 1992;56:89–94. [PMC free article] [PubMed]
- 3.Schneider RR, Hunter DB. Nursing disease in mink. Scientifur 1992;16:239–242.
- 4.Schneider RR, Hunter DB, Waltner-Toews D. Nursing disease in mink: ranch level epidemiology. Prev Vet Med 1992;14:181–194.
- 5.Clausen TN, Hansen O. Electrolytes in mink with nursing sickness. Acta Physiol Scand 1989;136(24A):P9.
- 6.Schneider RR, Hunter DB. A survey of the cause of mortality in adult mink, with emphasis on the lactation period. Can Vet J 1993;34:103–108. [PMC free article] [PubMed]
- 7.Schneider RR, Hunter DB, Waltner-Toews D. Nursing disease in mink: individual level epidemiology. Prev Vet Med 1992;14:167–179.
- 8.Wamberg S, Clausen TN, Olesen CR, Hansen O. Nursing sickness in lactating mink (Mustela vison). II. Pathophysiology and changes in body fluid composition. Can J Vet Res 1992;56:95–101. [PMC free article] [PubMed]
- 9.Schneider RR. Diseases of the lactation period. In: Hunter RB, Lemieux N, eds. Mink... biology, health and disease. Canada Mink Breeders' Association. Guelph: Graphic and Print Services, University of Guelph, 1996:12:1–8.
- 10.Korhonen H, Mononen J, Haapanen K, Harri M. Factors influencing reproductive performance, kit growth and pre-weaning survival in farmed mink. Scientifur 1991;15:43–48.
- 11.Hansen O, Wamberg S, Clausen T. Failure of loop diuretics to induce nursing sickness in mink at weaning. Can J Vet Res 1996;60:277–280. [PMC free article] [PubMed]
- 12.Tauson A-H, Olafsson BL, Elnif J, Treuthardt J, Ahlstrøm Ø. Minkens och rävens mineralförsörjning. (Mineral requirements of mink and foxes, in Swedish). NJF Report Nr. 79. Copenhagen: Jordbrugsforlaget, 1992.
- 13.Clausen TN, Wamberg S, Hansen O. Effects of dietary salt supplementation on clinical and subclinical nursing sickness in lactating mink (Mustela vison). In: Frindt A, Brzozowski M, eds. Proceedings from the 6th International Scientific Congress in Fur Animal Production: Animal Production Review, Applied Science Reports Vol. 28, Nutrition, Pathology and Disease, 1996:87–91.
- 14.Rouvinen-Watt K, White MB, Clarke N, Cormier M. Impact of feed supplements on diet palatability by mink. NJF seminar, October 2–4, Vuokatti, Finland, 2002.
- 15.Layton HN. Development of digestive capabilities and improvement of diet utilization by mink pre- and post-weaning with emphasis on gastric lipase [MSc thesis]. Truro, Nova Scotia: Nova Scotia Agricultural College, Halifax, Nova Scotia: Dalhousie University, 1998. 148p.
- 16.Sabine G. Personal communication, January 19, 2002.
- 17.Clausen TN, Damgaard B. Sodium chloride in the feed in the nursing period. NJF seminar, October 2–4, Vuokatti, Finland, 2002.
- 18.Clausen TN, Hansen O, Wamberg S. Plasma cortisol concentration as an indicator of stress in mink dams at weaning. Scientifur 1999;23:271–273.
- 19.Sørensen B, Clausen TN, Wamberg S, Hansen O. Physiological changes in mink (Mustela vison) dams subjected to weaning at different times during lactation. Acta Agric Scand, Sect A, Animal Sci 2001;51:148–154.
- 20.Børsting CF, Gade A. Glucose homeostasis in mink (Mustela vison). A review based on interspecies comparisons. Original Review. Scientifur 2000;24:9–18.
- 21.Berestov V. Carbohydrates. In: Brandt A, ed. Haematology and Clinical Chemistry of Fur Animals — A Current Treatise. Jyväskylä, Finland: Gummerus Kirjapaino Oy, 1989:64–65.
- 22.Aulerich RJ, Powell DC, Bursian SJ. Handbook of Biological Data for Mink. Department of Animal Science: Michigan State University, East Lansing, Michigan, USA 1999.
- 23.Petersen IM, Sand O, Sørensen PG. Effects of starvation on the activities of key enzymes of the glycolysis and the gluconeogenesis in liver of mink. NJF seminar, October 4–6, Gothenburg, Sweden, 1995.
- 24.Sørensen PG, Petersen IM, Sand O. Activities of carbohydrate and amino acid metabolizing enzymes from liver of mink (Mustela vison) and preliminary observations on steady state kinetics of the enzymes. Comp Biochem Physiol 1995;112:59–64. [DOI] [PubMed]
- 25.Fink R, Børsting CF. Quantitative glucose metabolism in lactating mink (Mustela vison) — effects of dietary levels of protein, fat and carbohydrates. Acta Agric Scand, Sect A, Anim Sci 2002;52:34–42.
- 26.Buddington RK, Chen JW, Diamond JM. Dietary regulation of intestinal brush-border sugar and amino acid transport in carnivores. Am J Physiol 1991;261:793–801. [DOI] [PubMed]
- 27.Maskell IE, Johnson JV. Digestion and Absorption. In: Burger I, ed. The Waltham Book of Companion Animal Nutrition. New York: Pergamon Press, 1996:25–44.
- 28.Olesen CR, Clausen TN, Wamberg S. Compositional changes in mink (Mustela vison) milk during lactation. Norw J Agric Sci 1992;9:308–314.
- 29.Wamberg S, Tauson A-H. Daily milk intake and body water turnover in suckling mink (Mustela vison) kits. Comp Biochem Physiol Part A 1998;119:931–939. [DOI] [PubMed]
- 30.Børsting C, Damgaard B. The intermediate glucose metabolism in the nursing period of the mink. NJF-seminar No. 253, October 4–6, Gothenburg, Sweden, 1995.
- 31.Damgaard BM, Børsting CF. Glucose tolerance test in mink. NJF-Seminar No. 253, October 4–6, Gothenburg, Sweden, 1995.
- 32.Legrand-Defretin V. Differences between cats and dogs: a nutritional review. Proc Nutr Soc 1994;53:15–24. [DOI] [PubMed]
- 33.Fink R, Tauson A-H, Børsting CF. Dietary protein, fat and carbohydrate supply to lactating mink (Mustela vison). NJF-seminar No. 331, October 1–3, Snekkersten, Denmark, 2001.
- 34.Pölönen I, Scott R, Oldfield J. Mink diet energy during pre-weaning and early post-weaning periods. Scientifur 1993;17:47–51.
- 35.Rouvinen K, Kiiskinen T. Influence of dietary fat source on the body fat composition of mink (Mustela vison) and blue fox (Alopex lagopus). Acta Agric Scand 1989;39:279–288.
- 36.Korhonen H, Niemelä P. Effect of feeding level during autumn and winter on breeding weight and result in single and pair-housed minks. Agric Food Sci Finl 1997;6:305–312.
- 37.Wamberg S, Olesen CR, Hansen HO. Influence of dietary sources of fat on lipid synthesis in mink (Mustela vison) mammary tissue. Comp Biochem Physiol 1992;103:199–204. [DOI] [PubMed]
- 38.Seimiya Y, Kikuchi F, Tanaka S, Oshima K. Pathological observations of nursing sickness in mink. Jpn J Vet Sci 1988;50:255–257. [DOI] [PubMed]
- 39.Hansen NE, Finne L, Skrede A, Tauson A-H. Energiforsyningen hos mink og rœv. (Energy requirements of mink and foxes, in Danish). NJF Report Nr. 63. Copenhagen: DSR Forlag, Landbohøjskola, 1991.
- 40.Korhonen H, Niemelä P. Winter energetics and feeding activities in the male mink. Scientifur 1993;17:137–142.
- 41.Tauson A-H. Pre-mating body weight changes and reproductive performance in female mink. Acta Agric Scand 1984;34:177–187.
- 42.Tauson A-H, Aldén E. Different feeding intensity levels to mink. 2. Effects on female reproductive performance, pre-weaning kit growth and longevity of females. Swedish J Agric Res 1985;15:97–107.
- 43.Tauson A-H. Different feeding intensity levels to mink. 2. Effects on male reproductive performance. Swedish J Agric Res 1985;15:77–85.
- 44.Kemp B, Martens RPCH, Hazeleger W, Soede NM, Noordhuizen JPTM. The effect of different feeding levels during pregnancy on subsequent breeding results in mink (Mustela vison). J Anim Physiol a Anim Nutr 1993;69:115–119.
- 45.Frayn KN. Adipose tissue and the insulin resistance syndrome. Proc Nutr Soc 2001;60:375–380. [DOI] [PubMed]
- 46.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001;60:329–339. [DOI] [PubMed]
- 47.Samra JS. Regulation of lipid metabolism in adipose tissue. Proc Nutr Soc 2000;59:441–446. [DOI] [PubMed]
- 48.Takahashi Y, Ide T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br J Nutr 2000;84:175–184. [PubMed]
- 49.Griffiths HR. The influence of diet on protein oxidation. Nutr Res Rev 2002;15:3–17. [DOI] [PubMed]
- 50.Rosebrough RW, Poch SM, Russell BA, Richards MP. Dietary protein may regulate lipid metabolism. Feedstuffs 2002;7:11–23.
- 51.Ganong WF. Review of Medical Physiology. Los Angeles: Lange Medical Publications, 1979.
- 52.Burkholder WJ, Toll PW. Obesity. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, eds. Small Animal Clinical Nutrition. 4th edition. Marceline: Mark Morris Institute, Walsworth Publishing Company, 2000:401–430.
- 53.Zicker SC, Ford RB, Nelson RW, Kirk CA. Endocrine and lipid disorders. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, eds. Small Animal Clinical Nutrition. 4th edition. Marceline: Mark Morris Institute, Walsworth Publishing Company, 2000:849–885.
- 54.Fox JG, Marini RP. Diseases of the endocrine system. In: Fox JG, ed. Biology and Diseases of the Ferret. 2nd edition Baltimore: Williams & Wilkins, 1998:291–305.