Abstract
Recently, a radioimmunoassay (RIA) for measurement of canine pancreatic lipase immunoreactivity (cPLI) in serum was developed and validated. However, RIAs require frequent use of radioactive materials. Therefore, the goal of this project was to develop and validate an enzyme-linked immunosorbent assay (ELISA) for cPLI. After purifying cPL, we developed and purified antiserum against cPL in rabbits. The purified antibody was bound to microtitre plates and used to capture antigen. A portion of the purified antibody was biotinylated and used to identify the captured antigen. Streptavidin labelled with horseradish peroxidase and a horseradish peroxidase substrate were used for detection. The assay was validated by determination of sensitivity, working range, linearity, accuracy, precision, and reproducibility. The reference interval for serum cPLI was determined by the central 95th percentile in 74 clinically healthy dogs: 2.2 to 102.1 μg/L. The sensitivity and the upper limit of the working range were 0.1 and 999.2 μg/L, respectively. The ratios of observed to expected values for dilutional parallelism for 6 serum samples ranged from 0.0 to 148.8%; the ratios for spiking recovery for 4 serum samples ranged from 90.4 to 112.6%, assuming 55% recovery of the cPL. Coefficients of variation for intra- and interassay variability for 6 different serum samples were 2.4, 3.4, 4.1, 5.8, 7.4, and 10.0% and 5.9, 7.7, 11.6, 13.9, 23.5, and 46.2%, respectively. We conclude that the ELISA described here is sufficiently sensitive, linear, accurate, precise, and reproducible for clinical application. Evaluation of its clinical usefulness for the diagnosis of exocrine pancreatic disorders in dogs is under way.
Introduction
Serum lipase activity has been used to evaluate pancreatic function and disorders in humans and dogs for several decades (1,2,3,4,5,6). However, it lacks both sensitivity and specificity in humans and dogs with exocrine pancreatic disorders (1,7,8,9,10,11,12,13). The lack of specificity is due to the fact that many different cell types, such as mucous pit and neck cells in the gastric mucosa, hepatocytes, and adipocytes, synthesize and secrete lipases, and kinetic assays cannot distinguish between lipases of different cellular origins (14). Recently, a radioimmunoassay (RIA) for the measurement of canine pancreatic lipase immunoreactivity (cPLI) in serum has been developed and validated (15). Although RIAs are well suited for the measurement of concentrations of polypeptides in biologic fluids, they have a major disadvantage: they require the use of radioactive materials (16). Notwithstanding the overall safety of radioiodination and RIAs, other alternatives may be preferable given the concerns of laboratory personnel, the public perception of potential risks whenever radioactive materials are used, and regulatory restraints placed on laboratory facilities using this technology. Therefore, the goal of this project was to develop and analytically validate an enzyme-linked immunosorbent assay (ELISA) for the measurement of cPLI.
Materials and methods
Purification of cPL
Classic cPL, most commonly referred to as cPL, was purified as described previously (17). Briefly, pancreata were collected from research dogs that had been sacrificed for unrelated research projects. Pancreatic tissue was delipidated with the use of organic solvents. The delipidated extract was further purified by extracting the enzymes in a Tris buffer (Sigma Chemicals, St. Louis, Missouri, USA) containing 2 protease inhibitors, benzaminidine (Sigma Chemicals) and phenylmethylsulfonyl fluoride (Sigma Chemicals), then using anion-exchange chromatography, gel-filtration chromatography, and cation-exchange chromatography (17).
Production and purification of antiserum against cPL
Antiserum directed against cPL was developed in 2 New Zealand white rabbits by repeated inoculation of purified cPL emulsified with TiterMax Gold (CytRx Corporation, Atlanta, Georgia, USA) for the first 2 injections and with incomplete Freund's adjuvant (Sigma Chemicals) for 9 more injections. The amount of protein injected varied according to the titre in the animal (data not shown).
The polyclonal antiserum was purified by affinity chromatography. Briefly, an affinity chromatography column (HiTrap; Amersham–Pharmacia Biotech, Piscataway, New Jersey, USA) for cPL was prepared according to the manufacturer's instructions. Antiserum was applied to the column after lipoprotein precipitation and a change of buffer to 75 mM Tris-HCl and 150 mM NaCl, pH 8.0. After the absorbance (280 nm) of the eluent had returned to baseline levels the column was washed with 100 mM glycine (Sigma Chemicals), 500 mM NaCl, pH 3.0. The buffer of the purified polyclonal antibody was changed to phosphate-buffered saline (PBS), pH 7.2 [100 mM sodium phosphate, 150 mM NaCl, pH 7.2 (BupHTM dry-blend buffers; Pierce Chemical Company, Rockford, Illinois, USA)], and the antibody concentration adjusted to approximately 1 mg/mL. The purified polyclonal antibody was stored at −80°C.
For biotinylation, some of the purified monospecific polyclonal antibody in PBS, pH 7.2, was injected into a dialysis cassette (Pierce Chemical Company) and an approximately 20-fold molar excess of biotin (Pierce Chemical Company) added. After incubation for 30 min at room temperature (approximately 20°C), the material was dialyzed 3 times against 800 mL of PBS, pH 7.2, for 1 h at 4°C. Biotinylation efficiency was determined by use of a 2-(4'- hydroxyazobenzene) benzoic acid avidin assay kit (Pierce Chemical Company). This procedure was repeated until a biotinylation coefficient of approximately 3 to 4 was reached. The concentration of the biotinylated antibody was adjusted to 1 mg/mL and aliquots of 100 μL were frozen.
Development and optimization of ELISA for measurement of cPLI
A sandwich ELISA was developed. ELISA plates were coated with the capture antibody, and nonspecific binding sites were blocked identically for all plates. First, 96-well flat-bottom ELISA plates (Combiplate8; Labsystems Oy, Atlsinki, Finland) were coated with affinity-purified monospecific anti-cPL-antibody (cPL-AB), 200 ng/well in carbonate–bicarbonate buffer, pH 9.4 (BupHTM dry blend buffers), 100 μL/well. The plates were incubated for 1 h at 37°C with constant shaking (Stat Fax 2200; Awareness Technology Inc., Palm City, Florida, USA) and washed 4 times (Columbus plate washer; Tecan US Inc., Durham, North Carolina, USA) with PBS, pH 7.2, 200 μL/well. Nonspecific binding sites were blocked with a milk-free blocking solution (Superblock in PBS; Pierce Chemical Company), 200 μL/well. The plates were incubated for 1 h at 37°C with constant shaking and washed 4 times as described previously.
All plates were set up in the same fashion. Standard solutions were applied in duplicates from the highest to the lowest standard solution. Then blanks, 3 control samples with different cPLI concentrations, and unknown samples were applied. The standards were prepared by a 1:2 serial dilution of a solution of 3.0 μg/L cPL in PBS, pH 7.2, containing 1% bovine serum albumin (BSA; Sigma Chemicals) and 0.05% polyoxyethylene sorbitan monolaurate (Tween 20; Sigma Chemicals). Standards of 3.000, 1.500, 0.750, 0.375, 0.188, 0.094, 0.047, 0.024, and 0.012 μg/L were produced and frozen in aliquots of 300 μL at −20°C. The standards were thawed immediately before loading. For blanks, PBS, pH 7.2, containing 1% BSA and 0.05% Tween 20 was used. Control samples were prepared in a 1:200 dilution with PBS, pH 7.2, containing 1% BSA and 0.05% Tween 20. Unknown samples were also initially prepared in a 1:200 dilution of PBS, pH 7.2, containing 1% BSA and 0.05% Tween 20; those with raw results of less than 0.025 μg/L (corresponding to less than 5 μg/L of serum cPLI) were re-evaluated in the following assay in a dilution of 1:20 with PBS, pH 7.2, containing 1% BSA and 0.05% Tween 20. Wells were each loaded with 100 μL of the designated solution. The plates were incubated for 1 h at 37°C without shaking and washed 4 times as described previously.
For detection of the captured antigen, plates were incubated with the secondary antibody solution containing biotinylated anti-cPL antibody at 100 ng/well in 100 μL/well of PBS, pH 7.2, containing 1% BSA and 0.05% Tween 20. After incubation for 1 h at 37°C with constant shaking the plates were washed 4 times as described previously and incubated with 100 μL/well of horseradish-peroxidase-labelled streptavidin solution (50 ng/mL) in PBS, pH 7.2, containing 1% BSA and 0.05% Tween 20. The plates were incubated for another hour at 37°C with constant shaking, washed 4 times, and then developed for 12 min with 100 μL/well of a 3,3',5,5'- tetramethylbenzidine dihydrochloride substrate solution (ImmunoPure Turbo TMB; Pierce Chemical Company). The reaction was stopped by adding a solution of 4 M acetic acid (Sigma Chemicals) and 0.5 M sulfuric acid (Sigma Chemicals), 100 μL/well. The plates were read at a wavelength of 450 nm (UV MAX; Molecular Devices, Sunnyvale, California, USA). Standard curves were calculated with use of a 4-parameter curve fit: y = (A − D)/[1 + (x/C)B] + D, where D is the y value corresponding to the asymptote at high values on the x axis, A is the y value corresponding to the asymptote at low values on the x axis, C is the x value corresponding to the midpoint between A and D, and B describes how rapidly the curve makes its transition from the asymptotes in the centre. All 4 parameters are calculated with an algorithm based on the Levenberg–Marquardt method (SOFTMAX PRO; Molecular Devices).
During optimization the influence of several parameters was analyzed. Different concentrations of primary antibody, secondary antibody, and horseradish-peroxidase-labelled streptavidin were compared. Different washing buffers and washing protocols were analyzed, and the effects of using different buffers to dilute the reagents were studied. Various incubation times and protocols for standards and samples were also evaluated. In the interest of space none of the results of these experiments are presented here, and only the optimized ELISA procedure is described.
Validation of ELISA for measurement of cPLI
The assay was validated by determination of assay sensitivity, working range, dilutional parallelism, spiking recovery, intra-assay variability, and interassay variability. Dilutional parallelism, spiking recovery, and intra- and interassay variability were determined with the use of samples diluted 1:200 or 1:20 with PBS, pH 7.2, containing 1% BSA and 0.05% Tween 20. All serum samples used for assay validation were stored at −20°C until used.
Assay sensitivity was determined by calculating the mean response of 10 sets of blanks and evaluating the mean plus 3 standard deviations (s) on the standard curve (18). The lower limit of the working range was defined as the sensitivity. The upper limit of the working range was determined by the apparent value of an absorbance, which equals the mean maximum absorbance minus 3 s, as determined from the mean absorbance in 10 duplicate wells containing approximately 100 μg/L of cPL. For validation of the assay at different dilutions, we used 4 serum samples diluted 1:200 and 2 serum samples diluted 1:20. All serum samples were single random samples that were available from other studies. Dilutional parallelism was determined by evaluating each sample at its initial strength (1:200 or 1:20) and at dilutions of 1:2, 1:4, and 1:8. Spiking recovery was determined by adding 0.0, 0.023, 0.047, 0.094, 0.188, 0.375, and 0.750 μg/L to each of the 6 diluted serum samples. Intra-assay variability was determined by evaluating the 6 diluted serum samples 10 times within the same assay run [% CV = (s/mean)*100, where CV = coefficient of variation]. Interassay variability was determined by evaluating the 6 diluted serum samples in 10 consecutive assay runs [% CV = (s/mean)*100]. A reference interval for serum cPLI was established from the central 95th percentile (2.5th to 97.5th percentile) of the serum cPLI values in 74 clinically healthy dogs (45 dogs belonging to several research colonies and 29 pet dogs). The dogs were adults (> 1 y of age) of different breeds and sexes. None had any clinical signs reported by the care taker or the owner and had an unremarkable physical examination.
Serum cPLI values obtained with the ELISA were compared with values obtained with a previously described RIA (15). Correlation between the 2 methods was assessed by the Spearman correlation test for nonparametric data (Prism; Graph Pad, San Diego, California, USA). The dogs used for this analysis were the dogs used for establishing the reference interval for the ELISA. The serum cPLI value for 1 of the dogs was removed from further analysis because the value measured by RIA exceeded the working range of the assay, and sufficient serum for repeated analysis of a diluted sample was not available. Therefore, correlation was assessed in only 73 paired serum samples from clinically healthy dogs.
Results
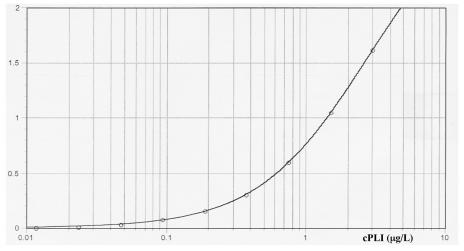
The assay resulted in reproducible standard curves (Figure 1). The sensitivity and lower limit of the working range of cPLI in serum was determined to be 0.0018 μg/L, or 1.8 ng/L. The maximum and upper limit of the working range was determined to be 4.996 μg/L. Taking into account the dilution of serum samples, this translates into a working range of 0.4 to 999.2 μg/L when serum samples are diluted at 1:200 and 0.1 to 99.2 μg/L when serum samples are diluted 1:20. Therefore, the overall working range of the assay is 0.1 to 999.2 μg/L.
Figure 1. Representative standard curve from enzyme-linked immunosorbent assay (ELISA) for canine pancreatic lipase immunoreactivity (cPLI), calculated with a 4-parameter curve fit: y = (A − D)/[1 + (x/C)B] + D, where A = 0.007, B = 1.114, C = 2.656, and D = 3.048. The y axis displays the absorbance at a wavelength of 450 nm.
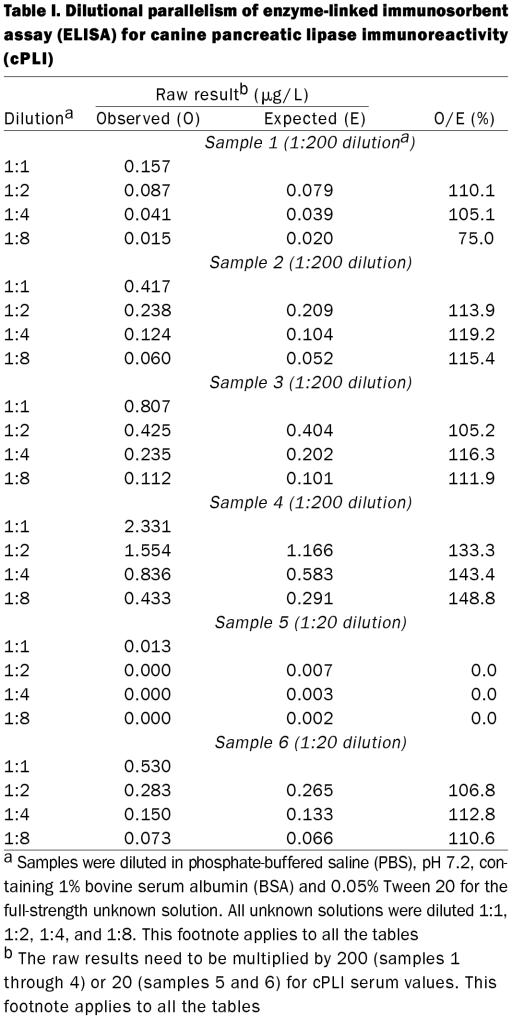
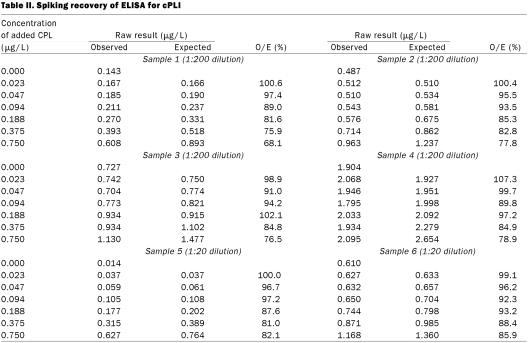
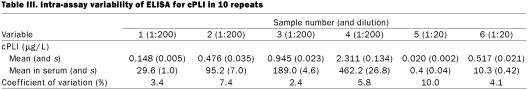
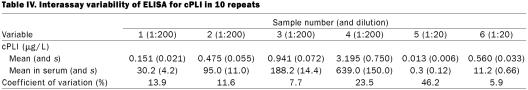
Observed to expected ratios for dilutional parallelism of 4 serum samples diluted 1:200 for the full-strength solution ranged from 75.0 to 148.8% (Table I, samples 1 through 4). Observed to expected ratios for spiking recovery of 4 serum samples diluted 1:200 ranged from 68.1 to 107.3%, with a mean of 89.7% (s 10.1%) (Table II, samples 1 through 4). Coefficients of variation for intra-assay variability for the 4 serum samples diluted 1:200 were 3.4, 7.4, 2.4, and 5.8% (Table III, samples 1 through 4). Coefficients of variation for interassay variability for the 4 serum samples diluted 1:200 were 13.9, 11.6, 7.7, and 23.5% (Table IV, samples 1 through 4).
Table I.
Table II.
Table III.
Table IV.
Observed to expected ratios for dilutional parallelism of 2 serum samples diluted 1:20 for preparation of the full-strength solution ranged from 0.0 to 112.8% (Table I, samples 5 and 6). Observed to expected ratios for spiking recovery of 2 serum samples diluted 1:20 ranged from 81.0 to 100.0% (Table II, samples 5 and 6). Coefficients of variation for intra-assay variability for 2 serum samples diluted 1:20 were 10.0 and 4.1% (Table III, samples 5 and 6). Coefficients of variation for interassay variability for 2 serum samples diluted 1:20 were 46.2 and 5.9% (Table IV, samples 5 and 6).
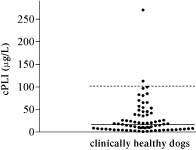
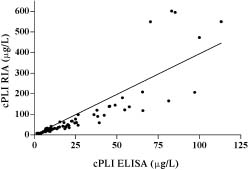
The median serum cPLI concentration in the 74 clinically healthy dogs as determined by ELISA (Figure 2) was 16.3 μg/L (mean 29, s 38.9, range 1.4−270.6 μg/L). The reference interval, calculated as the central 95th percentile, was 2.2 to 102.1 μg/L. The serum cPLI concentration in 73 of the 74 clinically healthy dogs used for establishing the reference interval for the ELISA was also assayed by RIA. Both data sets failed normality testing. The Spearman test for nonparametric data showed close correlation between the RIA and ELISA values for serum cPLI in the 73 dogs (Spearman r = 0.9708; Figure 3). The correlation is described by y = 3.942x, where y = serum cPLI as measured by RIA and x = serum cPLI as measured by ELISA.
Figure 2. Serum cPLI in 74 clinically healthy dogs, as measured by ELISA. The solid line shows the median, and the broken line shows the upper limit of the reference interval.
Figure 3. Correlation of cPLI values obtained with radioimmunoassay (RIA) and ELISA in 73 of the clinically healthy dogs; the data-point of the extreme outlier for cPLI-RIA was removed before analysis. The linear regression line is described by y = 3.942x. With the Spearman test for nonparametric data, r = 0.9708, indicating correlation between the 2 parameters.
Discussion
An ELISA for the quantification of cPLI in serum was developed, optimized, and validated. During optimization we determined that incubation of standards and samples with constant shaking led to much lower and more variable responses. It may be that this is due to the higher density of macromolecules in serum samples than in standard solutions and that with constant shaking other macromolecules push antigen molecules from a favourable position for antibody binding before a bond can be established. Therefore, shaking during incubation of samples and standards was discontinued.
The assay was initially validated with unknown samples diluted 1:10 with PBS, pH 7.2, containing 1% BSA and 0.05 Tween 20. However, the assay parameters were unacceptable, and linearity for samples in areas of the working range was reproducible only when samples were diluted 1:200 with the same buffer. After quantification of a series of random serum samples it became clear that many samples in the lower area of the working range could not be accurately assayed when diluted 1:200. Therefore, the assay was also validated with such samples diluted 1:20.
Dilutional parallelism of all 6 samples, diluted 1:200 or 1:20, was 0.0 to 148.8%. This may seem unacceptable. However, the results for sample 5 at the 1:20 dilution can be disregarded because the full-strength concentration was extremely low. Also, the expected value of sample 1 (dilution 1:200) at a dilution of 1:8 of the full-strength sample was less than 0.025 μg/L and would not have been measured in the 1:200 dilution but in the 1:20 dilution. Finally, sample 4, measured in the full-strength sample, was at 50% of the maximum. When the value for a 1:2 dilution is assumed to be the full-strength concentration for that sample, then the ratios of observed to expected values are 107.6, 111.3, and 105.2% for the following dilution steps. Hence it can be concluded that the assay is sufficiently linear for clinical use, with decreased linearity for extremely low and extremely high cPLI concentrations.
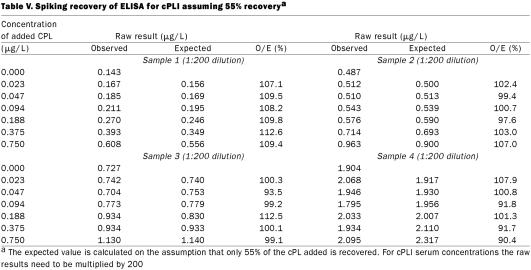
The results of spiking recovery are interesting, in that the recovery continuously decreased as a larger concentration of cPL was added to the samples for all 6 serum samples and both dilutions. Failure to recover cPL added to the serum samples would suggest that some of the added cPL was rendered undetectable. There are several possible explanations. For example, cPL could be bound by lipids in the serum, which might render the cPL molecules undetectable. Alternatively, lipase could be bound by some serum proteins. Whatever the mechanism, other immunoassays that have been established were complicated by the same lack of complete recovery (18). To determine whether this complication would preclude successful use of the assay, the most important question to ask is whether the decrease in recovery is consistent. When a recovery of 55% of the cPL added to the samples was assumed, recovery ratios ranged from 90.4 to 112.6% (mean 102.3, s 6.6%) for the 4 samples diluted 1:200 (Table V). The decrease in recovery was consistent among the samples. Thus, the assay we have described is not accurate from a purely analytic point of view, as it does not correctly reflect the number of molecules present. However, for an assay to be clinically useful, analytic accuracy is not required. Because the recovery is consistent among the samples, they remain comparable clinically, and the assay is sufficiently accurate for clinical use.
Table V.
Intra- and interassay coefficients of variation are a measure of the variability of the result for the same sample evaluated repeatedly in the same assay run and in separate assay runs, respectively. The goal for any assay is the smallest possible coefficient of variation. Although there are no generally accepted minimal performance requirements for immunoassays, it is generally accepted that intra- or interassay variability of less than 10 to 15% is acceptable. However, more importantly, the variability needs to be evaluated on the basis of the medical needs for the assay. With our ELISA, the coefficients of variation for intra-assay variability for the 6 samples were 10.0, 4.1, 3.4, 7.4, 2.4, and 5.8% (for the samples with the lowest to the highest cPLI concentrations). All of these values are 10% or less, which shows that the assay is sufficiently precise for clinical use. The coefficients of variation for interassay variability for the 6 samples were 46.2, 5.9, 13.9, 11.6, 7.7, and 23.5% (for the samples with the lowest to the highest cPLI concentrations). This shows that the assay is reproducible for most of the working range, with decreased reproducibility in the very low and the very high areas of the range. Thus, 2 samples in the middle of the working range had CVs of 10% or less and 2 had CVs greater than 10% but less than 15%. For the sample with the lowest cPLI value the adjusted serum concentration ranged between 0.0 and 0.4 μg/L (mean 0.26 μg/L). Considering the lower limit of the reference interval, 2.2 μg/L, established in clinically healthy dogs, such variation would most likely not be significant for clinical samples. This hypothesis remains to be tested in clinical studies evaluating the usefulness of this ELISA in the diagnosis of exocrine pancreatic insufficiency in the dog. Also, the sample with the highest serum cPLI concentration (mean 639 μg/L) had a high CV (23.5%). The results for this sample ranged from 467.2 to 914 μg/L. This degree of variation may appear unacceptable. However, if one considers the upper limit of the reference interval, 102.1 μg/L, it seems likely that this degree of variation will not be of clinical significance. However, this speculation must be verified by further studies assessing the clinical utility of this assay for the diagnosis of canine pancreatitis.
The reference interval of 2.2 to 102.1 μg/L, established in 74 clinically healthy dogs, differs from the one established for serum cPLI measured by RIA in 47 clinically healthy dogs: 4.4 to 276.1 μg/L (15). One possible explanation is that the reference intervals for the RIA and the ELISA were established in 2 different populations of dogs. However, our comparison of serum cPLI concentrations measured by RIA and by ELISA in the same dogs showed that the RIA values were consistently higher than the ELISA values. It may appear counterintuitive that serum cPLI concentrations are significantly different when measured by the 2 assays. However, as others (19) have noted, immunoassays are not truly analytic, and different immunologic assays for the same substance may produce different results. More importantly, in our study, the results from the 2 assays correlated closely, with a Spearman r = 0.9708, indicating that both assays do evaluate the same function. The median serum cPLI-RIA concentration was significantly higher than the median serum cPLI-ELISA concentration. One possible explanation is that there is more steric hindrance when pancreatic lipase is fixed to the bottom of the wells of the ELISA plates, leading to lower results. However, the analytic result of a cPLI assay is of little clinical interest, and the assay that gives more accurate results is not necessarily more clinically useful. Which, if either, of the 2 assays would be more useful clinically will depend on issues of reproducibility, ease of performance, cost, long-term stability, and, most importantly, ability to distinguish normal dogs from dogs with exocrine pancreatic disorders. Evaluation of the clinical utility of the ELISA described here in the diagnosis of exocrine pancreatic insufficiency and pancreatitis in dogs as well as of the effect of common interferences, such as lipemia and hemolysis, remains to be done.
In summary, an ELISA for the measurement of cPLI in dog serum was developed and analytically validated. A reference interval of 2.2 to 102.1 μg/L was established in 74 clinically healthy dogs. Serum cPLI concentrations measured by ELISA are significantly lower than serum cPLI concentrations measured by RIA, but the results are closely correlated. Further work to assess the clinical utility of the ELISA that we have described for the diagnosis of exocrine pancreatic disorders in the dog are needed and are being conducted.
Footnotes
Acknowledgments
This work was supported by a research grant from the Ralston Purina Company, St. Louis, Missouri, USA.
Address all correspondence and reprint requests to Dr. Jörg M. Steiner; telephone: (979) 862-4046; fax: (979) 458-2230; e-mail: jsteiner@cvm.tamu.edu
This material was presented as a research abstract at the 2001 ACVIM forum in Denver, Colorado, USA.
Received October 4, 2002. Accepted January 23, 2003.
References
- 1.Strombeck DR, Farver T, Kaneko JJ. Serum amylase and lipase activities in the diagnosis of pancreatitis in dogs. Am J Vet Res 1981;42:1966–1970. [PubMed]
- 2.Brobst D, Ferguson AB, Carter JM. Evaluation of serum amylase and lipase activity in experimentally induced pancreatitis in the dog. J Am Vet Med Assoc 1970;157:1697–1702. [PubMed]
- 3.Mia AS, Koger HD, Tierney MM. Serum values of amylase and pancreatic lipase in healthy mature dogs and dogs with experimental pancreatitis. Am J Vet Res 1978;39:965–969. [PubMed]
- 4.Ventrucci M, Gullo L, Daniele C, et al. Comparative study of serum pancreatic isoamylase, lipase, and trypsin-like immunoreactivity in pancreatic disease. Digestion 1983;28:114–121. [DOI] [PubMed]
- 5.Kolars JC, Ellis CJ, Levitt MD. Comparison of serum amylase pancreatic isoamylase and lipase in patients with hyperamylasemia. Dig Dis Sci 1984;29:289–293. [DOI] [PubMed]
- 6.Flamion B, Delhaye M, Horanyi Z, et al. Comparison of elastase-1 with amylase, lipase, and trypsin-like immunoreactivity in the diagnosis of acute pancreatitis. Am J Gastroenterol 1987;82:532–535. [PubMed]
- 7.Tetrault GA. Lipase activity in serum measured with Ektachem is often increased in nonpancreatic disorders. Clin Chem 1991;37:447–451. [PubMed]
- 8.Donnelly JG, Ooi DS, Burns BF, et al. Chronic increased serum lipase without evidence of pancreatitis: Tumor-derived lipase? Clin Chem 1996;42:462–464. [PubMed]
- 9.Simpson KW, Simpson JW, Lake S, et al. Effect of pancreatectomy on plasma activities of amylase, isoamylase, lipase and trypsin-like immunoreactivity in dogs. Res Vet Sci 1991;51:78–82. [DOI] [PubMed]
- 10.Eto K, Pairent FW, Appert HE, et al. Renal excretion of amylase and lipase by dogs. Arch Surg 1969;98:241–244. [DOI] [PubMed]
- 11.Polzin DJ, Osborne CA, Stevens JB, et al. Serum amylase and lipase activities in dogs with chronic primary renal failure. Am J Vet Res 1983;44:404–410. [PubMed]
- 12.Bedrak E. Blood serum enzyme activity of dogs exposed to heat stress and muscular exercise. J Appl Physiol 1965;20:587–590. [DOI] [PubMed]
- 13.Parent J. Effects of dexamethasone on pancreatic tissue and on serum amylase and lipase activities in dogs. J Am Vet Med Assoc 1982;180:743–746. [PubMed]
- 14.Petersen SB, Drabløs F. A sequence analysis of lipases, esterases, and related proteins. In: Woolley P, Petersen SB, eds. Lipases — their structure, biochemistry, and application. Cambridge, England: Cambridge University Press, 1994:23–48.
- 15.Steiner JM, Williams DA. Development and validation of a radioimmunoassay for the measurement of canine pancreatic lipase immunoreactivity (cPLI) in serum. J Vet Intern Med 2000;14:378. [DOI] [PubMed]
- 16.Bailey GS. Radioimmunoassay of peptides and proteins. Methods Mol Biol 1994;32:449–459. [DOI] [PubMed]
- 17.Steiner JM, Williams DA. Purification of classical pancreatic lipase from dog pancreas. Biochimie 2002;84:1243–1251. [DOI] [PubMed]
- 18.Steiner JM, Williams DA, Moeller EM, Melgarejo T. Development and validation of an enzyme-linked immunosorbent assay for feline trypsin-like immunoreactivity. Am J Vet Res 2000;61:620–623. [DOI] [PubMed]
- 19.Ekins R. Immunoassay standardization. Scand J Clin Lab Invest 1991;51(Suppl 205):33–46. [DOI] [PubMed]