Abstract
We have previously reported that cannabidiol (CBD) lowers the incidence of diabetes in young non-obese diabetes-prone (NOD) female mice. In the present study we show that administration of CBD to 11–14 week old female NOD mice, which are either in a latent diabetes stage or with initial symptoms of diabetes, ameliorates the manifestations of the disease. Diabetes was diagnosed in only 32% of the mice in the CBD-treated group, compared to 86% and 100% in the emulsifier-treated and untreated groups, respectively. In addition, the level of the proinflammatory cytokine IL-12 produced by splenocytes was significantly reduced, whereas the level of the anti-inflammatory IL-10 was significantly elevated following CBD-treatment. Histological examination of the pancreata of CBD-treated mice revealed more intact islets than in the controls. Our data strengthen our previous assumption that CBD, known to be safe in man, can possibly be used as a therapeutic agent for treatment of type 1 diabetes.
Keywords: Cannabinoids, Cannabidiol, Diabetes, NOD mice, Cytokines
1. Introduction
Type 1 diabetes mellitus (T1DM - insulin-dependent diabetes) is an autoimmune disease resulting in destruction of insulin-producing pancreatic β cells, a process which is assumed to be mediated mainly by CD4 Th1 and CD8 T lymphocytes (Atkinson and Leiter, 1999; Mundrup-Poulsen et al., 2003). The NOD mouse is the animal used most often for pre-clinical evaluation of prophylactic and therapeutic treatments of diabetes. When the NOD mouse is not treated, it develops a disease with characteristics similar to autoimmune insulitis in man (Anderson and Bluestone, 2005).
Insulitis is an inflammation of the islands of Langerhans and is the initial lesion of insulin-dependent diabetes mellitus, during which leukocytes, lymphocytes in particular, surround and infiltrate the islets.
While numerous approaches have successfully been used to prevent diabetes in young NOD mice, the amelioration of diabetes after onset, or around onset time, is much more difficult to achieve. Importantly, some of the therapies found to be effective in this mice model, have subsequently shown efficacy in clinical trials. Raz et al. have reported immunomodulation with DiaPep277 in a phase 2 clinical trial (Raz et al., 2001) and Keymeulen et al. have successfully employed anti CD3 antibodies (Keymeulen et al., 2005).
Cannabidiol (CBD) is a potent anti-inflammatory agent. It is effective in supressing IFN-γ and TNF-α production and progression of autoimmune Th1-mediated rheumatoid arthritis by inhibition of T cell proliferation (Malfait et al., 2000). This observation led us to investigate the possible effects of CBD on additional autoimmune diseases. Recently we demonstrated that CBD lowers the incidence of diabetes in young NOD mice. It also significantly reduces the plasma levels of the proinflammatory cytokines IFN-γ and TNF-α, lowers Th1-associated cytokine production in vitro and augments Th2-associated cytokines IL-4 and IL-10 (Weiss et al., 2006). Following this observation, which shows that CBD partly prevents initiation of diabetes, we decided to explore the possibility that CBD can also prevent the development of overt or latent diabetes.
In our present report we found that CBD significantly inhibited insulitis in 11–14 week old NOD female mice.
2. Materials and methods
2.1 Mice
Non-obese female diabetic NOD/LtJ mice, 11–14-week-old, were obtained from Harlan, Israel. Female NOD mice develop diabetes after 12–18 weeks. BALB/c female mice (11–14 weeks old) were also obtained from the same supplier. Mice were fed standard laboratory animal chow ad libitum and were kept in pathogen free (SPF) animal house facility. The study was conducted in compliance with the international laws on animal experimentation and approved by the Ethical Committee of the Hebrew University Medical School.
2.2 Cannabidiol (CBD)
CBD was extracted from Cannabis resin (hashish) and purified as previously reported (Gaoni and Mechoulam, 1971). The crystalline CBD, melting point 66–67° C, presented a single peak on gas chromatography analysis. CBD was first dissolved in ethanol and then the same amount of Cremophor EL (Sigma) was added. This solution was then further diluted with saline so that the final solution was ethanol/cremophor/saline (1:1:18).
2.3 Experimental design
NOD female mice, , 11–14 week old, with impaired glucose tolerance and 14 week old mice with normal glucose tolerance were assigned to three groups (Gross et al., 1998). One group received CBD, the second group received Cremophor/ethanol/saline (vehicle) and the third group remained untreated. CBD was administered i.p. at a dose of 5 mg/kg/day, in 0.1 ml. The experiment was continued for 4 weeks, each mouse receiving 5 administrations per week. The mice were observed until 24 weeks of age.
The level of urine glucose was assayed once or twice a week by test strips (Medi-Test Combi 9 Macherey-Nagel, Duren) and was considered positive after the appearance of glucosuria (>1000mg/dl) in at least two determinations, according to the manufacturer’s information.. A level of 1000mg/dl glucose in the urine was found to correlate with >200 mg/dl. glucose in the blood.
2.4 Peritoneal macrophage culture
Peritoneal lavages from each mouse, containing approximately 40% macrophages (PMθ), were collected in phosphate buffer saline (PBS), centrifuged and the pellets were then resuspended and plated in 24-well platesperitoneal cells for two hours. The wells were washed to remove non-adherent cells and fresh DMEM with 10% fetal calf serum (FCS) was added to the cells, mostly macrophages >90%, for further over night incubation. Harvested supernatants were centrifuged to remove any cells and stored at −80°C until assayed for cytokines.
2.5 Splenic lymphocyte culture
Spleen cells from each mouse were removed aseptically, the cells were released, washed in PBS, resuspended in RPMI complete medium supplemented with 10% FCS, 100 U/ml penicillin, 100μg/ml streptomycin and 2mM L-glutamine (Beith Haemek, Israel), counted with Trypan-blue dye exclusion to distinguish the live cells (unstained) and dispensed into 24-well plates (Greiner Labs, Germany) at 2×106 cells/ml medium per well. Cells were stimulated with 2.5 μg/ml Concanavalin A (ConA) (Sigma St. Louis, MI, USA) and cultured at 37°C in a 5% CO2 humidified incubator. Supernatants were harvested after 48 hour incubation, cleared by centrifugation and stored at −80°C until assayed for cytokines.
2.6 Cytokine assay
Levels of cytokines in the plasma and in the supernatants of splenic lymphocytes and peritoneal macrophages of the mice were assayed by the “sandwich” ELISA technique. ELISA reagents were purchased as Opt. EIA Cytokine ELISA sets from BD Biosciences (San Diego, CA, USA) and were used according to the manufacturer’s protocol as previously described (Ji et al., 2003).
2.7 Histopathology
Pancreatic tissue was fixed in 4% buffered formalin, embedded in paraffin, and 5-μm sections were stained with hematoxylin and eosin. Twenty sections were screened and scored by two independent observers and were graded as follows: intact islets; partially infiltrated islets and peri-infiltrated islets; totally infiltrated islets; destroyed islets.
2.7 Statistical analysis
The statistical significance of the results of diabetes occurrence in Fig 1 was calculated by the Kaplan-Meier method, and statistical significant differences between the curves were analyzed by log rank. Differences between experimental and control values (Table 1) were analyzed for significance (p≤0.05) by one-tail distribution-free Mann-Whitney U test.
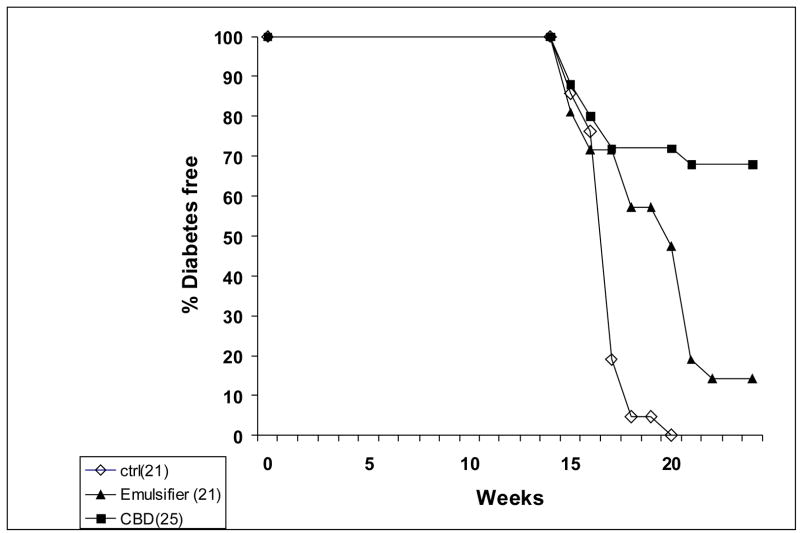
Figure 1.
Incidence of diabetes in NOD mice treated with 5 mg/kg CBD i.p., vehicle control and untreated mice. The incidence of diabetes in the CBD treated group, compared with vehicle-treated mice and untreated controls was significant p<0.001 (log rank test)
Table 1.
Cytokine levels in the plasma and supernatants of peritoneal macrophages and spleen cells from untreated and CBD treated mice at 24 weeks of age.
| In vivo treatment of NOD mice | number of mice | in vitro spleen cell activation | Cytokine evaluated | pg/ml Mean ±S.E | P= |
|---|---|---|---|---|---|
| CBD
Vehicle |
5
4 |
Plasma
Plasma |
IL-6
IL-6 |
2.0±2.0
100.7±44.1 |
0.016 |
| CBD
Vehicle |
4
4 |
PMθ
PMθ |
IL-6
IL-6 |
50.2±17.7
100.0±20.0 |
0.015 |
| CBD
Vehicle |
3
3 |
PMθ
PMθ |
IL-10
IL-10 |
234.7±85.3
1.0±1.0 |
0.05 |
| CBD
Vehicle |
4
4 |
Sp
Sp |
IL-10
IL-10 |
97.5±57.5
1.0±1.0 |
0.029 |
| CBD
Vehicle |
9
8 |
Sp
Sp |
TNFα
TNFα |
20.2±10.2
148.0±44.0 |
0.068 |
| CBD
Vehicle |
13
9 |
Sp
Sp |
IL-12
IL-12 |
36.9±15.3
114.0±38.1 |
0.018 |
| CBD
Vehicle |
5
4 |
Sp
Sp |
IL-4
IL-4 |
278±97.9
100±21.9 |
0.056 |
Differences between CBD and control values were analyzed for significance by one-tail distribution-free Mann-Whitney U test.
3. Results
3.1 CBD partly prevents the development of overt diabetes in NOD female mice
CBD was administered i.p. to 11–14 week old female NOD mice. Treatment was continued for 4 weeks (5 days a week) at a dose of 5 mg/kg/day. Treatment was then withdrawn and the mice were observed until 24 weeks of age. A remarkable reduction of diabetes development was observed in CBD-treated mice, compared with vehicle-treated mice and untreated controls (p<0.001, log rank test). At the end of the 24 weeks, only 8 of 25 (32%) of the CBD-treated mice developed glucosuria, compared with 18 of 21 (86%) vehicle-treated mice and 100% (21 mice) of the untreated controls (Fig 1).
3.2 Effect of CBD on cytokine profile
Cytokine concentration of IL-12, TNF-α, IL-6 and IL-10 in the supernatants of NOD splenocytes and macrophages (stimulated and unstimulated in vitro) as well as IL-6 in plasma were assessed by ELISA. A significant reduction of IL-6 level was noted in the plasma of CBD-treated mice (p=0.016), as well as in the levels of IL-6 and IL-12 in cell supernatants of CBD-treated NOD mice, compared with the levels in vehicle-treated mice (p= 0.015 and p=0.018, respectively). The level of TNF-α in splenocyte supernatants was also reduced, but the reduction was not significant (p=0.068). In contrast the levels of the Th2-associated cytokines, IL-10, in supernatants of macrophages and splenocytes and IL-4 secreted by splenocytes were higher, although in the case of IL-4, the enhancement was not significant (p=0.05, p= 0.029 and p=0.056 respectively) (Table 1). The changes in the cytokine profile suggest that CBD administration induces a Th1 → Th2 shift (Crane and Forrester, 2005; Chan et al., 1991),
3.3 Histology of pancreas
The morphological structure of intact islets from CBD-treated NOD mice was similar to normal islets, in contrast to the abundant mononuclear cell invasion into islets in vehicle-treated and untreated mice. The number of intact islets in pancreata taken from untreated (6 out of 73, 8%) and vehicle treated mice (12 out of 94, 13%) decreased, compared to CBD-treated mice (108 out of 140, 77%). As most islets in the control diabetic mice were fully destroyed and disappeared, the total number of islets counted was lower than that in the CBD-treated. (Table 2).
Table 2.
Histological analysis of pancreas tissue from mice treated with CBD, or with vehicle or untreated.
| Treatment | No. of scored islets | Intact islets | Total ruined islets | Full infiltrated islets | Partial infiltrated islets | Percent intact islets |
|---|---|---|---|---|---|---|
| Control | 73 | 6 | 29 | 28 | 5 | 8 |
| Vehicle | 94 | 12 | 30 | 43 | 7 | 13 |
| CBD | 140 | 108 | - | 12 | 15 | 77 |
Cell infiltration into islets (leading to insulitis) was also lower in the pancreata of CBD –treated mice when they were examined 6–8 weeks after the treatment was stopped. The morphological appearance of intact islets was similar to that of normal islets. In contrast, abundant mononuclear cell invasion into islets and destroyed islets were seen in the pancreata of vehicle and untreated controls (Fig 2).
Figure 2.
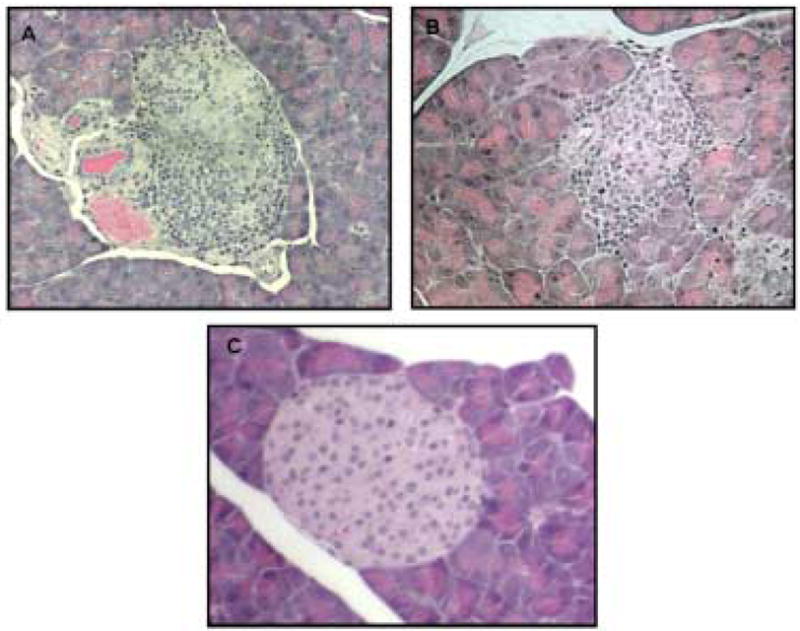
Representative histology of Hematoxylin-Eosin stained pancreases from (A) untreated control mice, mag. × 800, (B) vehicle treated mice, mag. × 800, and (C) CBD-treated mice, mag. × 800. Abundant mononuclear cell invasion into islets and pri-insulitis in the pancreata of untreated and vehicle treated mice (A and B). The pancreas of CBD-treated non-diabetic mice showed neither cell-infiltration nor islet destruction (C).
4. Discussion
Studying the levels of various cytokines secreted in vitro by macrophages and splenocytes derived from CBD-treated NOD mice demonstrated a decrease in production of IL-6, TNF-α and IL-12. In contrast the levels of IL-10 (by PMθ and splenocytes) and IL-4 (by splenocytes) were enhanced. However the changes in TNF-α and IL-4 levels indicated a trend only, as they did not achieve statistical significance. Numerous investigations have shown that suppression of proinflammatory cytokines TNF-α, IL-6 as well as IL-12 inhibits inflammatory damage. Moreover, the increase of IL-10 production, which suppresses proinflammatory cytokines production, is known to abrogate inflammatory manifestation.
We have previously shown that treatment of NOD mice (6–12 weeks old) with CBD, leads to augmentation of IL-4 and IL-10 production by splenocytes and decrease in IFN-γ (Weiss et al., 2006). We assumed that this phenomenon demonstrates a Th1 to Th2 cytokine shift, a mechanism that may prevent development of diabetes (Elenkov et al. 2005).
The results of the present study indicate that treatment of 11–14 weeks old female NOD mice, either in a latent diabetes stage (after 14 weeks) or with initial symptoms of diabetes (appearing up to 14 weeks), with CBD for 4 weeks, led to sustained inhibition of insulitis. CBD treatment induced a qualitative modification of the islets infiltrates by mononuclear cells, inhibited specific destruction of the islets and eventually prevented diabetes (Fig 2, Table 2). CBD treatment reduced diabetes from 86% in untreated mice to 32% in CBD-treated mice. The direct effect of CBD on glucose load tests (i.e. 2 gr/kg i.p.) in BALB/c versus NOD mice showed no difference in treatment with saline compared to CBD. This result show no direct effect of CBD on glucose levels in the blood. It was also demonstrated that CBD treatment inhibited IL-12 production by splenocytes. IL-12 is a cytokine that plays a major role in autoimmunity including diabetes (Rothe et al.,1996; Trembleau et al., 1995; Rabinovitch et al., 1996; Trichieri et al., 1995). Macrophages and dendritic cells are known to produce IL-12, which triggers generation of IFNγ (Verma et al., 2006).
Th1 cytokines activate cytotoxic T cells and stimulate macrophages to produce proinflammatory cytokines and nitrogen free radicals (NO). Macrophages and dendritic cells are known to produce IL-12, which triggers generation of IFNγ (Verma et al., 2006). All these molecules contribute to islet destruction. These results indicate that, CBD suppression of macrophage IL-12 production can inhibit Th1 mediated autoimmunity.
The difference between Th1 and Th2 cells in diabetes are discussed in various studies (Rabinovitch et a.,, 2003, Maierhoff G, 2002, Suarez-Pinzon 2001). The aim in treatment of diabetes is to decrease cytokine levels of the Th1 related cytokines IFNγ and IL-12 and to increase Th2 related cytokines IL-4, IL-10.
The data in our NOD study suggest that IL-6 participates in islet inflammation, and CBD, by significant suppression of IL-6 production, decreases islet destruction. Additional factors, such as proinflammatory cytokines and mediators presumably participate in the pathological events.
Furthermore, in this study, we show that immune cells from CBD-treated mice enhance in vitro IL-4 and IL-10 cytokine production. These cytokine alterations correlated with disease suppression. Several reports support that immunological interventions, which enhance Th2 immune responses in NOD mice are protective against the disease. For example, treatment of NOD mice with IL-4, a Th2 cytokine, prevents overt diabetes (Rapaport et al., 1993; Cameron et al., 1997; Tominaga et al., 1998) and local expression of IL-4 in pancreatic beta cells in transgenic mice inhibits insulitis (Mueller et al., 1996). Shi et al. (2006) have demonstrated that abrogation of recurrent autoimmunity requires host IL-4. In addition, levels of IL-10, a Th2 associated cytokine, correlate with protection (Mueller et al., 1996) and IL-4 and IL-10 inhibit diabetes by suppressing Th1 cytokine production in islet grafts (Rabinovitch et al., 1995).
Histological examination of the pancreas indicated the absence of insulitis in 77% of the islets in the CBD-treated group, compared to 13% in the pancreas taken from vehicle treated mice; thus, β cell destruction in the CBD-treated mice was markedly suppressed (Table 2, Fig 2C).
We have previously shown that CBD treatment of young NOD mice preserved more than 85% of the islets as determined by histological analysis (Weiss et al., 2006). We also reported that CBD inhibited TNF-α production in vitro and in vivo (Malfait et al., 2000) and have noted the same trend now in a different system. We have also previously seen that nitric oxide formation is decreased in vitro (Ben-Shabat et al., 2006). As TNF-α and nitric oxide are important mediators of IL-1β induced β-cell damage (Corbett et al., 1991) it is possible that this inhibition represents one of the protective pathways through which CBD acts.
It was recently suggested by Carrier et al.(2006) that CBD has anti-inflammatory properties due to its binding capacity to A2A adenosine receptor, which leads to inhibition of adenosine uptake and enhancement of endogenous adenosine signaling. It was further demonstrated that adenosine transport inhibitors suppress the production in vivo of TNF-α, one of the key cytokines of inflammation. Thus the observed CBD effect in diabetes may be due, at least partly, to its binding and activation of the A2A receptor. Indeed, there are several reports which indicate that A2A receptor activation is involved in causing diabetes although in some cases it may represent the result of diabetes (Grden et al., 2005; Thong and Graham., 2002; Awad et al., 2006).
It is possible that CBD acts by antagonism of cannabinoid agonists. Bermudez-Siva et al. (2006) demonstrated that activation of cannabinoid CB1 receptors induces glucose intolerance in mice and Juan-Pico et al. (2006) reported that the endocannabinoid 2-arachidonoyl glycerol (2-AG), through CB2 receptors, affects Ca2+ signals in β-cells and as a consequence decreases insulin secretion. As CBD was recently found to be an antagonist of CB1 and CB2 receptor agonists (Thomas et al., 2007) it is possible that via this route it affects the development of diabetes in NOD mice. Indeed the CB1 cannabinoid receptor antagonist, rimonabant, was reported to lead to lower plasma glucose and insulin levels, as well as improved insulin resistance in animals and to an improvement in glycemic control in prediabetic and type 2 diabetic patients (Gelfand and Cannon, 2006).
We have also demonstrated that in vivo CBD treatment augments IL-4 and IL-10 production, which can prevent the development of diabetes through a Th1 → Th2 cytokine shift, as other studies have noted (Weiss et al., 2006).
An extensive list of therapeutic strategies has been shown to provide some protection from the development of overt diabetes in the NOD mouse model. A number of clinically available immunomodulatory compounds, such as cyclosporine, FK506 and Rapamycin may suppress the disease progression, but some of them are toxic or they can augment the risk of infection and tumorigenesis (Kai et al., 1993; Baeder et al., 1992).
Successful induction of remission in 17 of 25 diabetic mice by CBD treatment long after cessation of treatment, indicates that CBD exerts long lasting beneficial influence on β-cell mass and glycemia. We assume that by effectively controlling autoimmune effector T-cells or by influencing cytokine balance resulting in a Th1 → Th2 shift, CBD caused amelioration of autoimmune insulitis and allowed endogenous precursor cells to slowly give rise to increased β-cell mass and thus maintain normoglycemia.
Although insulin replacement therapies provide chronic care for people with type 1 diabetes, this treatment does not restore normal glucose control and is associated with significant risk of hypoglycemia. Controlling autoimmunity may represent an alternative therapeutic pathway aimed at restoring pancreatic cell mass and function.
CBD is not psychoactive and has anti-inflammatory and anti autoimmune properties. Based on the above presented results, on the previously documented anti-inflammatory effects of CBD (Malfait et al., 2000) and on its clinical safety (Cunha et al., 1980), it seems reasonable to consider the use of CBD for controlling type 1 diabetes at an early stage of the disease.
Acknowledgments
We acknowledge support by the US National Institute on Drug Abuse (DA-9789 to RM) and the Concern Foundation, Los Angeles to RG.
Abbreviations
- CBD
cannabidiol
- ConA
concanavalin A
- FCS
fetal calf serum
- NO
nitric oxide
- NOD
non-obese diabetic mice
- PMθ
peritoneal macrophages
- SPF
special pathogen free
- T1DM
type 1 diabetes mellitus
- Th1
T helper 1
- Th2
T helper 2
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- Awad AS, Huang L, Ye H, Duong ET, Bolton WK, Linden J, Okusa MD. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- Baeder WL, Sredy J, Sehgal SN, Chang JY, Adams LM. Rapamycin prevents the onset of insulin-dependent diabetes mellitus (IDDM) in NOD mice. Clin Exp Immunol. 1992;89:174–178. doi: 10.1111/j.1365-2249.1992.tb06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shabat S, Hanus LO, Katzavian G, Gallily R. New cannabidiol derivatives: Synthesis, binding to cannabinoid receptors and evaluation of their antiinflammatory activity. J Med Chem. 2006;49:1113–1117. doi: 10.1021/jm050709m. [DOI] [PubMed] [Google Scholar]
- Bermudez-Siva FJ, Serrano A, Diaz-Molina FJ, Sanchez Vera I, Juan-Pico P, Nadal A, Fuentes E, Rodriguez de Fonseca F. Activation of cannabinoid CB1 receptors induces glucose intolerance in rats. Eur J Pharmacol. 2006;531:282–284. doi: 10.1016/j.ejphar.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cameron MJ, Arreaza GA, Zucker P, Chensue SW, Strieter RM, Chakrabarti S, Delovitch TL. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J Immunol. 1997;159:4686–4692. [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci (USA) 2006;103:7895–7900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HA, Wolf SF, Young D, Clark SC, Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JA, Lancaster JR, Sweetland MA. Interleukin-1 beta-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. Role of nitric oxide in interleukin-1 beta-induced inhibition of insulin secretion. J Biol Chem. 1991;266:21351–21354. [PubMed] [Google Scholar]
- Crane IJ, Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit Rev Immunol. 2005;25:75–102. doi: 10.1615/critrevimmunol.v25.i2.10. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, Mechoulam R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–185. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–69. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. The isolation and structure of Δ1-THC and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93:217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- Gelfand EV, Cannon CP. Rimonabant: a cannabinoid receptor type 1 blocker for management of multiple cardiometabolic risk factors. J Am Coll Cardiol. 2006;47:1919–1926. doi: 10.1016/j.jacc.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Grden M, Podgorska M, Szutowicz A, Pawelczyk T. Altered expression of adenosine receptors in heart of diabetic rat. J Physiol Pharmacol. 2005;56:587–597. [PubMed] [Google Scholar]
- Gross DJ, Weiss L, Reibstein I, Van den Brand J, Okamoto H, Clark A. Amelioration of diabetes in nonobese diabetic mice with advanced disease by linomide-induced immunoregulation combined with Reg protein treatment. Endocrinology. 1998;139(5):2369–74. doi: 10.1210/endo.139.5.5997. [DOI] [PubMed] [Google Scholar]
- Ji YH, Weiss L, Zeira Abdul-Hai AM, Har-Noy M, Slavin S. Allogeneic cell-mediated immunotherapy of leukemia with immune donor lymphocytes to upregulate antitumor effects and downregulate antihost responces. Bone Marrow Transplant. 2003;32:495–504. doi: 10.1038/sj.bmt.1704150. [DOI] [PubMed] [Google Scholar]
- Juan-Pico P, Fuentes E, Bermudes-Silva FJ, Javier Diaz-Molina F, Ripoll C, Rodriguez de Fonseca F, Nadal A. Cannabinoid receptors regulate Ca2+ signals and insulin secretion in pancreatic β-cells. Cell Calcium. 2006;39:155–162. doi: 10.1016/j.ceca.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Kai N, Motojima K, Tsunoda T, Kanematsu T. Prevention of insulitis and diabetes in nonobese diabetic mice by administration of FK506. Transplantation. 1993;55:936–940. doi: 10.1097/00007890-199304000-00045. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3 antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The non-psychoactive cannabis-constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci (USA) 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup-Poulsen T. Beta cell death and protection. Ann NY Acad Sci. 2003;1005:32–42. doi: 10.1196/annals.1288.005. [DOI] [PubMed] [Google Scholar]
- Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 1996;184:1093–1099. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch A, Suarez-Pinzon WL, Sorensen O, Bleackley RC, Power RF, Rajotte RV. Combined therapy with interleukin-4 and interleukin-10 inhibits autoimmune diabetes recurrence in syngeneic islet-transplanted nonobese diabetic mice. Analysis of cytokine mRNA expression in the graft. Transplantation. 1995;60:368–374. doi: 10.1097/00007890-199508270-00012. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A, Suarez-Pinzon WL, Sorensen O. Interleukin 12 mRNA expression in islets correlates with beta-cell destruction in NOD mice. J Autoimmun. 1996;9:645–651. doi: 10.1006/jaut.1996.0084. [DOI] [PubMed] [Google Scholar]
- Rapoport MJ, Jaramillo A, Zipris D, Lazarus AH, Serreze DV, Leiter EH, Cyopick P, Danska JS, Delovitch TL. Interleukin 4 reverses T-cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- Rothe H, Burkart V, Faust A, Kolb H. Interleukin-12 gene expression is associated with rapid development of diabetes mellitus in non-obese diabetic mice. Diabetologia. 1996;39:119–122. doi: 10.1007/BF00400422. [DOI] [PubMed] [Google Scholar]
- Shi Q, Wang D, Hadley GA, Farber DL, Bartlett ST. Abrogation of recurrent autoimmunity in the NOD mouse: A critical role for host interleukin 4. Surgery. 2006;140:281–288. doi: 10.1016/j.surg.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB(1) and CB(2) receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong FS, Graham TE. The putative roles of adenosine in insulin- and exercise-mediated regulation of glucose transport and glycogen metabolism in skeletal muscle. Can J Appl Physiol. 2002;27:152–178. doi: 10.1139/h02-011. [DOI] [PubMed] [Google Scholar]
- Tominaga Y, Nagata M, Yasuda H, Okamoto N, Arisawa K, Moriyama H, Miki M, Yokono K, Kasuga M. Administration of IL-4 prevents autoimmune diabetes but enhances pancreatic insulitis in NOD mice. Clin Immunol Immunopathol. 1998;86:209–218. doi: 10.1006/clin.1997.4471. [DOI] [PubMed] [Google Scholar]
- Trembleau S, Penna G, Bosi E, Mortara A, Gately MK, Adorini L. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J Exp Med. 1995;81:817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- Verma ND, Boyd R, Robinson C, Plain KM, Tran GT, Hall BM. Interleukin-12p70 prolongs allograft survival by induction of interferon gamma and nitric oxide production. Transplantation. 2006;82:1324–1333. doi: 10.1097/01.tp.0000239519.56358.c1. [DOI] [PubMed] [Google Scholar]
- Weiss L, Zeira M, Reich S, Har-Noy M, Mechoulam R, Slavin S, Gallily R. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39:143–151. doi: 10.1080/08916930500356674. [DOI] [PubMed] [Google Scholar]