Abstract
Human immunodeficiency virus-associated distal-symmetric neuropathy (HIV-DSP) is the most common neurological complication of HIV infection. The pathophysiology of HIV-DSP is poorly understood and no treatment is available for this entity. The dorsal root ganglia (DRG) are the principal sites of neuronal damage and are associated with reactive mononuclear phagocytes as well as HIV-infected macrophages. To determine the role of HIV-infected macrophages in the pathogenesis of HIV-DSP, we developed a technique for culturing human DRG’s. When the dissociated DRG neurons were exposed to supernatants from macrophages infected with CXCR4 or CCR5 tropic HIV-1 strains axonal retraction was observed without neuronal cell death but there was mitochondrial dysfunction in the neuronal cell body. Even though CXCR4 and CCR5 were expressed on the DRG neurons, the effects were independent of these receptors. Antioxidants rescued the neuronal cell body but not the axon from the toxic effects of the culture supernatants. Further, peripheral nerves of HIV-infected patients obtained at autopsy did not show evidence of increased oxidative stress. These observations suggest a differential effect on the axon and cell body. Different mechanisms of injury may be operative in these two structures.
Keywords: neuropathy, dorsal root ganglia, macrophages, neurotoxicity, axonal retraction, mitochondria, chemokine, AIDS
Introduction
Human immunodeficiency virus (HIV)-associated polyneuropathy has become the most common neurological complication of HIV infection (24). More than half of the individuals with advanced HIV infection display signs of a neuropathy (19, 31). Of all forms of acquired immunodeficiency syndrome (AIDS)-associated neuropathies, distal-symmetric polyneuropathy (DSP) is the most frequent subtype, occurring especially in the later stages of AIDS (24). It manifests as a painful sensory neuropathy and is an important cause of morbidity in this population. Indeed, the use of antiretroviral agents can in themselves cause a peripheral neuropathy that may be clinically indistinguishable from HIV-DSP(5). Despite its importance, it remains perhaps the most understudied complication of HIV infection.
The pathogenesis of HIV-DSP remains unclear. The most characteristic pathological feature is the distal degeneration of long axons (24) accompanied by macrophage infiltration (1, 24) and either the absence or modest loss of neurons in the sensory dorsal root ganglia (DRG) (8, 11, 27). Furthermore, there is degeneration within the centrally directed extension of sensory DRG neurons (27). These findings have led to the hypothesis that the primary pathology could be at the level of the sensory neurons in the DRG, leading secondarily to a dying back process with axonal degeneration.
The presence of HIV-infected perivascular macrophages has been shown in the DRG of patients with and without DSP. These studies demonstrated the presence of HIV proviral DNA, mRNA, p24 antigen in these cells (2, 20, 29, 38). Another consistent neuropathological abnormality in the DRG appears to be the presence of activated macrophages, which express MHC antigens and pro-inflammatory cytokines (20, 24, 38). HIV-DSP correlates with the degree of macrophage activation (13). Taken together, this has led to the hypothesis that activated macrophages play an important role in the pathogenesis of peripheral neuropathy. Two possible neuropathogenetic mechanisms have been proposed; the direct effect of HIV or HIV proteins on DRGs and the indirect neurotoxicity of products secreted by activated macrophages.
HIV-infection of the central nervous system is mainly CCR5-dependent and macrophage-tropic (15, 23). Nonetheless neurological symptoms such as HIV-DSP typically occur late in the course of disease progression, when there is an emergence of CXCR4-tropic and dual tropic HIV isolates that use both CCR5 and CXCR4 receptors. Macrophages can support efficient replication by a subset of primary CXCR4 viruses (32), but it is not known whether late-emerging CXCR4 isolates are macrophage tropic and mediate at least some of their pathogenetic effects by infecting macrophages. Reactive macrophages in the DRG may release pro-inflammatory cytokines, reactive oxygen species and excitotoxins, which could result in damage to axons and nerve cells.
We reasoned that since HIV infects only humans, the best model to study the mechanisms of HIV mediated neuropathy would be to use human DRG cultures and HIV-infected human macrophages. We thus developed techniques to culture human DRG and exposed them to supernatants from macrophages infected with CXCR4 or CCR5 tropic strains of HIV. We monitored the cell bodies and neurites for morphological and functional changes, including indicators of oxidative stress. We also determined the role of chemokine receptors in mediating these changes.
Materials and Methods
Characterization of HIV infection and macrophages in DRG
Paraffin and free-floating sections from lumbar DRGs obtained at autopsy from three patients with history of HIV-associated neuropathy and three controls were processed for immunocytochemistry. The distribution of macrophage infiltration in the DRG were assessed by immunostaining of paraffin sections with anti-CD68 antibodies (Dako Inc., Carpinteria, CA). Double immunostaining with anti-HIV-p24 and anti-GFAP antibodies (Dako Inc., Carpinteria, CA) allowed evaluation of productive infection and the topographic association with Schwann cells stained with anti-GFAP antibodies. Double immunostained sections were visualized by confocal microscopy (Zeiss LSM 510 Meta, Germany).
Preparation and culture of Dorsal Root Ganglion cells
DRGs were obtained from human fetuses of 6–16 weeks gestational age with consent from women undergoing elective termination of pregnancy and approval by the Johns Hopkins University Institutional Review Board. DRGs were mechanically dissected under aseptic conditions from the intervertebral foramina by using a dissecting microscope (Nikon SMZ660), following which they were treated with 2.5% trypsin/deoxyribonuclease I (Invitrogen, Carlsbad, CA) solution for 10 min at 37ºC, centrifuged for 6 min at 1000 rpm and resuspended in DRG culture medium (DMEM/F12 (Gibco, Rockville, MD) with 10% heat-inactivated fetal bovine serum (FBS; Sigma, St Louis, MO), 100 U/ml of penicillin G, 100 μg/ml of streptomycin, 250 ng/ml of amphotericin B (Gibco, Rockville, MD), 50 μg/ml of gentamycin (Quality Biological, Inc., Gaithersburg, MD), 15 nM nerve growth factor-beta (Sigma, St Louis, MO), 25 μg/ml insulin (Sigma, St Louis, MO), 1 μg/ml linoleic acid-albumin bovine serum albumin (Sigma, St Louis, MO) and 1× B27 (Gibco, Rockville, MD). Dissociated cells (50,000 cells/well) were directly plated onto poly-D lysine-coated plates and incubated in a humidified chamber with 5% CO2 atmosphere at 37 ºC. The mixed neural cell population contained about 5–10% neurons, 15–30% Schwann cells and 60–80% fibroblast cells as determined by immunostaining with antisera to βIII-tubulin, glial fibrillary acidic protein (GFAP) or anti-fibroblast surface antibody. DRG cultures were maintained for at least 5 days and monitored every day for viability by light microscopy before conducting the experiments below. Experiments were done when the supporting cell layer consisting of Schwann cells and fibroblasts was almost confluent. With this as a criterion, there were no significant differences between the cultures as estimated by light microscopy.
Culture and infection of THP-1 cells and Monocyte-derived Macrophages
THP-1, a human monocytic leukemia cell line (34) was obtained from American Type Culture Collection (Rockville, MD). Cells were grown in suspension in DMEM medium (Gibco, Rockville, MD) containing 10% FBS, 100 U/ml of penicillin G, 100 μg/ml of streptomycin, 250 ng/ml of amphotericin B and cultured in 75 cm2 tissue flasks (Corning, NY) in a humidified 5% CO2 atmosphere at 37ºC. 5 × 106 cells were infected with 1 ml of cell-free inoculum of HIV-1 IIIB (p24=10ng/ml) (25, 28), a CXCR4 tropic strain, for 1 h at 37ºC in a water bath. Cells were washed to remove unabsorbed virus and fresh medium was added. HIV-p24 antigen was determined in tissue culture supernatants by an enzyme-linked immunosorbent assay (ELISA) kit (Beckman Coulter, Inc., Fullerton, CA) every 3 to 5 days over 4 weeks. HIV viral load was determined using a Roche Amplicor version 1.5 kit. To obtain serum-free supernatant, the medium was replaced by serum-free DMEM for 16 h. Cell viability of infected THP-1 cells was evaluated by trypan blue exclusion at the time of collection of the supernatant, and was at least 90%. After centrifugation, the serum-free supernatant was harvested and passed through a 0.2 μm membrane filter (Corning, NY) to remove any cell debris. The culture supernatant collected on day 14 (p24=245 ng/ml; viral load = 8,770,720 copies/ml) was used for further experiments. It was stored in liquid nitrogen until the time of experimental treatment and thawed immediately before use. Uninfected THP-1 cells were cultured in a similar manner and used as a control.
Monocyte derived macrophages (MDM) were isolated from human peripheral blood mononuclear cells (PBMC) of healthy volunteers (New York Blood Center, NY) by using a ficoll-hypaque gradient. After isolation, 1 × 108 cells were seeded into a T75 flask in RPMI 1640 (Gibco, Rockville, MD) supplemented with 20% FBS, 10% pooled human serum from HIV-seronegative donors (Gemini Bio-Products, Woodland, CA), 2 mM L-glutamine, 100 U/ml of penicillin and 100 μg/ml of streptomycin (Sigma, St Louis, MO). After a confluent monolayer was established, MDM were inoculated with cell-free HIV-1 Ba-L (p24=12 ng/ml) (9), a macrophage tropic strain, for 1 h, then washed and cultured in RPMI 1640 supplemented with 20% FBS, 2 mM L-glutamine, 100 U/ml of penicillin and 100 μg/ml of streptomycin. Culture supernatants were monitored for p24 antigen by ELISA (Beckman Coulter, Inc., Fullerton, CA) at selected time points, collected and stored in liquid nitrogen. To obtain serum free supernatant the medium in the MDM cultures was replaced with RPMI 1640 supplemented with 2 mM L-glutamine, 100 U/ml of penicillin and 100 μg/ml of streptomycin for 16 to 18 hours. The culture supernatant collected on day 14 (p24=275 ng/ml; viral load = 35,367,800 copies/ml) was used for further experiments. Uninfected MDM from the same donor were treated in a similar manner and supernatants were used as a control. Both viral strains were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 IIIB from Dr. Robert Gallo and HIV-1 Ba-L from Dr. Suzanne Gartner, Dr. Mikulas Popovic and Dr. Robert Gallo.
Determination of Toxicity
At the time of experimental treatment, the DRG culture medium was replaced with Locke’s buffer containing 154 mM NaCl, 5.6 mM KCl, 2.3 mM CaCl2, 1 mM MgCl2, 3.6 mM NaHCO3, 5 mM glucose, and 5 N-2-hydroxyethylpiperazine-N′-2-ethansulfonic acid (pH 7.2). The cells were incubated with either different dilutions of the supernatant from infected or uninfected THP-1 cells or MDM and, depending on the experiment, with polyclonal antisera to CXCR4 at 1:100 (v/v) (catalog number PX 172A, Cell Sciences, Inc. Canton, MA), polyclonal antisera to CCR5 antisera at 1:100 (v/v) (catalog number PX164A, Cell Sciences, Inc. Canton, MA) or 10 μM trolox (alpha-tocopherol). At these concentrations the antisera block the effects of SDF-1 and RANTES on changes in intracellular calcium in neurons (data not shown). The concentration of trolox was chosen according to previously published experiments (35). After 24 h of incubation, the cells were fixed in 4% paraformaldehyde, immunostained with a monoclonal antibody to βIII tubulin (Promega, Madison, WI) and labeled with Hoechst 33342-trihydrochloride trihydrate (15 μg/ml, Molecular Probes, Eugene, OR). Alexa Fluor-568 conjugated to goat anti-mouse (Molecular Probes, Eugene, OR) was used as secondary antiserum. Total neuritic length was measured in micrometers using a Nikon fluorescence microscope (OpenLab software, Improvision, Lexington, MA). For this purpose, random pictures of the slide were taken and every neurite, for which the beginning and the end point could be clearly identified, was measured. Each experimental condition was done in triplicate wells and repeated three to six times in cultures from different fetuses. With this method about 15 neurons per cover slip per condition were analyzed.
The DNA binding dye Hoechst 33342 was used to measure apoptotic cell death. Neurons were differentiated from other cell types based on positive βIII-tubulin immunostaining and morphological characteristics. Pyknotic or fragmented chromatin was considered apoptotic. Nuclei were visualized under epifluorescence illumination (λex= 340 nm and barrier filter 510 nm) with a 40× objective. About 200 neurons were analyzed for each experimental condition and each experiment was repeated three to six times. Neurons were counted and the percentage of apoptotic neurons was calculated. In each of the assays, to eliminate any investigator bias, the investigator was blinded to the treatment conditions.
Reactive oxygen species
DRG cultures were plated on 35 mm or 24-well glass bottom tissue culture plates and cultured for at least 7 days. After this time cells were exposed for 24 h to either different dilutions of the supernatant of infected or uninfected THP-1 cells or MDM and depending on the experiment 10 μM trolox. Hydrogen peroxide (H2O2) was measured using dihydrorhodamine 123 (DHR, Molecular Probes, Eugene, OR), which is specifically oxidized by H2O2 into 2 molecules of rhodamine. Neuronal cultures were incubated with DHR at a final concentration of 10 μM for 30 min at 37ºC and then washed with Locke’s buffer. The fluorescence emission pattern of DHR-labeled neurons was immediately observed under confocal microscopy (Zeiss LSM 410 or 510 Meta, Germany) (λex= 488 nm and λem= 529 nm). To measure the DHR intensity, random pictures of the cultures were taken with the confocal microscope. Single neuronal cell bodies were traced and the was area analyzed (Image J software, NIH, USA). Each experiment was performed in triplicate and repeated 3 to 7 times.
Mitochondrial membrane potential
JC-1 is a fluorescent dye that accumulates in the mitochondrial matrix depending upon the membrane potential. In mitochondria with low potential, JC-1 shows green fluorescence, whereas at hyperpolarized potentials JC-1 monomers exceed a critical concentration and form J-aggregates which cause a shift in the fluorescence maximum, resulting in a red emission peak.
DRG cells on 35 mm dishes or 24-well glass bottom tissue culture plates were cultured for at least 5 days before use in the experimental treatments described above. After 24 h of exposure to culture supernatants, the cells were incubated for 30 min with the JC-1 dye (10 μM) at 37ºC and then washed with Locke’s buffer. Optical measurements were acquired immediately under confocal microscopy (Zeiss LSM 510 Meta, Germany) in both the green and red regions of the spectrum. Stacks of images were collected at 0.2 μm in the plane of the optical axis by using a 40× oil immersion objective. Random pictures of the cultures were taken with the confocal microscope and the intensity was measured at both wavelengths by tracing the neuronal cell body and separately 2 to 3 cm of the proximal axon in the appropriate stack and the ratio of the intensity at both wavelengths was calculated (Image J software, NIH, USA). Each experiment was performed in triplicate and repeated 3 to 4 times.
Nitrosylated proteins by slot blot and Western blotting
Frozen sciatic nerve tissue was obtained from the National Neuro-AIDS Tissue Consortium (NNTC)/Manhattan HIV Brain Bank. Tissue was obtained from HIV-seropositive subjects with (n=5) or without clinical signs of a neuropathy (n=6) or HIV-seronegative control subjects without neuropathy (n=6). Control subjects were screened by chart reviews to ensure that they were neurologically normal by clinical examination and metabolic panels and complete blood cell counts established that they did not have any other cause of neuropathy. All patients were confirmed to be HIV-seronegative by analysis of a postmortem serum sample. The diagnosis of a neuropathy in the HIV-seropositive individuals was made ante-mortem by a detailed neurological examination.
A small tissue sample was cut from the nerve, and each sample was immediately homogenized with ice cold RIPA buffer (1% SDS, 1% triton X100, 150 mM NaCl, 1 mM sodium phosphate, 2 mM EDTA, sodium deoxycholate, protease inhibitor (Sigma, St. Louis, MO) and sonicated on ice. After high-speed centrifugation tissue extracts were stored at −20°C and thawed immediately before use. For immunochemical detection of 3-nitrotyrosine residues (3-NT) in sciatic nerve homogenates, a slot blot technique was used. After removing the detergents from the homogenates with a commercial kit (OrgoSol™, Detergent-Out™, Genotech, St. Louis MO), the protein concentration was measured using the BCA kit (Pierce, Rockford, IL) following manufacture’s instructions. A total of 70 μg of homogenate protein was loaded onto nitrocellulose in a slot-blot microfiltration unit (Bio-Rad Laboratories, Hercules, CA). For 3-NT determination, a monoclonal antibody (Upstate, Lake Placid, NY) was used. Images were scanned and the area under the curve was analyzed (ImageJ software, NIH, USA).
Characterization of the CXCR4 and CCR5 distribution in human DRG neurons
To characterize the CXCR4 and CCR5 distribution, the DRG cultures were fixed in 4% paraformaldehyde, blocked with 1× PBS containing 10% normal goat serum, and double immunostained with monoclonal anti-βIII tubulin antibody (Promega, Madison, WI) and either polyclonal antisera to CXCR4 at a dilution of 1:100 (v/v) (Cell Sciences, Inc. Canton, MA) or polyclonal antisera to CCR5 at a dilution of 1:100 (v/v) (Cell Sciences, Inc. Canton, MA). Following incubation with the primary antibody overnight at 4ºC the cells were washed and incubated with Alexa Fluor-568 conjugated to goat anti-mouse (Molecular Probes, Eugene, OR) and Alexa Fluor-488 conjugated to goat anti-rabbit (Molecular Probes, Eugene, OR). Slides were mounted with Gel/Mount (Biomeda Inc., Foster City, CA) and viewed using a confocal microscope (Zeiss LSM 510 Meta, Germany). Each receptor staining was done in triplicate and repeated 3 times. 100 neurons were analyzed for each antisera in each experiment and the receptor distribution was expressed as a percentage of total cells.
Statistical analysis
Statistical analysis was performed using GraphPad InStat version 3.06 and GraphPad Prism version 4.01 for Windows (GraphPad Software, San Diego, CA). The results from each set of experiments were averaged and standardized. The data were expressed as the mean ± SEM. Comparisons between experimental groups (infected versus matched uninfected control supernatant) were performed with Student’s t test for paired observations or ANOVA. Categorical variables were calculated with the Fisher’s test. Differences were considered statistically significant for p values < 0.05.
Results
HIV infection in human DRG cells from patients with history of HIV peripheral neuropathy
We assessed DRG from HIV patients who had a history of AIDS and peripheral neuropathy and found marked perineuronal as well as interstitial infiltration of CD68- and HLA class II-positive cells, consistent with the presence of macrophages (figure 1A). Many of the immunostained cells exhibited macrophage morphology and the immunostaining with anti-HLA-DR confirmed their profile of activation. Double immunostaining with anti-HIV-p24 and anti-GFAP demonstrated productive HIV infection within cells with macrophage morphology localized in both perineuronal and interstitial compartments (figure 1B). No evidence of p24 immunostaining was seen in the Schwann cells.
Figure 1. HIV infection in human DRG cells from patients with history of HIV-peripheral neuropathy.
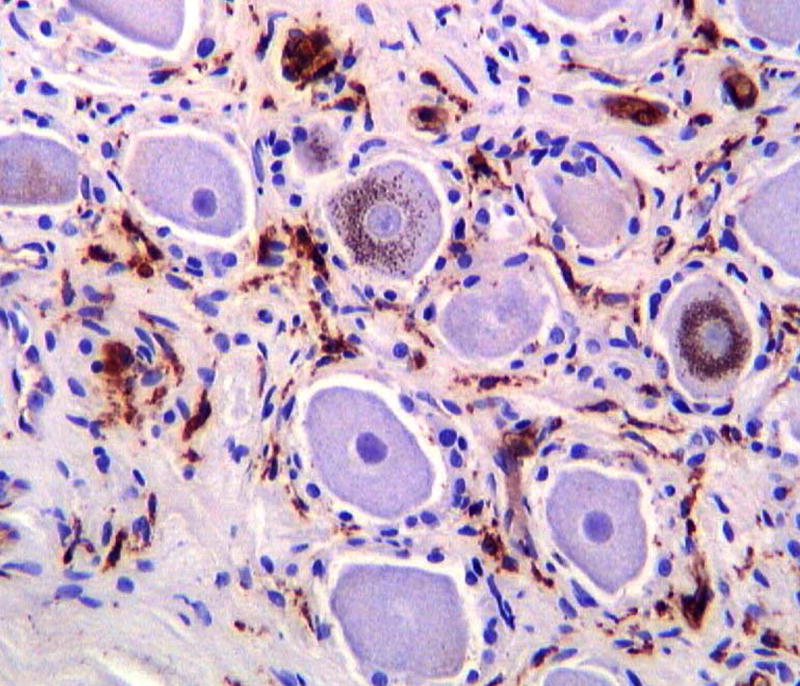
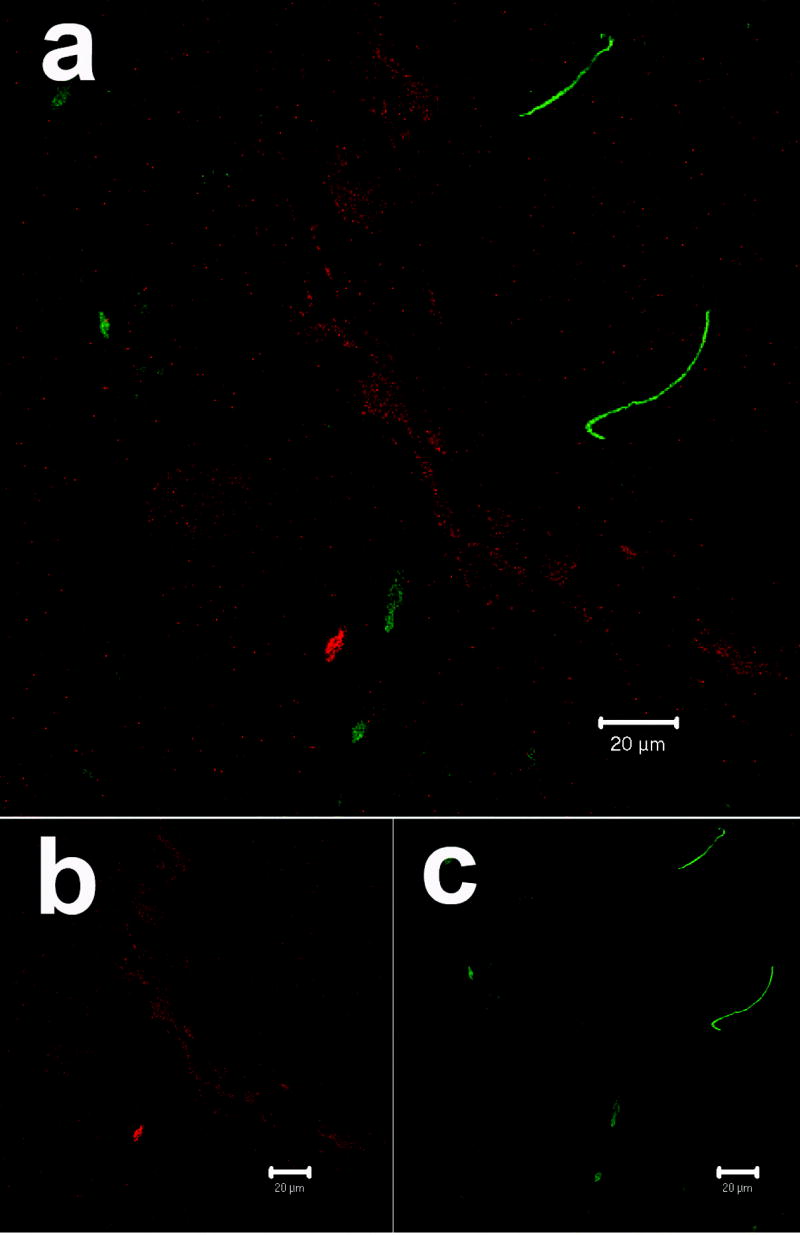
A) Macrophage infiltration of the DRG visualized with anti-CD68 immunostaining and counterstained with Cresyl-violet
B) Distribution of HIV-p24 antigen (red) in the DRG as visualized by confocal microscopy. The HIV-p24 was localized in the perineuronal compartment and within cells with macrophage morphology. Schwann cells and their processes (green) visualized with anti-GFAP antibodies lack any co-localization with HIV-p24.
Supernatants from HIV-1 IIIB- and HIV-1Ba-L-infected cells induce neuritic retraction in DRG neurons
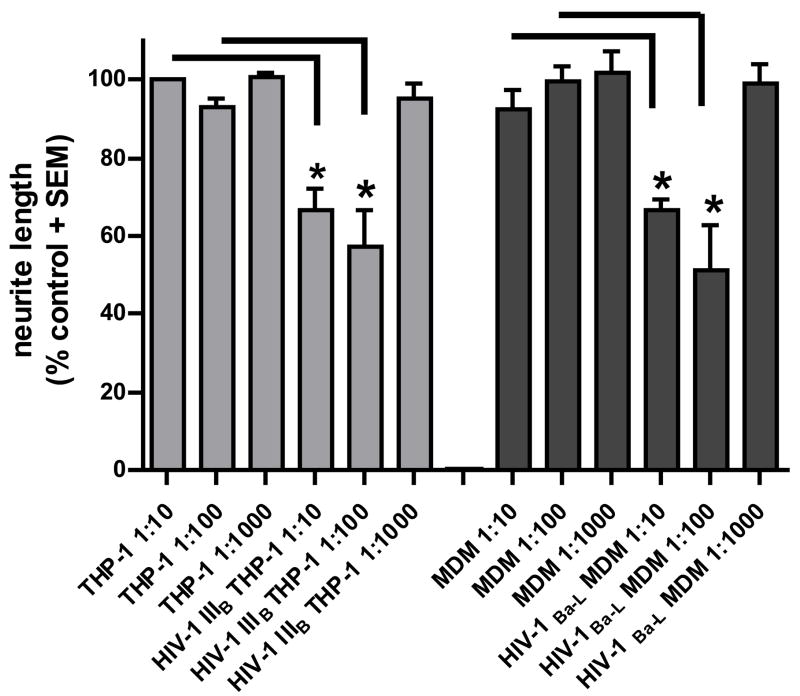
To address the pathophysiology of HIV-DSP we established human DRG cultures and determined whether supernatants from HIV-1IIIB- and HIV-1 Ba-L-infected monocytic cells could induce neuritic retraction in human DRG neurons. We incubated human fetal DRG cells with the culture supernatants at a final dilution of 1:10, 1:100 and 1:1000 for 24 h in Locke’s buffer. Reduction of the mean total neuritic length was consistently observed at dilutions of 1:10 and 1:100 (figure 2). We observed nearly similar amounts of neurite shortening (35–45%) with supernatants from HIV-1IIIB-infected cells and HIV-1Ba-L-infected MDM. Interestingly, there was no significant difference between the effects at a 1:10 or 1:100 dilutions of the supernatants with either viral strain. Culture supernatants at 1:1000 dilution, however, failed to cause any neurite shortening (figure 2).
Figure 2. Effect of supernatants from HIV infected monocytic cells on neuritic length of DRG neurons.
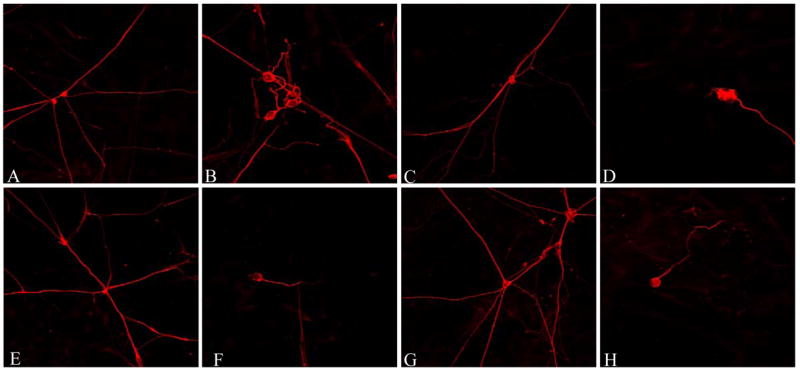
Supernatants from HIV-1IIIB THP-1 infected cells and HIV-1Ba-L infected MDM induce neuritic retraction in human DRG neurons. Confocal images of anti-βIII tubulin immunostained DRG cultures treated for 24 hours with supernatants from uninfected THP-1 cells (A, 1:10 dilution; C, 1:100 dilution), HIV-1IIIB (B, 1:10 dilution; D, 1:100 dilution), uninfected MDM (E, 1:10 dilution; G, 1:100 dilution) and HIV-1Ba-L supernatant (F, 1:10 dilution; H, 1:100 dilution). (I) Reduction of the total neuritic length per axon is shown as standardized data. * Supernatant from infected monocytic cells versus the matched control, p < 0.05.
We observed a differential susceptibility of the neurons. Neurons which were completely integrated in a neuronal network appeared to have better preserved neurite length, whereas neurons growing at the edge of a neuronal network or as single non integrated neurons like the ones illustrated in figure 2, showed neurite retraction after exposure to supernatants from HIV-infected cells. Neurite retraction was often accompanied by morphological changes, such as segmentation. It also appeared that there were more “single nonintegrated neurons” in cultures that had been exposed to the supernatants from HIV-1 infected cells, suggesting, that they may have lost their contact to the other neurons due to neurite degeneration. The results shown in figure 2I include all neurons without differentiation.
We next determined whether neurite trimming was accompanied by significant neuronal cell death. The general neuronal apoptotic rate depended on the fetus from which the cells were derived and varied between 0.5 and 10% with no significant difference between neurons exposed to supernatants from HIV-infected monocytic cells versus supernatants from uninfected cells. There was one exception, however. In neurons from one fetus, supernatants from HIV-1 IIIB and HIV-1 Ba-L infected cells at dilutions 1:100 caused significant neuronal cell death (HIV-1 IIIB 12.7% and HIV-1 Ba-L 17.1%) compared to the matched uninfected control (THP-1, 3% and MDM, 2.5%, p<0.05).
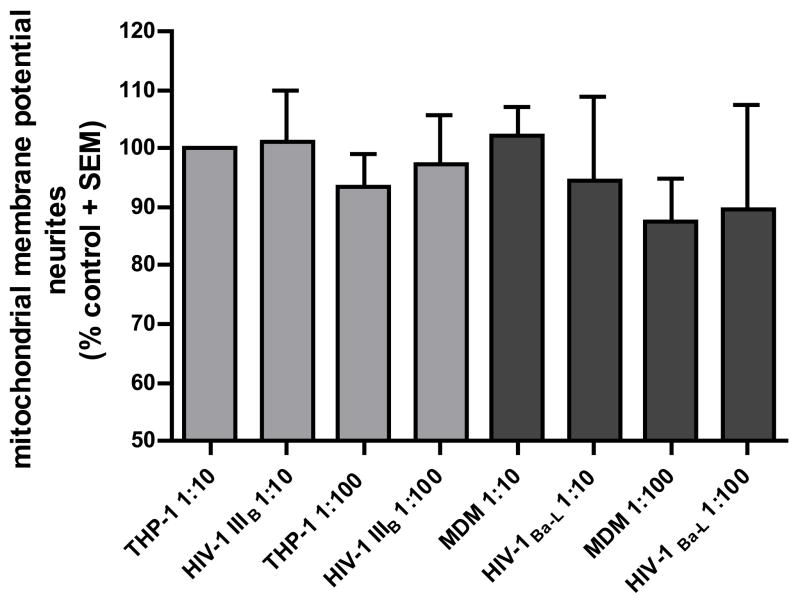
Supernatants from HIV-1IIIB- and HIV-1Ba-L-infected cells induce neuronal mitochondrial toxicity in DRG cultures
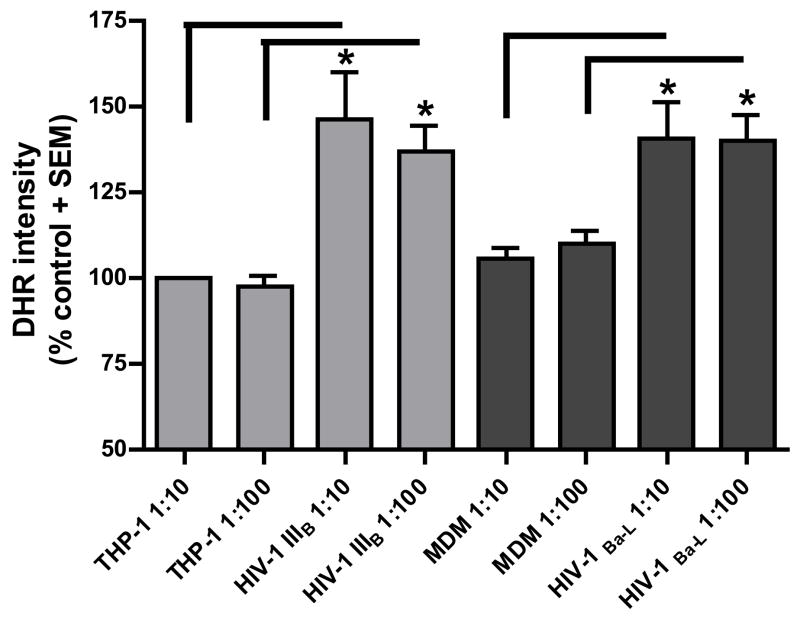
Reactive oxygen species (ROS) production was measured by DHR labeling of neurons in the presence of supernatants from HIV infected macrophages. Dose-independent, but significantly higher amounts of ROS in the neuronal cell body were observed with supernatants from both HIV-1IIIB- and HIV-1Ba-L-infected cells (figure 3A). The DHR intensity in cells treated with infected macrophages conditioned media was 35–45% higher compared to the controls. Mitochondrial membrane potential was also measured using a JC-1 assay. Human fetal DRG cultures were exposed to supernatants from infected and uninfected monocytic cells at dilutions of 1:10 and 1:100 for 24 h in Locke’s buffer. A decrease in mitochondrial membrane depolarization occurred after exposure to the supernatants from HIV-infected monocytic cells. A small (<20%) but significant change in mitochondrial membrane potential was observed with supernatants from both HIV-1IIIB-a nd HIV-1Ba-L-infected cells. Consistent with observations above, there was no significant difference between the 1:10 and 1:100 dilutions with either HIV strain (figure 3B). This suggests that the small changes in mitochondrial membrane potential may be sufficient to trigger ROS formation.
Figure 3. Effect of supernatants from HIV infected monocytic cells on mitochondrial function of DRG cell bodies.
Mitochondrial free radical production and membrane potential was measured in human DRG cell bodies following treatment with supernatants from THP-1 cells infected with HIV-1IIIB and MDM infected with HIV-1Ba-L.
A) Increase in DHR intensity is shown as standardized data. * Supernatant from infected monocytic cells versus the matched control, p < 0.05. Each data bar represents approximately 100 analyzed neurons
B) Mitochondrial membrane potential (red/green ratio) is shown as standardized data. * Supernatant from infected monocytic cells versus the matched control, p < 0.05. Each data bar represents at least 150 analyzed neurons.
Effect of trolox on HIV-1IIIB- and HIV-1Ba-L-induced neurotoxicity
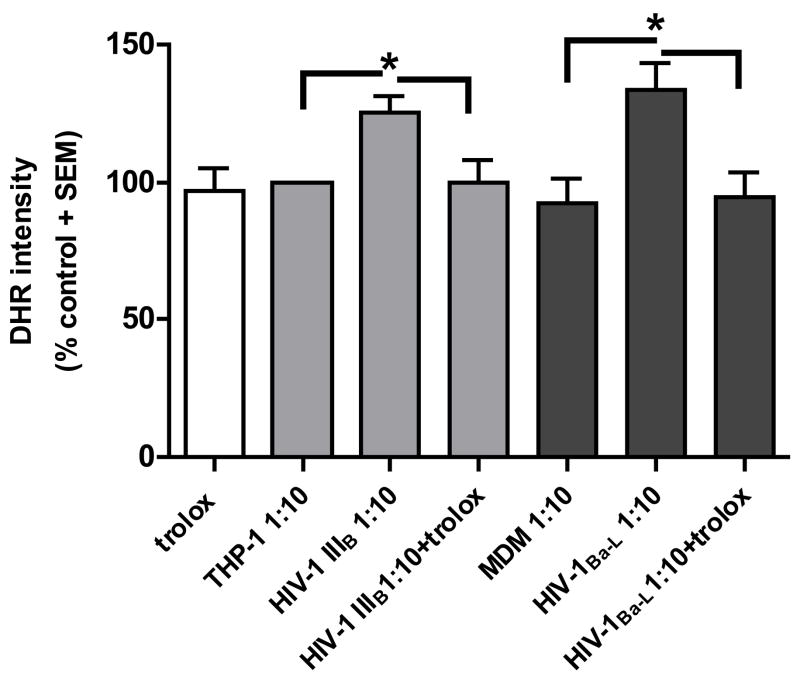
We analyzed whether trolox (10 μM) could prevent the accumulation of ROS in human neuronal DRG cultures. When tested alone, trolox, did not have a significant influence on the DHR intensity in the neuronal cell body suggesting that the culture conditions alone did not induce oxidative stress. When incubated together with supernatants from HIV-1IIIB- and HIV-1Ba-L-infected cells at a dilution of 1:10, trolox prevented the oxidative stress (figure 4A).
Figure 4. Effect of trolox on HIV-1IIIB and HIV-1 Ba-L induced neurotoxicity.
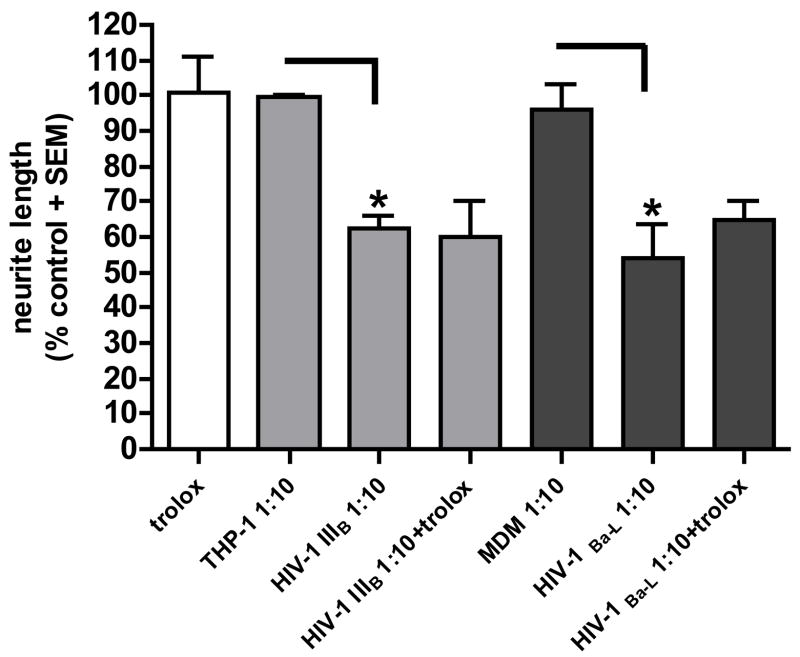
A) Trolox blocked oxidative stress in the neuronal cell body. DHR intensity is shown as standardized data. * Supernatant from infected monocytic cells versus the matched control, p < 0.05. Each data bar represents approximately 100 analyzed neurons. B) Trolox failed to prevent neuritic trimming in human DRG neurons. Reduction of the total neuritic length per axon is shown as standardized data. * Supernatant from infected monocytic cells versus the matched control, p < 0.05. There was no significant difference in the neuritic length of neurons treated with a 1:10 dilution of the supernatants from HIV-infected cells and trolox compared to the supernatants alone.
To determine whether blocking ROS accumulation could also prevent axonal retraction in the neurons, we tested trolox together with the supernatants of HIV-1IIIB- and HIV-1 Ba-L-infected monocytic cells at a dilution 1:10 for 24 h, immunostained the neurons with anti-βIII tubulin and measured the axonal length. Trolox failed to prevent neurite trimming (figure 4B).
Mitochondrial dysfunction in axons
Since trolox did not prevent axonal retraction, we compared the mitochondrial function in the neuronal cell body and neurites following exposure to supernatants from HIV-infected monocytic cells using the JC-1 assay. Interestingly, while significant changes in the mitochondrial membrane potential were noted in the neuronal cell body, similar changes did not occur in the neurites (figure 5).
Figure 5. Effect of supernatants from HIV-infected monocytic cells on axonal mitochondrial potential.
Supernatants from HIV-1IIIB THP-1 infected cells and HIV-1Ba-L infected MDM had no significant effect on the mitochondrial membrane potential in axons of neurons derived from human dorsal root ganglia. Each data bar represents approximately 150 analyzed axons.
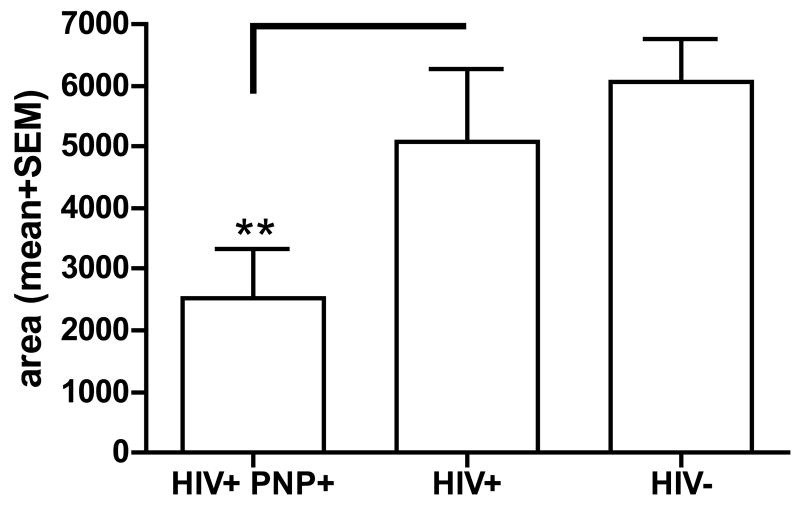
To explore these observations further, we compared the amount of nitrosylated proteins in sciatic nerve obtained at autopsy from HIV-infected individuals with peripheral neuropathy with the amounts from neuropathy-free HIV-infected subjects and HIV-seronegative controls. The three groups did not differ in age and gender but differed in racial distribution (table 1). There was no significant difference in the amount of nitrosylated proteins between the control subjects and the HIV-seropositive individuals without neuropathy. Only small amounts of 3-NT -modified proteins were observed in HIV-infected subjects with neuropathy, while 4/6 patients with HIV infection without a peripheral neuropathy and 3/6 HIV-seronegative subjects had strong bands on the slot blot, indicative of 3-NT production (figure 6A). The HIV-infected subjects with neuropathy had significantly lower amounts of 3-NT compared to the HIV-seronegative subjects (figure 6B).
Table 1.
Demographic characteristics of the subjects who were compared for 3-NT protein residues
| HIV+ SUBJECTS WITH PNP (N=5) | HIV+ SUBJECTS WITHOUT PNP (N=6) | CONTROL SUBJECTS (N=6) | P | |
|---|---|---|---|---|
| Age in years (mean/SD) | 47.2 ± 15 | 45.2 ± 4.8 | 49.7 ± 8.7 | 0.74 |
| Male | 60% | 50% | 67% | |
| Race (AA/W/Hispanic) | 3/2/0 | 2/1/3 | 1/0/5 |
AA African-American; W White
Figure 6. Detection of 3NT modified proteins in peripheral nerve of HIV infected patients.
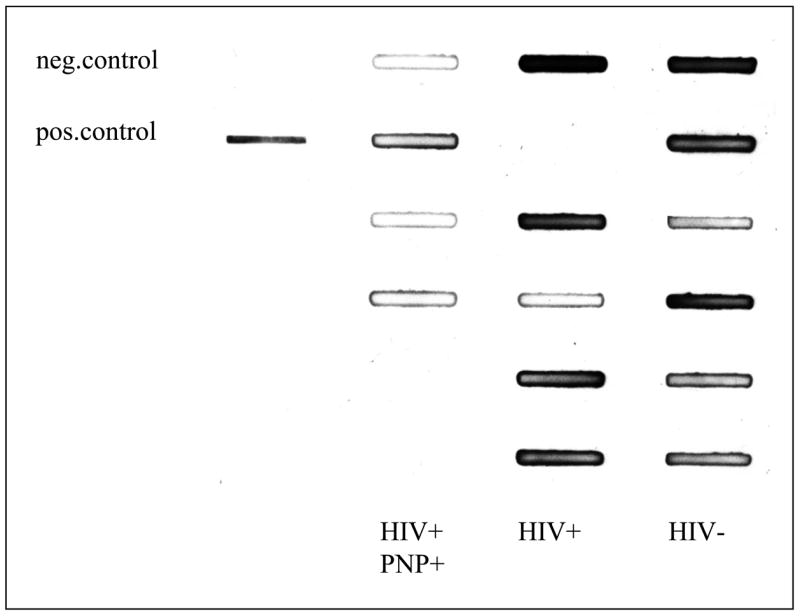
A) Sciatic nerve extracts were analyzed by slot blot by using an antisera to 3-NT. The left row represents a negative (BSA) and a positive control (nitrosylated BSA), the second row shows 5 sciatic homogenate samples from HIV-seropositive individuals with neuropathy. The slots in the third row were loaded with samples from HIV-seropositive subjects without neuropathy and the right row with samples from the HIV-seronegative controls
B) The bar graph represents the mean area±SEM. ** p< 0.05 compared to the HIV-negative controls.
Characterization of chemokine receptor distribution in DRG neurons and prevention of axonal retraction by antisera to the chemokine receptors
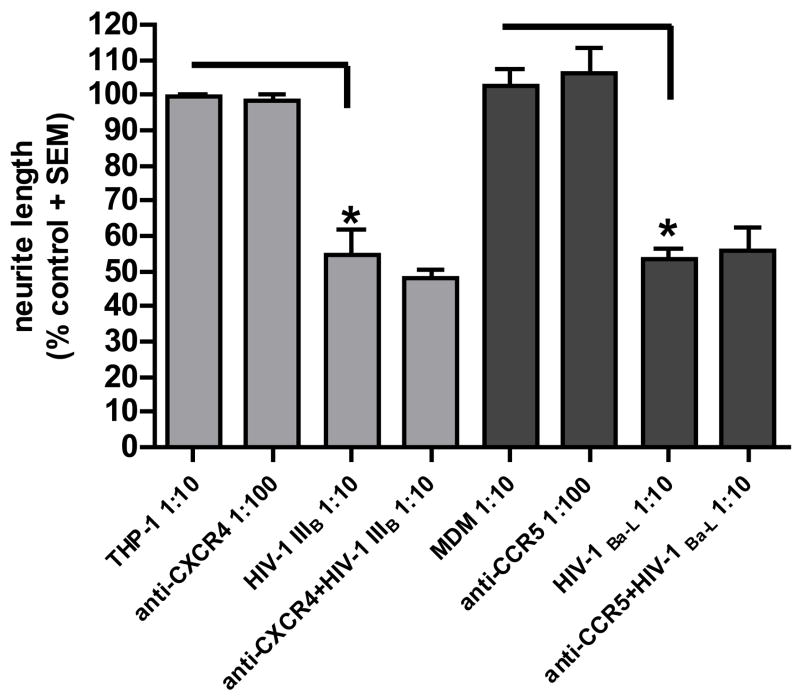
Immunostaining of the DRG cultures with antisera to CXCR4 and CCR5 chemokine receptors showed that these receptors were expressed on all neurons in similar amounts (figure 7 A and B). Nonetheless, neutralizing antibodies to CXCR4 or CCR5 did not ameliorate neurite retraction (figure 7C) mediated by the supernatants from HIV-1IIIB and HIV-1Ba-L infected macrophages, suggesting that viral proteins and/or host factors can cause neurotoxicity independent of these chemokine receptors.
Figure 7. Chemokine receptor expression and effect of chemokine receptor antibodies on neuritic retraction induced by HIV-infected monocytic cells.
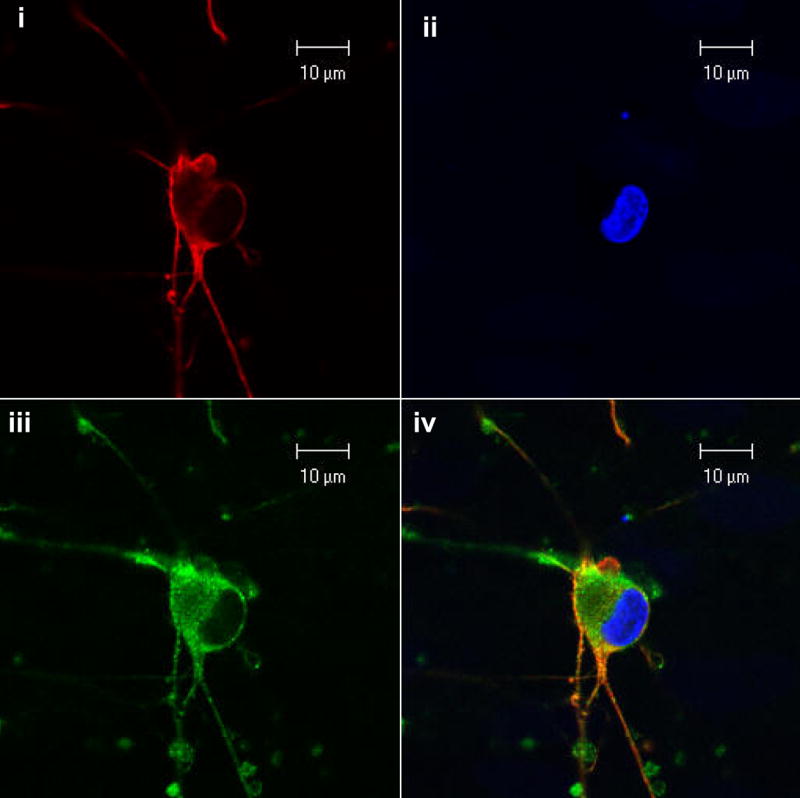

A) Human DRG neurons were (i) immunostained for CXCR4 showing red fluorescence. (ii) Hoechst 33342 stain was used to visualize the nuclei. (iii) Immunostaining with anti-βIII-tubulin antisera shows green fluorescence. (iv) Shows the merged photomicrograph of all three panels
B) Human DRG neurons were (i) immunostained for CCR5 showing red fluorescence. (ii) Hoechst 33342 stain was used to visualize the nuclei. (iii) Immunostaining with anti-βIII-tubulin antisera shows green fluorescence. (iv) Shows the merged photomicrograph of all three panels
C) Anti-CXCR4 and anti-CCR5 failed to prevent neurite retraction induced by supernatants from THP-1 cells infected with HIV-1IIIB or MDM infected with HIV-1Ba-L. Reduction of the total neurite length per axon is shown as standardized data. * Supernatant from infected monocytic cells versus the matched control, p < 0.05.
Discussion
In this study, we found that the DRG of HIV infected patients was infiltrated with HIV infected and activated macrophages; therefore, we have developed a biologically relevant human in vitro model of HIV-DSP. We established techniques for culturing mixed human DRG neurons with Schwann cells and then exposed them to supernatants from HIV-infected macrophages. We demonstrated that the supernatants of macrophages infected with CXCR4 and CCR5 tropic HIV-1 strains with comparable levels of HIV-p24 induced neuronal retraction in human DRG neurons and oxidative stress in the neuronal cell body. Humans in contrast to inbred rodent models are genetically heterogeneous and genetic factors may contribute to the development of HIV-DSP (4). We noticed that occasional DRG cultures were exquisitely sensitive to toxicity induced by the supernatants from HIV infected macrophages. Although technical factors cannot be entirely excluded, it is possible that host genetic factors may be a contributory factor.
Previous studies in which the HIV env was sequenced from the peripheral nerve showed a predominance of CCR5-dependent and macrophage tropic HIV-1, although dual tropic viruses were also identified (13). Most laboratory-adapted CXCR4 viruses, such as HIV-1IIIB, do not replicate efficiently in macrophages (37). Nevertheless, macrophages can support efficient replication by a subset of primary CXCR4 viruses (32, 36). We thus compared the neurotoxic potential of extracellular products released from HIV infected monocytic cells infected with wither a CCR5 or CXCR4 tropic strain. We found that both viral strains produced similar amounts of toxicity in several different assays of neuronal injury. In each of these assays, similar amounts of toxicity were observed with dilutions of 1:10 and 1:100 of the culture supernatants while no toxicity was observed at 1:1000 dilution, suggesting that the toxic substance(s) was present at low concentrations despite very high viral loads in the supernatants.
Chemokine receptors CXCR4 and CCR5 are also expressed on neurons and glial cells and their respective endogenous ligands SDF-1 and RANTES can mediate neurotoxicity by interaction of these receptors(14, 30). Further, the HIV envelope protein gp120 may also interact with these receptors to cause neurotoxicity in DRG neurons(14, 22). By immunostaining we found that both CXCR4 and CCR5 were expressed on the human DRG neurons in nearly equal amounts. We were unable to block the neurotoxic effects of supernatants, however, from HIV-infected monocytic cells with neutralizing antisera to these receptors suggesting that either substances other than these ligands may be responsible for the neurotoxic effects in this experimental system or that gp120 may cause neurotoxicity by mechanisms independent of its interactions with chemokine receptors. Although we have not yet identified the precise substance responsible for the neurotoxic effects on the DRG neurons, previous studies with brain derived neurons have implicated several other cytokines, and viral proteins in mediating these effects (reviewed in (17)). Immunopathological studies in DRG from patients with DSP have shown activated macrophages with concomitant immunostaining for pro-inflammatory cytokines such as TNF-α, interferon-γ and IL-6 (20, 38) as well as evidence for upregulation of nitric-oxide (NO) (20). Experimental studies show that HIV infected macrophages also release glutamate (12)and excitatory amino acid receptors may be expressed on DRG neurons(3, 16). However, HIV infected macrophages may also release other neurotoxic substances such as Ntox (10)and HIV proteins such as Tat(21). It has not been clear, however, which of these factors contribute to neuronal pathology in the DRG during HIV-infection.
Macrophages are the prime mediators of oxidative stress although human mononuclear cells may have little capacity to synthesize nitric oxide (6). Oxidative stress can also be induced by several HIV viral proteins and cytokines implicated in neuronal injury (33). Hence, we monitored the production of oxidative stress in the DRG neurons following exposure to supernatants from HIV-infected macrophages. We observed that in the neuronal cell body of the DRG neurons ROS were produced and mitochondrial membrane potential was reduced, which was prevented by the use of trolox as an antioxidant. Trolox has been shown to prevent mitochondrial toxicity in neuronal cultures exposed to the CSF from HIV-infected individuals (35). In contrast, the neurites did not show any changes in mitochondrial potential and trolox did not prevent neuritic retraction. We further measured 3-NT levels in the sciatic nerve of HIV-infected patients as a measure of oxidative stress. The HIV-infected patients with peripheral neuropathies did not show evidence of increased 3-NT proteins, instead, higher levels were noted in asymptomatic patients. Although these observations do not rule out the possibility that oxidative damage may occur at nerve terminals, it does raise the possibility that axonal injury may occur independent of oxidative stress. Similar observations have been made in patients with HIV dementia, where indices of oxidative stress are increased early in the disease and diminished in patients with severe HIV dementia(35). These findings suggest that the injury to the neuronal cell body and the axon may be differentially regulated. This is supported by observations that the signaling pathways mediating axonal degeneration might be distinct from those mediating neuronal apoptosis and dying back axonal degeneration may occur as a result of a caspase-independent pathways(7, 18, 26). But importantly, our observations suggest that antioxidants may not be sufficient to prevent peripheral neuropathy in HIV-infected patients.
Taken together we have shown that the supernatants of macrophages infected with either CCR5 or CXCR4 tropic HIV-1 strains mediate axonal retraction without causing neuronal cell death, but can cause mitochondrial dysfunction in the neuronal cell body in human DRG neuronal cultures. The toxic effect of the supernatants on the axons was not prevented by antioxidants while they protected the cell body. Further, peripheral nerves of HIV infected patients did not show evidence of increased oxidative stress. These observations suggest a differential effect on the axon and cell body and that different mechanisms of injury may be operative in these two structures. Further, even though CXCR4 and CCR5 were expressed on the DRG neurons, these effects were independent of these receptors, suggesting that factors other than these chemokine receptors should be considered in the neuropathogenesis of HIV-DSP.
Acknowledgments
This work was supported by grants from the German Research Foundation (KH); and R01NS43990 (A Nath) and DA-K08-16160 (CAP) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bradley WG, Shapshak P, Delgado S, Nagano I, Stewart R, Rocha B. Morphometric analysis of the peripheral neuropathy of AIDS. Muscle Nerve. 1998;21:1188–1195. doi: 10.1002/(sici)1097-4598(199809)21:9<1188::aid-mus10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Brannagan TH, 3rd, Nuovo GJ, Hays AP, Latov N. Human immunodeficiency virus infection of dorsal root ganglion neurons detected by polymerase chain reaction in situ hybridization. Ann Neurol. 1997;42:368–372. doi: 10.1002/ana.410420315. [DOI] [PubMed] [Google Scholar]
- 3.Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;501:780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- 4.Corder EH, Robertson K, Lannfelt L, Bogdanovic N, Eggertsen G, Wilkins J, Hall C. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med. 1998;4:1182–1184. doi: 10.1038/2677. [see comments] [DOI] [PubMed] [Google Scholar]
- 5.Cornblath DR, Hoke A. Recent advances in HIV neuropathy. Curr Opin Neurol. 2006;19:446–450. doi: 10.1097/01.wco.0000245366.59446.57. [DOI] [PubMed] [Google Scholar]
- 6.Denis M. Human monocytes/macrophages: NO or no NO? J Leukoc Biol. 1994;55:682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers MD. Deconstructing the axon: Wallerian degeneration and the ubiquitin-proteasome system. Trends Neurosci. 2004;27:3–6. doi: 10.1016/j.tins.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Esiri MM, Morris CS, Millard PR. Sensory and sympathetic ganglia in HIV-1 infection: immunocytochemical demonstration of HIV-1 viral antigens, increased MHC class II antigen expression and mild reactive inflammation. J Neurol Sci. 1993;114:178–187. doi: 10.1016/0022-510x(93)90295-a. [DOI] [PubMed] [Google Scholar]
- 9.Gartner S, Markovits P, Markovitz DM, Betts RF, Popovic M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. Jama. 1986;256:2365–2371. [PubMed] [Google Scholar]
- 10.Giulian D, Yu J, Tom D, Li J, Wendt E, Lin SN, Schwarcz R, Noonan C. Study of receptor mediated neurotoxins released by HIV-1 infected mononuclear phagocytes found in human brain. Journal of Neuroscience. 1996;16:3139–3153. doi: 10.1523/JNEUROSCI.16-10-03139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin JW, Wesselingh SL, Griffin DE, Glass JD, McArthur JC. Peripheral nerve disorders in HIV infection. Similarities and contrasts with CNS disorders. Res Publ Assoc Res Nerv Ment Dis. 1994;72:159–182. [PubMed] [Google Scholar]
- 12.Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol. 2001;117:97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- 13.Jones G, Zhu Y, Silva C, Tsutsui S, Pardo CA, Keppler OT, McArthur JC, Power C. Peripheral nerve-derived HIV-1 is predominantly CCR5-dependent and causes neuronal degeneration and neuroinflammation. Virology. 2005;334:178–193. doi: 10.1016/j.virol.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54:287–296. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- 15.Korber BT, Kunstman KJ, Patterson BK, Furtado M, McEvilly MM, Levy R, Wolinsky SM. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CJ, Bardoni R, Tong CK, Engelman HS, Joseph DJ, Magherini PC, MacDermott AB. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron. 2002;35:135–146. doi: 10.1016/s0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- 18.Melli G, Hoke A. Models of HIV-associated Neuropathies Differ in Pathogenic Site of Action. 12th conference on retroviruses and opportunistic infections; 2005. [Google Scholar]
- 19.Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, Ryan E, Yakoushina T, Khan S, Mahboob R, Naseer M, Dorfman D, Sharp V. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004;61:546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 20.Nagano I, Shapshak P, Yoshioka M, Xin KQ, Nakamura S, Bradley WG. Parvalbumin and calbindin D-28 k immunoreactivity in dorsal root ganglia in acquired immunodeficiency syndrome. Neuropathol Appl Neurobiol. 1996;22:293–301. doi: 10.1111/j.1365-2990.1996.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 21.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 22.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohagen A, Devitt A, Kunstman KJ, Gorry PR, Rose PP, Korber B, Taylor J, Levy R, Murphy RL, Wolinsky SM, Gabuzda D. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J Virol. 2003;77:12336–12345. doi: 10.1128/JVI.77.22.12336-12345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;6:21–27. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- 25.Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 26.Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 27.Rance NE, McArthur JC, Cornblath DR, Landstrom DL, Griffin JW, Price DL. Gracile tract degeneration in patients with sensory neuropathy and AIDS. Neurology. 1988;38:265–271. doi: 10.1212/wnl.38.2.265. [DOI] [PubMed] [Google Scholar]
- 28.Ratner L, Gallo RC, Wong-Staal F. HTLV-III, LAV, ARV are variants of same AIDS virus. Nature. 1985;313:636–637. doi: 10.1038/313636c0. [DOI] [PubMed] [Google Scholar]
- 29.Rizzuto N, Cavallaro T, Monaco S, Morbin M, Bonetti B, Ferrari S, Galiazzo-Rizzuto S, Zanette G, Bertolasi L. Role of HIV in the pathogenesis of distal symmetrical peripheral neuropathy. Acta Neuropathol (Berl) 1995;90:244–250. doi: 10.1007/BF00296507. [DOI] [PubMed] [Google Scholar]
- 30.Rostasy K, Egles C, Chauhan A, Kneissl M, Bahrani P, Yiannoutsos C, Hunter DD, Nath A, Hedreen JC, Navia BA. SDF-1alpha is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. J Neuropathol Exp Neurol. 2003;62:617–626. doi: 10.1093/jnen/62.6.617. [DOI] [PubMed] [Google Scholar]
- 31.Schifitto G, McDermott MP, McArthur JC, Marder K, Sacktor N, Epstein L, Kieburtz K. Incidence of and risk factors for HIV-associated distal sensory polyneuropathy. Neurology. 2002;58:1764–1768. doi: 10.1212/wnl.58.12.1764. [DOI] [PubMed] [Google Scholar]
- 32.Simmons G, Reeves JD, McKnight A, Dejucq N, Hibbitts S, Power CA, Aarons E, Schols D, De Clercq E, Proudfoot AE, Clapham PR. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J Virol. 1998;72:8453–8457. doi: 10.1128/jvi.72.10.8453-8457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner J, Haughey N, Li W, Venkatesan A, Anderson C, Reid R, Malpica T, Pocernich C, Butterfield DA, Nath A. Oxidative stress and therapeutic approaches in HIV dementia. Antioxid Redox Signal. 2006;8:2089–2100. doi: 10.1089/ars.2006.8.2089. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 35.Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G, Butterfield DA, Buch S, Narayan O, Sinai A, Geiger J, Berger JR, Elford H, Nath A. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- 36.Valentin A, Trivedi H, Lu W, Kostrikis LG, Pavlakis GN. CXCR4 mediates entry and productive infection of syncytia-inducing (X4) HIV-1 strains in primary macrophages. Virology. 2000;269:294–304. doi: 10.1006/viro.1999.0136. [DOI] [PubMed] [Google Scholar]
- 37.Yi Y, Rana S, Turner JD, Gaddis N, Collman RG. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772–777. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshioka M, Shapshak P, Srivastava AK, Stewart RV, Nelson SJ, Bradley WG, Berger JR, Rhodes RH, Sun NC, Nakamura S. Expression of HIV-1 and interleukin-6 in lumbosacral dorsal root ganglia of patients with AIDS. Neurology. 1994;44:1120–1130. doi: 10.1212/wnl.44.6.1120. [DOI] [PubMed] [Google Scholar]