Abstract
Nerve root compression produces chronic pain and altered spinal neuropeptide expression. This study utilized controlled transient loading in a rat model of painful cervical nerve root compression to investigate the dependence of mechanical allodynia on load magnitude. Injury loads (0-110mN) were applied quasistatically using a customized loading device, and load thresholds to produce maintained mechanical allodynia were defined. Bilateral spinal expression of substance P (SP) and calcitonin gene-related peptide (CGRP) was assessed 7 days following compression using immunohistochemistry to determine relationships between these neuropeptides and compression load. A three-segment change point model was implemented to model allodynia responses and their relationship to load. Load thresholds were defined at which ipsilateral and contralateral allodynia were produced and sustained. The threshold for increased allodynia was lowest for acute (day 1) ipsilateral responses (26.29mN), while thresholds for allodynia on day 7 were similar for the ipsilateral (38.16mN) and contralateral forepaw (38.26mN). CGRP, but not SP, significantly decreased with load; the thresholds for ipsilateral and contralateral CGRP decreases corresponded to 19.52mN and 24.03mN, respectively. These thresholds suggest bilateral allodynia may be mediated by spinal mechanisms, and that these mechanisms depend on the magnitude of load.
Keywords: nerve root, cervical, load, neck pain, threshold
Introduction
The annual incidence of neck pain among adults ranges from 14-50% (Côté et al., 2004; Fejer et al., 2005) affecting up to 71% of people in their lifetime (Côté et al., 1998, 2000). Individuals may suffer from chronic pain throughout their lives, resulting in large societal costs (Rempel et al., 1992; Côté et al., 2004). Cervical spine motions decrease the intervertebral foramen (Nuckley et al., 2002) causing transient impingement of the nerve roots and radicular pain when motions exceed the normal range, as can occur in accidents and sports injuries (Krivickas and Wilbourn, 2000; Torg et al., 2002).
Nerve root compression can produce tissue damage, edema, membrane leakage, and Wallerian degeneration, as well as changes in neuropeptide expression and spinal glial activation (Olmarker et al., 1989; Pedowitz et al., 1992; Colburn et al., 1997, 1999; Hashizume et al., 2000; Winkelstein et al., 2001a; Kobayashi et al., 2005a,b). These responses result in bilateral sensitivity (Hubbard and Winkelstein, 2005) and central sensitization: a heightened responsiveness and reduced threshold for afferent inputs to the central nervous system (Woolf and Walters, 1991; Ji et al., 2003). Canine lumbar root compression for 1 hour produces endoneurial edema but only for loads above 15gf (147.2mN) (Kobayashi et al., 1993, 2002), suggesting a load threshold for producing neural pathology. While those studies provide insight into physiologic responses for nerve root loading and their dependence on biomechanics, injury mechanics and cellular/molecular outcomes have not been investigated in the context of pain. Models of persistent pain from root compression have not used controlled loading mechanics (Winkelstein et al., 2001b, 2002; Winkelstein and DeLeo, 2002, 2004; Sekiguchi et al., 2003, 2004). Previous work in our lab has shown that transient compression of the rat cervical nerve root with 10gf (98.1mN) produces persistent mechanical allodynia, and suggested that 10gf is above the load threshold for producing bilateral behavioral hypersensitivity in rats (Hubbard and Winkelstein, 2005). Currently, there is an incomplete understanding of the role of compression severity in transient nerve root loading for producing either acute or persistent mechanical allodynia, and no study has identified tissue loading thresholds for behavioral outcomes in the cervical spine.
The neuropeptides substance P (SP) and calcitonin gene-related peptide (CGRP) have been studied extensively for their contributions to nociception (Oku et al., 1987; Cridland and Henry, 1989; Kawamura et al., 1989; Levine et al., 1993; Bennett et al., 2000; Kobayashi et al., 2004, 2005a,b; Aoki et al., 2005). SP is released from axon terminals upon C-fiber stimulation (Malcangio et al., 2000), and its expression in the superficial laminae of the spinal cord decreases after lumbar neural injuries (Munglani et al., 1996; Malmberg and Basbaum, 1998; Allen et al., 1999). CGRP affects nociception by promoting the release, and slowing the metabolism, of SP (Allen et al., 1999; Meert et al., 2003). Allodynia increases following intrathecal CGRP administration and decreases after administration of a CGRP antagonist (Oku et al., 1987; Cridland and Henry, 1989; Bennett et al., 2000). Despite studies characterizing the temporal expression of spinal SP and CGRP (Cahill and Coderre, 2002; Jang et al., 2004), the dependence of these neuropeptides on nerve root compression load and persistent behavioral hypersensitivity is not fully understood.
In this study, a statistical model is used to identify load thresholds governing allodynia and spinal neuropeptide responses. To quantify changes in behavioral outcomes, mechanical allodynia (response to a stimulus which is not normally painful) is measured, which has clinical relevance as a pain outcome and is a sensitive measure in rodent models (Colburn et al., 1999; Tabo et al., 1999; Bennett et al., 2000). It is hypothesized that bilateral mechanical allodynia will depend on load magnitude above a defined threshold. We further hypothesize that altered spinal SP and CGRP expression can be modeled by similar load thresholds, which will match the thresholds for mechanical allodynia.
Materials and Methods
Experiments were performed using male Holtzman rats (250-350 grams; Harlan Sprague-Dawley, Indianapolis, IN) housed with a 12-12 hour light-dark cycle and free access to food and water. All experimental procedures were approved by an Institutional Animal Care and Use Committee.
Surgical Procedures
Surgical procedures were performed under halothane inhalation anesthesia (4% induction, 2% maintenance) and were adapted from published methods (Hubbard and Winkelstein, 2005; Rothman et al., 2005). After the spinal cord and right roots were exposed, a customized loading device with microcompression platens (0.7mm width) applied compression to the C7 dorsal root proximal to the dorsal root ganglion (DRG) (Figure 1A). Platen displacement was measured by an LVDT (5mm travel distance, 0.25% sensitivity; RDP, Pottstown, PA), and the applied load was recorded by a load cell (490mN, 0.15mN resolution; Omega, Stamford, CT). Mechanical data were recorded at 10Hz using LabVIEW (National Instruments, Austin, TX). Digital video (Qimaging, Burnaby, British Columbia) monitored the nerve root during compression.
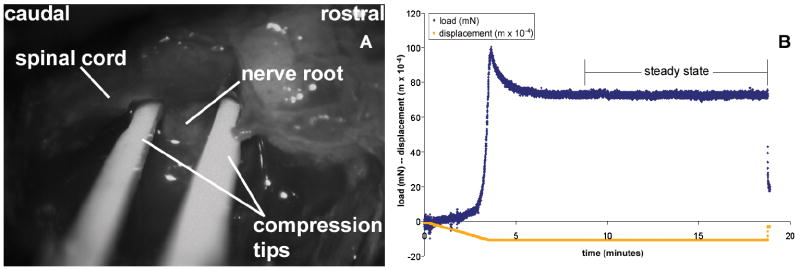
Figure 1.
(A) Surgical exposure of right C7 dorsal root showing anatomy and compression platens inserted prior to load application. (B) Mechanical data (load & displacement) during compression of the C7 nerve root (rat #194). Steady state load (71.32mN) was defined as the average load measured between 5 and 15 minutes after the peak load was reached.
The right C7 dorsal root was compressed transversely through its diameter to prescribed loads ranging between 0-110mN (n=25), with loads distributed across that range. Due to considerable stress relaxation for dynamic compression of neural tissue (Miller et al., 2000; Gefen and Margulies, 2004; Coats and Margulies, 2006; Cheng and Bilston, 2007), quasistatic loading (0.004mm/s) was implemented. Upon reaching a predetermined peak load, platen displacement was held for 15 minutes. The load relaxed approximately 20% within the first 5 minutes of the hold period (Figure 1B). Steady state load was reported as the average load measured between 5 and 15 minutes during compression. Sham procedures were performed with the same surgical technique but without tissue compression (n=4). Wounds were closed using 3-0 polyester suture and surgical staples.
Mechanical Allodynia
All rats were evaluated for bilateral forepaw mechanical allodynia on days 1, 3, 5, and 7 following surgical procedures (Ramer et al., 2000; Lee et al., 2004; Hubbard and Winkelstein, 2005; Rothman et al., 2005). Prior to surgery, baseline measurements were recorded for two consecutive days. A single tester performed all allodynia testing and was blinded to the applied compression load.
For each session, following 20 minutes of acclimation to the testing environment, rats were stimulated on the plantar surface of each forepaw using three von Frey filaments (13.7mN, 19.6mN, 39.2mN) (Stoelting, Wood Dale, IL). Each testing session consisted of three rounds of 10 stimulations on each forepaw, separated by 10 minutes. The total number of paw withdrawals was summed for each forepaw with each filament.
Spinal Cord Tissue Preparation & Immunohistochemistry
Rats were euthanized on day 7 by an overdose of sodium pentobarbital (40mg/kg) and transcardially perfused with 200ml of phosphate buffered saline (PBS) followed by 200ml of 4% paraformaldehyde in PBS. Spinal cord tissue was harvested and placed in 4% paraformaldehyde for 1 hour, then in 30% sucrose for 5 days before freeze-mounting in OCT (Fisher, Fairlawn, NJ) and storage at -80°C. Axial sections (20μm) immediately rostral to the C7 root insertion were sectioned for free-floating immunohistochemistry. Sections were blocked in 2% normal goat or donkey serum. Polyclonal antibodies against CGRP (1:4000; Bachem, San Carlos, CA) and SP (1:2000; Chemicon, Temecula, CA) were applied to tissue sections in PBS-T (0.3% triton). Goat anti-rabbit (1:1000; Vector, Burlingame, CA) or donkey anti-rabbit (1:1250; Chemicon, Temecula, CA) secondary antibodies were applied for CGRP or SP immunostaining, respectively. All antibody dilutions were previously optimized (Rothman et al., 2005). Sections were exposed to 3,3-diaminobenzidine for color development (Vector Labs, Burlingame, CA).
Representative bilateral tissue sections for SP and CGRP from each rat were imaged at 50X to quantify immunostaining. Within a 350×750 pixel area including the superficial laminae, pixels with a staining intensity above a predetermined mean threshold for positive immunoreactivity in normal tissue were quantified (Abbadie et al., 1996; Malmberg and Basbaum, 1998; Rothman et al., 2005). For densitometry, reactive pixels were only detected in laminae I and II. The number of pixels above threshold was reported as a percentage of the total in the region and averaged for the sections analyzed for each rat.
Data & Statistical Analyses
Mechanical allodynia was analyzed by groups defined by average steady state compression loads. Rats were divided into four groups (n=6 or 7 per group) in addition to shams (n=4), based on average steady state load (±SD) (Table 1). Allodynia responses were averaged for each group to provide mean ipsilateral and contralateral responses and analyzed by a two-way analysis of variance (ANOVA) with repeated measures to determine significant effects of load over time. A one-way ANOVA with post-hoc Bonferroni correction compared means at each time point. These statistical analyses were performed using SYSTAT v10.2 (Richmond, CA). Allodynia data are presented as mean (±SEM), with significance at p<0.05.
Table 1.
Applied Nerve Root Compression Load
| Rat I.D. | Peak Load (mN) | Average Peak Load (mN)* | Steady State Load (mN) | Average Steady State Load (mN)* |
|---|---|---|---|---|
| 195 | 31.20 | 21.80 ± 6.31 | 5.30 | 10.89 ± 6.49 |
| 217 | 26.87 | 22.07 | ||
| 219 | 16.88 | 9.22 | ||
| 220 | 14.24 | 7.06 | ||
| 221 | 19.82 | 15.11 | ||
| 222 | 21.76 | 6.77 | ||
|
| ||||
| 175 | 89.37 | 57.35 ± 17.15 | 32.86 | 37.18 ± 7.28 |
| 176 | 54.35 | 37.96 | ||
| 198 | 54.35 | 37.08 | ||
| 200 | 61.41 | 38.55 | ||
| 201 | 62.36 | 51.31 | ||
| 218 | 34.08 | 33.65 | ||
| 223 | 45.56 | 28.55 | ||
|
| ||||
| 178 | 87.70 | 85.27 ± 10.29 | 74.26 | 66.71 ± 7.45 |
| 194 | 98.79 | 71.32 | ||
| 196 | 88.28 | 72.10 | ||
| 197 | 90.64 | 58.76 | ||
| 199 | 74.97 | 56.41 | ||
| 214 | 71.26 | 67.36 | ||
|
| ||||
| 177 | 114.09 | 108.04 ± 4.70 | 108.99 | 98.10 ± 6.96 |
| 212 | 106.07 | 97.90 | ||
| 213 | 108.75 | 92.31 | ||
| 215 | 105.19 | 90.64 | ||
| 216 | 101.61 | 95.65 | ||
| 224 | 112.51 | 103.20 | ||
average loads ± standard deviation
Thresholds to initiate and sustain allodynia were determined using injury load as a continuous variable. Applied load for shams was defined as 0mN. Ipsilateral and contralateral allodynia on days 1 and 7 and over the entire postoperative period (total allodynia) were specifically analyzed with respect to load. For each behavioral data set, a Bayesian three-segment change point model was fit to the load-allodynia relationship (Muggeo, 2003) because of its utility to fit the apparent segmented response of allodynia and load, with flat responses for the low-load and higher-load ranges. Such a model enables the identification of the change points between the segments, providing load thresholds for allodynia. The three segments included minimum and maximum allodynia responses, and a linear segment between the extremes. The first change point corresponded to the load above which allodynia was elevated over sham, representing a threshold to produce allodynia. The second change point indicated the load above which allodynia reached a maximum. Additional details of this model are in supplementary Appendix A.
Bilateral spinal SP and CGRP immunoreactivity were also analyzed with respect to load. This approach tested if the relationship between neuropeptide expression and load also followed the three-segment model. Further, if that model did fit the neuropeptide data, change points were determined for altered neuropeptide expression at day 7. Compressive load was compared to the average percent immunoreactivity for each neuropeptide in the ipsilateral and contralateral dorsal horns, separately. Thresholds are reported as a mean followed by the 95% credible interval in parentheses, with significance determined by the exclusion of 0 from the credible interval.
Results
Mechanical Allodynia
Average loads for the compression groups [10.89mN (±6.49), 37.18mN (±7.28), 66.71mN (±7.45), 98.10mN (±6.96)] were significantly different from each other (p<0.0001, Table 1). Qualitative trends in allodynia between groups were similar for testing with all filaments. Responses elicited by the 39.2mN filament were the most robust and are presented in detail here. Compression by 10.89 or 37.18mN did not produce ipsilateral allodynia significantly greater than sham; sham surgeries did not produce allodynia different from baseline on any day (Figure 2A). Following a 66.71mN load, ipsilateral allodynia was significantly elevated over sham on day 1 (p<0.023) and did not decrease between days 3 and 7. Compression with 98.10mN produced ipsilateral allodynia that was significantly increased over sham on days 1, 3, and 5 (p<0.02) and was not significantly different than that following a 66.71mN load on any day (Figure 2A). Ipsilateral mechanical allodynia produced by 66.71 and 98.10mN loads was significantly greater than that for the 10.89mN load group on days 1 and 5 (p<0.03).
Figure 2.
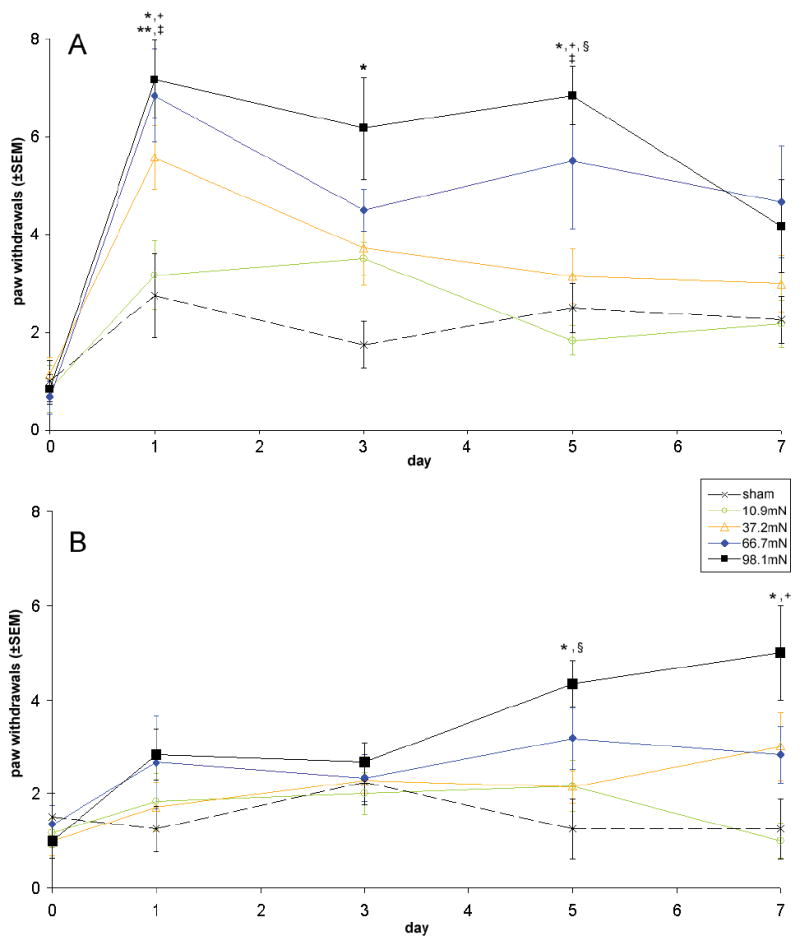
Ipsilateral (A) and contralateral (B) mechanical allodynia assessed with a 39.2mN von Frey filament, reported as frequency of paw withdrawals. Data are grouped into four average load groups according to steady state compression load. Similar trends were observed with the other filaments. A significant increase relative to sham is indicated by (*) for the 98.10mN group and (**) for the 66.71mN group. A significant increase relative to the 10.89mN group is indicated by (+) for the 98.10mN group and (‡) for the 66.71mN group. The 98.10mN group significantly elevated above the 37.18mN group is indicated by (§).
Contralateral mechanical allodynia was significantly elevated over sham only for 98.10mN compression, on days 5 and 7 (p<0.03; Figure 2B). Contralateral allodynia for a 98.10mN load was also significantly greater than that for 37.18 and 10.89mN compressions on days 5 (p<0.05) and 7 (p<0.005), respectively. Sham procedures did not produce any contralateral allodynia.
While bilateral allodynia was graded according to load group (Figure 2), a three-segment change point model quantified the relationship between allodynia and load (Table 2). Ipsilateral allodynia at day 1 demonstrated a robust increase between the first and third segments (Figure 3A). Ipsilateral allodynia was produced above a load of 26.29mN (1.57, 65.43) at day 1 (Table 2, Figure 3A). The second change point was identified at 48.95mN (22.66, 92.12), indicating the load above which allodynia was maximum. The corresponding increase in allodynia between the first and third segments [3.73 paw withdrawals, (2.01, 5.39)] was statistically significant (Table 2). Similarly, for ipsilateral paw withdrawals on day 7 and for total allodynia (Figure 3B & 3C), the corresponding first and second change point loads were 38.16mN (2.75, 92.70) and 54.35mN (6.57, 104.38) for day 7, and 22.86mN (1.18, 65.04) and 76.32mN (47.48, 106.14) for total allodynia (Table 2). While the increase in ipsilateral allodynia between the two change points was not significant at day 7, the increase in total allodynia was significant. Contralateral allodynia at day 1 did not vary with load (Table 2, Figure 3D); however, both day 7 and total allodynia were well fit (i.e. a significant increase in allodynia between change points) by the three-segment model and significantly increased with load (Table 2, Figure 3E & 3F). At day 7, contralateral allodynia greater than sham (0mN) was produced above 38.26mN (2.55,85.45) and plateaued for loads over 66.02mN (26.78, 103.30) (Figure 3E). The analogous thresholds for total allodynia were slightly higher than those for day 7: 52.09mN (5.89, 73.97) and 76.81mN (49.64, 102.12) (Table 2, Figure 3F).
Table 2.
Change Point Model Parameters
| Outcome Measure | First Chang Point (mN) | Second Change Point (mN) | Change in Outcome Measure (paw withdrawals, % positive pixels) | |
|---|---|---|---|---|
| Allodynia | ipsilateral – day 1 | 26.29 | 48.95 | 3.73† |
| ipsilateral – day 7 | 38.16 | 54.36 | 1.73 | |
| ipsilateral – total | 22.86 | 76.32 | 13.60† | |
| contralateral – day 1 | 48.36 | 57.98 | 0.96 | |
| contralateral – day 7 | 38.26 | 66.02 | 2.94† | |
| contralateral – total | 52.09 | 76.81 | 6.85† | |
|
| ||||
| Spinal Neuropeptide Reactivity | SP – ipsilateral | 36.00 | 46.11 | -2.32 |
| SP – contralateral | 41.01 | 50.03 | -1.53 | |
| CGRP – ipsilateral | 19.52 | 28.35 | -3.04† | |
| CGRP – contralateral | 24.03 | 34.53 | -3.37† | |
SP = substance P; CGRP = calcitonin gene-related peptide
significant change in outcome measure between first and second change points
Figure 3.
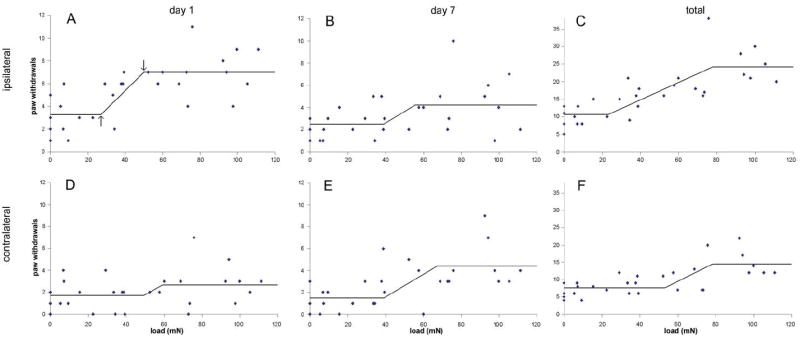
Mechanical allodynia on days 1 (A, D) and 7 (B, E) after compression, and total allodynia (C, F) as a function of applied nerve root load magnitude. Ipsilateral (A-C) and contralateral (D-F) mechanical allodynia are shown for stimulation with a 39.2mN von Frey filament. Superimposed on each plot is the corresponding three-segment change point model with the segments having no difference from sham, a linear increase in allodynia with load, and the maximum allodynia responses. The first and second change points are indicated by arrows in panel (A).
Spinal Neuropeptides
Bilateral dorsal horn SP and CGRP immunostaining generally decreased with increases in applied root load (Figure 4). Sham surgeries did not alter either neuropeptide relative to uninjured, naïve rats. Also, immunostaining in the deeper laminae was unchanged for all procedures. While the three-segment model did capture the minor decrease in SP immunoreactivity with load, this decrease was neither robust nor significant (Table 2, Figure 4A-C). However, bilateral changes in CGRP immunoreactivity did depend significantly on load, with a response to low loads similar to sham, followed by a linear decrease as load increased, reaching a lower plateau. The change points for ipsilateral and contralateral decreases in CGRP immunoreactivity were 19.52mN (2.16, 46.21) and 24.03mN (3.14, 46.40), respectively (Table 2).
Figure 4.
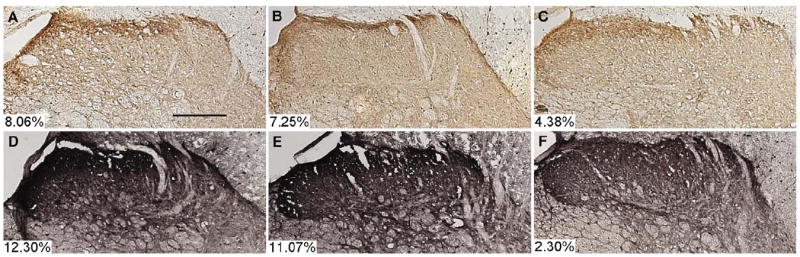
Representative images for the ipsilateral spinal cord dorsal horns (laminae I-IV) labeled for substance P (A-C) or calcitonin gene-related peptide (D-F) immunoreactivity at day 7 after nerve root compression: sham (A, D), 10.89mN (B, E), and 98.10mN (C, F). Positive immunolabeling assessed by densitometry was confined primarily to laminae I and II. Individual densitometry measures for these images are indicated in each panel. A slight decrease in substance P staining after compression with a high load is apparent (C), and a more robust decrease is evident for CGRP immunoreactivity (F). Contralateral immunostaining followed similar trends for all groups. Scale bar in (A) = 200μm.
Discussion
This is the first study to quantify the effect of transient nerve root compression magnitude on modulating behavioral outcomes, or to establish load thresholds for persistent mechanical allodynia and sustained changes in spinal neuropeptides. In general, loads to initiate ipsilateral allodynia (i.e. day 1) were less than those to produce maintained allodynia (i.e. day 7, total allodynia) (Table 2, Figure 3A-C). Differences in these thresholds for acute and maintained allodynia indicate that some loads may be sufficient only to transiently affect sensitivity while greater loads may initiate mechanisms that lead to persistent hypersensitivity. The dependence of allodynia on load at day 7 was not significant primarily due to the relative variability in responses observed at that time point. Considering results from the entire testing period, a significant increase in total allodynia was determined using the model (Table 2). From both the day 7 and total allodynia relationships to load, nerve root compression as low as 22.86mN can lead to persistent allodynia. Above 76.32mN, allodynia is not further modulated by applied load (Table 2, Figure 3A-C), corroborating previous work showing no difference in allodynia for 10gf (98.10mN) or 60gf (588.60mN) compression loads (Hubbard and Winkelstein, 2005). Contralateral allodynia exhibited a late onset, consistent with previous studies (Tabo et al., 1999; Hunt et al., 2001; Rutkowski et al., 2002; Araujo et al., 2003; Hubbard and Winkelstein, 2005). The load threshold for contralateral allodynia at day 7 (38.26mN) matched that to produce ipsilateral allodynia (38.16mN) (Table 2). The load of 38.26mN required to produce bilateral allodynia at day 7 after compression implies that persistent pain may not be maintained for loading below this threshold. Further, the manifestation of contralateral allodynia at day 7 suggests that a central, spinal mechanism may contribute to the maintenance of pain.
The three-segment model is supported for use with the current application based on both experimental and statistical evidence (Spiegelhalter et al., 2002). A previous study applying compression loads above 100mN suggested that a non-linear relationship may exist between nerve root load and resulting allodynia (Hubbard and Winkelstein, 2005). This implied that a critical load for establishing allodynia may exist, beyond which behavioral sensitivity is not modulated by load. Using a statistical measure for model appropriateness (deviance information criterion), it was found that for all cases relating allodynia to load, a linear regression model was not superior to the change point model. Moreover, the change points defined by this model identify potential load thresholds at which compression of the nerve root can produce and maximize mechanical allodynia (Table 2). It must be noted that for cases in which loads are applied only within the linear region between the change points, a linear regression would be a simpler, and possibly more appropriate, model.
Spinal SP and CGRP demonstrated differences at day 7 following transient nerve root compression. The thresholds for decreased ipsilateral CGRP immunoreactivity were less than those for contralateral changes at day 7 (Table 2). Additionally, bilateral CGRP thresholds were below those thresholds for producing allodynia on day 7 (Table 2), suggesting that additional spinal responses likely contribute to pain maintenance. Spinal SP did not significantly depend on load but did correspond closely with thresholds for allodynia at day 7 (Table 2), and may indicate different mechanistic roles for SP and CGRP in pain. Nonetheless, the dependence of CGRP immunoreactivity on load strongly implicates a decrease in this neuropeptide in the superficial laminae as contributing, at least partially, to sustained allodynia.
Previous investigations of spinal SP or CGRP provide conflicting evidence for their involvement in the onset and maintenance of pain. Although our findings corroborate reports of decreases in SP or CGRP in the superficial laminae following neural injury (Munglani et al., 1996; Malmberg and Basbaum, 1998; Swamydas et al., 2004; Kobayashi et al., 2005a,b), increased CGRP has also been implicated in mediating elevated behavioral sensitivity (Oku et al., 1987; Cridland and Henry, 1989; Christensen and Hulsebosch, 1997; Bennett et al., 2000). Decreases in spinal neuropeptides may result from increased peptide utilization in the spinal cord, increased receptor internalization, decreased synthesis in the DRG, or breakdown of anterograde axonal transport at the injury site (Kobayashi et al., 2005a,b; Allen et al., 1999; Cahill and Coderre, 2002). In our study, damage to unmyelinated afferents may have reduced axonal transport in the dorsal root, leading to a depletion of CGRP in the superficial laminae. Alternatively, axonal damage may have affected production of SP and CGRP in the DRG as a result of neuronal dysfunction or diminished neurotrophic transport (Kirita et al., 2007). With previous studies suggesting neuropeptide expression in the dorsal horn is unchanged or increased as early as 1 day after nerve root compression (Kobayashi et al., 2005a; Rothman et al., 2005), the depletion of CGRP in the superficial laminae at day 7 may reflect a spinal accommodation following an initial increase in spinal CGRP. Assessment of temporal expression of neuropeptides in the dorsal horns is necessary to define their roles following painful injury. Axonal damage assessment in the compressed nerve root and local neuropeptide and neurotrophin localization following transient compression are also needed to understand the mechanistic relationship between these injury pathways. Lastly, studies are needed to quantify early expression of these and other regulatory proteins in the spinal cord and DRG in order to elucidate the specific mechanisms dependent on load for painful nerve root injury.
The compression device used in this study applied displacements that produced loads across the range of 0-110mN via quasistatic motor-controlled displacement. These measured loads were smaller and more repeatable than those imposed by prefabricated clips or forceps used previously in lumbar radiculopathy models (Kobayashi et al., 1993, 2002; Sekiguchi et al., 2003). Two-second forceps compression in a rat model produced ipsilateral allodynia for at least one week, but without contralateral allodynia (Sekiguchi et al., 2003). The lack of contralateral hypersensitivity in that model emphasizes the role of mechanics in producing varied behavioral outcomes. In models of chronic nerve root ligation, a linear relationship between allodynia and tissue strain was suggested, providing a strain-threshold for persistent allodynia (Winkelstein et al., 2001b, 2002; Winkelstein and DeLeo, 2002, 2004). However, those studies assumed that the ligation applied constant strain over the post-operative period. The present study utilized platen displacement to apply desired compression loads; tissue strains were not calculated here. However, the strain threshold for persistent mechanical allodynia following chronic lumbar root ligation (Winkelstein and DeLeo, 2004) was determined to be much lower (22.2% tissue compression) than the tissue deformations through the diameter of the root estimated in the present model (~90%), suggesting that the load threshold for painful transient root compression is higher than that for chronic compression. Moreover, allodynia at day 7 after transient compression indicates that behavioral hypersensitivity persists even after the removal of the painful stimulus. Maintened allodynia has also been demonstrated previously in a model of transient compression, with behavioral hypersensitivity lasting at least two weeks after injury (Rothman et al., 2007).
In summary, this injury model enables examination of the in vivo mechanics for painful nerve root compression and defines predictive load thresholds governing behavioral hypersensitivity and sustained cellular responses. Further, this model offers potential utility for identifying specific thresholds to activate other pathways responding to persistent pain (e.g. macrophage recruitment, cytokine release, neurotrophin release), both locally and in the central nervous system. Results for ipsilateral mechanical allodynia support a higher threshold for maintenance of pain compared to its onset (Table 2, Figure 3A-C). The similarity in thresholds for ipsilateral and contralateral allodynia at day 7 further supports central mechanisms as contributing to persistent pain. This was substantiated by bilateral decreases in spinal CGRP at day 7 for loads above 24.03mN. By defining pain symptoms and spinal neuropeptide expression in the context of injury mechanics, these findings define potential factors contributing to cervical radiculopathy and supply evidence for a direct role of mechanics in modulating painful outcomes for transient cervical nerve root compression.
Supplementary Material
Acknowledgments
This work was funded by grant support from the National Institute of Arthritis, Musculoskeletal, and Skin Diseases (#AR047564-02), the Catharine Sharpe Foundation, and fellowship funding from the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. Journal of Neurophysiology. 1999;81:1379–1390. doi: 10.1152/jn.1999.81.3.1379. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, Saito T, Moriya H. Expression and co-expression of VR1, CGRP, and IB4-binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine. 2005;30:1496–1500. doi: 10.1097/01.brs.0000167532.96540.31. [DOI] [PubMed] [Google Scholar]
- Araujo MC, Sinnott CJ, Strichartz GR. Multiple phases of relief from experimental mechanical allodynia by systemic lidocaine: responses to early and late infusions. Pain. 2003;103:21–29. doi: 10.1016/s0304-3959(02)00350-0. [DOI] [PubMed] [Google Scholar]
- Bacon DW, Watts DG. Estimating transition between 2 intersecting straight lines. Biometrika. 1971;58:525–534. [Google Scholar]
- Bennett AD, Chastain KM, Hulsebosch CE. Alleviation of mechanical and thermal allodynia by CGRP(8-37) in a rodent model of chronic central pain. Pain. 2000;86:163–175. doi: 10.1016/s0304-3959(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Coderre TJ. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre- or post-treatment with a neurokinin-1 antagonist. Pain. 2002;95:277–285. doi: 10.1016/S0304-3959(01)00410-9. [DOI] [PubMed] [Google Scholar]
- Coats B, Margulies SS. Material properties of porcine parietal cortex. Journal of Biomechanics. 2006;39:2521–2525. doi: 10.1016/j.jbiomech.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Cheng S, Bilston LE. Unconfined compression of white matter. Journal of Biomechanics. 2007;40:117–124. doi: 10.1016/j.jbiomech.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Hulsebosch CE. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Experimental Neurology. 1997;147:463–475. doi: 10.1006/exnr.1997.6608. [DOI] [PubMed] [Google Scholar]
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. Journal of Neuroimmunology. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Experimental Neurology. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- Côté P, Cassidy JD, Carroll L. The Saskatchewan health and back pain survey: the prevalence of neck pain and related disability in Saskatchewan adults. Spine. 1998;23:1689–1698. doi: 10.1097/00007632-199808010-00015. [DOI] [PubMed] [Google Scholar]
- Côté P, Cassidy JD, Carroll L. The factors associated with neck pain and its related disability in the Saskatchewan population. Spine. 2000;25:1109–1117. doi: 10.1097/00007632-200005010-00012. [DOI] [PubMed] [Google Scholar]
- Côté P, Cassidy JD, Carroll LJ, Kristman V. The annual incidence and course of neck pain in the general popoulation: a population-based cohort study. Pain. 2004;112:267–273. doi: 10.1016/j.pain.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Cowles MK, Carlin BP. Markov chain monte carlo convergence diagnostics: a comparative review. Journal of the American Statistical Association. 1996;91:883–904. [Google Scholar]
- Cridland RA, Henry JL. Intrathecal administration of CGRP in the rat attenuates a facilitation of the tail flick reflex induced by either substance P or noxious cutaneous stimulation. Neuroscience Letters. 1989;102:241–246. doi: 10.1016/0304-3940(89)90085-2. [DOI] [PubMed] [Google Scholar]
- Fejer R, Jordan A, Hartvigsen J. Categorising the severity of neck pain: establishment of cut-points for use in clinical and epidemiological research. Pain. 2005;119:176–182. doi: 10.1016/j.pain.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Gefen A, Margulies SS. Are in vivo and in situ brain tissues mechanically similar? Journal of Biomechanics. 2004;37:1339–1352. doi: 10.1016/j.jbiomech.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Gelfand AE, Smith AFM. Sampling-based approaches to calculating marginal densities. Journal of the American Statistical Association. 1990;85:398–409. [Google Scholar]
- Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine. 2000;25:1206–1217. doi: 10.1097/00007632-200005150-00003. [DOI] [PubMed] [Google Scholar]
- Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine. 2005;30:1924–1932. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- Hunt JL, Winkelstein BA, Rutkowski MD, Weinstein JN, DeLeo JA. Repeated injury to the lumbar nerve roots produces enhanced mechanical allodynia and persistent spinal neuroinflammation. Spine. 2001;19:2073–2079. doi: 10.1097/00007632-200110010-00005. [DOI] [PubMed] [Google Scholar]
- Jang J, Nam T, Paik K, Leem J. Involvement of peripherally released substance P and calcitonin gene-related peptide in mediating mechanical hyperalgesia in a traumatic neuropathy model of the rat. Neuroscience Letters. 2004;360:129–132. doi: 10.1016/j.neulet.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosciences. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Kuraishi Y, Minami M, Satoh M. Antinociceptive effect of intrathecally administered antiserum against calcitonin gene-related peptide on thermal and mechanical noxious stimuli in experimental hyperalgesic rats. Brain Research. 1989;497:199–203. doi: 10.1016/0006-8993(89)90990-6. [DOI] [PubMed] [Google Scholar]
- Kirita T, Takebayashi T, Mizuno S, Takeuchi H, Kobayashi T, Fukao M, Yamashita T, Tohse N. Electrophysiologic changes in dorsal root ganglion neurons and behavioral changes in a lumbar radiculopathy model. Spine. 2007;32:E65–72. doi: 10.1097/01.brs.0000252202.85377.96. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yoshizawa H, Hachiya Y, Ukai T, Morita T. Vasogenic edema induced by compression injury to the spinal nerve root. Distribution of intravenously injected protein tracers and gadolinium-enhanced magnetic resonance imaging. Spine. 1993;18:1410–1424. [PubMed] [Google Scholar]
- Kobayashi S, Yoshizawa H. Effect of mechanical compression on the vascular permeability of the dorsal root ganglion. Journal of Orthopaedic Research. 2002;20:730–739. doi: 10.1016/S0736-0266(01)00170-X. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression Part 2: morphological and immunohistochemical changes of dorsal root ganglion. Journal of Orthopaedic Research. 2004;22:180–188. doi: 10.1016/S0736-0266(03)00132-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Kokubo Y, Uchida K, Yayama T, Takeno K, Negoro K, Nakajima H, Baba H, Yoshizawa H. Effect of lumbar nerve root compression on primary sensory neurons and their central branches: changes in the nociceptive neuropeptides substance P and somatostatin. Spine. 2005a;30:276–282. doi: 10.1097/01.brs.0000152377.72468.f4. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Sasaki S, Shimada S, Kaneyasu M, Mizukami Y, Kitade I, Ogawa M, Kawahara H, Baba H, Yoshizawa H. Changes of calcitonin gene-related peptide in primary sensory neurons and their central branch after nerve root compression of the dog. Archives of Physical Medicine and Rehabilitation. 2005b;86:527–533. doi: 10.1016/j.apmr.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Krivickas LS, Wilbourn AJ. Peripheral nerve injuries in athletes: a case series of over 200 injuries. Seminars in Neurology. 2000;20:225–232. doi: 10.1055/s-2000-9832. [DOI] [PubMed] [Google Scholar]
- Lee KE, Thinnes JH, Gokhin DS, Winkelstein BA. A novel rodent neck pain model of facet-mediated behavioral hypersensitivity: implications for persistent pain and whiplash injury. Journal of Neuroscience Methods. 2004;137:151–159. doi: 10.1016/j.jneumeth.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. Journal of Neuroscience. 1993;13:2273–2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcangio M, Ramer MS, Boucher TJ, McMahon SB. Intrathecally injected neurotrophins and the release of substance P from the rat isolated spinal cord. European Journal of Neuroscience. 2000;12:139–144. doi: 10.1046/j.1460-9568.2000.00890.x. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain. 1998;76:215–222. doi: 10.1016/s0304-3959(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Meert TF, Vissers K, Geenen F, Kontinen VK. Functional role of exogenous administration of substance P in chronic constriction injury model of neuropathic pain in gerbils. Pharmacology Biochemistry and Behavior. 2003;76:17–25. doi: 10.1016/s0091-3057(03)00187-4. [DOI] [PubMed] [Google Scholar]
- Miller K, Chinzei K, Orssengo G, Bednarz P. Mechanical properties of brain tissue in-vivo: experiment and computer simulation. Journal of Biomechanics. 2000;33:1369–1376. doi: 10.1016/s0021-9290(00)00120-2. [DOI] [PubMed] [Google Scholar]
- Muggeo VM. Estimating regression models with unknown break-points. Statistics in Medicine. 2003;22:3055–3071. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- Munglani R, Harrison SM, Smith GD, Bountra C, Birch PJ, Elliot PJ, Hunt SP. Neuropeptide changes persist in spinal cord despite resolving hyperalgesia in a rat model of mononeuropathy. Brain Research. 1996;743:102–108. doi: 10.1016/s0006-8993(96)01026-8. [DOI] [PubMed] [Google Scholar]
- Nuckley DJ, Konodi MA, Raynak GC, Ching RP, Mirza SK. Neural space integrity of the lower cervical spine: effect of normal range of motion. Spine. 2002;27:587–595. doi: 10.1097/00007632-200203150-00006. [DOI] [PubMed] [Google Scholar]
- Oku R, Satoh M, Fujii N, Otaka A, Yajima H, Takagi H. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Research. 1987;403:350–354. doi: 10.1016/0006-8993(87)90074-6. [DOI] [PubMed] [Google Scholar]
- Olmarker K, Rydevik B, Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression. Spine. 1989;6:569–573. [PubMed] [Google Scholar]
- Pedowitz RA, Garfin SR, Massie JB, Hargens AR, Swenson MR, Myers RR, Rydevik BL. Effects of magnitude and duration of compression on spinal nerve root conduction. Spine. 1992;17:194–199. doi: 10.1097/00007632-199202000-00013. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Rempel DM, Harrison RJ, Barnhart S. Work-related cumulative trauma disorders of the upper extremity. Journal of the American Medical Association. 1992;267:838–842. [PubMed] [Google Scholar]
- Rothman SM, Hubbard RD, Lee KE, Winkelstein BA. Detection, transmission, and perception of pain. In: Slipman CW, Derby R, Simeone FA, Mayer TG, editors. Interventional Spine: An Algorithmic Approach. Saunders; 2007. [Google Scholar]
- Rothman SM, Kreider RA, Winkelstein BA. Spinal neuropeptide responses in persistent and transient pain following cervical nerve root injury. Spine. 2005;30:2491–2496. doi: 10.1097/01.brs.0000186316.38111.4b. [DOI] [PubMed] [Google Scholar]
- Rutkowski MD, Winkelstein BA, Hickey WF, Pahl JL, DeLeo JA. Lumbar nerve root injury induces central nervous system neuroimmune activation and neuroinflammation in the rat: relationship to painful radiculopathy. Spine. 2002;27:1604–1613. doi: 10.1097/00007632-200208010-00003. [DOI] [PubMed] [Google Scholar]
- Sekiguchi Y, Kikuchi S, Myers RR, Campana WM. ISSLS Prize Winner: Erythropoietin inhibits spinal neuronal apoptosis and pain following nerve root crush. Spine. 2003;28:2577–2584. doi: 10.1097/01.BRS.0000096674.12519.12. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Kikuchi S, Myers RR. Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain. Spine. 2004;29:1105–1111. doi: 10.1097/00007632-200405150-00011. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, Van der Linde A. Bayesian measures of model complexity and fit (with Discussion) Journal of the Royal Statistical Society Series B (Statistical Methodology) 2002;64:583–616. [Google Scholar]
- Swamydas M, Skoff A, Adler J. Partial sciatic nerve transection causes redistribution of pain-related peptides and lowers withdrawal threshold. Experimental Neurology. 2004;188:444–451. doi: 10.1016/j.expneurol.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Tabo E, Jinks SL, Eisele JH, Jr, Carstens E. Behavioral manifestations of neuropathic pain and mechanical allodynia, and changes in spinal dorsal horn neurons, following L4-L6 dorsal root constriction in rats. Pain. 1999;80:503–520. doi: 10.1016/S0304-3959(98)00243-7. [DOI] [PubMed] [Google Scholar]
- Torg JS, Guille JT, Jaffe S. Injuries to the cervical spine in American football players. Journal of Bone and Joint Surgery. 2002;84:112–122. doi: 10.2106/00004623-200201000-00017. [DOI] [PubMed] [Google Scholar]
- Winkelstein BA, DeLeo JA. Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: considerations for persistent pain. Brain Research. 2002;956:294–301. doi: 10.1016/s0006-8993(02)03560-6. [DOI] [PubMed] [Google Scholar]
- Winkelstein BA, DeLeo JA. Mechanical thresholds for initiation and persistence of pain following nerve root injury: mechanical and chemical contributions and injury. Journal of Biomechanical Engineering. 2004;126:258–263. doi: 10.1115/1.1695571. [DOI] [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. Journal of Comparative Neurology. 2001a;439:127–139. [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Weinstein JN, DeLeo JA. Quantification of neural tissue injury in a rat radiculopathy model: comparison of local deformation, behavioral outcomes, and spinal cytokine mRNA for two surgeons. Journal of Neuroscience Methods. 2001b;111:49–57. doi: 10.1016/s0165-0270(01)00445-9. [DOI] [PubMed] [Google Scholar]
- Winkelstein BA, Weinstein JN, DeLeo JA. The role of mechanical deformation in lumbar radiculopathy. Spine. 2002;27:27–33. doi: 10.1097/00007632-200201010-00009. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Walters ET. Common patterns of plasticity contributing to nociceptive sensitization in mammals and Aplysia. Trends in Neuroscience. 1991;14:74–78. doi: 10.1016/0166-2236(91)90024-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.